Construction and Preclinical Evaluation of a Recombinant Attenuated Measles Vaccine Candidate of the H1a Genotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses and Animals
2.2. Construction of Full-Length Genome Plasmids of Recombinant Measles Virus
2.3. Rescue of Recombinant Measles Virus
2.4. Growth Characteristics of Recombinant Measles Virus
2.5. Scanning Electron Microscopy
2.6. Immunization of Mice
2.7. Immunization of Guinea Pigs
2.8. Challenge of IFNα/βR−/− (A129) Mice
2.9. Immunization of Rhesus Macaques
2.10. Animal Ethics Statement
2.11. Micro-Neutralization Assay
2.12. Viral Genome Sequencing and Bioinformatic Analysis
2.13. Statistical Analysis
3. Results
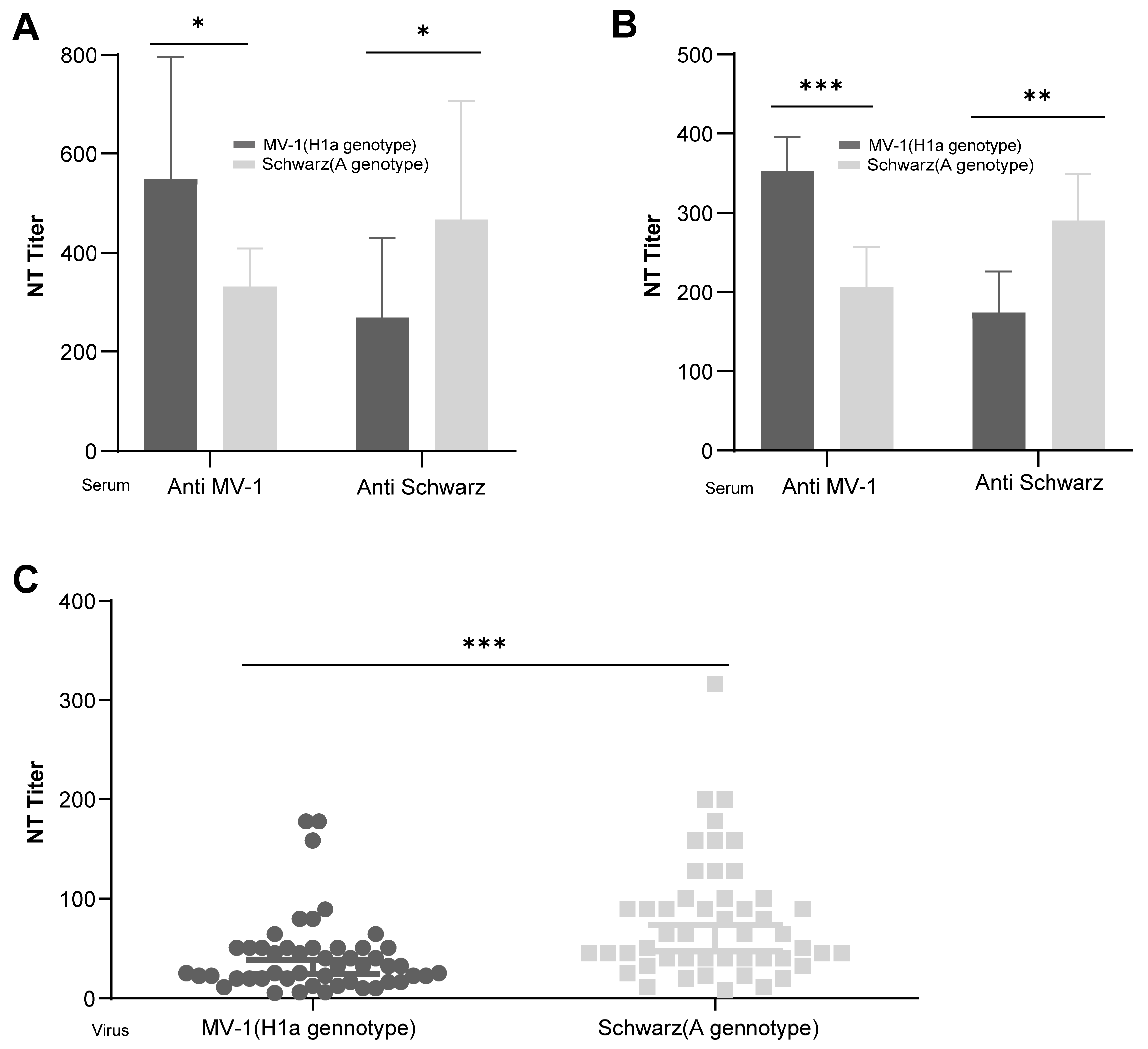
3.1. Differential Immunogenicity Between Measles Virus Genotypes A and H1a: Genotype-Specific Neutralization Potency of Post-Immunization Sera
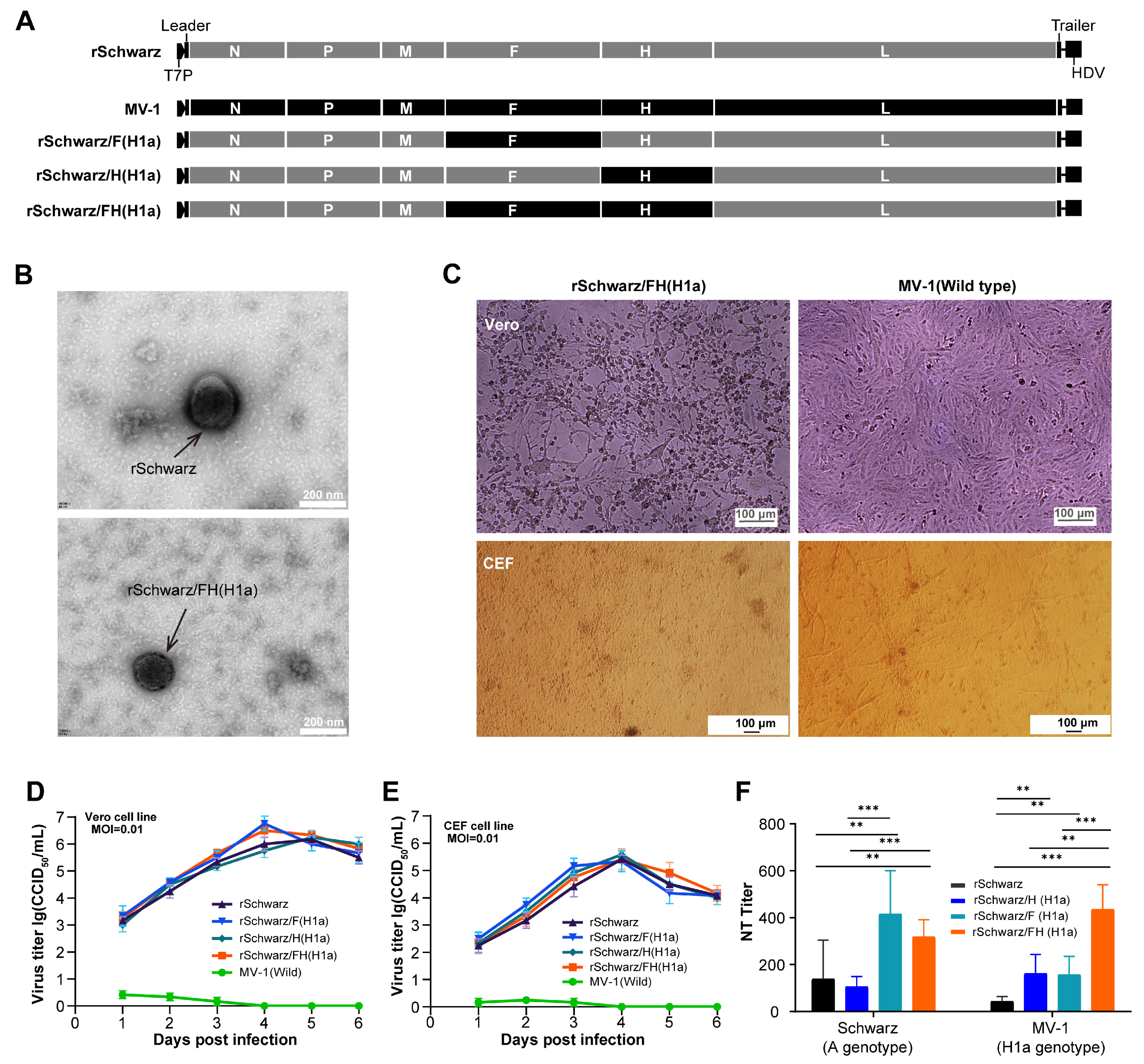
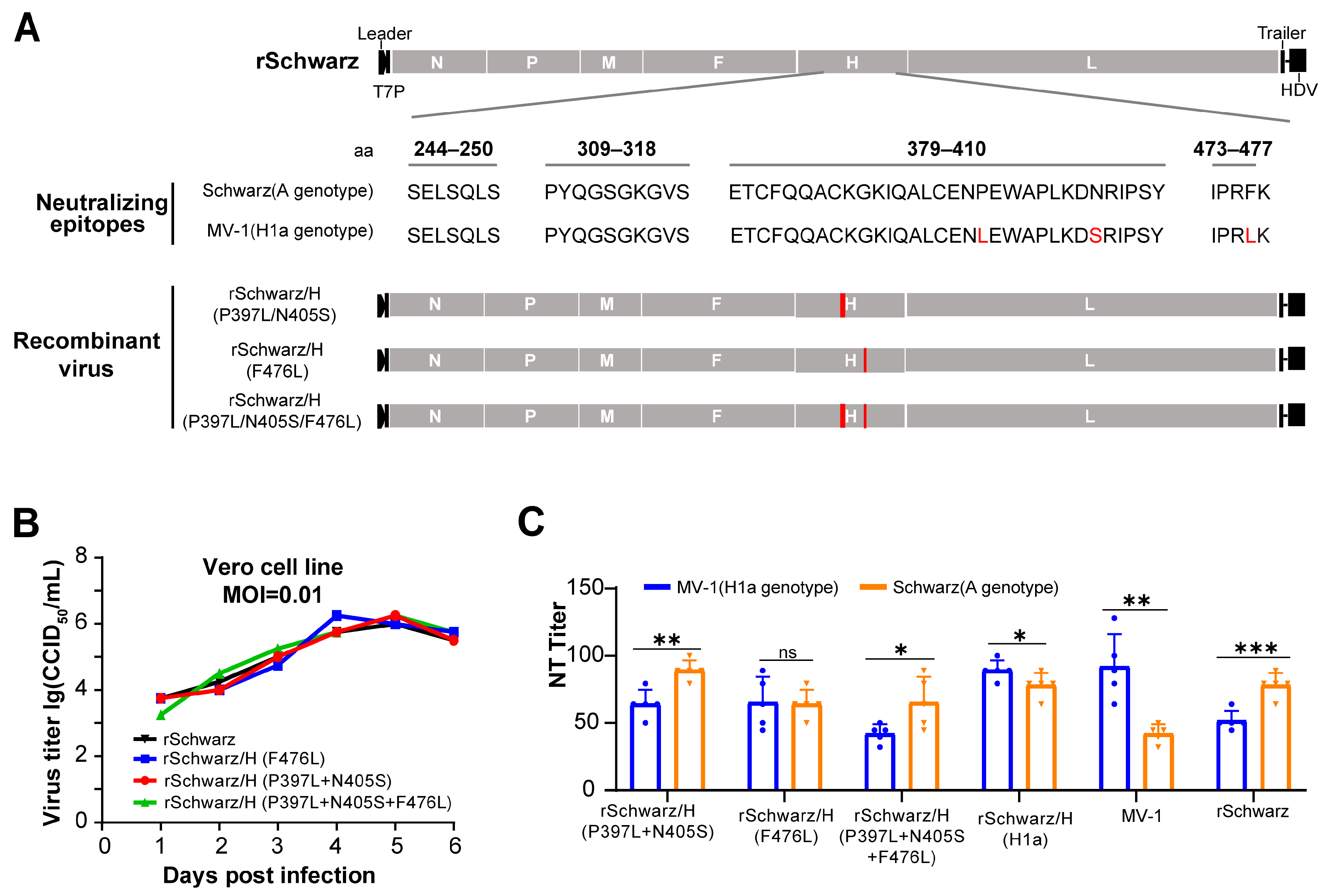
3.2. rSchwarz/FH(H1a) Exhibits Improved Immunogenicity, with the H Protein Residue 476 Playing a Key Role in Cross-Protection Between Genotypes H1a and A Measles Virus
3.3. rSchwarz/FH(H1a) Induces High Titers of Neutralizing Antibodies Against Multiple Epidemic Genotype Strains of Measles Virus Compared to rSchwarz
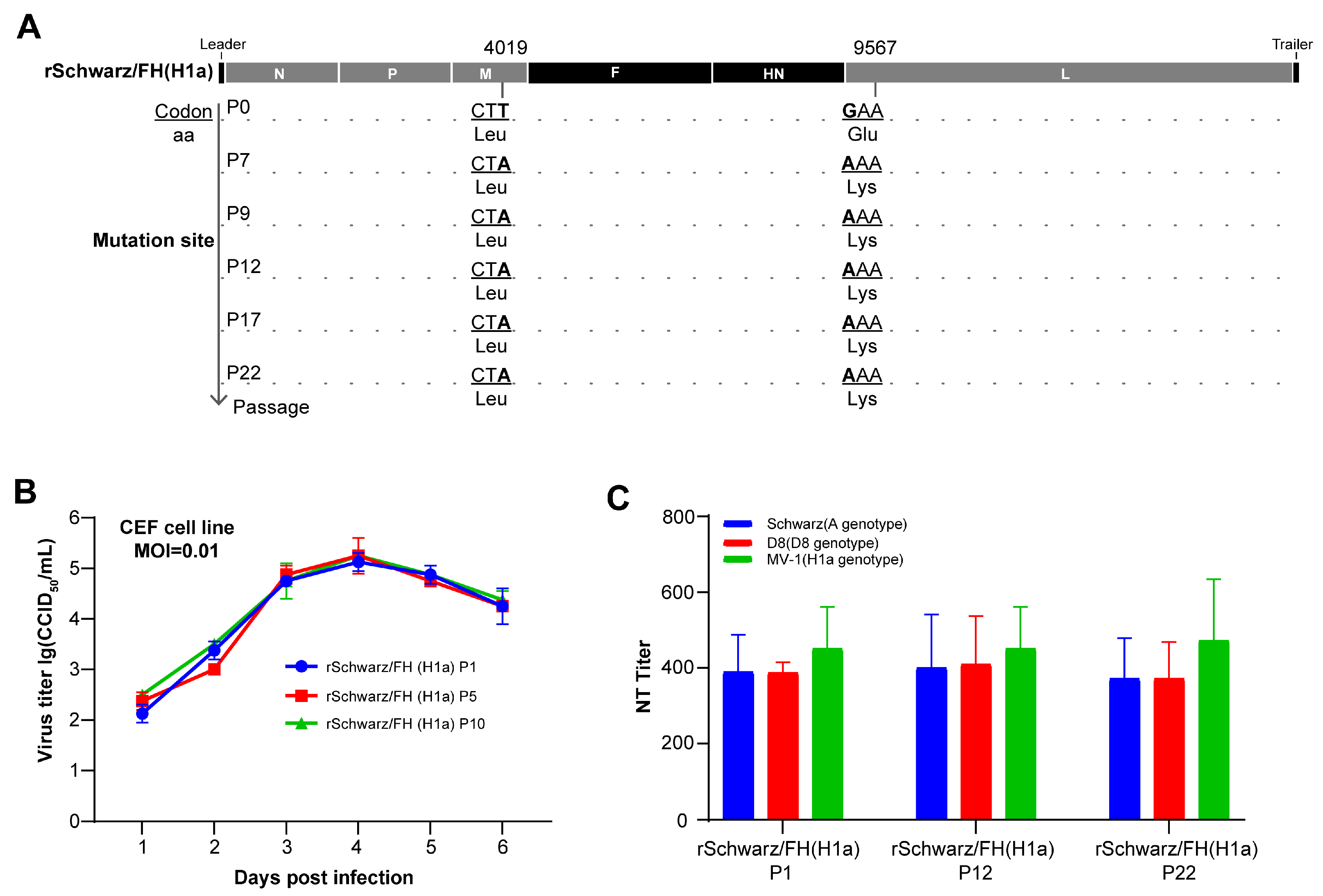
3.4. rSchwarz/FH(H1a) Exhibits Good Genetic Stability and Immune Persistence
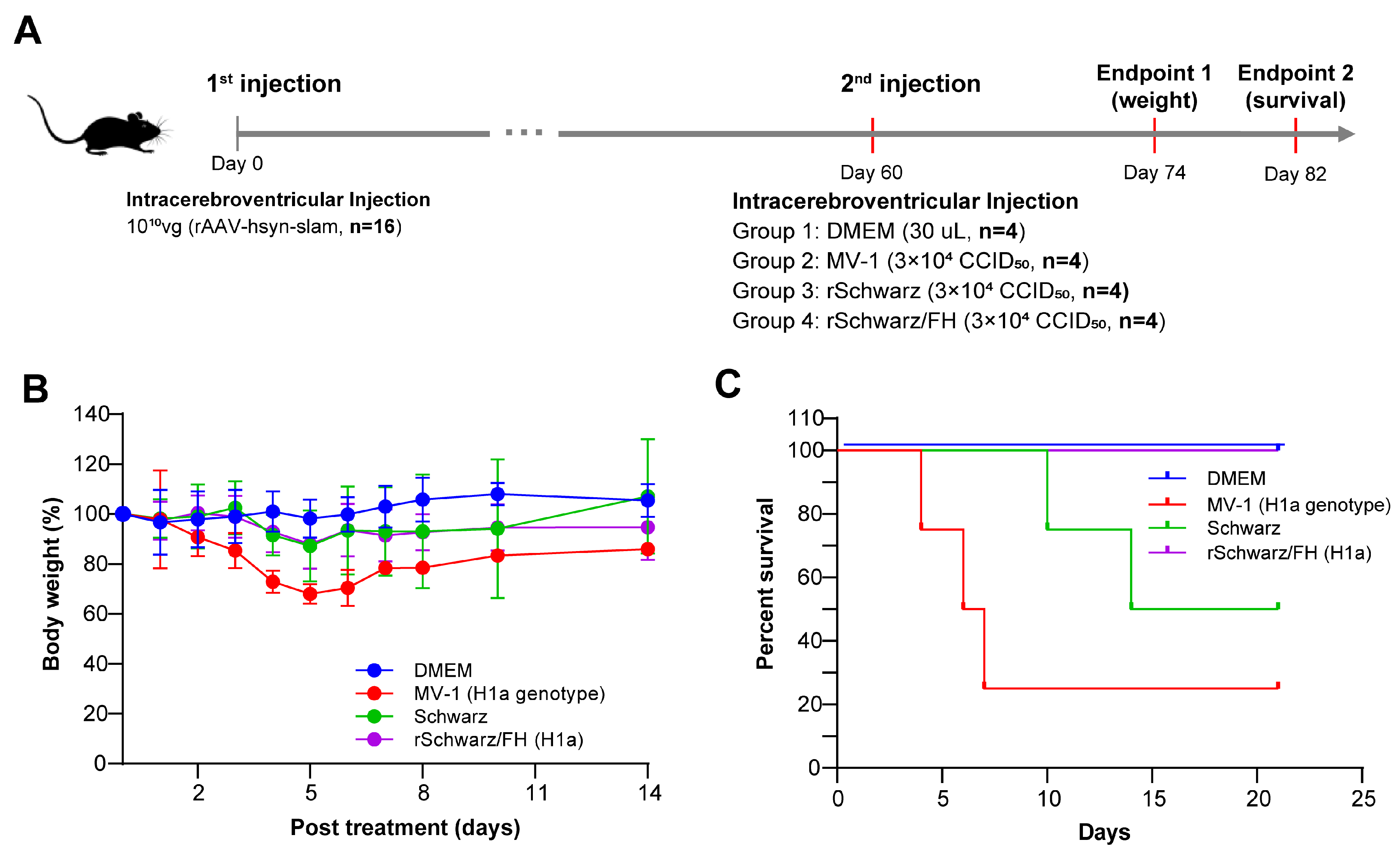
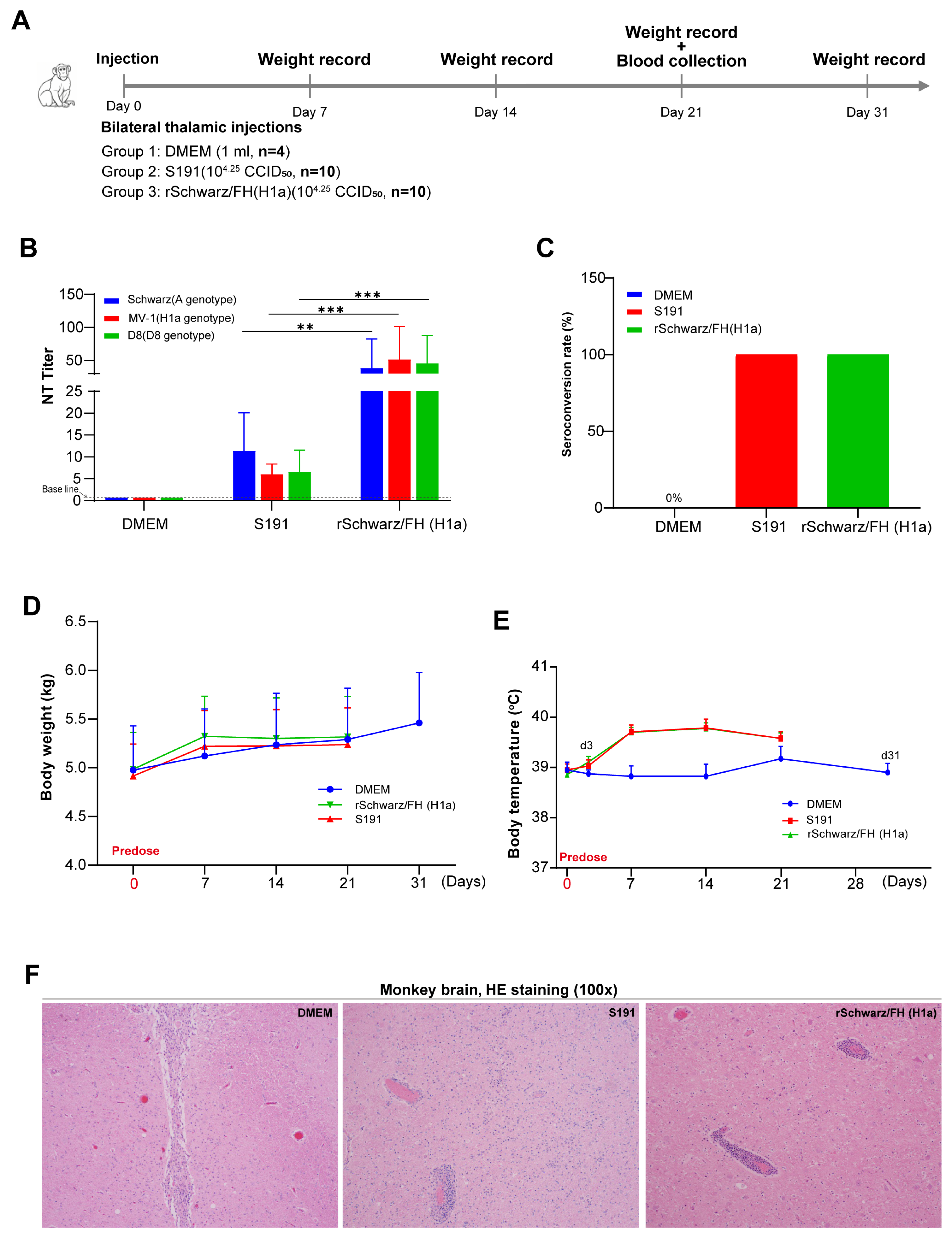
3.5. rSchwarz/FH(H1a) Exhibits Valid Safety in Mice and Rhesus Macaques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hübschen, J.M.; Gouandjika-Vasilache, I.; Dina, J. Measles. Lancet 2022, 399, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Nomenclature for describing the genetic characteristics of wild-type measles viruses (update). Wkly. Epidemiol. Rec. 2001, 76, 249–251.
- Update of the nomenclature for describing the genetic characteristics of wild-type measles viruses: New genotypes and reference strains. Wkly. Epidemiol. Rec. 2003, 78, 229–232.
- New genotype of measles virus and update on global distribution of measles genotypes. Wkly. Epidemiol. Rec. 2005, 80, 347–351.
- eClinicalMedicine. Concerning global rise in measles cases. eClinicalMedicine 2024, 68, 102502. [Google Scholar] [CrossRef]
- Hendriks, J.; Blume, S. Measles vaccination before the measles-mumps-rubella vaccine. Am. J. Public Health 2013, 103, 1393–1401. [Google Scholar] [CrossRef]
- Tidjani, O.; Grunitzky, B.; Guérin, N.; Lévy-Bruhl, D.; Lecam, N.; Xuereff, C.; Tatagan, K. Comparative serological efficacy of the measles vaccine strains Edmonston-Zagreb, Schwarz and AIK-C in Togolese infants of 4–5 months and 8–10 months. Bull. Soc. Pathol. Exot. 1991, 84, 873–884. [Google Scholar]
- Mühlebach, M.D. Vaccine platform recombinant measles virus. Virus Genes 2017, 53, 733–740. [Google Scholar] [CrossRef]
- Wang, Z.; Hangartner, L.; Cornu, T.I.; Martin, L.R.; Zuniga, A.; Billeter, M.A.; Naim, H.Y. Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine 2001, 19, 2329–2336. [Google Scholar] [CrossRef]
- Lorin, C.; Segal, L.; Mols, J.; Morelle, D.; Bourguignon, P.; Rovira, O.; Mettens, P.; Silvano, J.; Dumey, N.; Le Goff, F.; et al. Toxicology, biodistribution and shedding profile of a recombinant measles vaccine vector expressing HIV-1 antigens, in cynomolgus macaques. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 1211–1225. [Google Scholar] [CrossRef]
- Park, S.I.; Park, S.; Lee, K.; Kwak, H.W.; Kim, Y.K.; Park, H.J.; Bang, Y.J.; Kim, J.Y.; Kim, D.; Seo, K.W.; et al. Intranasal immunization with the recombinant measles virus encoding the spike protein of SARS-CoV-2 confers protective immunity against COVID-19 in hamsters. Vaccine 2024, 42, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Swett-Tapia, C.; Bogaert, L.; de Jong, P.; van Hoek, V.; Schouten, T.; Damen, I.; Spek, D.; Wanningen, P.; Radošević, K.; Widjojoatmodjo, M.N.; et al. Recombinant measles virus incorporating heterologous viral membrane proteins for use as vaccines. J. Gen. Virol. 2016, 97, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Liniger, M.; Zuniga, A.; Morin, T.N.; Combardiere, B.; Marty, R.; Wiegand, M.; Ilter, O.; Knuchel, M.; Naim, H.Y. Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine 2009, 27, 3299–3305. [Google Scholar] [CrossRef]
- Stebbings, R.; Li, B.; Lorin, C.; Koutsoukos, M.; Février, M.; Mee, E.T.; Page, M.; Almond, N.; Tangy, F.; Voss, G. Immunogenicity of a recombinant measles HIV-1 subtype C vaccine. Vaccine 2013, 31, 6079–6086. [Google Scholar] [CrossRef]
- Reading, R. A randomized, controlled trial of an aerosolized vaccine against measles. Child Care Health Dev. 2015, 41, 638–639. [Google Scholar] [CrossRef]
- Joyce, J.C.; Collins, M.L.; Rota, P.A.; Prausnitz, M.R. Thermostability of Measles and Rubella Vaccines in a Microneedle Patch. Adv. Ther. 2021, 4, 2100095. [Google Scholar] [CrossRef]
- Kaić, B.; Tešović, G. Measles outbreak: A warning sign of troubles ahead. Croat. Med. J. 2019, 60, 393–396. [Google Scholar] [CrossRef]
- Yang, W. Transmission dynamics of and insights from the 2018-2019 measles outbreak in New York City: A modeling study. Sci. Adv. 2020, 6, eaaz4037. [Google Scholar] [CrossRef]
- Minta, A.A.; Ferrari, M.; Antoni, S.; Portnoy, A.; Sbarra, A.; Lambert, B.; Hatcher, C.; Hsu, C.H.; Ho, L.L.; Steulet, C.; et al. Progress Toward Measles Elimination—Worldwide, 2000–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1262–1268. [Google Scholar] [CrossRef]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (R(0)) of measles: A systematic review. Lancet Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef]
- Hill, H.A.; Yankey, D.; Elam-Evans, L.D.; Chen, M.; Singleton, J.A. Vaccination Coverage by Age 24 Months Among Children Born in 2019 and 2020—National Immunization Survey-Child, United States, 2020–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. Worrying global decline in measles immunisation. Lancet Microbe 2022, 3, e9. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, J.; Huang, H.; Hu, Y.; Bian, J.; Xu, D.; Li, F. Measles incidence rate and a phylogenetic study of contemporary genotype H1 measles strains in China: Is an improved measles vaccine needed? Virus Genes 2011, 43, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Fatemi Nasab, G.S.; Salimi, V.; Abbasi, S.; Adjami Nezhad Fard, F.; Mokhtari Azad, T. Comparison of neutralizing antibody titers against outbreak-associated measles genotypes (D4, H1 and B3) in Iran. Pathog. Dis. 2016, 74, ftw089. [Google Scholar] [CrossRef]
- Vaidya, S.R.; Kumbhar, N.S.; Andhare, G.K.; Pawar, N.; Walimbe, A.M.; Kinikar, M.; Kasibhatla, S.M.; Kulkarni-Kale, U. Neutralizing Antibody Response to Genotypically Diverse Measles Viruses in Clinically Suspected Measles Cases. Viruses 2023, 15, 2243. [Google Scholar] [CrossRef]
- Bankamp, B.; Kim, G.; Hart, D.; Beck, A.; Ben Mamou, M.; Penedos, A.; Zhang, Y.; Evans, R.; Rota, P.A. Global Update on Measles Molecular Epidemiology. Vaccines 2024, 12, 810. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Zhu, Z.; Liu, C.; Mao, N.; Ji, Y.; Wang, H.; Jiang, X.; Li, C.; Tang, W.; et al. Genetic characterization of the hemagglutinin genes of wild-type measles virus circulating in china, 1993–2009. PLoS ONE 2013, 8, e73374. [Google Scholar] [CrossRef]
- Masters, N.B.; Wagner, A.L.; Ding, Y.; Zhang, Y.; Boulton, M.L. Assessing measles vaccine failure in Tianjin, China. Vaccine 2019, 37, 3251–3254. [Google Scholar] [CrossRef]
- Vlaicu, O.; Banica, L.; Hohan, R.; Surleac, M.; Florea, D.; Miron, V.D.; Tudor, A.; Săndulescu, O.; Drăgănescu, A.C.; Oțelea, D.; et al. Antigenic Divergence from the Seasonal Vaccine of the Influenza Virus Strains Circulating in Romania During Three Successive Seasons (2021–2024). Microorganisms 2024, 12, 2363. [Google Scholar] [CrossRef]
- van der Straten, K.; Guerra, D.; van Gils, M.J.; Bontjer, I.; Caniels, T.G.; van Willigen, H.D.G.; Wynberg, E.; Poniman, M.; Burger, J.A.; Bouhuijs, J.H.; et al. Antigenic cartography using sera from sequence-confirmed SARS-CoV-2 variants of concern infections reveals antigenic divergence of Omicron. Immunity 2022, 55, 1725–1731.e4. [Google Scholar] [CrossRef]
- Tahara, M.; Bürckert, J.P.; Kanou, K.; Maenaka, K.; Muller, C.P.; Takeda, M. Measles Virus Hemagglutinin Protein Epitopes: The Basis of Antigenic Stability. Viruses 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Ito, Y.; Brindley, M.A.; Ma, X.; He, J.; Xu, S.; Fukuhara, H.; Sakai, K.; Komase, K.; Rota, P.A.; et al. Functional and structural characterization of neutralizing epitopes of measles virus hemagglutinin protein. J. Virol. 2013, 87, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Ertl, O.T.; Wenz, D.C.; Bouche, F.B.; Berbers, G.A.; Muller, C.P. Immunodominant domains of the Measles virus hemagglutinin protein eliciting a neutralizing human B cell response. Arch. Virol. 2003, 148, 2195–2206. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Kajikawa, M.; Maita, N.; Takeda, M.; Kuroki, K.; Sasaki, K.; Kohda, D.; Yanagi, Y.; Maenaka, K. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc. Natl. Acad. Sci. USA 2007, 104, 19535–19540. [Google Scholar] [CrossRef]
- Finsterbusch, T.; Wolbert, A.; Deitemeier, I.; Meyer, K.; Mosquera, M.M.; Mankertz, A.; Santibanez, S. Measles viruses of genotype H1 evade recognition by vaccine-induced neutralizing antibodies targeting the linear haemagglutinin noose epitope. J. Gen. Virol. 2009, 90, 2739–2745. [Google Scholar] [CrossRef]
- Nossal, G.J. Inactivated measles vaccine and the risk of adverse events. Bull. World Health Organ. 2000, 78, 224–225. [Google Scholar]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Arai, T.; Terao-Muto, Y.; Uchida, S.; Lin, C.; Honda, T.; Takenaka, A.; Ikeda, F.; Sato, H.; Yoneda, M.; Kai, C. The P gene of rodent brain-adapted measles virus plays a critical role in neurovirulence. J. Gen. Virol. 2017, 98, 1620–1629. [Google Scholar] [CrossRef]
- Satoh, Y.; Higuchi, K.; Nishikawa, D.; Wakimoto, H.; Konami, M.; Sakamoto, K.; Kitagawa, Y.; Gotoh, B.; Jiang, D.P.; Hotta, H.; et al. M protein of subacute sclerosing panencephalitis virus, synergistically with the F protein, plays a crucial role in viral neuropathogenicity. J. Gen. Virol. 2021, 102, 001682. [Google Scholar] [CrossRef]
- Bankamp, B.; Hodge, G.; McChesney, M.B.; Bellini, W.J.; Rota, P.A. Genetic changes that affect the virulence of measles virus in a rhesus macaque model. Virology 2008, 373, 39–50. [Google Scholar] [CrossRef]
- Kweder, H.; Ainouze, M.; Brunel, J.; Gerlier, D.; Manet, E.; Buckland, R. Measles Virus: Identification in the M Protein Primary Sequence of a Potential Molecular Marker for Subacute Sclerosing Panencephalitis. Adv. Virol. 2015, 2015, 769837. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.B.; Thomas, D.; Lewicki, H.; Billeter, M.A.; Oldstone, M.B. V and C proteins of measles virus function as virulence factors in vivo. Virology 2000, 267, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ferren, M.; Horvat, B.; Mathieu, C. Measles Encephalitis: Towards New Therapeutics. Viruses 2019, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ayata, M.; Takeuchi, K.; Takeda, M.; Ohgimoto, S.; Kato, S.; Sharma, L.B.; Tanaka, M.; Kuwamura, M.; Ishida, H.; Ogura, H. The F gene of the Osaka-2 strain of measles virus derived from a case of subacute sclerosing panencephalitis is a major determinant of neurovirulence. J. Virol. 2010, 84, 11189–11199. [Google Scholar] [CrossRef]
- Duprex, W.P.; Duffy, I.; McQuaid, S.; Hamill, L.; Cosby, S.L.; Billeter, M.A.; Schneider-Schaulies, J.; ter Meulen, V.; Rima, B.K. The H gene of rodent brain-adapted measles virus confers neurovirulence to the Edmonston vaccine strain. J. Virol. 1999, 73, 6916–6922. [Google Scholar] [CrossRef]
- Blixenkrone-Møller, M.; Bernard, A.; Bencsik, A.; Sixt, N.; Diamond, L.E.; Logan, J.S.; Wild, T.F. Role of CD46 in measles virus infection in CD46 transgenic mice. Virology 1998, 249, 238–248. [Google Scholar] [CrossRef][Green Version]
- Sellin, C.I.; Davoust, N.; Guillaume, V.; Baas, D.; Belin, M.F.; Buckland, R.; Wild, T.F.; Horvat, B. High pathogenicity of wild-type measles virus infection in CD150 (SLAM) transgenic mice. J. Virol. 2006, 80, 6420–6429. [Google Scholar] [CrossRef]
- Mura, M.; Ruffié, C.; Billon-Denis, E.; Combredet, C.; Tournier, J.N.; Tangy, F. hCD46 receptor is not required for measles vaccine Schwarz strain replication in vivo: Type-I IFN is the species barrier in mice. Virology 2018, 524, 151–159. [Google Scholar] [CrossRef]
- Naniche, D.; Yeh, A.; Eto, D.; Manchester, M.; Friedman, R.M.; Oldstone, M.B. Evasion of host defenses by measles virus: Wild-type measles virus infection interferes with induction of Alpha/Beta interferon production. J. Virol. 2000, 74, 7478–7484. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Liu, Y.; Zhang, Y.; Niu, B.; Wang, H.; Guo, Y.; Wang, J.; Ruan, J.; Xie, G.; Wang, Z.; et al. Construction and Preclinical Evaluation of a Recombinant Attenuated Measles Vaccine Candidate of the H1a Genotype. Vaccines 2025, 13, 571. https://doi.org/10.3390/vaccines13060571
Xie L, Liu Y, Zhang Y, Niu B, Wang H, Guo Y, Wang J, Ruan J, Xie G, Wang Z, et al. Construction and Preclinical Evaluation of a Recombinant Attenuated Measles Vaccine Candidate of the H1a Genotype. Vaccines. 2025; 13(6):571. https://doi.org/10.3390/vaccines13060571
Chicago/Turabian StyleXie, Lixia, Yuanbao Liu, Yajing Zhang, Biao Niu, Hui Wang, Yue Guo, Jinliang Wang, Juncheng Ruan, Guandong Xie, Zhiguo Wang, and et al. 2025. "Construction and Preclinical Evaluation of a Recombinant Attenuated Measles Vaccine Candidate of the H1a Genotype" Vaccines 13, no. 6: 571. https://doi.org/10.3390/vaccines13060571
APA StyleXie, L., Liu, Y., Zhang, Y., Niu, B., Wang, H., Guo, Y., Wang, J., Ruan, J., Xie, G., Wang, Z., Fu, Z., An, Q., & Tian, D. (2025). Construction and Preclinical Evaluation of a Recombinant Attenuated Measles Vaccine Candidate of the H1a Genotype. Vaccines, 13(6), 571. https://doi.org/10.3390/vaccines13060571





