A Similar Nonclinical Safety Evaluation of Prev(e)nar 13 in a Multi-Dose Formulation Containing the Preservative 2-Phenoxyethanol
Abstract
1. Introduction
| Vaccine | Age Group | Vaccine Type | 2-PE Dose | Route/ Dose (mL) | Company |
|---|---|---|---|---|---|
| IPOL [12] | 6 weeks and older | IPV | 0.50% a | IM, SC/0.5 | Sanofi |
| Adacel b [11] | 10 to 64 years | Tdap | 3.3 mg/dose | IM/0.5 | Sanofi |
| Daptacel b [13] | 6 weeks through 6 years | DTaP | 3.3 mg/dose | IM/0.5 | Sanofi |
| Pentacel b [14] | 6 weeks through 4 years | DTaP-IPV-Hib | 3.3 mg/dose | IM/0.5 | Sanofi |
| Quadracel b [15] | 4 through 6 years | DTap-IPV | 3.3 mg/dose | IM/0.5 | Sanofi |
| Vaccine | Age Group | Vaccine Type | 2-PE Dose | Route/ Dose (mL) | Company |
|---|---|---|---|---|---|
| Prev(e)nar 13 MDV [4,16] | 6 weeks and older | Pneumococcal | 4 mg/dose | IM/0.5 | Pfizer |
| Tetravac [17] | 2 months to 12 years, with additional booster recommended between the ages of 4 and 13 | DTaP-IPV | 2.5 µL 0.5% v/v a 2.75 mg/dose | IM/0.5 | Sanofi |
| Revaxis [18] | 6 years or older | Td-IPV | NA b | IM/0.5 | Sanofi |
| Repevax [19] | 3 years or older | DTaP-IPV | NA b | IM/0.5 | Sanofi |
| Avaxim (pediatric) [20] | 1 to 15 years c | HepA | 2.5 µL 0.5% v/v a 2.75 mg/dose | IM/0.5 | Sanofi |
| Avaxim [21,22] | 12 years or older d | HepA | 2.5 µL 0.5% v/v a 2.75 mg/dose | IM/0.5 | Sanofi |
| IMOVAX Polio [23] | 2 months or older | IPV | ≤1.0% e | SC/0.5 | Sanofi |
| ViVAXIM [24] | 16 years or older | HepA-Typhoid | 2.5 µL 0.25% v/v a 2.75 mg/dose | IM/1 | Sanofi |
| Td Adsorbed [25] | 7 years and older | Td | 0.6% v/v a 3.3 mg/dose | IM/0.5 | Sanofi |
| Kinrix [26] | 4 through 6 years | DTaP-IPV | ≤2.5 mg/dose | IM/0.5 | GSK |
| HEXASIL [27] | 6 weeks and older | DTwP-HepB-IPV-Hib | 0.5% e | IM/0.5 | Serum Institute of India Pvt. Ltd. |
| Eupolio Inj. [28] | 6 weeks and older | Sabin IPV | 2.5 mg/dose | IM/0.5 | LG Chem Ltd. |
| Picovax [29] | 6 weeks and older | IPV | 0.5% w/v 2.5 mg/dose | IM/0.5 | AJ Vaccines A/S |
| Poliomyelitis Vaccine (Inactivated) [30] | 6 weeks and older | IPV | 2.5 mg/dose | IM, SC/0.5 | Serum Institute of India Pvt. Ltd. |
| Poliomyelitis Vaccine [31] | Children and adults | IPV | 5 mg/mL 2.5 mg/dose | IM, SC/0.5 | Bilthoven Biologicals B.V. |
| ShanIPV [32] | 6 weeks and older | IPV | 2.5 µL/dose 2.75 mg/dose | IM, SC/0.5 | Sanofi |
| Poliomyelitis Vaccine (Vero Cell), Inactivated, Sabin Strains [33] | 2 months and older | SIPV | NA b | IM/0.5 | Sinovac Biotech Co. Ltd. |
| Synflorix [34] | 6 weeks to 5 years | Pneumococcal | 10 mg/mL 5 mg/dose | IM/0.5 | GSK |
| SKYTyphoid Multi Inj. [35] | 6 months to 45 years | Typhoid | 5 mg/dose | IM/0.5 | SK Bioscience Co., Ltd. |
| TYPHIBEV [36] | 6 months to 45 years | Typhoid | 5 mg/dose | IM/0.5 | Biological E. Limited |
| Typbar-TCV [37] | 6 months to 45 years | Typhoid | 5 mg/dose | IM/0.5 | Bharat Biotech International Limited |
| ZyVac [38] | 6 months to 45 years | Typhoid | 0.5 mg/dose | IM/0.5 | Zydus Lifesciences Limited |
| Vaccine | Age Group | Vaccine Type | 2-PE Dose | Route/ Dose (mL) | Company |
|---|---|---|---|---|---|
| Havrix (pediatric) [39,40] | 1 through 18 years | HepA | 0.5% w/v a 2.5 mg/dose | IM/0.5 | GSK |
| Havrix (adult) [39,40] | 19 years or older | HepA | 0.5% w/v a 5 mg/dose | IM/1 | GSK |
| Twinrix (adult) [41,42] | 16 years or older | HepA-HepB | 5 mg/dose | IM/1 | GSK |
| Pediarix [43,44] | 6 weeks through 6 years | DTaP-HepB-IPV | 2.5 mg/dose | IM/0.5 | GSK |
| Infanrix Hexa [45,46] | 6 weeks to 2 years | DTaP-IPV-Hib-HepB | 2.5 mg/dose | IM/0.5 | GSK |
| Discontinued 2-PE-containing vaccines | |||||
| LYMErix [47] b | 15 to 70 years | Lyme Disease | 2.5 mg/dose | IM/0.5 | GSK |
| Infanrix [48,49] b | 6 weeks through 6 years | DTaP | 2.5 mg/dose | IM/0.5 | GSK |
| Poliorix [50] | 6 weeks or older | IPV | NA d | IM/0.5 | GSK |
| ViATIM [51] c | 16 years or older | HepA-Typhoid | NA | Slow IM, SC/1 | Sanofi |
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Test and Control Articles
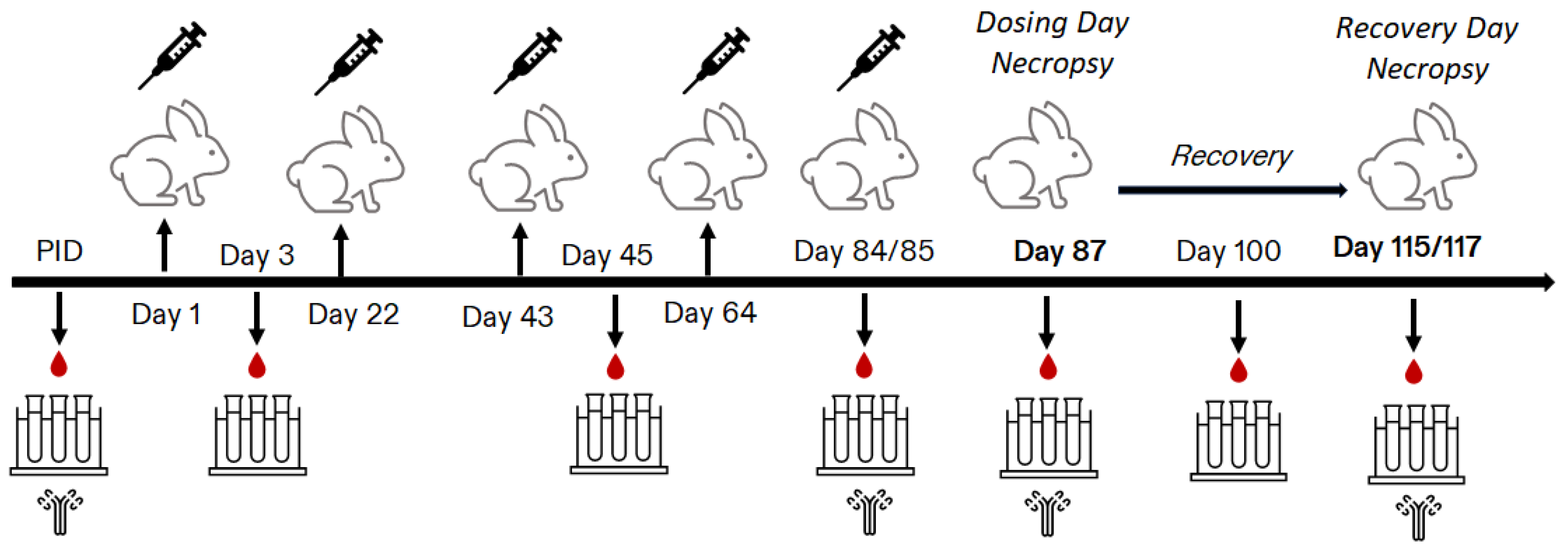
2.3. Study Design
2.4. In-Life Assessments
2.5. Blood Sample Collections for Clinical Pathology
2.6. Post-Mortem Assessments
2.7. Serology Analysis
2.8. Statistical Analysis
3. Results
3.1. In-Life Findings
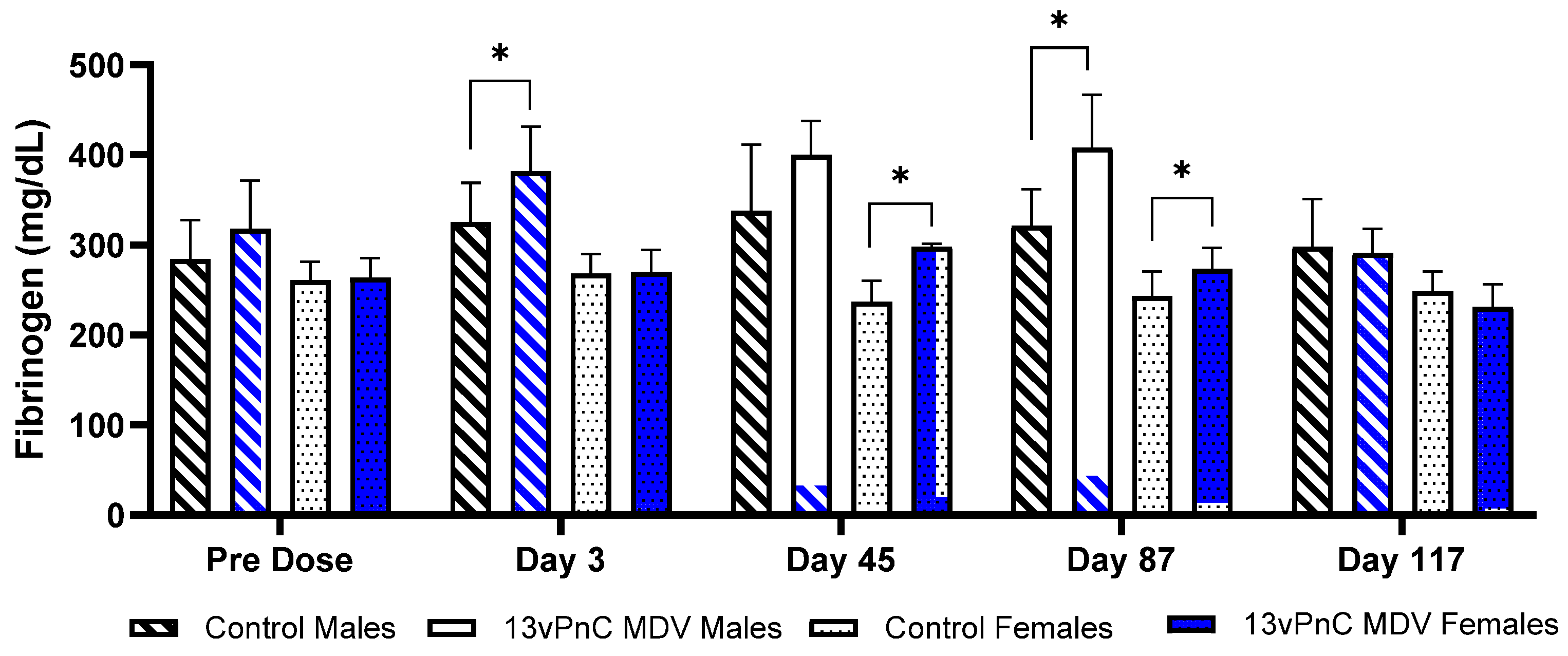
3.2. Clinical Pathology
3.3. Organ Weight/Macroscopic and Microscopic Observatoins
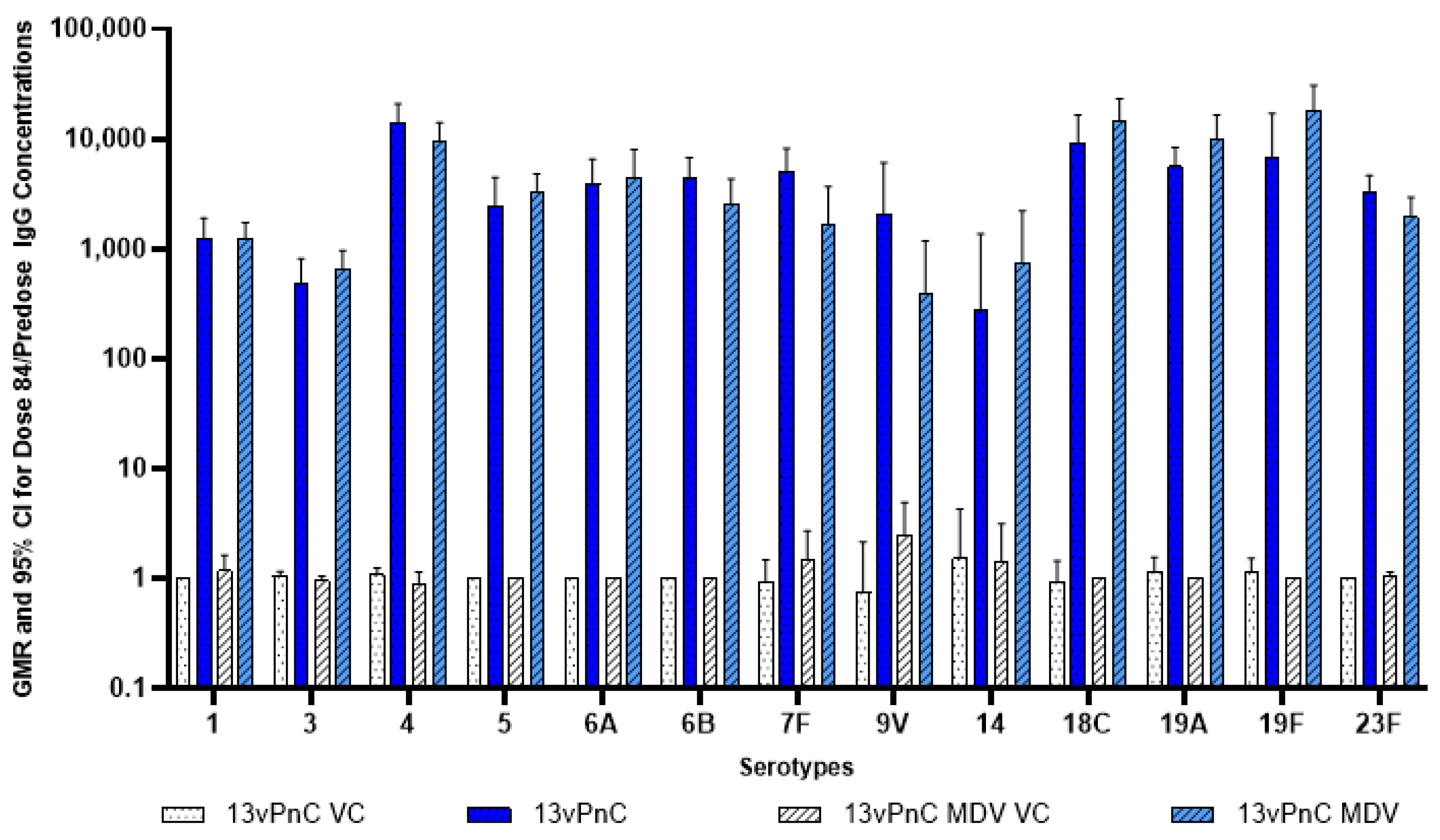
3.4. Serology
4. Discussion
4.1. Nonclinical Safety Data in Vaccines Containing 2-PE
4.2. Other Nonclinical Safety Data on 2-PE
4.3. Clinical Safety Data in Vaccines and Dermal Products Containing 2-PE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadioglu, A.; Taylor, S.; Iannelli, F.; Pozzi, G.; Mitchell, T.J.; Andrew, P.W. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect. Immun. 2002, 70, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [CrossRef] [PubMed]
- Prevenar 13 [Summary of Product Characteristics]. Revised: April 2024. Available online: https://www.ema.europa.eu/en/documents/product-information/prevenar-13-epar-product-information_en.pdf (accessed on 28 December 2024).
- Khandke, L.; Yang, C.; Krylova, K.; Jansen, K.U.; Rashidbaigi, A. Preservative of choice for Prev(e)nar 13 in a multi-dose formulation. Vaccine 2011, 29, 7144–7153. [Google Scholar] [CrossRef] [PubMed]
- Idoko, O.T.; Mboizi, R.B.; Okoye, M.; Laudat, F.; Ceesay, B.; Liang, J.Z.; Le Dren-Narayanin, N.; Jansen, K.U.; Gurtman, A.; Center, K.J.; et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine (PCV13) formulated with 2-phenoxyethanol in multidose vials given with routine vaccination in healthy infants: An open-label randomized controlled trial. Vaccine 2017, 35, 3256–3263. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, S.K.; Ramanan, P.V.; Sapru, A.; Sundaram, B.; Shah, B.H.; Kaul, D.; Karthik Nagesh, N.; Kalina, W.V.; Chand, R.; Ding, M.; et al. Safety and immunogenicity of a multidose vial formulation of 13-valent pneumococcal conjugate vaccine administered with routine pediatric vaccines in healthy infants in India: A phase 4, randomized, open-label study. Vaccine 2021, 39, 6787–6795. [Google Scholar] [CrossRef] [PubMed]
- Lowe, I.; Southern, J. The antimicrobial activity of phenoxyethanol in vaccines. Lett. Appl. Microbiol. 1994, 18, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Safety (SCCS). Opinion on Phenoxyethanol. SCCS/1575/16. 2016. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_195.pdf (accessed on 6 January 2025).
- Dreno, B.; Zuberbier, T.; Gelmetti, C.; Gontijo, G.; Marinovich, M. Safety review of phenoxyethanol when used as a preservative in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Adacel [Package Insert]. Revised 1 January 2023. Available online: https://www.fda.gov/media/119862/download (accessed on 5 January 2025).
- IPOL [Package Insert]. Revised: May 2022. Available online: https://www.fda.gov/media/75695/download (accessed on 5 January 2025).
- Daptacel [Package Insert]. Revised: xx/202x. Available online: https://www.fda.gov/media/74035/download (accessed on 5 January 2025).
- Pentacel [Package Insert]. Revised: xx/202x. Available online: https://www.fda.gov/media/74385/download (accessed on 5 January 2025).
- Quadracel [Package Insert]. Revised: xx/202x. Available online: https://www.fda.gov/media/91640/download (accessed on 5 January 2025).
- PREVNAR 13 Multidose Vial. [WHO-Prequalification of Medical Products]. Revised January 2019. Available online: https://extranet.who.int/prequal/sites/default/files/vwa_vaccine/pq_309_Prevenar13_MDV_Pfizer_PI-2019.pdf (accessed on 5 January 2025).
- TETRAVAC [Summary of Product Characteristics]. Revised: 5 April 2024. Available online: https://www.medicines.ie/medicines/tetravac-34982/spc (accessed on 5 January 2025).
- Revaxis [Summary of Product Characteristics]. Revised: 6 January 2025. Available online: https://www.medicines.org.uk/emc/product/5581 (accessed on 10 January 2025).
- Repevax [Summary of Product Characteristics]. Revised: 4 January 2025. Available online: https://www.medicines.org.uk/emc/product/5580 (accessed on 5 January 2025).
- Avaxim-Pediatric [Product Monograph]. Published: 8 June 2015. Available online: https://pdf.hres.ca/dpd_pm/00030794.PDF (accessed on 5 January 2025).
- Avaxim [Summary of Product Characteristics]. Revised: 6 January 2025. Available online: https://www.medicines.org.uk/emc/product/1394/smpc/ (accessed on 10 January 2025).
- Avaxim [Product Monograph]. Revised: September 2015. Available online: https://pdf.hres.ca/dpd_pm/00032192.PDF (accessed on 5 January 2025).
- IMOVAX Polio [Product Monograph]. Revised: December 2010. Available online: https://pdf.hres.ca/dpd_pm/00012948.PDF (accessed on 5 January 2025).
- ViVAXIM [Product Monograph]. Revised: September 2015. Available online: https://pdf.hres.ca/dpd_pm/00032177.PDF (accessed on 5 January 2025).
- Td Absorbed [Product Monograph]. Revised: October 2012. Available online: https://pdf.hres.ca/dpd_pm/00020609.PDF (accessed on 5 January 2025).
- Kinrix [Package Insert]. Revised X/2023. Available online: https://www.fda.gov/media/80128/download (accessed on 5 January 2025).
- HEXASIL. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/hexasiil-0 (accessed on 5 January 2025).
- Eupolio Inj. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/eupolio-inj (accessed on 5 January 2025).
- Picovax. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/picovax (accessed on 5 January 2025).
- Poliomyelitis Vaccine (Inactivated). [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/poliomyelitis-vaccine-inactivated (accessed on 5 January 2025).
- Poliomyelitis Vaccine. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/poliomyelitis-vaccine (accessed on 5 January 2025).
- ShanIPV. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/shanipvtm-0 (accessed on 5 January 2025).
- Poliomyelitis Vaccine (Vero Cell), Inactivated, Sabin Strains. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/poliomyelitis-vaccine-vero-cell-inactivated-sabin-strains-1 (accessed on 5 January 2025).
- Synflorix. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/synflorix-0 (accessed on 5 January 2025).
- SKYTyphoid Multi Inj. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/skytyphoid-multi-inj (accessed on 5 January 2025).
- TYPHIBEV. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/typhibevr-0 (accessed on 5 January 2025).
- Typbar-TCV. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/typbar-tcv-0 (accessed on 5 January 2025).
- ZyVac. [WHO-Prequalification of Medical Products]. Available online: https://extranet.who.int/prequal/vaccines/p/zyvacr (accessed on 5 January 2025).
- US Food and Drug Administration. Summary for Basis of Approval—Havrix. Available online: https://wayback.archive-it.org/7993/20170112211916/http:/www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM244562.pdf (accessed on 5 January 2025).
- Havrix [Package Insert]. Revised: XX/XXXX. Available online: https://www.fda.gov/media/119388/download (accessed on 5 January 2025).
- Twinrix [Package Insert]. Revised: April 2023. Available online: https://www.fda.gov/media/119351/download (accessed on 5 January 2025).
- Centers for Disease Control and Prevention. Notice to Readers: FDA Approval for a Combined Hepatitis A and B Vaccine. Published: 21 September 2001. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5037a4.htm (accessed on 5 January 2025).
- Pediarix [Package Insert]. Revised April 2023. Available online: https://www.fda.gov/media/79830/download (accessed on 5 January 2025).
- US Food and Drug Administration. Review Memorandum: BLA STN 103907 (99-0800). Published: 12 November 2001. Available online: http://wayback.archive-it.org/7993/20170723144354/https:/www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM106897.pdf (accessed on 5 January 2025).
- Infanrix Hexa [Summary of Product Characteristics]. Revised: 31 August 2010. Available online: https://www.ema.europa.eu/en/documents/product-information/infanrix-hexa-epar-product-information_en.pdf (accessed on 5 January 2025).
- European Medicines Agency. Scientific Discussion. Infanrix Hexa. Last Updated: 2004. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/infanrix-hexa-epar-scientific-discussion_en.pdf (accessed on 5 January 2025).
- Lymerix [Prescribing Information]. Available online: https://wayback.archive-it.org/7993/20170405164754/https:/www.fda.gov/ohrms/dockets/ac/01/briefing/3680b2_03.pdf (accessed on 5 January 2025).
- US Food and Drug Administration. Summary for Basis of Approval—Infanrix. Available online: http://wayback.archive-it.org/7993/20170723024627/https:/www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM244609.pdf (accessed on 5 January 2025).
- Infanrix [Package Insert]. Revised X/2023. Available online: https://www.fda.gov/media/75157/download (accessed on 5 January 2025).
- PoliorixTM. [WHO-Package Insert]. Revised: June 2010. Available online: https://pubmedinfo.org/wp-content/uploads/2018/03/poliorix_-_ang_-_6_stron_-_gsk_-_2010_-_who.pdf (accessed on 5 January 2025).
- Viatim [Summary of Product Characteristics]. Last updated: 2 September 2019. Available online: https://www.medicines.org.uk/emc/product/1534/smpc (accessed on 5 January 2025).
- National Academy of Sciences (US). The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Draize, J.H. Dermal toxicity. In Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics; Food and Drug Administration, Association of Food and Drug Officials of the United States: Topeka, KS, USA, 1965; pp. 46–59. [Google Scholar]
- Rohde, C.M.; Lindemann, C.; Giovanelli, M.; Sellers, R.S.; Diekmann, J.; Choudhary, S.; Ramaiah, L.; Vogel, A.B.; Chervona, Y.; Muik, A.; et al. Toxicological Assessments of a Pandemic COVID-19 Vaccine-Demonstrating the Suitability of a Platform Approach for mRNA Vaccines. Vaccines 2023, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, R.; Eriksson, H. Aluminium adjuvants in vaccines—A way to modulate the immune response. Semin. Cell Dev. Biol. 2021, 115, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Breslin, W.J.; Phillips, J.E.; Lomax, L.G.; Bartels, M.J.; Dittenber, D.A.; Calhoun, L.L.; Miller, R.R. Hemolytic activity of ethylene glycol phenyl ether (EGPE) in rabbits. Fundam. Appl. Toxicol. 1991, 17, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Zaman, S.F.; Zaman, F.; Aziz, A.; Faisal, S.B.; Traskine, M.; Habib, M.A.; Ruiz-Guinazu, J.; Borys, D. Immunologic non-inferiority and safety of the investigational pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (PHiD-CV) 4-dose vial presentation compared to the licensed PHiD-CV 1-dose vial presentation in infants: A phase III randomized study. Vaccine 2018, 36, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M.; Bond, N.H.; Maccato, M.; Pinell, P.; Hammill, H.A.; Swamy, G.K.; Walter, E.B.; Jackson, L.A.; Englund, J.A.; Edwards, M.S.; et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA 2014, 311, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.; Landthaler, M.; Stolz, W. Generalized eczema in an 18-month-old boy due to phenoxyethanol in DPT vaccine. Contact Dermat. 1998, 38, 50–51. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Scientific Discussion. Infanrix Penta. Last Updated: 10 July 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/infanrix-penta-epar-scientific-discussion_en.pdf (accessed on 27 December 2020).
- Georgitis, J.W.; Fasano, M.B. Allergenic components of vaccines and avoidance of vaccination-related adverse events. Curr. Allergy Asthma Rep. 2001, 1, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Buhrer, C.; Bahr, S.; Siebert, J.; Wettstein, R.; Geffers, C.; Obladen, M. Use of 2% 2-phenoxyethanol and 0.1% octenidine as antiseptic in premature newborn infants of 23–26 weeks gestation. J. Hosp. Infect. 2002, 51, 305–307. [Google Scholar] [CrossRef] [PubMed]
| Finding | Male | Female | ||||
|---|---|---|---|---|---|---|
| Dosage Group | Dosage Group | |||||
| Saline Control | Vehicle Control | 13vPnC | Saline Control | Vehicle Control | 13vPnC | |
| Injection Site (Dosing-Phase Necropsy) a | 5 | 5 | 5 | 5 | 5 | 5 |
| Degeneration/Necrosis, Myofibers | 0 | 1 | 3 | 1 | 1 | 2 |
| Minimal | 0 | 0 | 1 | 0 | 1 | 0 |
| Mild | 0 | 1 | 1 | 1 | 0 | 2 |
| Moderate | 0 | 0 | 1 | 0 | 0 | 0 |
| Inflammation, Chronic | 0 | 2 | 4 | 0 | 3 | 5 |
| Minimal | 0 | 0 | 0 | 0 | 1 | 3 |
| Mild | 0 | 2 | 2 | 0 | 2 | 0 |
| Moderate | 0 | 0 | 2 | 0 | 0 | 2 |
| Injection Site (Recovery-Phase Necropsy) a | 5 | 5 | 5 | 5 | 5 | 5 |
| Degeneration/Necrosis | 0 | 1 | 2 | 0 | 1 | 3 |
| Minimal | 0 | 1 | 2 | 0 | 1 | 2 |
| Mild | 0 | 0 | 0 | 0 | 0 | 1 |
| Finding | Male | Female | ||||
|---|---|---|---|---|---|---|
| Dosage Group | Dosage Group | |||||
| Vehicle Control | 13vPnC MDV Vehicle Control | 13vPnC MDV | Vehicle Control | 13vPnC MDV Vehicle Control | 13vPnC MDV | |
| Spleen (Dosing-Phase Necropsy) a | 5 | 5 | 5 | 5 | 5 | 5 |
| Germinal Centers, Increased | ||||||
| Minimal | 0 | 2 | 3 | 0 | 0 | 5 |
| Mild | 0 | 0 | 1 | 0 | 0 | 0 |
| Spleen (Recovery-Phase Necropsy) a | 5 | 5 | 5 | 5 | 5 | 5 |
| Germinal Centers, Increased | ||||||
| Minimal | 0 | 0 | 2 | 0 | 0 | 3 |
| Intramuscular Site (Dosing-Phase Necropsy) a | 5 | 5 | 5 | 5 | 5 | 5 |
| Degeneration/Necrosis, Myofibers | ||||||
| Minimal | 3 | 0 | 2 | 2 | 4 | 2 |
| Mild | 0 | 0 | 1 | 0 | 0 | 1 |
| Moderate | 1 | 1 | 0 | 0 | 0 | 0 |
| Inflammation, Chronic | ||||||
| Minimal | 2 | 4 | 2 | 4 | 4 | 4 |
| Mild | 1 | 1 | 3 | 1 | 0 | 0 |
| Moderate | 2 | 0 | 0 | 0 | 0 | 1 |
| Intramuscular Site (Recovery-Phase Necropsy) a | 5 | 5 | 5 | 5 | 5 | 5 |
| Degeneration/Necrosis | ||||||
| Minimal | 1 | 3 | 1 | 1 | 0 | 0 |
| Inflammation, Chronic | ||||||
| Minimal | 2 | 4 | 3 | 4 | 3 | 4 |
| Mild | 2 | 0 | 1 | 0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chervona, Y.; Shen, W.; Choudhary, S.; Markiewicz, V.; Giardina, P.C.; Rohde, C.M. A Similar Nonclinical Safety Evaluation of Prev(e)nar 13 in a Multi-Dose Formulation Containing the Preservative 2-Phenoxyethanol. Vaccines 2025, 13, 486. https://doi.org/10.3390/vaccines13050486
Chervona Y, Shen W, Choudhary S, Markiewicz V, Giardina PC, Rohde CM. A Similar Nonclinical Safety Evaluation of Prev(e)nar 13 in a Multi-Dose Formulation Containing the Preservative 2-Phenoxyethanol. Vaccines. 2025; 13(5):486. https://doi.org/10.3390/vaccines13050486
Chicago/Turabian StyleChervona, Yana, Wen Shen, Shambhunath Choudhary, Victoria Markiewicz, Peter C. Giardina, and Cynthia M. Rohde. 2025. "A Similar Nonclinical Safety Evaluation of Prev(e)nar 13 in a Multi-Dose Formulation Containing the Preservative 2-Phenoxyethanol" Vaccines 13, no. 5: 486. https://doi.org/10.3390/vaccines13050486
APA StyleChervona, Y., Shen, W., Choudhary, S., Markiewicz, V., Giardina, P. C., & Rohde, C. M. (2025). A Similar Nonclinical Safety Evaluation of Prev(e)nar 13 in a Multi-Dose Formulation Containing the Preservative 2-Phenoxyethanol. Vaccines, 13(5), 486. https://doi.org/10.3390/vaccines13050486






