The New Era of Pneumococcal Vaccination in Adults: What Is Next?
Abstract
1. Introduction
1.1. Virulence
1.2. Antibiotic Resistance
2. Epidemiology and Burden
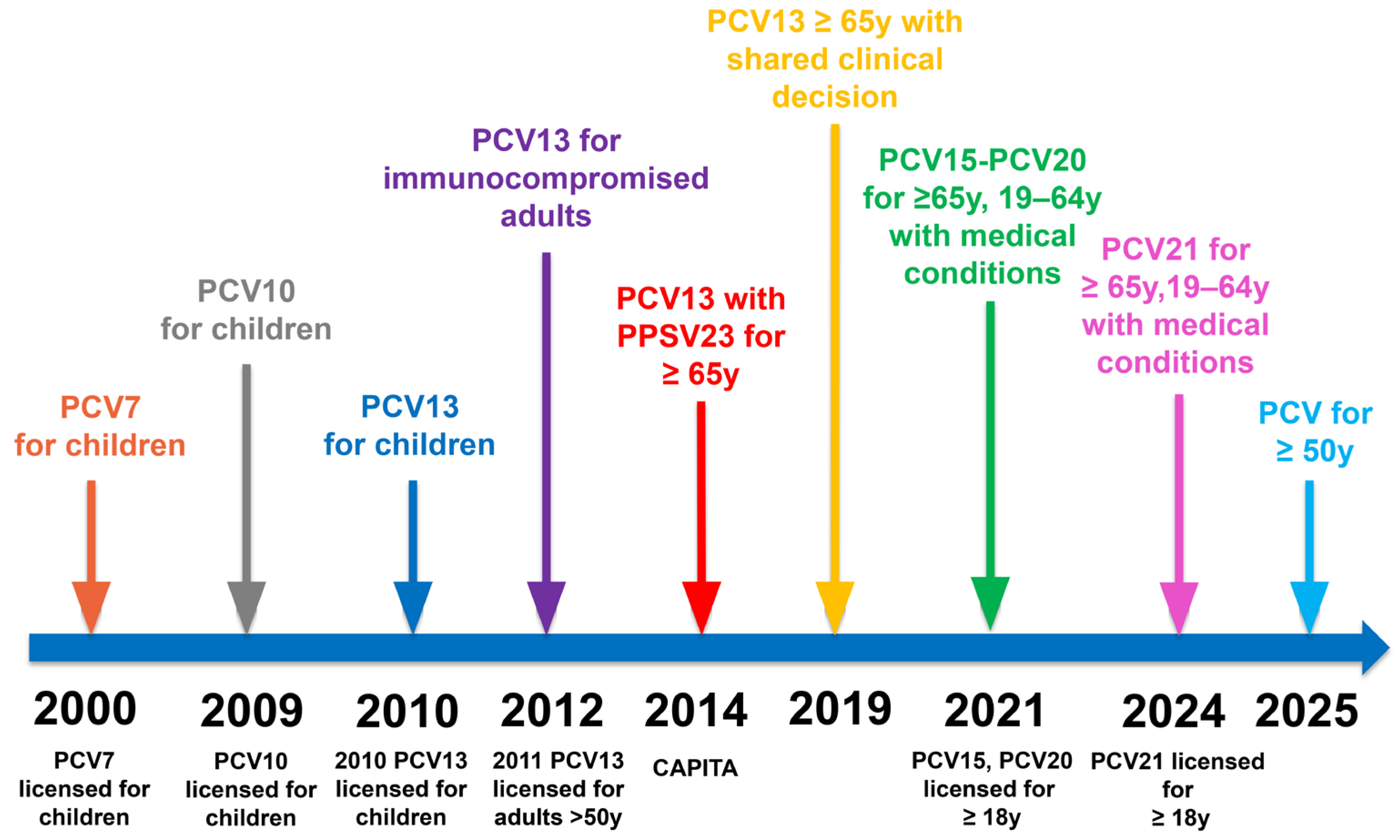
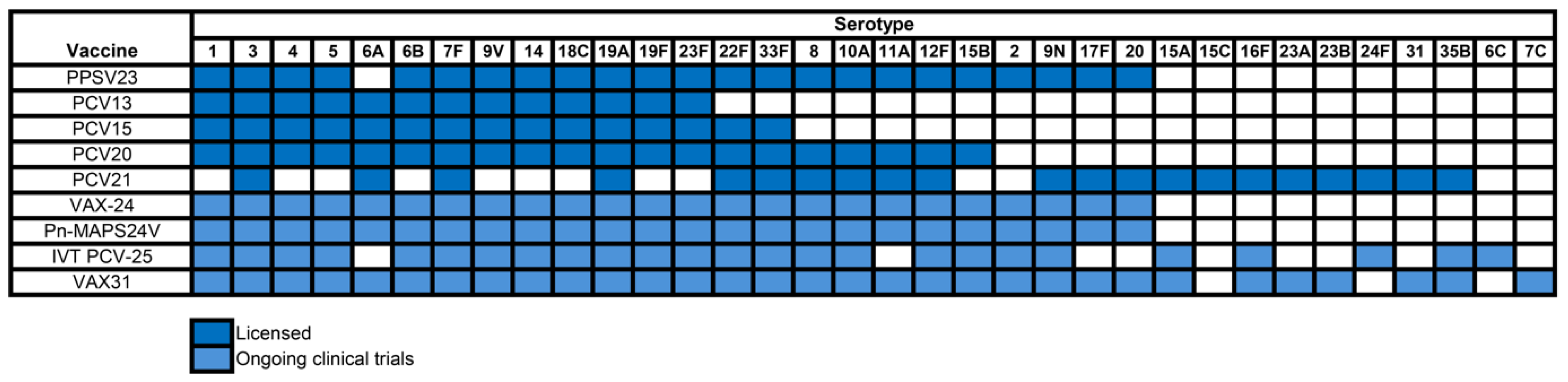
3. Evolution of Pneumococcal Vaccines
4. Recent Developments in Pneumococcal Vaccine Technology
4.1. Examples of Conjugate Vaccines with Novel Conjugation Strategies
4.2. Emerging Pneumococcal Vaccine Technologies Beyond Conjugate Vaccines
4.2.1. Protein-Based Vaccines
4.2.2. Whole-Cell Vaccines
4.2.3. mRNA and Nanoparticle Platforms
5. Effectiveness of Licensed Pneumococcal Vaccines
6. Cost-Effectiveness of Pneumococcal Vaccination
7. Pneumococcal Vaccination Coverage Rates in Adults
8. Pneumococcal Vaccine Recommendations
8.1. United States
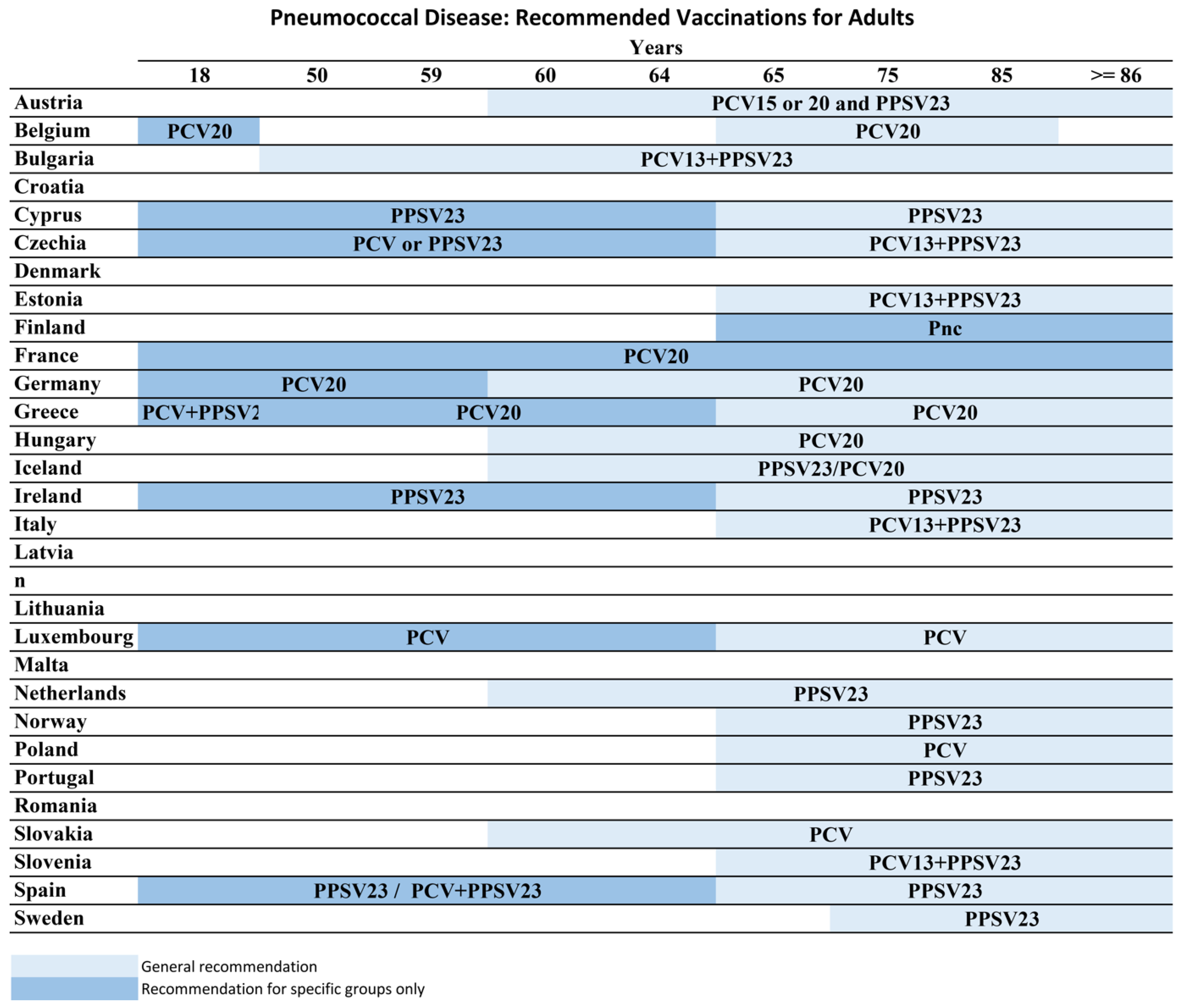
8.2. Europe
8.3. WHO Regions
8.4. Türkiye
9. Surveillance Gaps, Equity, and Ethical Considerations
9.1. Surveillance Gaps
9.2. Equity and Ethics
10. Conclusions
Funding
Conflicts of Interest
References
- Simell, B.; Auranen, K.; Kayhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; Pneumococcal Carriage, G. The fundamental link between pneumococcal carriage and disease. Expert. Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, P.; Esposito, S.; Schito, G.C.; Marchese, A.; Cavagna, R.; Principi, N.; Hercules Project Collaborative Group. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children: Implications for the use of heptavalent pneumococcal conjugate vaccine. Emerg. Infect. Dis. 2002, 8, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Suaya, J.A.; Mendes, R.E.; Sings, H.L.; Arguedas, A.; Reinert, R.R.; Jodar, L.; Isturiz, R.E.; Gessner, B.D. Streptococcus pneumoniae serotype distribution and antimicrobial nonsusceptibility trends among adults with pneumonia in the United States, 2009–2017. J. Infect. 2020, 81, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Luck, J.N.; Tettelin, H.; Orihuela, C.J. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front. Cell Infect. Microbiol. 2020, 10, 613287. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Mitchell, T.J. Streptococcus pneumoniae: Virulence factors and variation. Clin. Microbiol. Infect. 2010, 16, 411–418. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- Aceil, J.; Avci, F.Y. Pneumococcal Surface Proteins as Virulence Factors, Immunogens, and Conserved Vaccine Targets. Front. Cell Infect. Microbiol. 2022, 12, 832254. [Google Scholar] [CrossRef]
- WHO. Ranking of Other Drug-Resistant Bacterial Infections. In Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Wang, J.L.; Lai, C.C.; Ko, W.C.; Hsueh, P.R. Global trends in non-susceptibility rates of Streptococcus pneumoniae isolates to ceftriaxone: Data from the antimicrobial testing leadership and surveillance (ATLAS) programme, 2016–2021. Int. J. Antimicrob. Agents 2024, 63, 107072. [Google Scholar] [CrossRef]
- Johnson, C.N.; Wilde, S.; Tuomanen, E.; Rosch, J.W. Convergent impact of vaccination and antibiotic pressures on pneumococcal populations. Cell Chem. Biol. 2024, 31, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.W.; Gladstone, R.A.; van Tonder, A.J.; Lees, J.A.; du Plessis, M.; Benisty, R.; Givon-Lavi, N.; Hawkins, P.A.; Cornick, J.E.; Kwambana-Adams, B.; et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: An international whole-genome sequencing study. Lancet Infect. Dis. 2019, 19, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.E.; Shutt, K.A.; Moore, M.R.; Beall, B.W.; Bennett, N.M.; Craig, A.S.; Farley, M.M.; Jorgensen, J.H.; Lexau, C.A.; Petit, S.; et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 2009, 360, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Fujisawa, T.; Ito, Y.; Chang, B.; Matsumura, Y.; Yamamoto, M.; Nagao, M.; Suga, S.; Ohnishi, M.; Ichiyama, S. Spread of Meropenem-Resistant Streptococcus pneumoniae Serotype 15A-ST63 Clone in Japan, 2012–2014. Emerg. Infect. Dis. 2018, 24, 275–283. [Google Scholar] [CrossRef]
- Kim, G.R.; Kim, E.Y.; Kim, S.H.; Lee, H.K.; Lee, J.; Shin, J.H.; Kim, Y.R.; Song, S.A.; Jeong, J.; Uh, Y.; et al. Serotype Distribution and Antimicrobial Resistance of Streptococcus pneumoniae Causing Invasive Pneumococcal Disease in Korea Between 2017 and 2019 After Introduction of the 13-Valent Pneumococcal Conjugate Vaccine. Ann. Lab. Med. 2023, 43, 45–54. [Google Scholar] [CrossRef]
- Casanova, C.; Kuffer, M.; Leib, S.L.; Hilty, M. Re-emergence of invasive pneumococcal disease (IPD) and increase of serotype 23B after easing of COVID-19 measures, Switzerland, 2021. Emerg. Microbes Infect. 2021, 10, 2202–2204. [Google Scholar] [CrossRef]
- Olarte, L.; Kaplan, S.L.; Barson, W.J.; Romero, J.R.; Lin, P.L.; Tan, T.Q.; Hoffman, J.A.; Bradley, J.S.; Givner, L.B.; Mason, E.O.; et al. Emergence of Multidrug-Resistant Pneumococcal Serotype 35B among Children in the United States. J. Clin. Microbiol. 2017, 55, 724–734. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.L.R.I. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- World Health Statistics 2024: Monitoring Health for the SDGs, Sustainable Development Goals; Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://iris.who.int/bitstream/handle/10665/376869/9789240094703-eng.pdf (accessed on 17 April 2025).
- Kang, L.; Jing, W.; Liu, J.; Liu, M. Trends of global and regional aetiologies, risk factors and mortality of lower respiratory infections from 1990 to 2019: An analysis for the Global Burden of Disease Study 2019. Respirology 2023, 28, 166–175. [Google Scholar] [CrossRef]
- Safiri, S.; Mahmoodpoor, A.; Kolahi, A.A.; Nejadghaderi, S.A.; Sullman, M.J.M.; Mansournia, M.A.; Ansarin, K.; Collins, G.S.; Kaufman, J.S.; Abdollahi, M. Global burden of lower respiratory infections during the last three decades. Front. Public. Health 2022, 10, 1028525. [Google Scholar] [CrossRef]
- GBD 2021 Lower Respiratory Infections and Antimicrobial Resistance Collaborators. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Lee, J.W.; Falsey, A.R.; Demont, C.; Nyawanda, B.O.; Cai, B.; Fuentes, R.; Stoszek, S.K.; et al. Global and Regional Burden of Hospital Admissions for Pneumonia in Older Adults: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222, S570–S576. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2019. 2019. Available online: www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf (accessed on 17 February 2025).
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2022. 2022. Available online: https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf (accessed on 17 February 2025).
- European Centre for Disease Prevention and Control. Invasive Pneumococcal Disease—Annual Epidemiological Report for 2022; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2022. [Google Scholar]
- Bar-Zeev, N.; Mtunthama, N.; Gordon, S.B.; Mwafulirwa, G.; French, N. Minimum incidence of adult invasive pneumococcal disease in Blantyre, Malawi an urban African setting: A hospital based prospective cohort study. PLoS ONE 2015, 10, e0128738. [Google Scholar] [CrossRef] [PubMed]
- Bewick, T.; Sheppard, C.; Greenwood, S.; Slack, M.; Trotter, C.; George, R.; Lim, W.S. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 2012, 67, 540–545. [Google Scholar] [CrossRef]
- Kobayashi, M.; Leidner, A.J.; Gierke, R.; Xing, W.; Accorsi, E.; Moro, P.; Kamboj, M.; Kuchel, G.A.; Schechter, R.; Loehr, J.; et al. Expanded Recommendations for Use of Pneumococcal Conjugate Vaccines Among Adults Aged ≥50 Years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 1–8. [Google Scholar] [CrossRef]
- Robinson, K.A.; Baughman, W.; Rothrock, G.; Barrett, N.L.; Pass, M.; Lexau, C.; Damaske, B.; Stefonek, K.; Barnes, B.; Patterson, J.; et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. JAMA 2001, 285, 1729–1735. [Google Scholar] [CrossRef]
- Sankilampi, U.; Honkanen, P.O.; Bloigu, A.; Leinonen, M. Persistence of antibodies to pneumococcal capsular polysaccharide vaccine in the elderly. J. Infect. Dis. 1997, 176, 1100–1104. [Google Scholar] [CrossRef]
- Barrett, D.J. Human immune responses to polysaccharide antigens: An analysis of bacterial polysaccharide vaccines in infants. Adv. Pediatr. 1985, 32, 139–158. [Google Scholar] [CrossRef]
- Black, S.; Shinefield, H.; Fireman, B.; Lewis, E.; Ray, P.; Hansen, J.R.; Elvin, L.; Ensor, K.M.; Hackell, J.; Siber, G.; et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 2000, 19, 187–195. [Google Scholar] [CrossRef]
- Wessels, M.R.; Paoletti, L.C.; Rodewald, A.K.; Michon, F.; DiFabio, J.; Jennings, H.J.; Kasper, D.L. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect. Immun. 1993, 61, 4760–4766. [Google Scholar] [CrossRef]
- Avci, F.Y. Novel strategies for development of next-generation glycoconjugate vaccines. Curr. Top. Med. Chem. 2013, 13, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.G.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Lynfield, R.; Reingold, A.; Cieslak, P.R.; Pilishvili, T.; Jackson, D.; et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 2003, 348, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Prymula, R.; Schuerman, L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert. Rev. Vaccines 2009, 8, 1479–1500. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 258–261. [Google Scholar]
- Centers for Disease Control and Prevention. Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 394–395. [Google Scholar]
- Matanock, A.; Lee, G.; Gierke, R.; Kobayashi, M.; Leidner, A.; Pilishvili, T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1069–1075. [Google Scholar] [CrossRef]
- Tomczyk, S.; Bennett, N.M.; Stoecker, C.; Gierke, R.; Moore, M.R.; Whitney, C.G.; Hadler, S.; Pilishvili, T.; Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 822–825. [Google Scholar]
- Hanquet, G.; Krizova, P.; Dalby, T.; Ladhani, S.N.; Nuorti, J.P.; Danis, K.; Mereckiene, J.; Knol, M.J.; Winje, B.A.; Ciruela, P.; et al. Serotype Replacement after Introduction of 10-Valent and 13-Valent Pneumococcal Conjugate Vaccines in 10 Countries, Europe. Emerg. Infect. Dis. 2022, 28, 137–138. [Google Scholar] [CrossRef]
- Platt, H.L.; Cardona, J.F.; Haranaka, M.; Schwartz, H.I.; Narejos Perez, S.; Dowell, A.; Chang, C.J.; Dagan, R.; Tamms, G.M.; Sterling, T.; et al. A phase 3 trial of safety, tolerability, and immunogenicity of V114, 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in adults 50 years of age and older (PNEU-AGE). Vaccine 2022, 40, 162–172. [Google Scholar] [CrossRef]
- Klein, N.P.; Peyrani, P.; Yacisin, K.; Caldwell, N.; Xu, X.; Scully, I.L.; Scott, D.A.; Jansen, K.U.; Gruber, W.C.; Watson, W. A phase 3, randomized, double-blind study to evaluate the immunogenicity and safety of 3 lots of 20-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults 18 through 49 years of age. Vaccine 2021, 39, 5428–5435. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Leidner, A.J.; Gierke, R.; Farrar, J.L.; Morgan, R.L.; Campos-Outcalt, D.; Schechter, R.; Poehling, K.A.; Long, S.S.; Loehr, J.; et al. Use of 21-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 793–798. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Malley, R.; Lipsitch, M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011, 378, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.; Kossyvaki, V.; Galvez, P.; Mendez, C. Pneumococcal Serotype Evolution and Burden in European Adults in the Last Decade: A Systematic Review. Microorganisms 2023, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, B.J.; Gertz, R.E., Jr.; Gladstone, R.A.; Walker, H.; Sherwood, L.K.; Jackson, D.; Li, Z.; Law, C.; Hawkins, P.A.; Chochua, S.; et al. Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin. Microbiol. Infect. 2016, 22, 60.e9–60.e29. [Google Scholar] [CrossRef]
- Findlow, H.; Borrow, R. Interactions of conjugate vaccines and co-administered vaccines. Hum. Vaccin. Immunother. 2016, 12, 226–230. [Google Scholar] [CrossRef]
- Duke, J.A.; Avci, F.Y. Emerging vaccine strategies against the incessant pneumococcal disease. NPJ Vaccines 2023, 8, 122. [Google Scholar] [CrossRef]
- Feemster, K.; Buchwald, U.K.; Banniettis, N.; Joyce, J.G.; Velentgas, P.; Chapman, T.J.; Yildirim, I. Immunogenicity of Current and Next-Generation Pneumococcal Conjugate Vaccines in Children: Current Challenges and Upcoming Opportunities. Open Forum Infect. Dis. 2024, 11, ofae220. [Google Scholar] [CrossRef]
- Dagan, R.; Poolman, J.; Siegrist, C.A. Glycoconjugate vaccines and immune interference: A review. Vaccine 2010, 28, 5513–5523. [Google Scholar] [CrossRef]
- Burrage, M.; Robinson, A.; Borrow, R.; Andrews, N.; Southern, J.; Findlow, J.; Martin, S.; Thornton, C.; Goldblatt, D.; Corbel, M.; et al. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 2002, 70, 4946–4954. [Google Scholar] [CrossRef]
- Morimoto, K.; Masuda, S. Pneumococcal vaccines for prevention of adult pneumonia. Respir. Investig. 2025, 63, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, B.; Chen, Q.; Wang, C.; Wang, B.; Ye, Q.; Xu, Y. Pneumococcal vaccines in China. Hum. Vaccin. Immunother. 2025, 21, 2460274. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Considerations for Pneumococcal Conjugate Vaccine (PCV) Product Choice; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Adigweme, I.; Futa, A.; Saidy-Jah, E.; Edem, B.; Akpalu, E.; Dibbasey, T.; Sethna, V.; Dhere, R.; Kampmann, B.; Bengt, C.; et al. Immunogenicity and safety of a 10-valent pneumococcal conjugate vaccine administered as a 2 + 1 schedule to healthy infants in The Gambia: A single-centre, double-blind, active-controlled, randomised, phase 3 trial. Lancet Infect. Dis. 2023, 23, 609–620. [Google Scholar] [CrossRef]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef]
- Wassil, J.; Sisti, M.; Fairman, J.; Davis, M.; Fierro, C.; Bennett, S.; Johnson, D.; Migone, T.S.; Nguyen, K.; Sauer, P.; et al. Evaluating the safety, tolerability, and immunogenicity of a 24-valent pneumococcal conjugate vaccine (VAX-24) in healthy adults aged 18 to 64 years: A phase 1/2, double-masked, dose-finding, active-controlled, randomised clinical trial. Lancet Infect. Dis. 2024, 24, 308–318. [Google Scholar] [CrossRef]
- Wassil, J.; Sisti, M.; Fairman, J.; Rankin, B.; Clark, J.; Bennett, S.; Johnson, D.; Migone, T.S.; Nguyen, K.; Paschenko, A.; et al. A phase 2, randomized, blinded, dose-finding, controlled clinical trial to evaluate the safety, tolerability, and immunogenicity of a 24-valent pneumococcal conjugate vaccine (VAX-24) in healthy adults 65 years and older. Vaccine 2024, 42, 126124. [Google Scholar] [CrossRef]
- Safety, Tolerability, and Immunogenicity Study of a 31-Valent Pneumococcal Conjugate Vaccine (VAX-31) in Adults. Available online: https://clinicaltrials.gov/study/NCT06151288 (accessed on 25 March 2025).
- Dose-Ranging Study to Evaluate a 25-Valent Pneumococcal Conjugate Vaccine. Available online: https://clinicaltrials.gov/study/NCT06077656?cond=25valent%20pneumococcal%20conjugate&rank=2 (accessed on 25 March 2025).
- Datta, A.; Kapre, K.; Andi-Lolo, I.; Kapre, S. Multi-valent pneumococcal conjugate vaccine for global health: From problem to platform to production. Hum. Vaccin. Immunother. 2022, 18, 2117949. [Google Scholar] [CrossRef]
- Harding, C.M.; Nasr, M.A.; Scott, N.E.; Goyette-Desjardins, G.; Nothaft, H.; Mayer, A.E.; Chavez, S.M.; Huynh, J.P.; Kinsella, R.L.; Szymanski, C.M.; et al. A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun. 2019, 10, 891. [Google Scholar] [CrossRef]
- Lagousi, T.; Basdeki, P.; Routsias, J.; Spoulou, V. Novel Protein-Based Pneumococcal Vaccines: Assessing the Use of Distinct Protein Fragments Instead of Full-Length Proteins as Vaccine Antigens. Vaccines 2019, 7, 9. [Google Scholar] [CrossRef]
- Oliveira, G.S.; Oliveira, M.L.S.; Miyaji, E.N.; Rodrigues, T.C. Pneumococcal Vaccines: Past Findings, Present Work, and Future Strategies. Vaccines 2021, 9, 1338. [Google Scholar] [CrossRef]
- Hill, S.; Entwisle, C.; Pang, Y.; Joachim, M.; McIlgorm, A.; Dalton, K.; Burbidge, P.; Colaco, C.; Brown, J.; Goldblatt, D.; et al. Immunogenicity and mechanisms of action of PnuBioVax, a multi-antigen serotype-independent prophylactic vaccine against infection with Streptococcus pneumoniae. Vaccine 2018, 36, 4255–4264. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.A.; Chang, L.J.; Sheng, X.; Hopfer, R.; Team, P.P.R.S. Safety and immunogenicity of a trivalent recombinant PcpA, PhtD, and PlyD1 pneumococcal protein vaccine in adults, toddlers, and infants: A phase I randomized controlled study. Vaccine 2015, 33, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, K.F.; de Souza, L.R.A.; da Silva Santos, B.S.A.; de Carvalho, K.R.A.; da Silva Messias, S.G.; de Faria Goncalves, A.P.; Kano, F.S.; Alves, P.A.; da Silva Campos, M.A.; Xavier, M.P.; et al. Intranasal influenza-vectored vaccine expressing pneumococcal surface protein A protects against Influenza and Streptococcus pneumoniae infections. NPJ Vaccines 2024, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- David, S.C.; Brazel, E.B.; Singleton, E.V.; Minhas, V.; Laan, Z.; Scougall, C.; Chen, A.Y.; Wang, H.; Gates, C.J.; McLean, K.T.; et al. A Nonadjuvanted Whole-Inactivated Pneumococcal Vaccine Induces Multiserotype Opsonophagocytic Responses Mediated by Noncapsule-Specific Antibodies. mBio 2022, 13, e0236722. [Google Scholar] [CrossRef]
- Malley, R.; Lipsitch, M.; Stack, A.; Saladino, R.; Fleisher, G.; Pelton, S.; Thompson, C.; Briles, D.; Anderson, P. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 2001, 69, 4870–4873. [Google Scholar] [CrossRef]
- Lu, Y.J.; Leite, L.; Goncalves, V.M.; Dias Wde, O.; Liberman, C.; Fratelli, F.; Alderson, M.; Tate, A.; Maisonneuve, J.F.; Robertson, G.; et al. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine 2010, 28, 7468–7475. [Google Scholar] [CrossRef]
- Keech, C.A.; Morrison, R.; Anderson, P.; Tate, A.; Flores, J.; Goldblatt, D.; Briles, D.; Hural, J.; Malley, R.; Alderson, M.R. A Phase 1 Randomized, Placebo-controlled, Observer-blinded Trial to Evaluate the Safety and Immunogenicity of Inactivated Streptococcus pneumoniae Whole-cell Vaccine in Adults. Pediatr. Infect. Dis. J. 2020, 39, 345–351. [Google Scholar] [CrossRef]
- Morais, V.; Texeira, E.; Suarez, N. Next-Generation Whole-Cell Pneumococcal Vaccine. Vaccines 2019, 7, 151. [Google Scholar] [CrossRef]
- Maeda, H.; Morimoto, K. Global distribution and characteristics of pneumococcal serotypes in adults. Hum. Vaccin. Immunother. 2025, 21, 2469424. [Google Scholar] [CrossRef]
- Mendes, D.; Averin, A.; Atwood, M.; Sato, R.; Vyse, A.; Campling, J.; Weycker, D.; Slack, M.; Ellsbury, G.; Mugwagwa, T. Cost-effectiveness of using a 20-valent pneumococcal conjugate vaccine to directly protect adults in England at elevated risk of pneumococcal disease. Expert. Rev. Pharmacoecon Outcomes Res. 2022, 22, 1285–1295. [Google Scholar] [CrossRef]
- Saokaew, S.; Rayanakorn, A.; Wu, D.B.; Chaiyakunapruk, N. Cost Effectiveness of Pneumococcal Vaccination in Children in Low- and Middle-Income Countries: A Systematic Review. Pharmacoeconomics 2016, 34, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.X.; Pham, H.L.; Bui, U.M.; Ho, H.T.; Bui, T.T. Cost-Effectiveness of the Pneumococcal Vaccine in the Adult Population: A Systematic Review. Healthcare 2024, 12, 2490. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Vaccination Coverage Among Adults in the United States, National Health Interview Survey, 2016. Available online: https://www.cdc.gov/adultvaxview/publications-resources/vaccination-coverage-adults-2016.html (accessed on 27 March 2025).
- International Longevity Centre UK. European Pneumococcal Vaccination: A Progress Report. 2021. Available online: https://ilcuk.org.uk/wp-content/uploads/2023/01/ILC-European-Pneumococcal-Vaccination.pdf (accessed on 27 March 2025).
- Satman, I.; Akalin, S.; Cakir, B.; Altinel, S.; The diaVAX Study Group. The effect of physicians’ awareness on influenza and pneumococcal vaccination rates and correlates of vaccination in patients with diabetes in Turkey: An epidemiological Study “diaVAX”. Hum. Vaccin. Immunother. 2013, 9, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Akin, L.; Kaya, M.; Altinel, S.; Durand, L. Cost of pneumococcal infections and cost-effectiveness analysis of pneumococcal vaccination at risk adults and elderly in Turkey. Hum. Vaccin. 2011, 7, 441–450. [Google Scholar] [CrossRef]
- ECDC Vaccine Scheduler: Pneumococcal Disease, Recommended vaccinations in European Countries. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1 (accessed on 27 March 2025).
- Fletcher, M.A.; Vojicic, J.; Daigle, D.; Taysi, B.; Haridy, H.; Abalos, M.G.; Del Carmen Morales, G. National recommendations for adult pneumococcal vaccination in countries of the WHO regions of Americas, Africa, Eastern Mediterranean, South East Asia, and Western Pacific. Vaccine 2024, 42, 126390. [Google Scholar] [CrossRef]
- Eişkin Bağışıklama Rehberi-2024, Türkiye Enfeksiyon Hastalıkları ve Klinik Mikrobiyoloji Uzmanlık Derneği. Available online: https://www.ekmud.org.tr/files/uploads/files/eriskin-bagisiklama-rehberi-2024.pdf (accessed on 27 March 2025).
- World Health Organization. Considerations for pneumococcal vaccination in older adults. Wkly. Epidemiol. Rec. 2021, 96, 217–228. [Google Scholar]
- Global Vaccine Action Plan 2011–2020. Geneva: World Health Organization. Available online: https://www.who.int/publications/i/item/global-vaccine-action-plan-2011-2020 (accessed on 21 April 2025).
- WHO. Immunization Agenda 2030: A Global Strategy To Leave No One Behind. Available online: https://cdn.who.int/media/docs/default-source/immunization/strategy/ia2030/ia2030-draft-4-wha_b8850379-1fce-4847-bfd1-5d2c9d9e32f8.pdf?sfvrsn=5389656e_69&download=true (accessed on 20 April 2025).
- Gavi, the Vaccine Alliance, Strategy 2021–2025: Saving Lives and Protecting Livelihoods. Available online: https://www.gavi.org/our-alliance/strategy/phase-5-2021-2025/equity-goal (accessed on 20 April 2025).
- World Health Organization. Routine vaccination coverage—Worldwide, 2023. Wkly. Epidemiol. Rec. 2024, 99, 641–652. [Google Scholar]
- WHO SAGE Values Framework. 2020. Available online: https://iris.who.int/bitstream/handle/10665/334299/WHO-2019-nCoV-SAGE_Framework-Allocation_and_prioritization-2020.1-eng.pdf?sequence=1 (accessed on 20 April 2025).
| Age | Comorbid Conditions | Immunocompromising Conditions |
|---|---|---|
| >50 y | Chronic heart disease | Chronic renal failure, nephrotic syndrome |
| Chronic liver disease | Immunodeficiencies | |
| Chronic lung disease | Iatrogenic immunosuppression | |
| Diabetes mellitus | Generalized malignancy | |
| Cochlear implant | HIV infection | |
| CSF leak | Hodgkin’s disease, leukemia, lymphoma, multiple myeloma | |
| Smoking | Solid organ transplant | |
| Alcoholism | Sickle cell disease or other hemoglobinopathies | |
| Nursing home residence | Congenital or acquired asplenia |
| Protein | Function | Rationale for Use |
|---|---|---|
| Pneumolysin (Ply) | Cytolytic toxin, pro-inflammatory | Highly conserved, induces strong neutralizing antibodies (used as toxoids in vaccines) |
| PspA (Pneum surface prtA) | Inhibits complement deposition | Surface-exposed, induces opsonic antibody, variable, families 1/2 cover ~99% of strains |
| PhtD (Hist triad prt D) | Zinc transport, adhesion | Surface-exposed, conserved; shown to be immunogenic and protective in animals |
| PsaA | Manganese-binding lipoprt (ABC transporter) | Adhesion, nutrient acquisition; conserved |
| PcpA | Cell wall surface protein (choline-binding) | Involved in colonization, induces mucosal and systemic immunity |
| PspC/CbpA | Complement-binding, adhesion to epithelial cells | Important in nasopharyngeal colonization |
| Pili proteins (e.g., RrgB) | Adhesion to host cells | Targeted in some vaccines (e.g., PnuBioVax) |
| Age/Group | Recommended Vaccine | Interval |
|---|---|---|
| ≥50 years (no prior PCV) | PCV15 + PPSV23/ PCV20/PCV21 | PPSV23 ≥ 1 year after PCV15 (8 weeks if high risk) |
| ≥50 years (received PCV13) | PCV20/PCV21 | ≥1 year after PCV13 |
| ≥50 years (received PPSV23) | PCV15/PCV20/PCV21 | ≥1 year after PPSV23 |
| ≥50 years (received PCV13 + PPSV23, both before 65) | PCV20/PCV21 | ≥5 years after last vaccine |
| ≥50 years (received PCV13 + PPSV23, after 65) | PCV20/ PCV21 (Clinical decision) | ≥5 years after last vaccine |
| 19–49 years with risk conditions (no prior PCV) | PCV15 + PPSV23/ PCV20/PCV21 | PPSV23 ≥ 1 year after PCV15 (8 weeks if high risk) |
| 19–49 years with risk conditions (received PCV13) | PCV20/PCV21 | ≥1 year after PCV13 |
| 19–49 years with risk conditions (received PPSV23) | PCV15/PCV20/PCV21 | ≥1 year after PPSV23 |
| 19–49 years with risk condition 1 (received PCV13 + PPSV23) | PCV20/PCV21 | ≥5 years after last vaccine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozisik, L. The New Era of Pneumococcal Vaccination in Adults: What Is Next? Vaccines 2025, 13, 498. https://doi.org/10.3390/vaccines13050498
Ozisik L. The New Era of Pneumococcal Vaccination in Adults: What Is Next? Vaccines. 2025; 13(5):498. https://doi.org/10.3390/vaccines13050498
Chicago/Turabian StyleOzisik, Lale. 2025. "The New Era of Pneumococcal Vaccination in Adults: What Is Next?" Vaccines 13, no. 5: 498. https://doi.org/10.3390/vaccines13050498
APA StyleOzisik, L. (2025). The New Era of Pneumococcal Vaccination in Adults: What Is Next? Vaccines, 13(5), 498. https://doi.org/10.3390/vaccines13050498






