Single-Domain Antibodies That Specifically Recognize Intact Capsids of Multiple Foot-and-Mouth Disease Serotype O Strains
Abstract
1. Introduction
- Screened previously isolated VHHs M81F and M208F that are clonally related to proven 146S-specific VHHs M210F and M170F [26], respectively.
- Yeast-produced VHHs Nb45F and Nb205F that were reported to be 146S-specific [40].
- Generated mutant VHH M918F by CDR3 mutagenesis of M170F.
- Isolated novel VHHs M907F, M910F, M912F, M915F, and M916F from phage-display immune libraries generated previously.
2. Materials and Methods
2.1. VHHs and FMDV Antigens
2.2. Generation of CDR3-Randomized M170 Phage-Display Library
2.3. Phage-Display Selection of VHHs
2.4. Yeast Production of VHHs
2.5. ELISAs
2.5.1. ELISA Using E. coli-Produced Phage-Displayed VHHs
2.5.2. ELISA Using E. coli-Produced Soluble VHHs
2.5.3. ELISA Analysis of SDG Fractions Using Yeast-Produced VHHs
2.5.4. ELISA Analysis of Particle Specificity of Yeast-Produced VHHs
2.5.5. Heterologous DAS-ELISA
2.5.6. Mapping of Antigenic Sites by Blocking/Competition ELISA
2.6. Affinity Measurements by Biolayer Interferometry
2.7. FMDV Phylogenetic Analysis
2.8. Molecular Visualization
3. Results
3.1. Generation and Selection of Novel 146S-Specific VHHs with Broader Strain Recognition
3.2. Yeast Production of VHHs
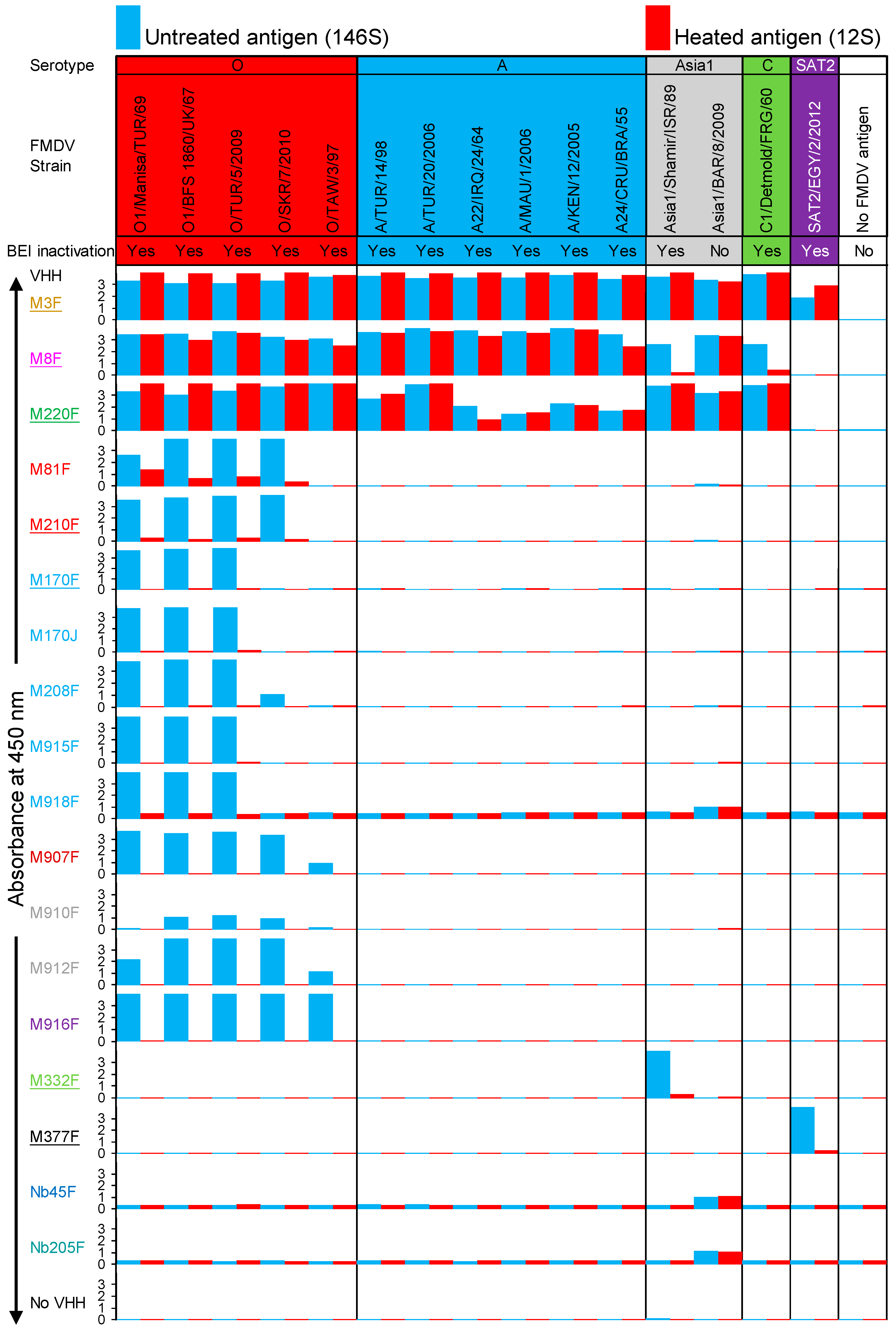
3.3. FMDV Strain and Particle Specificity of VHHs
3.4. FMDV Particle Specificity of VHHs
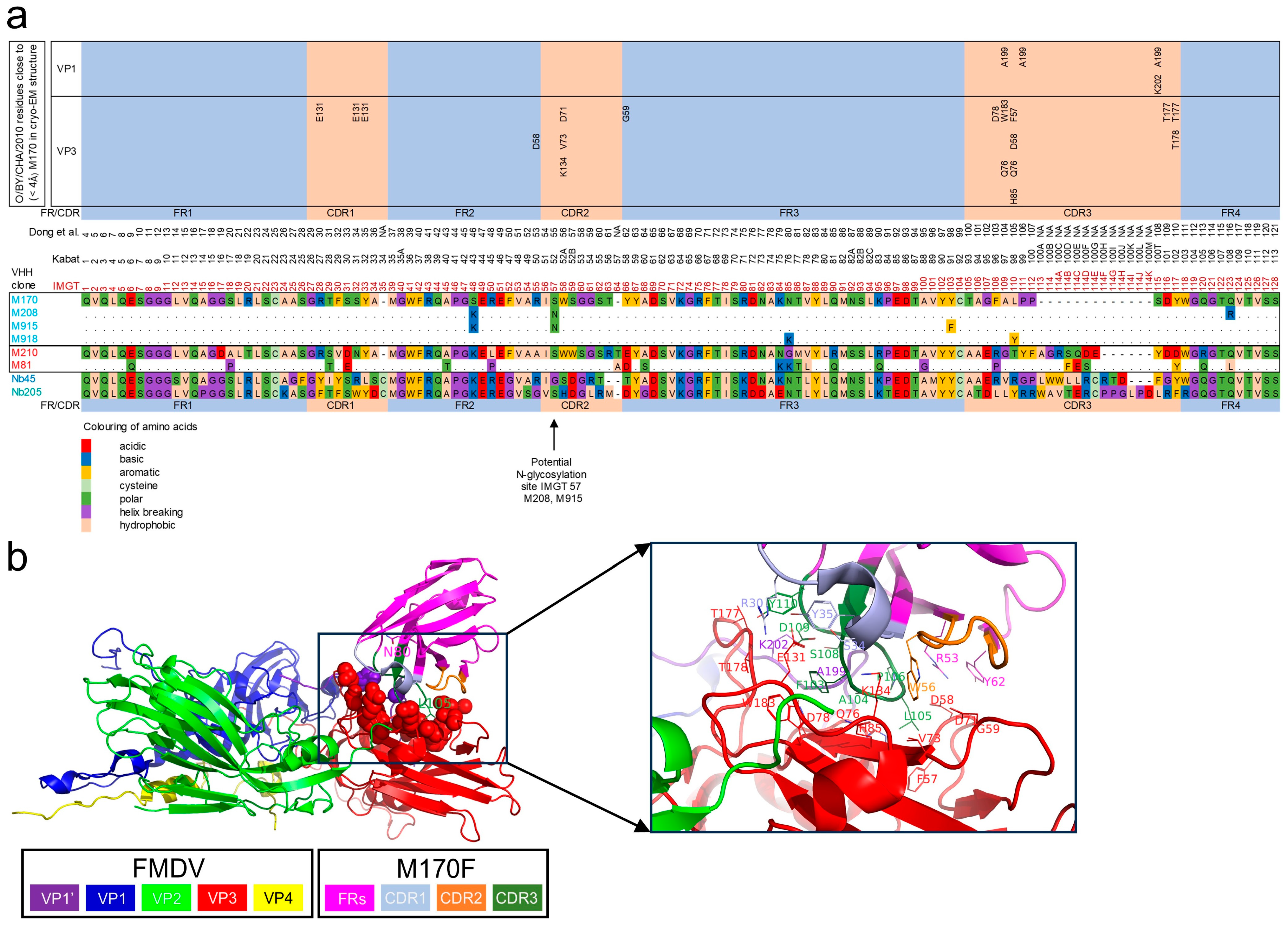
3.5. Epitope Mapping of VHHs by Competition ELISAs
3.6. VHH Binding Affinity for FMDV
3.7. Limited Validation of 146S-Specific ELISAs
4. Discussion
4.1. Isolation of 146S-Specific VHHs
4.2. Immunological Characterization of 146S-Specific VHHs
4.3. Application of Novel 146S-Specific VHHs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acharya, R.; Fry, E.; Stuart, D.; Fox, G.; Rowlands, D.; Brown, F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature 1989, 337, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Diaz-San Segundo, F.; Medina, G.N.; Stenfeldt, C.; Arzt, J.; de Los Santos, T. Foot-and-mouth disease vaccines. Vet. Microbiol. 2017, 206, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yu, S.; Wang, W.; Chen, W.; Wang, X.; Wu, K.; Li, X.; Fan, S.; Ding, H.; Yi, L.; et al. Development of Foot-and-Mouth Disease Vaccines in Recent Years. Vaccines 2022, 10, 1817. [Google Scholar] [CrossRef]
- Kotecha, A.; Seago, J.; Scott, K.; Burman, A.; Loureiro, S.; Ren, J.; Porta, C.; Ginn, H.M.; Jackson, T.; Perez-Martin, E.; et al. Structure-based energetics of protein interfaces guides foot-and-mouth disease virus vaccine design. Nat. Struct. Mol. Biol. 2015, 22, 788–794. [Google Scholar] [CrossRef]
- Mignaqui, A.C.; Ruiz, V.; Durocher, Y.; Wigdorovitz, A. Advances in novel vaccines for foot and mouth disease: Focus on recombinant empty capsids. Crit. Rev. Biotechnol. 2019, 39, 306–320. [Google Scholar] [CrossRef]
- Cartwright, B.; Chapman, W.G.; Brown, F. Serological and immunological relations between the 146S and 12S particles of foot-and-mouth disease virus. J. Gen. Virol. 1980, 50, 369–375. [Google Scholar] [CrossRef]
- Doel, T.R.; Chong, W.K. Comparative immunogenicity of 146S, 75S and 12S particles of foot-and-mouth disease virus. Arch. Virol. 1982, 73, 185–191. [Google Scholar] [CrossRef]
- Li, H.; Liu, P.; Dong, H.; Dekker, A.; Harmsen, M.M.; Guo, H.; Wang, X.; Sun, S. Foot-and-mouth disease virus antigenic landscape and reduced immunogenicity elucidated in atomic detail. Nat. Commun. 2024, 15, 8774. [Google Scholar] [CrossRef]
- Harmsen, M.M.; Fijten, H.P.D.; Westra, D.F.; Dekker, A. Stabilizing effects of excipients on dissociation of intact (146S) foot-and-mouth disease virions into 12S particles during storage as oil-emulsion vaccine. Vaccine 2015, 33, 2477–2484. [Google Scholar] [CrossRef]
- Paton, D.J.; Reeve, R.; Capozzo, A.V.; Ludi, A. Estimating the protection afforded by foot-and-mouth disease vaccines in the laboratory. Vaccine 2019, 37, 5515–5524. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Kim, H.; Park, S.Y.; Park, S.H.; Kim, J.S.; Park, J.W.; Park, J.H.; Ko, Y.J. Development of a Potent Stabilizer for Long-Term Storage of Foot-and-Mouth Disease Vaccine Antigens. Vaccines 2021, 9, 252. [Google Scholar] [CrossRef]
- Barteling, S.J.; Meloen, R.H. A simple method for the quantification of 140S particles of foot-and-mouth disease virus (FMDV). Arch. Gesamte. Virusforsch. 1974, 45, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Doel, T.R.; Mowat, G.N. An international collaborative study on foot and mouth disease virus assay methods. 2. Quantification of 146S particles. J. Biol. Stand. 1985, 13, 335–344. [Google Scholar] [CrossRef]
- Abu Elzein, E.M.E.; Crowther, J.R. The specific detection of foot-and-mouth disease virus whole particle antigen (140S) by enzyme labelled immunosorbent assay. J. Hyg. Camb. 1979, 83, 127–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crowther, J.R.; Abu-el Zein, E.M. Detection and quantification of foot and mouth disease virus by enzyme labelled immunosorbent assay techniques. J. Gen. Virol. 1979, 42, 597–602. [Google Scholar] [CrossRef]
- Ouldridge, E.J.; Barnett, P.V.; Hingley, P.J.; Rweyemamu, M.M. An indirect sandwich enzyme labelled immunosorbent assay for the detection of foot and mouth disease virus immunizing antigen in tissue culture harvests. J. Biol. Stand. 1984, 12, 339–351. [Google Scholar] [CrossRef]
- Alonso, A.; Darsie, G.C.; Teixeira, A.C.; Reis, J.L.; Mesquita, J.A. Application of monoclonal antibodies to quality control of foot-and-mouth disease vaccines. Vaccine 1994, 12, 682–686. [Google Scholar] [CrossRef]
- Crowther, J.R.; Reckziegel, P.O.; Prado, J.A. Quantification of whole virus particles (146S) of foot-and-mouth disease virus in the presence of virus subunits (12S), using monoclonal antibodies in a sandwich ELISA. Vaccine 1995, 13, 1064–1075. [Google Scholar] [CrossRef]
- Morioka, K.; Fukai, K.; Yoshida, K.; Yamazoe, R.; Onozato, H.; Ohashi, S.; Tsuda, T.; Sakamoto, K. Foot-and-mouth disease virus antigen detection enzyme-linked immunosorbent assay using multiserotype-reactive monoclonal antibodies. J. Clin. Microbiol. 2009, 47, 3663–3668. [Google Scholar] [CrossRef]
- Seki, C.; Robiolo, B.; Periolo, O.; Iglesias, M.; D’Antuono, A.; Maradei, E.; Barros, V.; La Torre, J.; Mattion, N. Rapid methodology for antigenic profiling of FMDV field strains and for the control of identity, purity and viral integrity in commercial virus vaccines using monoclonal antibodies. Vet. Microbiol. 2009, 133, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ma, J.W.; Sun, S.Q.; Guo, H.C.; Yang, Y.M.; Jin, Y.; Zhou, G.Q.; He, J.J.; Guo, J.H.; Qi, S.Y.; et al. Quantitative Detection of the Foot-And-Mouth Disease Virus Serotype O 146S Antigen for Vaccine Production Using a Double-Antibody Sandwich ELISA and Nonlinear Standard Curves. PLoS ONE 2016, 11, e0149569. [Google Scholar] [CrossRef]
- Van Maanen, C.; Terpstra, C. Quantification of intact 146S foot-and-mouth disease antigen for vaccine production by a double antibody sandwich ELISA using monoclonal antibodies. Biologicals 1990, 18, 315–319. [Google Scholar] [CrossRef]
- Yang, M.; Holland, H.; Clavijo, A. Production of monoclonal antibodies against whole virus particles of foot-and-mouth disease virus serotype O and A and their potential use in quantification of intact virus for vaccine manufacture. Vaccine 2008, 26, 3377–3382. [Google Scholar] [CrossRef]
- Gonzalez-Sapienza, G.; Rossotti, M.A.; Tabares-da Rosa, S. Single-Domain Antibodies As Versatile Affinity Reagents for Analytical and Diagnostic Applications. Front. Immunol. 2017, 8, 977. [Google Scholar] [CrossRef]
- Harmsen, M.M.; Van Solt, C.B.; Fijten, H.P.D.; Van Keulen, L.; Rosalia, R.A.; Weerdmeester, K.; Cornelissen, A.H.M.; De Bruin, M.G.M.; Eblé, P.L.; Dekker, A. Passive immunization of guinea-pigs with llama single-domain antibody fragments against foot-and-mouth disease. Vet. Microbiol. 2007, 120, 193–206. [Google Scholar] [CrossRef]
- Li, H.; Dekker, A.; Sun, S.; Burman, A.; Kortekaas, J.; Harmsen, M.M. Novel Capsid-Specific Single-Domain Antibodies with Broad Foot-and-Mouth Disease Strain Recognition Reveal Differences in Antigenicity of Virions, Empty Capsids, and Virus-Like Particles. Vaccines 2021, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.M.; Seago, J.; Perez, E.; Charleston, B.; Eble, P.L.; Dekker, A. Isolation of Single-Domain Antibody Fragments That Preferentially Detect Intact (146S) Particles of Foot-and-Mouth Disease Virus for Use in Vaccine Quality Control. Front. Immunol. 2017, 8, 960. [Google Scholar] [CrossRef]
- Clarke, J.D.; Duyvesteyn, H.M.E.; Perez-Martin, E.; Latisenko, U.; Porta, C.; Humphreys, K.V.; Hay, A.L.; Ren, J.; Fry, E.E.; van den Born, E.; et al. A broadly reactive ultralong bovine antibody that can determine the integrity of foot-and-mouth disease virus capsids. J. Gen. Virol. 2024, 105, 2032. [Google Scholar] [CrossRef] [PubMed]
- WOAH/FAO Foot-and-Mouth Disease Reference Laboratories Network. FMD Vaccine Producers. Available online: https://www.foot-and-mouth.org/fmd-vaccine-producers (accessed on 10 March 2025).
- Mitchell, L.S.; Colwell, L.J. Analysis of nanobody paratopes reveals greater diversity than classical antibodies. Protein Eng. Des. Sel. 2018, 31, 267–275. [Google Scholar] [CrossRef]
- Gordon, G.L.; Capel, H.L.; Guloglu, B.; Richardson, E.; Stafford, R.L.; Deane, C.M. A comparison of the binding sites of antibodies and single-domain antibodies. Front. Immunol. 2023, 14, 1231623. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.M.; Li, H.; Sun, S.; van der Poel, W.H.M.; Dekker, A. Mapping of foot-and-mouth disease virus antigenic sites recognized by single-domain antibodies reveals different 146S particle specific sites and particle flexibility. Front. Vet. Sci. 2023, 9, 1040802. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, P.; Bai, M.; Wang, K.; Feng, R.; Zhu, D.; Sun, Y.; Mu, S.; Li, H.; Harmsen, M.; et al. Structural and molecular basis for foot-and-mouth disease virus neutralization by two potent protective antibodies. Protein Cell 2022, 13, 446–453. [Google Scholar] [CrossRef]
- Yau, K.Y.; Dubuc, G.; Li, S.; Hirama, T.; Mackenzie, C.R.; Jermutus, L.; Hall, J.C.; Tanha, J. Affinity maturation of a V(H)H by mutational hotspot randomization. J. Immunol. Methods 2005, 297, 213–224. [Google Scholar] [CrossRef]
- Tiller, K.E.; Chowdhury, R.; Li, T.; Ludwig, S.D.; Sen, S.; Maranas, C.D.; Tessier, P.M. Facile Affinity Maturation of Antibody Variable Domains Using Natural Diversity Mutagenesis. Front. Immunol. 2017, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, J.M.; Desai, A.A.; Schardt, J.S.; Pornnoppadol, G.; Makowski, E.K.; Smith, M.D.; Kennedy, A.A.; Garcia de Mattos Barbosa, M.; Cascalho, M.; Lanigan, T.M.; et al. Directed evolution of potent neutralizing nanobodies against SARS-CoV-2 using CDR-swapping mutagenesis. Cell. Chem. Biol. 2021, 28, 1379–1388. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, C.; Levy, R.; Arndt, J.W.; Forsyth, C.M.; Razai, A.; Lou, J.; Geren, I.; Stevens, R.C.; Marks, J.D. Molecular evolution of antibody cross-reactivity for two subtypes of type A botulinum neurotoxin. Nat. Biotechnol. 2007, 25, 107–116. [Google Scholar] [CrossRef]
- Simons, J.F.; Lim, Y.W.; Carter, K.P.; Wagner, E.K.; Wayham, N.; Adler, A.S.; Johnson, D.S. Affinity maturation of antibodies by combinatorial codon mutagenesis versus error-prone PCR. mAbs 2020, 12, 1803646. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, J.; Cai, Z.; Du, L.; Hou, J.; Qiao, X.; Zheng, Q. Development of GEM-PA-nanotrap for purification of foot-and-mouth disease virus. Vaccine 2019, 37, 3205–3213. [Google Scholar] [CrossRef]
- Swindells, M.B.; Porter, C.T.; Couch, M.; Hurst, J.; Abhinandan, K.R.; Nielsen, J.H.; Macindoe, G.; Hetherington, J.; Martin, A.C. abYsis: Integrated Antibody Sequence and Structure-Management, Analysis, and Prediction. J. Mol. Biol. 2017, 429, 356–364. [Google Scholar] [CrossRef]
- Harmsen, M.M.; van Hagen-van Setten, M.; Willemsen, P.T.J. Small-Scale Secretory VHH Expression in Saccharomyces cerevisiae. Methods Mol. Biol. 2022, 2446, 159–179. [Google Scholar] [CrossRef]
- Lefranc, M.P.; Giudicelli, V.; Duroux, P.; Jabado-Michaloud, J.; Folch, G.; Aouinti, S.; Carillon, E.; Duvergey, H.; Houles, A.; Paysan-Lafosse, T.; et al. IMGT(R), the international ImMunoGeneTics information system(R) 25 years on. Nucleic Acids Res. 2015, 43, D413–D422. [Google Scholar] [CrossRef]
- Samuel, A.R.; Knowles, N.J. Foot-and-mouth disease type O viruses exhibit genetically and geographically distinct evolutionary lineages (topotypes). J. Gen. Virol. 2001, 82, 609–621. [Google Scholar] [CrossRef]
- Reeve, R.; Borley, D.W.; Maree, F.F.; Upadhyaya, S.; Lukhwareni, A.; Esterhuysen, J.J.; Harvey, W.T.; Blignaut, B.; Fry, E.E.; Parida, S.; et al. Tracking the Antigenic Evolution of Foot-and-Mouth Disease Virus. PLoS ONE 2016, 11, e0159360. [Google Scholar] [CrossRef] [PubMed]
- WOAH/FAO Foot-and-Mouth Disease Reference Laboratories Network. Foot-and-Mouth Disease Virus Serotypes, Topotypes and Lineages: FMDV Prototype Strains. Available online: https://www.foot-and-mouth.org/foot-and-mouth-disease-virus-serotypes-topotypes-and-lineages/fmdv-prototype-strains (accessed on 30 October 2024).
- Rosignoli, S.; Paiardini, A. Boosting the Full Potential of PyMOL with Structural Biology Plugins. Biomolecules 2022, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.M.; Fijten, H.P.D. Improved functional immobilization of llama single-domain antibody fragments to polystyrene surfaces using small peptides. J. Immunoass. Immunochem. 2012, 33, 234–251. [Google Scholar] [CrossRef]
- Mateu, M.G. Antibody recognition of picornaviruses and escape from neutralization: A structural view. Virus Res. 1995, 38, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef]
- Ryckaert, S.; Pardon, E.; Steyaert, J.; Callewaert, N. Isolation of antigen-binding camelid heavy chain antibody fragments (nanobodies) from an immune library displayed on the surface of Pichia pastoris. J. Biotechnol. 2010, 145, 93–98. [Google Scholar] [CrossRef]
- Kipriyanov, S.M.; Dubel, S.; Breitling, F.; Kontermann, R.E.; Little, M. Recombinant single-chain Fv fragments carrying C-terminal cysteine residues: Production of bivalent and biotinylated miniantibodies. Mol. Immunol. 1994, 31, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.; Schotte, P.; Descamps, F.; Boutton, C.; Casteels, P. Cysteine Linked Nanobody Dimers. Patent Application WO2016/097313, 2016. [Google Scholar]
- Yang, Y.; Li, H.; Li, Z.; Zhang, Y.; Zhang, S.; Chen, Y.; Yu, M.; Ma, G.; Su, Z. Size-exclusion HPLC provides a simple, rapid, and versatile alternative method for quality control of vaccines by characterizing the assembly of antigens. Vaccine 2015, 33, 1143–1150. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Li, Z.; Sun, L.; Ma, G.; Zhang, S.; Su, Z. Stabilization study of inactivated foot and mouth disease virus vaccine by size-exclusion HPLC and differential scanning calorimetry. Vaccine 2017, 35, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Spitteler, M.A.; Romo, A.; Magi, N.; Seo, M.G.; Yun, S.J.; Barroumeres, F.; Regulier, E.G.; Bellinzoni, R. Validation of a high performance liquid chromatography method for quantitation of foot-and-mouth disease virus antigen in vaccines and vaccine manufacturing. Vaccine 2019, 37, 5288–5296. [Google Scholar] [CrossRef]
- Kim, A.Y.; Park, S.Y.; Park, S.H.; Kim, J.Y.; Jin, J.S.; Kim, E.S.; Park, J.H.; Ko, Y.J. Comparison of High-Performance Liquid Chromatography with Sucrose Density Gradient Ultracentrifugation for the Quantification of Foot-and-Mouth Disease Vaccine Antigens. Vaccines 2022, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, Y.; Lin, X.; Zhao, Q.; Su, Z.; Ma, G.; Zhang, S. Size exclusion chromatography using large pore size media induces adverse conformational changes of inactivated foot-and-mouth disease virus particles. J. Chromatogr. A 2022, 1677, 463301. [Google Scholar] [CrossRef]
- Berryman, S.; Asfor, A.; Benham, E.; Howe, N.; Burman, A.; Brocchi, E.; Grazioli, S.; Tuthill, T.J. Foot-and-mouth disease vaccine quality: A universal test for intact viral capsids based on detection of VP4. Vaccine 2025, 51, 126845. [Google Scholar] [CrossRef]

| VHH a | Animal b | CDR3 Sequence | VHH Subfamily c | N-Glycosylation Site d | Reference e |
|---|---|---|---|---|---|
| Nb45F | Camel | AAERVRGPLWWLLRCRTDFGY | 4 | None | [40] |
| Nb205F | Camel | ATDLLYRRWAVTERCPPGLPDLRF | X | None | [40] |
| M3F | 6058 | AGDRSLTVVASSWRY | 1 | None | [26] |
| M3ggsVI-4Q6E | 6058 | AGDRSLTVVASSWRY | 1 | None | [28] |
| M8F | 6058 | NGLRASNAGWEPRFGT | 2 | None | [26] |
| M23F | 6058 | NLWSQWQSETY | 2 | None | [26] |
| M31F | 6058 | AAGLSIYSNTYYYTRGEEYTY | 1 | None | [26] |
| M81F | 6666 | AAEPGTYFAGRFESEYDY | 1 | None | [26] |
| M210F | 6666 | AAERGTYFAGRSQDEYDD | 1 | None | [26] |
| M220F | 7211 | AAGYRIDTQPMDRDFYYY | 1 | None | [26] |
| M332F | 3049 | AAAWSFRSDYGARLKSAYDF | 1 | None | [28] |
| M377F | 3050 | NALVLSSSWSEGDY | 2 | None | [28] |
| M170F | 7212 | TAGFALPPSDY | 1 | None | [26] |
| M170J | 7212 | TAGFALPPSDY | 1 | None | This study |
| M208F | 7212 | TAGFALPPSDY | 1 | 57 | [26] |
| M915F | 7212 | TAGFALPPSDY | 1 | 57 | This study |
| M918F | 7212 | TAGFAYPPSDY | 1 | None | This study |
| M907F | 7211 | AADNYHRSRYSGNYDYTDSWFGS | 1 | None | This study |
| M910F | 7212 | ARGAEAAGLGSHREYDYSY | 1 | None | This study |
| M912F | 7212 | ARGAEAAGWGSHHQYDYAY | 1 | None | This study |
| M916F | 7212 | AAEKSLILGTAVSGYDY | 1 | 57 | This study |
| O1/Manisa/TUR/69 | O/SKR/7/2010 | O/TAW/3/97 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EC0.5 (ng/mL) | EC | EC0.5 (ng/mL) | EC | EC0.5 (ng/mL) | EC | ||||
| VHH a | 146S | 12S | Ratio b | 146S | 12S | Ratio | 146S | 12S | Ratio |
| M3F | 1008 | 215 | 0.21 | 687 | 115 | 0.17 | 348 | 175 | 0.50 |
| M8F | 11 | 28 | 2.5 | 18 | 42 | 2.3 | 14 | 29 | 2.0 |
| M81F | 74 | 159 | 2.2 | 31 | >2000 c | >66 | >2000 | >2000 | ND d |
| M210F | 49 | >2000 | >41 | 37 | >2000 | >53 | >2000 | >2000 | ND |
| M170J | 43 | >2000 | >47 | >2000 | >2000 | ND | >2000 | >2000 | ND |
| M170F | 29 | >2000 | >70 | >2000 | >2000 | ND | >2000 | >2000 | ND |
| M208F | 15 | >2000 | >133 | 70 | >2000 | >29 | >2000 | >2000 | ND |
| M915F | 21 | >2000 | >94 | >2000 | >2000 | ND | >2000 | >2000 | ND |
| M918F e | 53 | >2000 | >38 | >2000 | >2000 | ND | >2000 | >2000 | ND |
| M907F | 32 | >2000 | >63 | 70 | >2000 | >29 | 578 | >2000 | >3.5 |
| M910F | 1611 | >2000 | >1.2 | 177 | >2000 | >11 | 1452 | >2000 | >1.4 |
| M912F | 223 | >2000 | >9 | 29 | >2000 | >69 | 300 | >2000 | >6.7 |
| M916F | 17 | >2000 | >117 | 14 | >2000 | >142 | 10 | >2000 | >208 |
| VHH a | FMDV Strain | KD (nM) | ka × 105 (1/Ms) | kd × 10−4 (1/s) | R2 |
|---|---|---|---|---|---|
| M81F | O1/Manisa/TUR/69 | 6.7 | 9.3 | 62 | 0.981 |
| M81F | O/SKR/7/2010 | 104 | 6.4 | 669 | 0.874 |
| M210F | O1/Manisa/TUR/69 | 1.7 | 24 | 40 | 0.983 |
| M210F | O/SKR/7/2010 | 4.4 | 25 | 113 | 0.980 |
| M170J without DTT b | O1/Manisa/TUR/69 | 11 | 4.0 | 44 | 0.964 |
| M170J | O1/Manisa/TUR/69 | 17 | 3.4 | 57 | 0.975 |
| M170F without DTT | O1/Manisa/TUR/69 | 8.5 | 3.4 | 29 | 0.991 |
| M170F | O1/Manisa/TUR/69 | 12 | 3.0 | 36 | 0.987 |
| M208F | O1/Manisa/TUR/69 | 13 | 5.5 | 70 | 0.976 |
| M208F | O/SKR/7/2010 | ND c | |||
| M915F | O1/Manisa/TUR/69 | 29 | 1.7 | 49 | 0.988 |
| M918F | O1/Manisa/TUR/69 | 18 | 1.3 | 24 | 0.984 |
| M907F | O1/Manisa/TUR/69 | 7.9 | 1.6 | 13 | 0.997 |
| M907F | O/SKR/7/2010 | 59 | 1.7 | 98 | 0.978 |
| M912F | O1/Manisa/TUR/69 | ND | |||
| M916F | O1/Manisa/TUR/69 | 264 | 1.9 | 512 | 0.995 |
| M916F | O/SKR/7/2010 | ND | |||
| M916F without DTT | O1/Manisa/TUR/69 | 3.1 | 5.8 | 18 | 0.842 |
| FMDV Strain | LOD a (µg/mL) | |||||
|---|---|---|---|---|---|---|
| M907F | M916F | |||||
| 146S | 146S + 12S b | 12S | 146S | 146S + 12S b | 12S | |
| O1/Manisa/TUR/69 | 0.015 | 0.018 | >2.0 c | 0.0059 | 0.0025 | >2.0 |
| O/SKR/7/2010 | 0.009 | 0.007 | >2.0 | 0.0010 | 0.0010 | >2.0 |
| O/TAW/3/97 | 0.022 | 0.017 | >2.0 | 0.0007 | 0.0017 | >2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmsen, M.M.; Gupta, N.; Dijkstra, Q.; Water, S.v.d.; Setten, M.v.; Dekker, A. Single-Domain Antibodies That Specifically Recognize Intact Capsids of Multiple Foot-and-Mouth Disease Serotype O Strains. Vaccines 2025, 13, 500. https://doi.org/10.3390/vaccines13050500
Harmsen MM, Gupta N, Dijkstra Q, Water Svd, Setten Mv, Dekker A. Single-Domain Antibodies That Specifically Recognize Intact Capsids of Multiple Foot-and-Mouth Disease Serotype O Strains. Vaccines. 2025; 13(5):500. https://doi.org/10.3390/vaccines13050500
Chicago/Turabian StyleHarmsen, Michiel M., Nishi Gupta, Quillan Dijkstra, Sandra van de Water, Marga van Setten, and Aldo Dekker. 2025. "Single-Domain Antibodies That Specifically Recognize Intact Capsids of Multiple Foot-and-Mouth Disease Serotype O Strains" Vaccines 13, no. 5: 500. https://doi.org/10.3390/vaccines13050500
APA StyleHarmsen, M. M., Gupta, N., Dijkstra, Q., Water, S. v. d., Setten, M. v., & Dekker, A. (2025). Single-Domain Antibodies That Specifically Recognize Intact Capsids of Multiple Foot-and-Mouth Disease Serotype O Strains. Vaccines, 13(5), 500. https://doi.org/10.3390/vaccines13050500







