Health Equity and Human Papillomavirus Vaccine Interventions for Adolescents: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Overview
2.2. Systematic Search
2.3. Selection Criteria
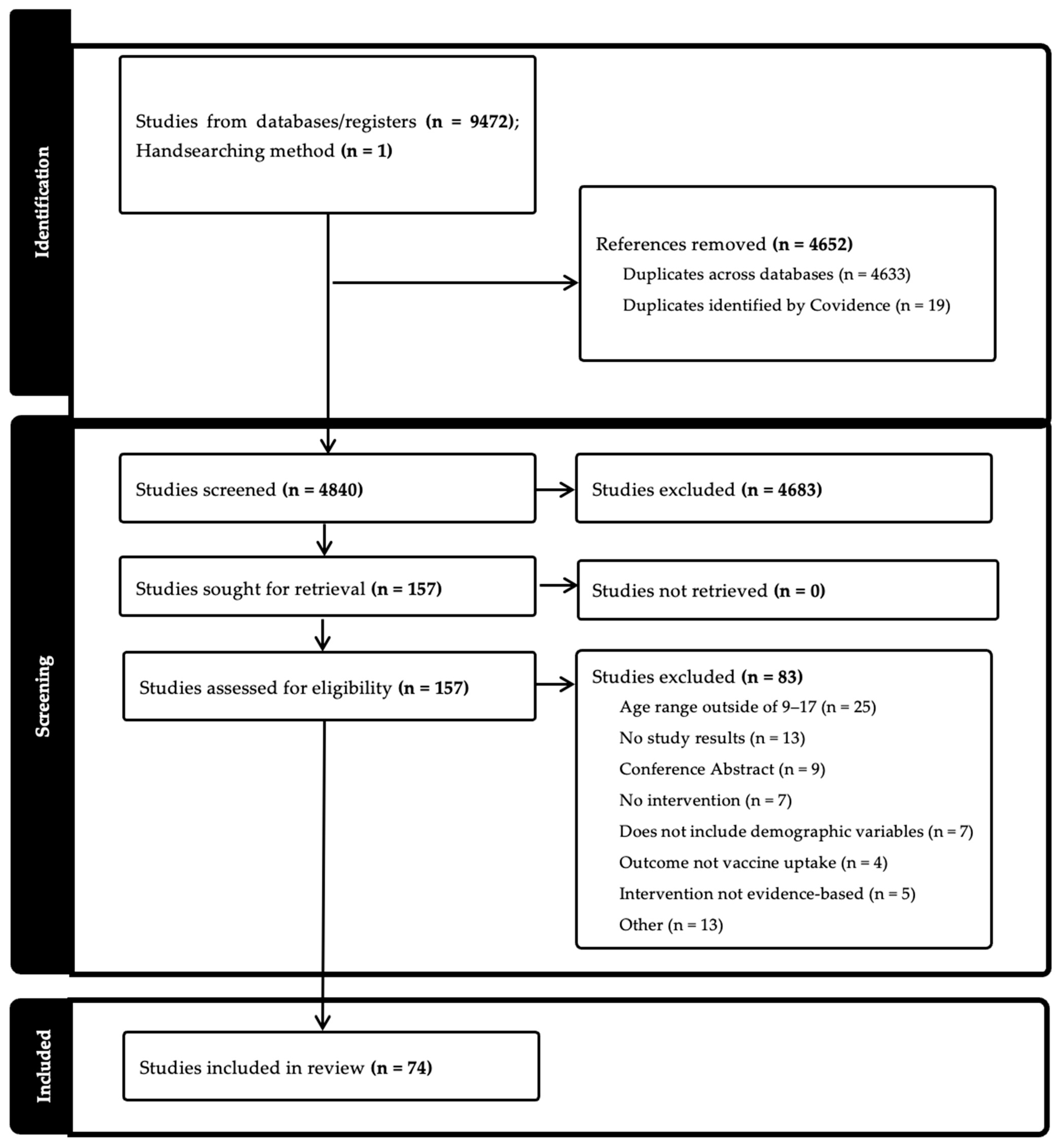
2.4. Screening and Eligibility
2.5. Data Extraction
3. Results
3.1. Summary of Findings
3.2. Methodology
3.3. Sex
3.4. Race/Ethnicity
3.5. Income/Socioeconomic Status
3.6. Geographic Region
3.7. Other Demographic Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Full Search String for Systematic Review
References
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Cancers Linked with HPV Each Year. Available online: https://www.cdc.gov/cancer/hpv/cases.html#cdc_generic_section_2-number-of-hpv-associated-cancer-cases-per-year (accessed on 10 March 2025).
- Centers for Disease Control and Prevention. Cancers by Age, Sex, Race, Ethnicity. Available online: https://gis.cdc.gov/Cancer/USCS/#/Demographics/ (accessed on 10 March 2025).
- Semprini, J.; Zahnd, W.; Brandt, H.M. What Cancers Explain the Growing Rural-Urban Gap in Human Papillomavirus-Associated Cancer Incidence? J. Rural Health 2025, 41, e12915. [Google Scholar] [CrossRef] [PubMed]
- Meites, E. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination—Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Trends Progress Report. Available online: https://progressreport.cancer.gov/prevention/hpv_immunization (accessed on 10 March 2025).
- Office of Disease Prevention and Health Promotion. Increase the Proportion of Adolescents Who Get Recommended Doses of the HPV Vaccine—IID-08. Healthy People 2030. Available online: https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08 (accessed on 10 March 2025).
- Centers for Disease Control and Prevention. Vaccination Coverage Among Adolescents (13–17). Available online: https://www.cdc.gov/teenvaxview/interactive/index.html (accessed on 10 March 2025).
- Rimer, B.K. HPV Vaccination for Cancer Prevention: Progress, Opportunities, and a Renewed Call to Action; Report to the President of the United States; President’s Cancer Panel: Bethesda, MD, USA, 2018. [Google Scholar]
- Rodriguez, S.A.; Mullen, P.D.; Lopez, D.M.; Savas, L.S.; Fernández, M.E. Factors Associated with Adolescent HPV Vaccination in the U.S.: A Systematic Review of Reviews and Multilevel Framework to Inform Intervention Development. Prev. Med. 2020, 131, 105968. [Google Scholar] [CrossRef]
- Avni-Singer, L.R.; Yakely, A.; Sheth, S.S.; Shapiro, E.D.; Niccolai, L.M.; Oliveira, C.R. Assessing Sociodemographic Differences in Human Papillomavirus Vaccine Impact Studies in the United States: A Systematic Review Using Narrative Synthesis. Public Health 2020, 178, 137–150. [Google Scholar] [CrossRef]
- American Medical Group Association. National HPV Vaccination Roundtable. HPV Vaccination Best Practices Learning Collaborative Summary Report and Lessons Learned. Available online: https://hpvroundtable.org/wp-content/uploads/2023/05/AMGA-HPV-Learning-Collaborative-Summary-Report-2021_FINAL-1.pdf (accessed on 10 March 2025).
- Open Science Framework Registries. Health Equity and HPV Vaccine Interventions Systematic Review. Public Registration, 9 October 2023. Available online: https://osf.io/7zk43 (accessed on 10 March 2025).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software; Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 10 March 2025).
- Agana-Norman, D.F.; Berenson, A.B.; Chang, M. Impact Assessment of a Provider-Targeted National Vaccine Messaging Campaign on Human Papillomavirus Vaccination Rates among US Adolescent Males. Prev. Med. 2022, 164, 107228. [Google Scholar] [CrossRef]
- Aragones, A.; Bruno, D.M.; Ehrenberg, M.; Tonda-Salcedo, J.; Gany, F.M. Parental Education and Text Messaging Reminders as Effective Community-Based Tools to Increase HPV Vaccination Rates among Mexican American Children. Prev. Med. Rep. 2015, 2, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S.; Zhu, H.; Rochefort, C.; Marks, E.; Fullington, H.M.; Rodriguez, S.A.; Kassa, S.; Tiro, J.A. Mechanisms of Self-Persuasion Intervention for HPV Vaccination: Testing Memory and Autonomous Motivation. Health Psychol. 2021, 40, 887–896. [Google Scholar] [CrossRef]
- Bastani, R.; Glenn, B.A.; Singhal, R.; Crespi, C.M.; Nonzee, N.J.; Tsui, J.; Chang, L.C.; Herrmann, A.K.; Taylor, V.M. Increasing HPV Vaccination among Low-Income, Ethnic Minority Adolescents: Effects of a Multicomponent System Intervention through a County Health Department Hotline. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 175–182. [Google Scholar] [CrossRef]
- Beck, A.; Bianchi, A.; Showalter, D. Evidence-Based Practice Model to Increase Human Papillomavirus Vaccine Uptake: A Stepwise Approach. Nurs. Womens Health 2021, 25, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Berenson, A.B.; Hirth, J.M.; Kuo, Y.F.; Starkey, J.M.; Rupp, R.E. Use of Patient Navigators to Increase HPV Vaccination Rates in a Pediatric Clinical Population. Prev. Med. Rep. 2020, 20, 101194. [Google Scholar] [CrossRef] [PubMed]
- Berenson, A.B.; Rupp, R.; Dinehart, E.E.; Cofie, L.E.; Kuo, Y.F.; Hirth, J.M. Achieving High HPV Vaccine Completion Rates in a Pediatric Clinic Population. Hum. Vaccin. Immunother. 2019, 15, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, T.A.; Broome, M.; Millman, J.; Epstein, J.; Derouin, A. Promoting Strategies to Increase HPV Vaccination in the Pediatric Primary Care Setting. J. Pediatr. Health Care 2022, 36, e36–e41. [Google Scholar] [CrossRef] [PubMed]
- Biehl, R.; Efre, A. Improving Human Papilloma Virus Vaccination Rates among Adolescents. J. Am. Assoc. Nurse Pract. 2023, 35, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Bonville, C.A.; Domachowske, J.B.; Suryadevara, M. A Quality Improvement Education Initiative to Increase Adolescent Human Papillomavirus (HPV) Vaccine Completion Rates. Hum. Vaccin. Immunother. 2019, 15, 1570–1576. [Google Scholar] [CrossRef]
- Bowden, M.; Yaun, J.; Bagga, B. Improving Human Papilloma Virus Vaccination Rates: Quality Improvement. Pediatr. Qual. Saf. 2017, 2, e048. [Google Scholar] [CrossRef]
- Brewer, N.T.; Hall, M.E.; Malo, T.L.; Gilkey, M.B.; Quinn, B.; Lathren, C. Announcements versus Conversations to Improve HPV Vaccination Coverage: A Randomized Trial. Pediatrics 2017, 139, e20161764. [Google Scholar] [CrossRef]
- Brodie, N.; McPeak, K.E. Improving Human Papilloma Virus Vaccination Rates at an Urban Pediatric Primary Care Center. Pediatr. Qual. Saf. 2018, 3, e098. [Google Scholar] [CrossRef]
- Buller, D.B.; Pagoto, S.; Henry, K.; Berteletti, J.; Walkosz, B.J.; Bibeau, J.; Baker, K.; Hillhouse, J.; Arroyo, K.M. Human Papillomavirus Vaccination and Social Media: Results in a Trial With Mothers of Daughters Aged 14–17. Front. Digit. Health 2021, 3, 683034. [Google Scholar] [CrossRef] [PubMed]
- Caskey, R.; Sherman, E.G.; Beskin, K.; Rapport, R.; Xia, Y.; Schwartz, A. A Behavioral Economic Approach to Improving Human Papillomavirus Vaccination. J. Adolesc. Health 2017, 61, 755–760. [Google Scholar] [CrossRef]
- Cassidy, B.; Braxter, B.; Charron-Prochownik, D.; Schlenk, E.A. A Quality Improvement Initiative to Increase HPV Vaccine Rates Using an Educational and Reminder Strategy With Parents of Preteen Girls. J. Pediatr. Health Care 2014, 28, 155–164. [Google Scholar] [CrossRef]
- Cates, J.R.; Crandell, J.L.; Diehl, S.J.; Coyne-Beasley, T. Immunization Effects of a Communication Intervention to Promote Preteen HPV Vaccination in Primary Care Practices. Vaccine 2018, 36, 122–127. [Google Scholar] [CrossRef]
- Cates, J.R.; Diehl, S.J.; Crandell, J.L.; Coyne-Beasley, T. Intervention Effects From a Social Marketing Campaign to Promote HPV Vaccination in Preteen Boys. Vaccine 2014, 32, 4171–4178. [Google Scholar] [CrossRef]
- Cates, J.R.; Fuemmeler, B.F.; Stockton, L.L.; Diehl, S.J.; Crandell, J.L.; Coyne-Beasley, T. Evaluation of a Serious Video Game to Facilitate Conversations About Human Papillomavirus Vaccination for Preteens: Pilot Randomized Controlled Trial. JMIR Serious Games 2020, 8, e16883. [Google Scholar] [CrossRef]
- Coley, S.; Hoefer, D.; Rausch-Phung, E. A Population-Based Reminder Intervention to Improve Human Papillomavirus Vaccination Rates Among Adolescents at Routine Vaccination Age. Vaccine 2018, 36 Pt B, 4904–4909. [Google Scholar] [CrossRef]
- Cox, J.E.; Bogart, L.M.; Elliott, M.N.; Starmer, A.J.; Meleedy-Rey, P.; Goggin, K.; Banerjee, T.; Samuels, R.C.; Hahn, P.D.; Epee-Bounya, A.; et al. Improving HPV Vaccination Rates in a Racially and Ethnically Diverse Pediatric Population. Pediatrics 2022, 150, e2021054186. [Google Scholar] [CrossRef]
- Daley, M.F.; Kempe, A.; Pyrzanowski, J.; Vogt, T.M.; Dickinson, L.M.; Kile, D.; Fang, H.; Rinehart, D.J.; Shlay, J.C. School-Located Vaccination of Adolescents With Insurance Billing: Cost, Reimbursement, and Vaccination Outcomes. J. Adolesc. Health 2014, 54, 282–288. [Google Scholar] [CrossRef]
- Dang, J.H.T.; McClure, S.; Gori, A.C.T.; Martens, T.; Mojadedi, A.; Smith, U.; Austin, C.J.; Chen, M.S., Jr. Implementation and Evaluation of a Multilevel Intervention to Increase Uptake of the Human Papillomavirus Vaccine Among Rural Adolescents. J. Rural Health 2023, 39, 136–141. [Google Scholar] [CrossRef]
- Davis, K.R.; Norman, S.L.; Olson, B.G.; Demirel, S.; Taha, A.A. A Clinical Educational Intervention to Increase HPV Vaccination Rates Among Pediatric Patients Through Enhanced Recommendations. J. Pediatr. Health Care 2022, 36, 589–597. [Google Scholar] [CrossRef]
- Dempsey, A.F.; Pyrznawoski, J.; Lockhart, S.; Barnard, J.; Campagna, E.J.; Garrett, K.; Fisher, A.; Dickinson, L.M.; O’Leary, S.T. Effect of a Health Care Professional Communication Training Intervention on Adolescent Human Papillomavirus Vaccination: A Cluster Randomized Clinical Trial. JAMA Pediatr. 2018, 172, e180016. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.E.; Zimet, G.D.; Xiao, S.; Tu, W.; Lindsay, B.; Church, A.; Downs, S.M. An Educational Intervention to Improve HPV Vaccination: A Cluster Randomized Trial. Pediatrics 2019, 143, e20181457. [Google Scholar] [CrossRef] [PubMed]
- Eldred, S.V.; Hamid, H.S.; Snider, J.C.; Weinberg, S.H.; Speck, N.; Reed, B.D.; Riley, M. A Medical Student-Driven “Vaccine Blitz” at a School-Based Health Center as an Effective Way to Improve Adolescent Vaccination Rates. Fam. Med. 2015, 47, 546–548. [Google Scholar] [PubMed]
- Fiks, A.G.; Grundmeier, R.W.; Mayne, S.; Song, L.; Feemster, K.; Karavite, D.; Hughes, C.C.; Massey, J.; Keren, R.; Bell, L.M.; et al. Effectiveness of Decision Support for Families, Clinicians, or Both on HPV Vaccine Receipt. Pediatrics 2013, 131, 1114–1124. [Google Scholar] [CrossRef]
- Fisher-Borne, M.; Preiss, A.J.; Black, M.; Roberts, K.; Saslow, D. Early Outcomes of a Multilevel Human Papillomavirus Vaccination Pilot Intervention in Federally Qualified Health Centers. Acad. Pediatr. 2018, 18, S79–S84. [Google Scholar] [CrossRef]
- Gilkey, M.B.; Grabert, B.K.; Heisler-MacKinnon, J.; Bjork, A.; Boynton, M.H.; Kim, K.; Alton Dailey, S.; Liu, A.; Todd, K.G.; Schauer, S.L.; et al. Coaching and Communication Training for HPV Vaccination: A Cluster Randomized Trial. Pediatrics 2022, 150, e2021052351. [Google Scholar] [CrossRef]
- Gilkey, M.B.; Heisler-MacKinnon, J.; Boynton, M.H.; Calo, W.A.; Moss, J.L.; Brewer, N.T. Impact of Brief Quality Improvement Coaching on Adolescent HPV Vaccination Coverage: A Pragmatic Cluster Randomized Trial. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 957–962. [Google Scholar] [CrossRef]
- Glenn, B.A.; Crespi, C.M.; Herrmann, A.K.; Nonzee, N.J.; Rosen, D.L.; Park, C.L.; Johnson, G.; Chang, L.C.; Singhal, R.; Taylor, V.M.; et al. Effectiveness and Feasibility of Three Types of Parent Reminders to Increase Adolescent Human Papillomavirus (HPV) Vaccination. Prev. Med. 2023, 169, 107448. [Google Scholar] [CrossRef]
- Glenn, B.A.; Nonzee, N.J.; Herrmann, A.K.; Crespi, C.M.; Haroutunian, G.G.; Sundin, P.; Chang, L.C.; Singhal, R.; Taylor, V.M.; Bastani, R. Impact of a Multi-Level, Multi-Component, System Intervention on HPV Vaccination in a Federally Qualified Health Center. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 1952–1958. [Google Scholar] [CrossRef]
- Gurfinkel, D.; Kempe, A.; Albertin, C.; Breck, A.; Zhou, X.; Vangala, S.; Beaty, B.; Rice, J.; Tseng, C.H.; Campbell, J.D.; et al. Centralized Reminder/Recall for Human Papillomavirus Vaccination: Findings From Two States-A Randomized Clinical Trial. J. Adolesc. Health 2021, 69, 579–587. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Zhu, W.; Baba, L.; Nguyen, M.; Berthoud, H.; Gundersen, G.; Hofstetter, A.M. Outreach and Reminders to Improve Human Papillomavirus Vaccination in an Integrated Primary Care System. Clin. Pediatr. 2018, 57, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.P.; Bernstein, J.; Pelton, S.; Belizaire, M.; Goff, G.; Horanieh, N.; Freund, K.M. Brief Client-Centered Motivational and Behavioral Intervention to Promote HPV Vaccination in a Hard-to-Reach Population: A Pilot Randomized Controlled Trial. Clin. Pediatr. 2016, 55, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Do, T.Q.N.; Hsu, E.; Schmeler, K.M.; Montealegre, J.R.; Rodriguez, A.M. School-Based Human Papillomavirus Vaccination Program for Increasing Vaccine Uptake in an Underserved Area in Texas. Papillomavirus Res. 2019, 8, 100189. [Google Scholar] [CrossRef] [PubMed]
- Kempe, A.; O’Leary, S.T.; Shoup, J.A.; Stokley, S.; Lockhart, S.; Furniss, A.; Dickinson, L.M.; Barnard, J.; Daley, M.F. Parental Choice of Recall Method for HPV Vaccination: A Pragmatic Trial. Pediatrics 2016, 137, e20152857. [Google Scholar] [CrossRef]
- Krantz, L.; Ollberding, N.J.; Beck, A.F.; Carol Burkhardt, M. Increasing HPV Vaccination Coverage Through Provider-Based Interventions. Clin. Pediatr. 2018, 57, 319–326. [Google Scholar] [CrossRef]
- Lennon, T.; Gundacker, C.; Nugent, M.; Simpson, P.; Magallanes, N.K.; West, C.; Willis, E. Ancillary Benefit of Increased HPV Immunization Rates Following a CBPR Approach to Address Immunization Disparities in Younger Siblings. J. Community Health 2019, 44, 544–551. [Google Scholar] [CrossRef]
- Mackey, J.K.; Thompson, K.; Abdulwahab, A.; Huntington, M.K. A Simple Intervention to Increase Human Papillomavirus Vaccination in a Family Medicine Practice. S. D. Med. 2019, 72, 438–441. [Google Scholar]
- Mayne, S.L.; duRivage, N.E.; Feemster, K.A.; Localio, A.R.; Grundmeier, R.W.; Fiks, A.G. Effect of Decision Support on Missed Opportunities for Human Papillomavirus Vaccination. Am. J. Prev. Med. 2014, 47, 734–744. [Google Scholar] [CrossRef]
- McLean, H.Q.; VanWormer, J.J.; Chow, B.D.W.; Birchmeier, B.; Vickers, E.; DeVries, E.; Meyer, J.; Moore, J.; McNeil, M.M.; Stokley, S.; et al. Improving Human Papillomavirus Vaccine Use in an Integrated Health System: Impact of a Provider and StaffIntervention. J. Adolesc. Health 2017, 61, 252–258. [Google Scholar] [CrossRef]
- O’Leary, S.C.; Frost, H.M. Does HPV Vaccination Initiation at Age 9 Improve HPV Initiation and Vaccine Series Completion Rates by Age 13? Hum. Vaccin. Immunother. 2023, 19, 2180971. [Google Scholar] [CrossRef] [PubMed]
- Parra-Medina, D.; Morales-Campos, D.Y.; Mojica, C.; Ramirez, A.G. Promotora Outreach, Education and Navigation Support for HPV Vaccination to Hispanic Women with Unvaccinated Daughters. J. Cancer Educ. 2015, 30, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Paskett, E.D.; Krok-Schoen, J.L.; Pennell, M.L.; Tatum, C.M.; Reiter, P.L.; Peng, J.; Bernardo, B.M.; Weier, R.C.; Richardson, M.S.; Katz, M.L. Results of a Multilevel Intervention Trial to Increase Human Papillomavirus (HPV) Vaccine Uptake Among Adolescent Girls. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Foley, S.; Hassan, A.; Jansen, E.; Preiss, S.; Isher-Witt, J.; Fisher-Borne, M. Impact of a Multilevel Quality Improvement Intervention Using National Partnerships on Human Papillomavirus Vaccination Rates. Acad. Pediatr. 2021, 21, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Legler, A.; Jansen, E.; Bernstein, J.; Pierre-Joseph, N.; Eun, T.J.; Biancarelli, D.L.; Schuch, T.J.; Leschly, K.; Fenton, A.T.H.R.; et al. Improving HPV Vaccination Rates: A Stepped-Wedge Randomized Trial. Pediatrics 2020, 146, e20192737. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.; Southard, E. Teaching It Forward: Educating Parents About HPV/HPV Vaccine. J.Doctoral Nurs. Pract. 2019, 12, 46–58. [Google Scholar] [CrossRef]
- Rand, C.M.; Brill, H.; Albertin, C.; Humiston, S.G.; Schaffer, S.; Shone, L.P.; Blumkin, A.K.; Szilagyi, P.G. Effectiveness of Centralized Text Message Reminders on Human Papillomavirus Immunization Coverage for Publicly Insured Adolescents. J. Adolesc. Health 2015, 56 (Suppl. S5), S17–S20. [Google Scholar] [CrossRef]
- Rand, C.M.; Vincelli, P.; Goldstein, N.P.N.; Blumkin, A.; Szilagyi, P.G. Effects of Phone and Text Message Reminders on Completion of the Human Papillomavirus Vaccine Series. J. Adolesc. Health 2017, 60, 113–119. [Google Scholar] [CrossRef]
- Rand, C.M.; Tyrrell, H.; Wallace-Brodeur, R.; Goldstein, N.P.N.; Darden, P.M.; Humiston, S.G.; Albertin, C.S.; Stratbucker, W.; Schaffer, S.J.; Davis, W.; et al. A Learning Collaborative Model to Improve Human Papillomavirus Vaccination Rates in Primary Care. Acad. Pediatr. 2018, 18, S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Richman, A.R.; Torres, E.; Wu, Q.; Carlston, L.; O’Rorke, S.; Moreno, C.; Olsson, J. Text and Email Messaging for Increasing Human Papillomavirus Vaccine Completion among Uninsured or Medicaid-Insured Adolescents in Rural Eastern North Carolina. J. Health Care Poor Underserved 2019, 30, 1499–1517. [Google Scholar] [CrossRef]
- Rickert, V.I.; Auslander, B.A.; Cox, D.S.; Rosenthal, S.L.; Rupp, R.E.; Zimet, G.D. School-Based HPV Immunization of Young Adolescents: Effects of Two Brief Health Interventions. Hum. Vaccin. Immunother. 2015, 11, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Santa Maria, D.; Markham, C.; Misra, S.M.; Coleman, D.C.; Lyons, M.; Desormeaux, C.; Cron, S.; Guilamo-Ramos, V. Effects of a Randomized Controlled Trial of a Brief, Student-Nurse Led, Parent-Based Sexual Health Intervention on Parental Protective Factors and HPV Vaccination Uptake. BMC Public Health 2021, 21, 585. [Google Scholar] [CrossRef] [PubMed]
- Scarinci, I.C.; Hansen, B.; Kim, Y.I. HPV Vaccine Uptake among Daughters of Latinx Immigrant Mothers: Findings from a Cluster Randomized Controlled Trial of a Community-Based, Culturally Relevant Intervention. Vaccine 2020, 38, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Shegog, R.; Savas, L.S.; Healy, C.M.; Frost, E.L.; Coan, S.P.; Gabay, E.K.; Preston, S.M.; Spinner, S.W.; Wilbur, M.; Becker, E.; et al. AVPCancerFree: Impact of a Digital Behavior Change Intervention on Parental HPV Vaccine-Related Perceptions and Behaviors. Hum. Vaccin. Immunother. 2022, 18, 2087430. [Google Scholar] [CrossRef] [PubMed]
- Smajlovic, A.; Toth, C.D. Quality Improvement Project to Increase Human Papillomavirus Two-Dose Vaccine Series Completion by 13 Years in Pediatric Primary Care Clinics. J. Adolesc. Health 2023, 72, 958–963. [Google Scholar] [CrossRef]
- Staras, S.A.; Vadaparampil, S.T.; Livingston, M.D.; Thompson, L.A.; Sanders, A.H.; Shenkman, E.A. Increasing Human Papillomavirus Vaccine Initiation Among Publicly Insured Florida Adolescents. J. Adolesc. Health 2015, 56 (Suppl. S5), S40–S46. [Google Scholar] [CrossRef]
- Staras, S.A.S.; Vadaparampil, S.T.; Thompson, L.A.; Scherr, C.; Gurka, M.J.; Filipp, S.L.; Shenkman, E.A. Postcard Reminders for HPV Vaccination Mainly Primed Parents for Providers’ Recommendations. Prev. Med. Rep. 2020, 20, 101188. [Google Scholar] [CrossRef]
- Steiner, C.R.; Dechant, J.; Brungo, L.; Cassidy, B. An Evidence-Based Protocol to Improve HPV Vaccine Initiation Rates at a County Immunization Clinic. J. Community Health Nurs. 2021, 38, 73–84. [Google Scholar] [CrossRef]
- Strasel, M.; VanLangen, K.M.; Benzer, J.; Geyer, A.; Jameson, A.P.; Dumkow, L.E. HPV Vaccination Rates in 9- and 10-Year-Olds Following a Pharmacist-Led Intervention. J. Am. Pharm. Assoc. (2003) 2023, 64, 278–282. [Google Scholar] [CrossRef]
- Szilagyi, P.; Albertin, C.; Gurfinkel, D.; Beaty, B.; Zhou, X.; Vangala, S.; Rice, J.; Campbell, J.D.; Whittington, M.D.; Valderrama, R.; et al. Effect of State Immunization Information System Centralized Reminder and Recall on HPV Vaccination Rates. Pediatrics 2020, 145, e20192689. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Albertin, C.; Humiston, S.G.; Rand, C.M.; Schaffer, S.; Brill, H.; Stankaitis, J.; Yoo, B.K.; Blumkin, A.; Stokley, S. A Randomized Trial of the Effect of Centralized Reminder/Recall on Immunizations and Preventive Care Visits for Adolescents. Acad. Pediatr. 2013, 13, 204–213. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Humiston, S.G.; Stephens-Shields, A.J.; Localio, R.; Breck, A.; Kelly, M.K.; Wright, M.; Grundmeier, R.W.; Albertin, C.; Shone, L.P.; et al. Effect of Training Pediatric Clinicians in Human Papillomavirus Communication Strategies on Human Papillomavirus Vaccination Rates: A Cluster Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Underwood, N.L.; Gargano, L.M.; Jacobs, S.; Seib, K.; Morfaw, C.; Murray, D.; Hughes, J.M.; Sales, J.M. Influence of Sources of Information and Parental Attitudes on Human Papillomavirus Vaccine Uptake Among Adolescents. J. Pediatr. Adolesc. Gynecol. 2016, 29, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Varman, M.; Sharlin, C.; Fernandez, C.; Vasudevan, J.; Wichman, C. Human Papilloma Virus Vaccination Among Adolescents in a Community Clinic Before and After Intervention. J. Community Health 2018, 43, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Vinci, D.M.; Ryan, J.; Howard, M.; Snider, D.; Strahan, B.; Smith, G.; McClain, R. Increasing Human Papillomavirus Vaccination in a Federally Qualified Health Center Organization Using a Systems-Based Intervention Integrating EHR and Statewide Immunization Information System. J. Community Health 2022, 47, 53–62. [Google Scholar] [CrossRef]
- White, L.S.; Maulucci, E.; Kornides, M.; Aryal, S.; Alix, C.; Sneider, D.; Gagnon, J.; Winfield, E.C.; Fontenot, H.B. HPV Vaccination Rates of 7th Grade Students After a Strong Recommending Statement from the School Nurse. J. Sch. Nurs. 2022, 40, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.A.; Dixon, B.E.; Xiao, S.; Tu, W.; Lindsay, B.; Sheley, M.; Dugan, T.; Church, A.; Downs, S.M.; Zimet, G. Physician Clinical Decision Support System Prompts and Administration of Subsequent Doses of HPV Vaccine: A Randomized Clinical Trial. Vaccine 2019, 37, 4414–4418. [Google Scholar] [CrossRef]
- Winer, R.L.; Gonzales, A.A.; Noonan, C.J.; Buchwald, D.S. A Cluster-Randomized Trial to Evaluate a Mother-Daughter Dyadic Educational Intervention for Increasing HPV Vaccination Coverage in American Indian Girls. J. Community Health 2016, 41, 274–281. [Google Scholar] [CrossRef]
- Woodall, W.G.; Zimet, G.; Kong, A.; Buller, D.; Reither, J.; Chilton, L.; Myers, V.; Starling, R. Vacteens.org: A Mobile Web App to Improve HPV Vaccine Uptake. Front. Digit. Health 2021, 3, 693688. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Raviotta, J.M.; Nowalk, M.P.; Moehling, K.K.; Reis, E.C.; Humiston, S.G.; Lin, C.J. Using the 4 Pillars™ Practice Transformation Program to Increase Adolescent Human Papillomavirus, Meningococcal, Tetanus-Diphtheria-Pertussis and Influenza Vaccination. Vaccine 2017, 35, 6180–6186. [Google Scholar] [CrossRef]
- Zorn, S.; Darville-Sanders, G.; Vu, T.; Carter, A.; Treend, K.; Raunio, C.; Vasavada, A. Multi-Level Quality Improvement Strategies to Optimize HPV Vaccination Starting at the 9-Year Well Child Visit: Success Stories from Two Private Pediatric Clinics. Hum. Vaccin. Immunother. 2023, 19, 2163807. [Google Scholar] [CrossRef]
- Villarroel, M.A.; Galinsky, A.M.; Lu, P.J.; Pingali, C. Human Papillomavirus Vaccination Coverage in Children Ages 9–17 Years: United States, 2022; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar]
- Do, E.K.; Rossi, B.; Miller, C.A.; Ksinan, A.J.; Wheeler, D.C.; Chukmaitov, A.; Fuemmeler, B.F. Area-Level Variation and Human Papillomavirus Vaccination Among Adolescents and Young Adults in the United States: A Systematic Review. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 13–21. [Google Scholar] [CrossRef]
- Kurani, S.; MacLaughlin, K.L.; Jacobson, R.M.; Sauver, J.L.S.; Jenkins, G.D.; Fan, C.; Rutten, L.J.F. Socioeconomic Disadvantage and Human Papillomavirus (HPV) Vaccination Uptake. Vaccine 2022, 40, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Humble, S.; Barnette, A.; Brandt, H.; Thompson, V.; Klesges, L.M.; Silver, M.I. Associations of Geographic-Based Socioeconomic Factors and HPV Vaccination Among Male and Female Children in Five US States. BMC Public Health 2024, 24, 702. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Gerend, M.A.; Boakye, E.A. Rural–Urban Differences in Human Papillomavirus Vaccination Among Young Adults in 8 US States. Am. J. Prev. Med. 2021, 60, 298–299. [Google Scholar] [CrossRef] [PubMed]
| HPV Round Table: HPV Vaccination Best Practices Learning Collaborative, Interventions | |
|---|---|
| Intervention Type and Descriptions | |
| Provider Education | Community meetings, pediatric provider meetings, guest speakers, weekly newsletters, encouraging articles, social media, and webinars. |
| Provider Incentives | Linking adolescent immunization to provider performance and compensation. |
| Patient Education | Incorporating patient education resources in exam rooms, flyers, letters, emails, texts, HER features, webinars, handouts, resources, and conversations. |
| Patient Outreach | Gap-closure and missed opportunity reports, personal phone calls, scripts to answer common questions, postcard campaigns, letters, calls, HER features such as reminder messages. |
| Patient Scheduling | Telehealth and virtual exam rooms, adding HPV vaccines to drive through flu vaccine settings, in-school onsite vaccine services, back-to-school campaigns, annual wellness visits, standing orders. |
| Electronic Health Record (EHR) Feature | Reports and alerting messages, overdue for visit reports, overdue for vaccination reports, alerts with real-time information during visits. |
| Author/ Year | Setting | Sample | Sample Size (Youth) | Study Design | Intervention Type | HPV Vaccine Outcome Variable | Demographics Analyzed by HPV Outcome Variable |
|---|---|---|---|---|---|---|---|
| Agana-Norman 2022 [16] | Other | Male youth | 97,587 | Non-experimental | Provider Education; Patient Education | Initiation and Completion | Race/ethnicity; Income/SES; Geography; Parental Education; Age |
| Aragones 2015 [17] | Community | Mexican/Mexican American parents | 69 | Quasi-experimental | Patient Education | Completion | Sex |
| Baldwin 2021 [18] | Clinic | Parents of pediatric patients | 161 | Experimental | Patient Education; Electronic Health Record (EHR) Feature | Vaccination status | - |
| Bastani 2022 [19] | Community | Youth Caregiver | 225 | Experimental | Patient Education | Initiation and Completion | - |
| Beck 2021 [20] | Clinic | Parents of youth 11–17 | 24 | Non-experimental | Patient Education | Initiation | - |
| Berenson 2019 [21] | Clinic | Families with children | 2162 | Non-experimental | Provider Education; Patient Education; Patient Scheduling; EHR Feature | Initiation and Completion | Sex; Race/ethnicity; Age |
| Berenson 2020 [22] | Clinic | Youth 9–17 | 21,395 | Quasi-experimental | Provider Education; Patient Education; Patient Scheduling | Whether visit included vaccination | Race/ethnicity; Age |
| Bernstein 2022 [23] | Clinic | Youth 11–12 | 128 | Non-experimental | Provider Education; Patient Education; Patient Outreach; EHR Feature; Standing Orders | Initiation | - |
| Biehl 2023 [24] | Clinic | Youth 11–17 | 1374 | Non-experimental | Provider Education; Patient Education; Patient Outreach; Standing Orders | Initiation and Completion | Sex; Insurance |
| Bonville 2019 [25] | Clinic | Youth 11–12; Providers | 232 | Non-experimental | Provider Education; Provider Incentives; EHR Feature | Initiation and Completion | - |
| Bowden 2017 [26] | Clinic | Youth 9–13 | 265 | Non-experimental | Provider Education; Patient Education; EHR Feature | Initiation | - |
| Brewer 2017 [27] | Clinic | Youth 11–17 | 37,796 | Experimental | Provider Education | Initiation and Completion | Sex; Insurance |
| Brodie 2018 [28] | Clinic | Youth 9–10 | 6703 | Non-experimental | Provider Education; EHR Feature | Initiation | - |
| Buller 2021 [29] | Online | Mothers and daughters 14–17 | 469 | Non-experimental | Patient Education | Initiation and Completion | - |
| Caskey 2017 [30] | Clinic | Youth 11–17; Parent/ guardian | 188 | Other: Quasi-randomized trial | Patient Outreach | Initiation and Completion | Sex; Race/ethnicity; Insurance |
| Cassidy 2014 [31] | Clinic | Parents of girls 11–12 | 53 | Quasi-experimental | Provider Education; Patient Education; Patient Scheduling; EHR Feature | Initiation and Completion | - |
| Cates 2014 [32] | Clinic; Community; Online | Boys 11–13 | 25,870 | Quasi-experimental | Provider Education; Patient Education | Initiation | Race/ethnicity; Age |
| Cates 2018 [33] | Clinic | Youth 9–14 | 147,294 | Other: Quasi-experimental | Provider Education; Provider Incentives; Patient Education | Initiation and Completion | Sex; Age |
| Cates 2020 [34] | Clinic; Online | Youth 11–12; Parents | 55 | Experimental | Patient Education | Initiation and Completion | - |
| Coley 2018 [35] | Community | Youth 11–13; Parent/ guardian | 303,965 | Experimental | Patient Education; Patient Outreach | Initiation and Completion | Sex |
| Cox 2022 [36] | Clinic | Youth 9–13 | 12,270 | Non-experimental | Provider Education; Patient Education; Patient Outreach; Patient Scheduling; EHR Feature; Standing Orders | Initiation and Completion | Sex; Race/ethnicity; Age |
| Daley 2014 [37] | School | Girls 6–8th grade | - | Experimental | Patient Outreach | Initiation and Completion | - |
| Dang 2023 [38] | Clinic | Youth 11–17 | 498 | Non-experimental | Provider Education; Patient Education; Patient Outreach; EHR Feature; Standing Orders | Initiation and Completion | - |
| Davis 2022 [39] | Clinic | Youth 12 | 39 | Non-experimental | Provider Education | HPV vaccination rate | Race/ethnicity; Insurance Status; Parental Age |
| Dempsey 2018 [40] | Clinic | Youth 11–17 | 43,132 | Experimental | Provider Education; Patient Education | Initiation and Completion | Sex; Age |
| Dixon 2019 [41] | Clinic | Parents/guardians of youth 11–17 | 1596 | Experimental | Patient Education; EHR Feature | Initiation and Completion | - |
| Eldred 2015 [42] | School | Middle school students | 184 | Quasi-experimental | Patient Education; Patient Outreach; Patient Scheduling | Initiation | Sex |
| Fiks 2013 [43] | Clinic | Girls 11–17 | 22,486 | Experimental | Provider Education; Patient Education; Patient Outreach; Patient Scheduling; EHR Feature | Initiation and Completion | Age |
| Fisher-Borne 2018 [44] | Clinic | Youth 11–12 | >20,000 | Non-experimental | Provider Education; Patient Outreach; EHR Feature; Standing Orders | Initiation and Completion | - |
| Gilkey 2022 [45] | Clinic | Youth 11–17 | 176,189 | Experimental | Provider Education; Provider Incentives; Standing Orders | Initiation and Completion | Geography; Age |
| Gilkey 2023 [46] | Clinic | Practices | 312,227 | Experimental | Provider Education | Initiation | Age |
| Glenn 2022 [47] | Clinic | Youth 11–17 | 14,738 | Experimental | Provider Education; Patient Outreach; Standing Orders | Initiation and Completion | Sex |
| Glenn 2023 [48] | Clinic | Youth 12; Caregiver | 877 | Quasi-experimental | Patient Outreach; Patient Scheduling | Receipt of next-dose vaccination | Sex |
| Gurfinkel 2021 [49] | Clinic | Youth 11–14 | 69,286 | Experimental | Patient Outreach | Initiation and Completion | Sex; Age |
| Henrikson 2018 [50] | Clinic | Parents of youth 10–12 | 1624 | Experimental | Patient Education; Patient Outreach | Initiation and Completion | Sex; Age |
| Joseph 2016 [51] | Clinic | Mothers of daughters 11–15 | 200 | Experimental | Patient Education | Initiation and Completion | - |
| Kaul 2019 [52] | School | 6th-, 7th-, and 8th-grade students | 2307 | Quasi-experimental | Patient Education | Initiation | Sex; Age |
| Kempe 2016 [53] | Clinic | Youth 11–17 | 929 | Experimental | Patient Outreach | Completion | Sex; Race/ethnicity; Age |
| Krantz 2018 [54] | Clinic | Youth 13–17; Providers | 314 | Non-experimental | Provider Education; Patient Scheduling; EHR Feature | Completion | Sex |
| Lennon 2019 [55] | Community | African American youth 13–17 | 118 | Quasi-experimental | Patient Education; Patient Outreach | Completion | - |
| Mackey 2019 [56] | Clinic; Other | Youth 11–12 | 247 | Non-experimental | Provider Education; Patient Education; EHR Feature | Initiation and Completion | Sex |
| Mayne 2014 [57] | Clinic | Girls 11–17 | 17,016 | Experimental | Provider Education; Patient Education; Patient Outreach; EHR Feature | HPV receipt | Geography |
| McLean 2017 [58] | Clinic | Youth 11–17 | 24, 658 | Quasi-experimental | Provider Education; Patient Outreach | Initiation and Completion | - |
| O’Leary 2023 [59] | Clinic | Youth 9–13 | 25,888 | Non-experimental | Provider Education; EHR Feature | Initiation and Completion | - |
| Parra-Medina 2015 [60] | Community | Hispanic women with daughters 11–17 | 372 | Quasi-experimental | Patient Education; Patient Outreach | Initiation and Completion | Parental Employment; Marital Status; Education; Insurance |
| Paskett 2016 [61] | Clinic; Other | Parent/guardian of girls 9–16 | 337 | Experimental | Provider Education; Patient Education | Initiation | - |
| Perkins 2020 [62] | Clinic | Youth 9–17; Parent/ guardian; Provider | 16,136 | Experimental | Provider Education | Initiation and Completion | Sex; Race/ethnicity; Geography; English Language |
| Perkins 2021 [63] | Clinic | Youth 13; Providers | 3283 | Non-experimental | Provider Education; Provider Incentives; Patient Outreach; EHR Feature; Standing Orders | Initiation and Completion | - |
| Potts 2019 [64] | Clinic | Parents of children 9–17 | 46 | Non-experimental | Patient Education | HPV vaccination rate. | Sex |
| Rand 2015 [65] | Clinic | Youth 11–16; Parents | 3812 | Experimental | Patient Outreach; Patient Scheduling | Initiation and Completion | Sex; Age |
| Rand 2017 [66] | Clinic | Youth 11–17; Parents | 749 | Experimental | Patient Outreach | Completion | Sex |
| Rand 2018 [67] | Clinic | Youth 11–17 | 43,435 | Non-experimental | Provider Education; Provider Incentives; EHR Feature; Standing Orders | Initiation and Completion | Sex |
| Richman 2019 [68] | Clinic | Parents of youth 9–17 | 257 | Experimental | Patient Education; Patient Outreach | Competition | Sex; Race/ethnicity; Income/SES; English Language |
| Rickert 2015 [69] | School; Clinic; Other | Parents of youth 11–15 | 445 | Experimental | Patient Education; Patient Outreach | Initiation and Completion | (All Parental) Sex; Race/ethnicity; Marital Status; Education; Insurance; Age |
| Santa Maria 2021 [70] | Community | Parents of youth 11–14 | 508 | Experimental | Patient Education; Patient Outreach; Patient Scheduling | Initiation and Completion | Sex |
| Scarinci 2020 [71] | Community | Latina immigrants with daughters 9–12 | 278 | Experimental | Patient Education; Patient Outreach | Completion | Income/SES |
| Shegog 2022 [72] | Clinic | Youth 11–17; Parents | 375 | Experimental | Patient Education; Patient Outreach; EHR Feature | Initiation | - |
| Smajlovic 2023 [73] | Clinic | Youth 13; Parents | 81,000 | Non-experimental | Provider Education; Patient Education; EHR Feature | Completion | Sex; Race/ethnicity |
| Staras 2015 [74] | Clinic | Youth 11–17; Parents | 6123 | Quasi-experimental | Provider Education; Patient Education; Patient Outreach | Initiation | Sex |
| Staras 2020 [75] | Other | Girls 11–17 | 2773 | Quasi-experimental | Patient Outreach | Initiation | - |
| Steiner 2021 [76] | Clinic | Youth 11–14; Registered nurses | 209 | Quasi-experimental | Provider Education; Patient Education; EHR Feature; Standing Orders | HPV Vaccination | Sex |
| Strasel 2023 [77] | Clinic | Youth 9–10 | 367 | Non-experimental | Provider Education; Patient Education; Patient Outreach; EHR Feature | Initiation | - |
| Szilagyi 2013 [78] | Clinic | Youth 10.5–17 | 4115 | Experimental | Patient Outreach | Initiation and Completion | Sex; Income/SES; Insurance; Geography; Age |
| Szilagyi 2020 [79] | Clinic | Youth 11–19 | 62,118 | Experimental | Patient Outreach | Initiation and Completion | Sex; Geography; Age |
| Szilagyi 2021 [80] | Clinic | Youth 11–17; Providers | 104,438 | Experimental | Provider Education | Initiation and Completion | Sex; Age |
| Underwood 2016 [81] | School | Girls under 18; Parent/guardian | 360 | Experimental | Patient Education; Patient Outreach | Initiation | Sex; Race/ethnicity; Age |
| Varman 2018 [82] | Clinic | Youth 9–17; Parent/ guardian; Providers | 3393 | Non-experimental | Provider Education; Patient Education; Patient Outreach | Initiation and Completion | Sex; Geography; Insurance |
| Vinci 2022 [83] | Clinic | Youth 11–16 | 8960 | Non-experimental | Provider Education; Patient Outreach; EHR Feature | Initiation and Completion | Sex; Age |
| White 2022 [84] | School | 7th grade students; Parents/guardians | 1686 | Quasi-experimental | Patient Education; Patient Outreach | Initiation | Sex; Race/ethnicity; Insurance |
| Wilkinson 2019 [85] | Clinic | Youth 11–17; Providers | 1285 | Experimental | EHR Feature | Initiation | Sex; Age |
| Winer 2016 [86] | Community | Mothers or legal guardians of girls 9–12 | 97 | Experimental | Patient Education | Initiation and Completion | Income/SES; Age |
| Woodall 2021 [87] | Clinic | Parents and daughters 11–14 | 82 | Experimental | Patient Education; Patient Outreach | Initiation and Completion | - |
| Zimmerman 2017 [88] | Clinic | Youth 11–17 | 9473 | Non-experimental | Provider Education; Patient Outreach; Standing Orders | Initiation and Completion | Sex; Race/ethnicity; Insurance; Age |
| Zorn 2023 [89] | Clinic | Youth 11–17 | 45,859 in May 2022 | Non-experimental | Provider Education; Patient Education; EHR Feature | Initiation and Completion | - |
| Author/Year * | Sex | Age | Race/ Ethnicity | Income/ SES | Geography | Insurance | Other |
|---|---|---|---|---|---|---|---|
| Agana-Norman 2022 [16] | - | S | S | S | S | - | S |
| Aragones 2015 [17] | NS | - | - | - | - | - | - |
| Berenson 2019 [21] | NS | S | S | - | - | - | - |
| Berenson 2020 [22] | - | S | S | - | - | - | - |
| Biehl 2023 [24] | NS | - | - | - | - | NS | - |
| Brewer 2017 [27] | S | ||||||
| Caskey 2017 [30] | S | - | S | - | - | NS | - |
| Cates 2014 [32] | - | S | NS | - | - | - | - |
| Cates 2018 [34] | S | S | - | - | - | - | - |
| Cox 2022 [36] | S | - | S | - | - | - | - |
| Davis 2022 [39] | - | NS | NS | - | - | NS | NS |
| Dempsey 2018 [40] | NS | - | - | - | - | - | - |
| Gilkey 2022 [45] | - | - | - | - | NS | - | - |
| Glenn 2022 [47] | S | - | - | - | - | - | - |
| Gurfinkel 2021 [49] | S | S | - | - | - | - | - |
| Henrikson 2018 [50] | - | NS | - | - | - | - | - |
| Kaul 2019 [52] | NS | S | - | - | - | - | - |
| Kempe 2016 [53] | NS | S | NS | - | - | - | - |
| Mayne 2014 [57] | - | - | - | - | S | - | - |
| Parra-Medina 2015 [60] | - | S | - | - | - | S | S |
| Perkins 2020 [62] | S | - | S | - | - | S | S |
| Rand 2015 [65] | NS | NS | - | - | - | - | - |
| Rand 2017 [66] | S | - | - | - | - | - | - |
| Richman 2019 [68] | NS | NS | NS | NS | - | - | NS |
| Rickert 2015 [69] | NS | S | S | - | - | NS | NS |
| Santa Maria 2021 [70] | NS | - | - | - | - | - | - |
| Scarinci 2020 [71] | - | - | - | NS | - | NS | NS |
| Szilagyi 2013 [78] | NS | NS | - | NS | - | NS | - |
| Szilagyi 2020 [79] | NS | NS | - | - | NS | - | - |
| Szilagyi 2021 [80] | NS | NS | - | - | - | - | - |
| Underwood 2016 [81] | S | NS | NS | - | - | - | - |
| Varman 2018 [82] | - | - | - | - | S | S | - |
| Vinci 2022 [83] | NS | S | - | - | - | - | - |
| Wilkinson 2019 [85] | NS | NS | - | - | - | - | - |
| Winer 2016 [86] | - | S | - | S | - | - | - |
| Zimmerman 2017 [88] | S | S | S | - | - | S | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maness, S.B.; Carpenter, L.C.; Akpan, I.; St. James, N.; Romero-Cely, D.; Harmon, G.J.C.; Cano, M.; Thompson, E.L. Health Equity and Human Papillomavirus Vaccine Interventions for Adolescents: A Systematic Review. Vaccines 2025, 13, 485. https://doi.org/10.3390/vaccines13050485
Maness SB, Carpenter LC, Akpan I, St. James N, Romero-Cely D, Harmon GJC, Cano M, Thompson EL. Health Equity and Human Papillomavirus Vaccine Interventions for Adolescents: A Systematic Review. Vaccines. 2025; 13(5):485. https://doi.org/10.3390/vaccines13050485
Chicago/Turabian StyleManess, Sarah B., Lois Coleman Carpenter, Idara Akpan, Nubwa St. James, Daniela Romero-Cely, G. J. Corey Harmon, Miranda Cano, and Erika L. Thompson. 2025. "Health Equity and Human Papillomavirus Vaccine Interventions for Adolescents: A Systematic Review" Vaccines 13, no. 5: 485. https://doi.org/10.3390/vaccines13050485
APA StyleManess, S. B., Carpenter, L. C., Akpan, I., St. James, N., Romero-Cely, D., Harmon, G. J. C., Cano, M., & Thompson, E. L. (2025). Health Equity and Human Papillomavirus Vaccine Interventions for Adolescents: A Systematic Review. Vaccines, 13(5), 485. https://doi.org/10.3390/vaccines13050485





