Plant-Derived Immunomodulatory Nanoadjuvants for Cancer Vaccines: Current Status and Future Opportunities
Abstract
1. Introduction
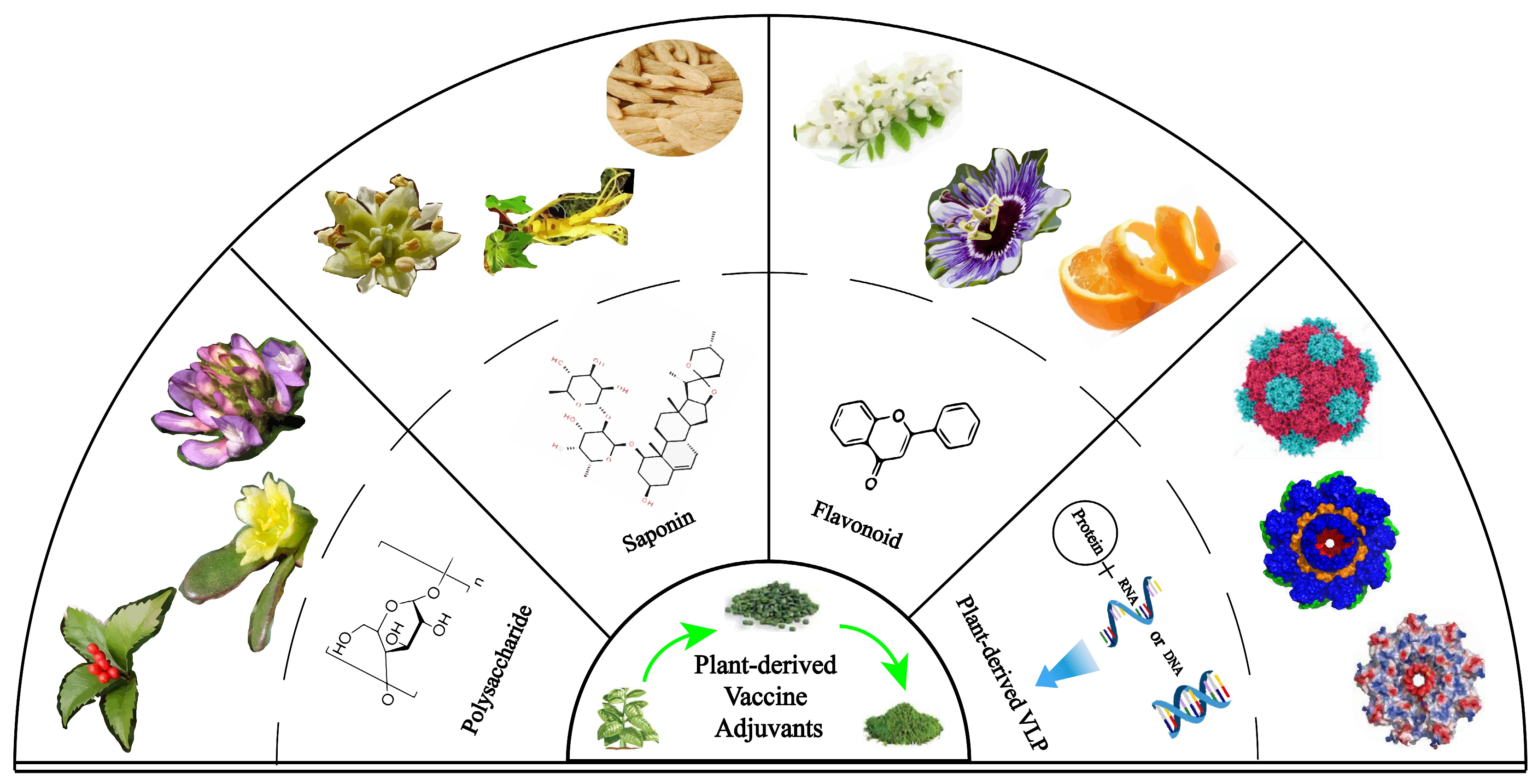
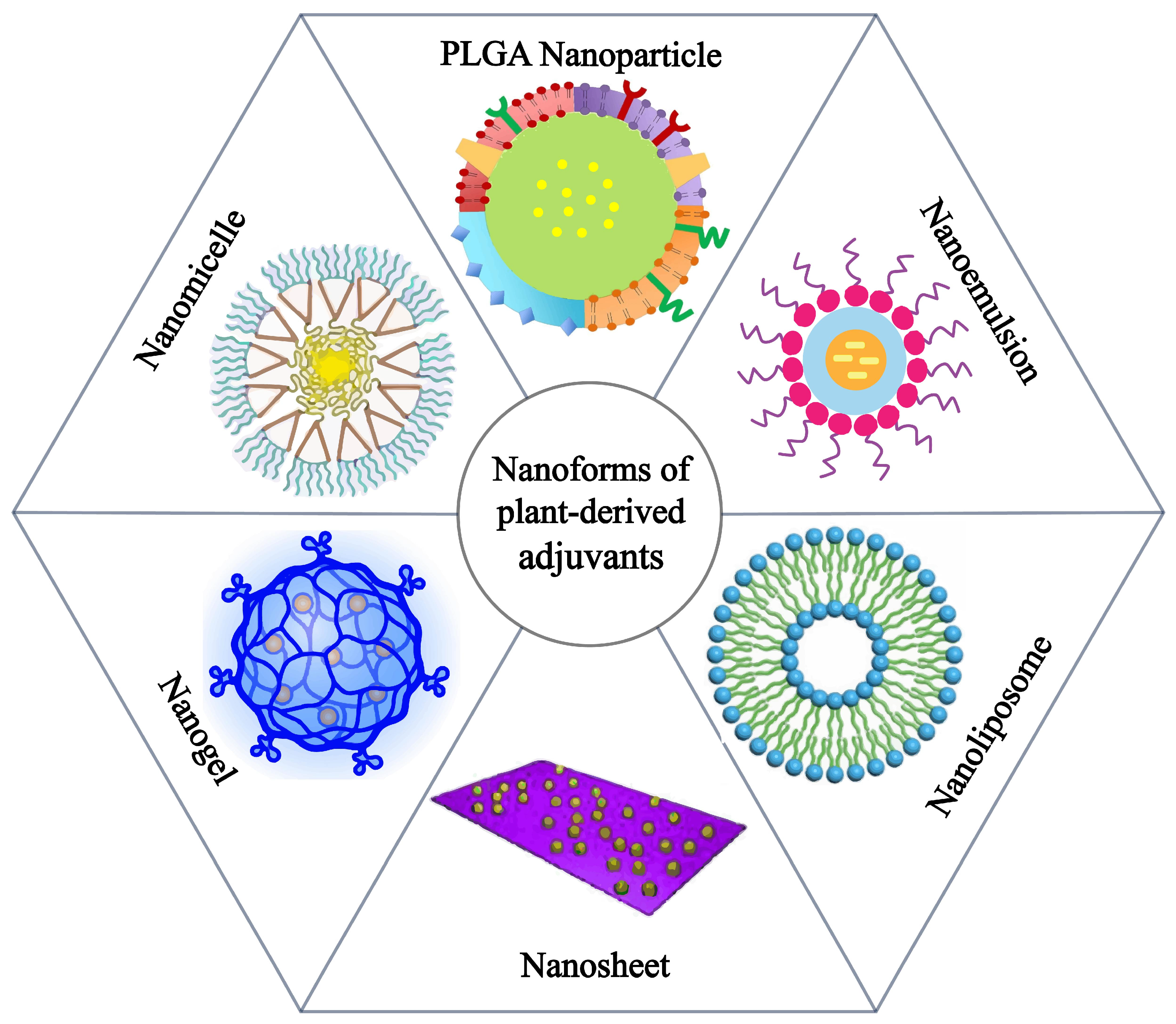
2. Application of Plant Nanoadjuvants in Cancer Vaccines
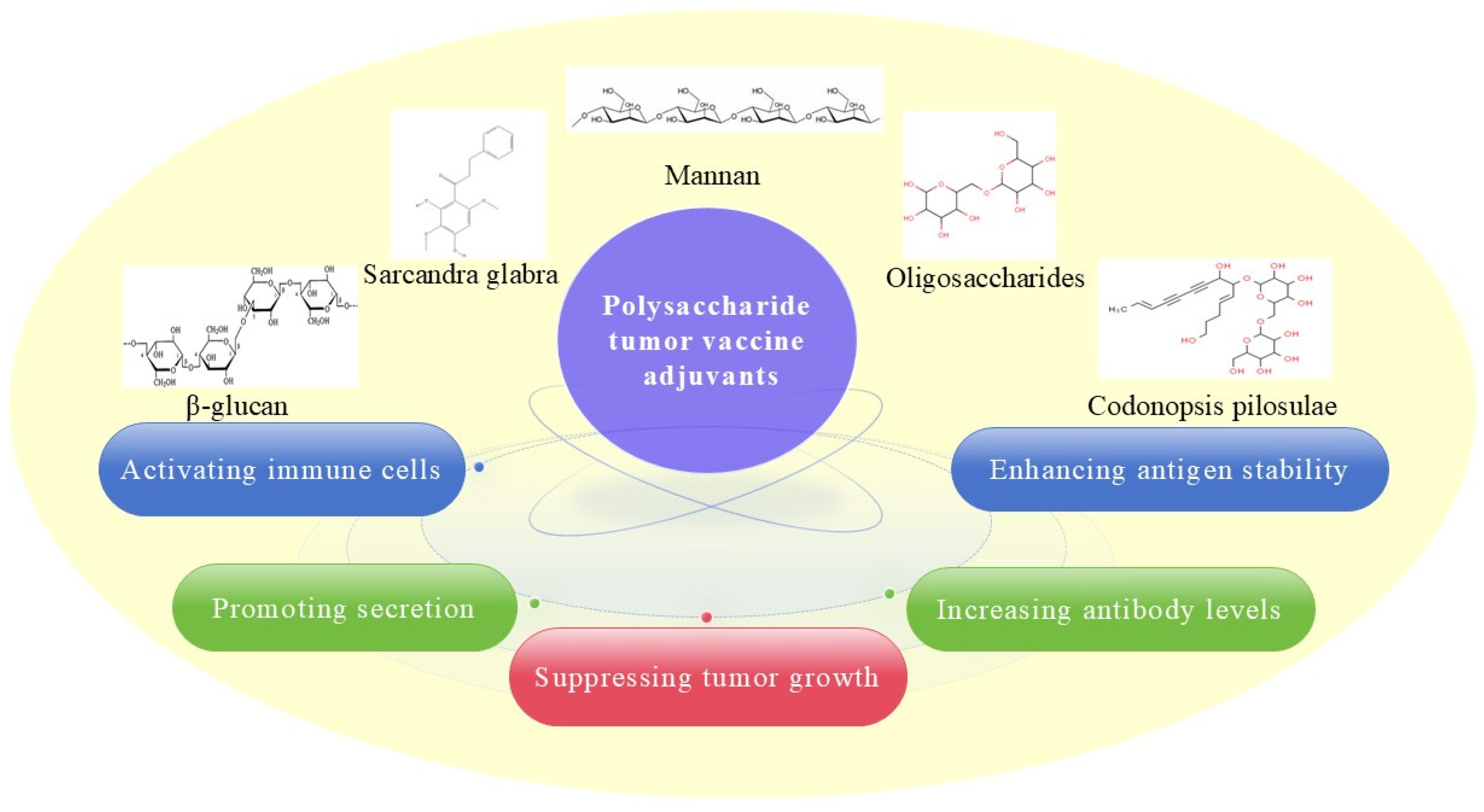
2.1. Polysaccharide Adjuvant and Its Nanoadjuvants
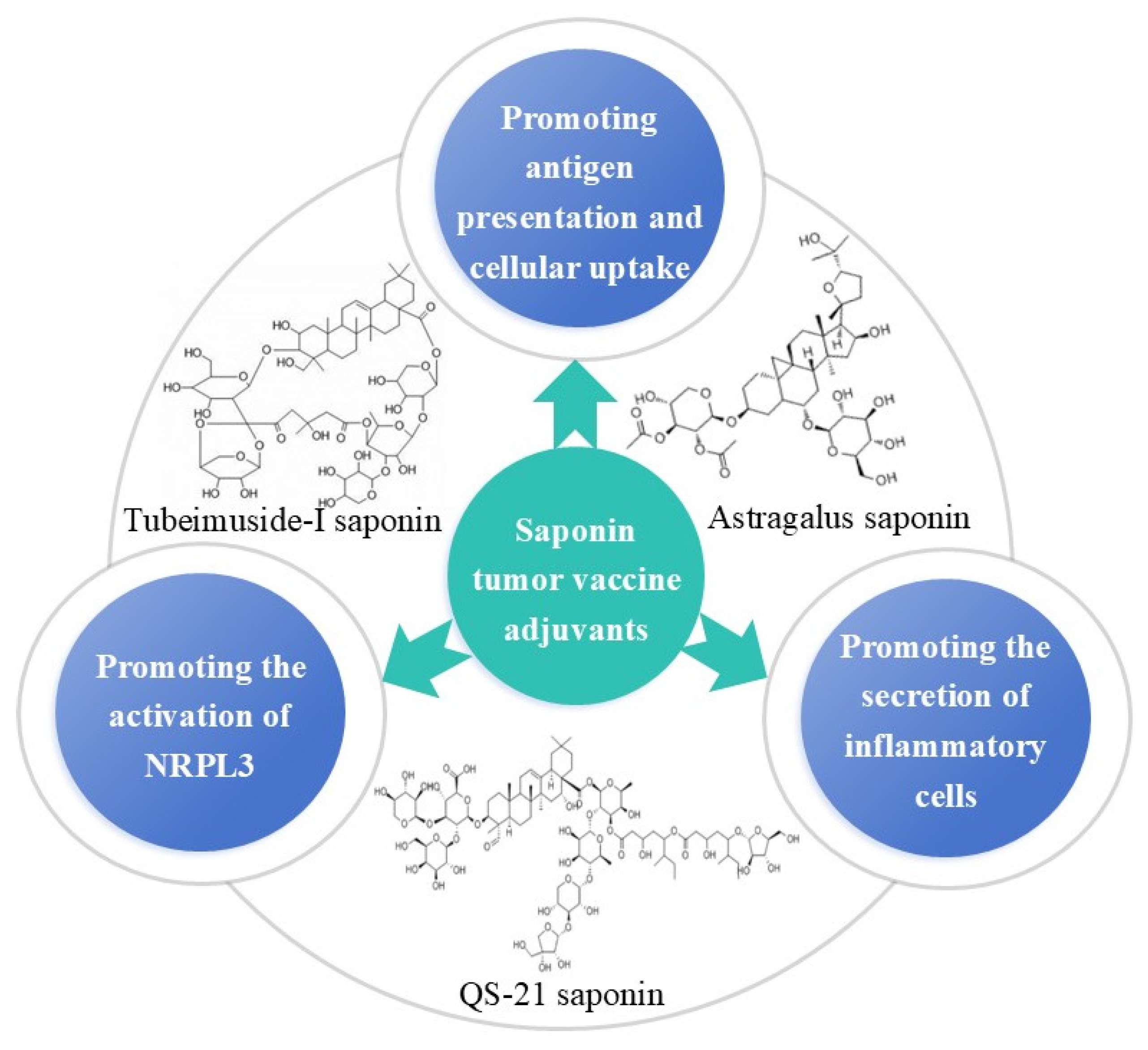
2.2. Saponin Adjuvant and Its Nanoadjuvants
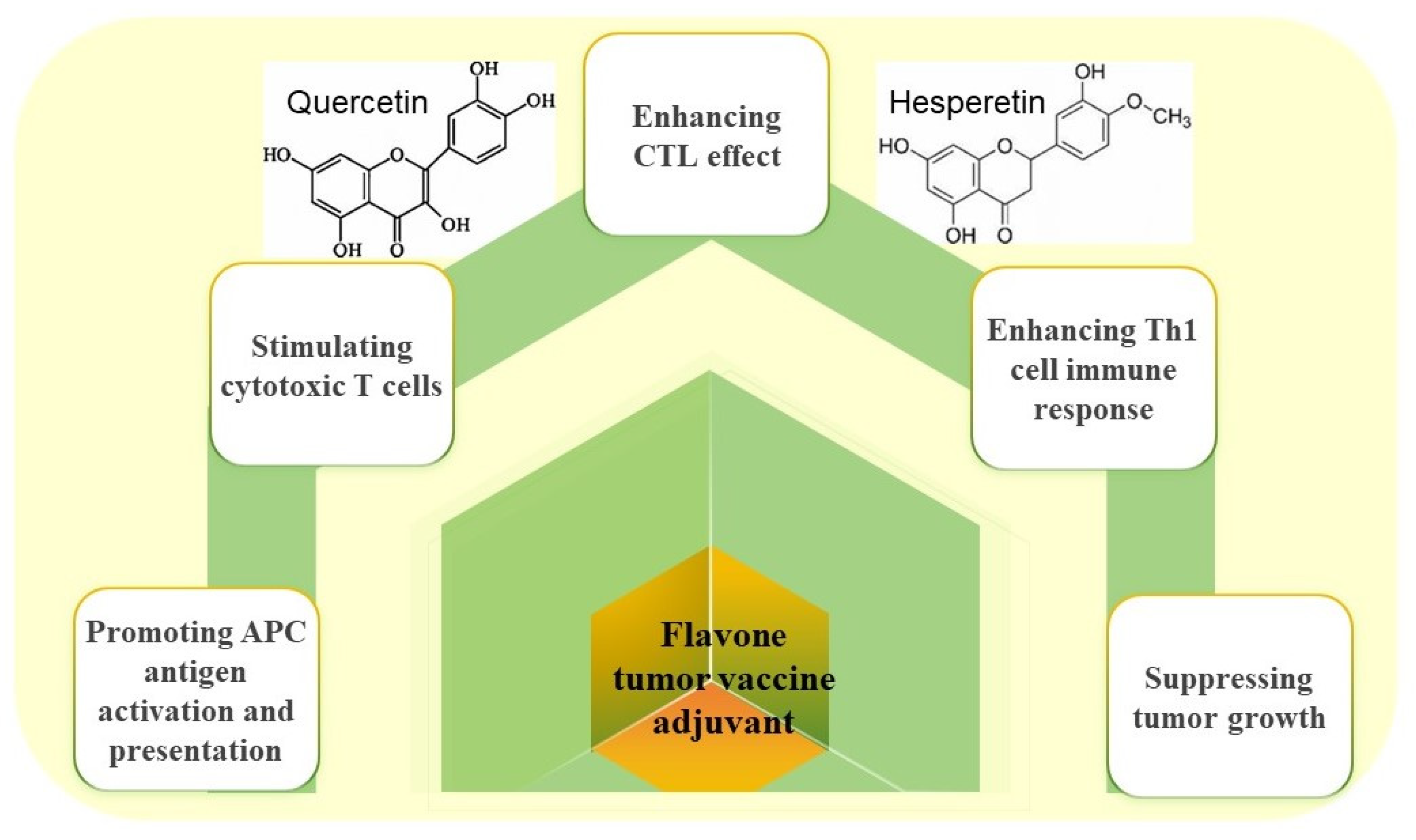
2.3. Flavonoid Adjuvant and Its Nanoadjuvants
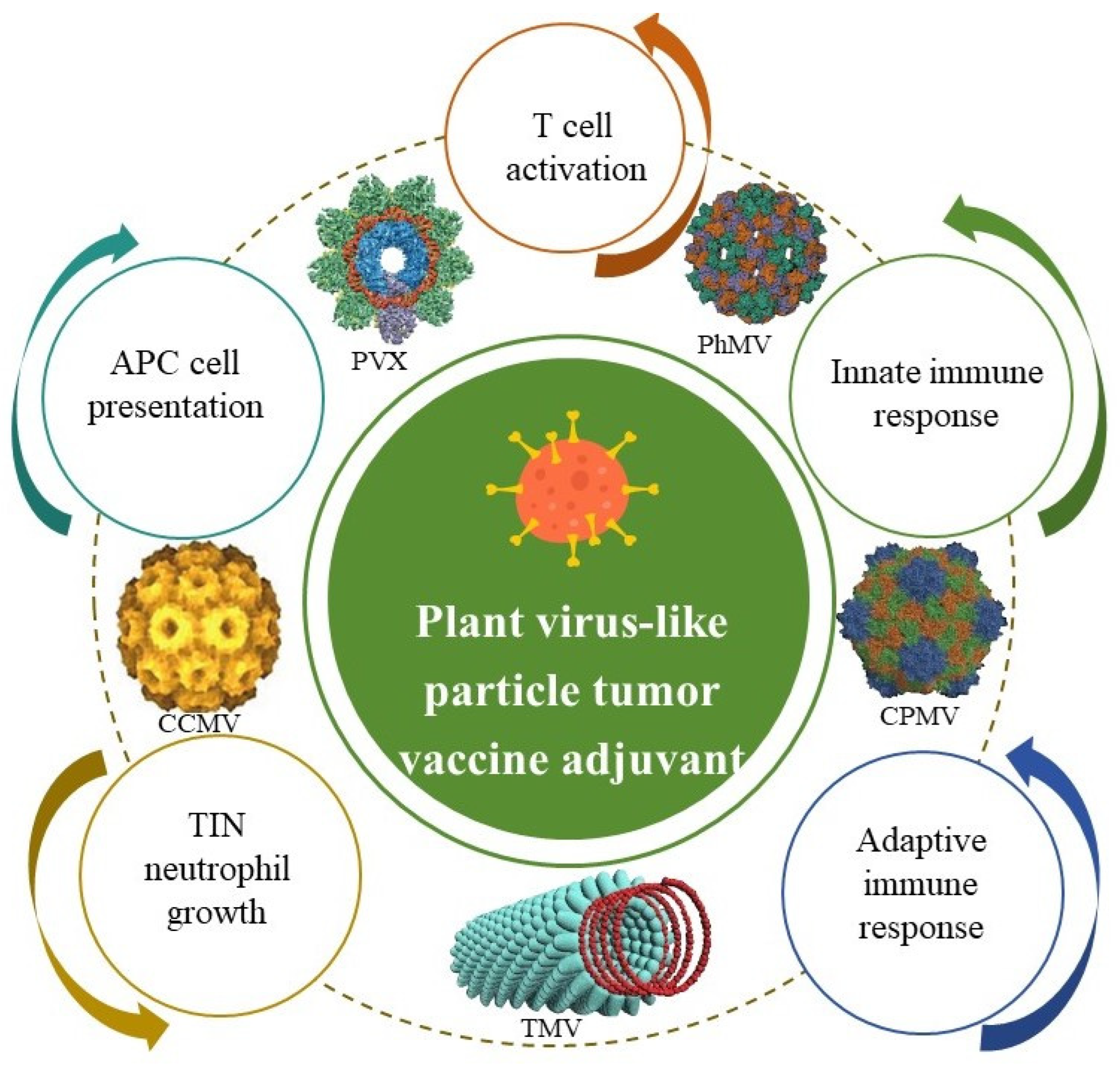
2.4. Plant-Derived Virus-like Particle Adjuvant and Its Nanoadjuvants
3. Limitations and Future Prospects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Mangsbo, S.; Nagarajan, D.; Habra, K.; Pockley, A.G.; McArdle, S.E.B. Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments. Front. Immunol. 2020, 11, 615240. [Google Scholar] [CrossRef] [PubMed]
- van Poelgeest, M.I.; van Seters, M.; van Beurden, M.; Kwappenberg, K.M.; Heijmans-Antonissen, C.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G.; Helmerhorst, T.J.M.; Offringa, R.; et al. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin. Cancer Res. 2005, 11, 5273–5280. [Google Scholar] [CrossRef]
- Kobayashi, A.; Weinberg, V.; Darragh, T.; Smith-McCune, K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 2008, 1, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Lim, Y.T. Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease. Clin. Exp. Vaccine Res. 2015, 4, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J. Vaccine adjuvants: Role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 2003, 8, 934–943. [Google Scholar] [CrossRef]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28, C25–C36. [Google Scholar] [CrossRef]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, C.Y.; Cheng, C.P.; Dai, S.H.; Chen, H.W.; Leng, C.H.; Chong, P.; Liu, S.J.; Huang, M.H. Degradable emulsion as vaccine adjuvant reshapes antigen-specific immunity and thereby ameliorates vaccine efficacy. Sci. Rep. 2016, 6, 36732. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Revuri, V.; Shah, M.; Larson, P.; Shao, Z.; Yu, D.; Prabha, S.; Griffith, T.S.; Ferguson, D.; Panyam, J. Combination of STING and TLR 7/8 Agonists as Vaccine Adjuvants for Cancer Immunotherapy. Cancers 2022, 14, 6091. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 155–172. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium adjuvants--in retrospect and prospect. Vaccine 2004, 22, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, F. Polysaccharides: Candidates of promising vaccine adjuvants. Drug Discov. Ther. 2015, 9, 88–93. [Google Scholar] [CrossRef]
- Fathi, M.; Zangabad, P.S.; Majidi, S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. BioImpacts BI 2017, 7, 269–277. [Google Scholar] [CrossRef]
- Almeida, C.R.; Serra, T.; Oliveira, M.I.; Planell, J.A.; Barbosa, M.A.; Navarro, M. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014, 10, 613–622. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Fonseca, A.C.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Águas, A.P.; Barbosa, J.N. Macrophage polarization following chitosan implantation. Biomaterials 2013, 34, 9952–9959. [Google Scholar] [CrossRef]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Gheybi, E.; Asoodeh, A.; Amani, J. Preparation of chitosan nanoparticle containing recombinant CD44v antigen and evaluation of its immunization capacity against breast cancer in BALB/c mice. BMC Cancer 2023, 23, 134. [Google Scholar] [CrossRef]
- Kensil, C.R.; Patel, U.; Lennick, M.; Marciani, D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991, 146, 431–437. [Google Scholar]
- Gin, D.Y.; Slovin, S.F. Enhancing Immunogenicity of Cancer Vaccines: QS-21 as an Immune Adjuvant. Curr. Drug Ther. 2011, 6, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Xiang, Z.; Xu, T.; He, M.; Xue, Q.; Song, H.; Gao, P.; Cong, Z. The chemistry and efficacy benefits of polysaccharides from Atractylodes macrocephala Koidz. Front. Pharmacol. 2022, 13, 952061. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Cheung, N.V.; Modak, S.; Mauguen, A.; Feng, Y.; Basu, E.; Roberts, S.S.; Ragupathi, G.; Kushner, B.H. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma with Prior Disease Progression. J. Clin. Oncol. 2021, 39, 215–226. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Mauguen, A.; Modak, S.; Ragupathi, G.; Basu, E.M.; Roberts, S.S.; Kushner, B.H.; Cheung, N.K. Effect of Oral β-Glucan on Antibody Response to Ganglioside Vaccine in Patients with High-Risk Neuroblastoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Kado, Y.; Kasho, N.; Harada, A. Cationic lipid potentiated the adjuvanticity of polysaccharide derivative-modified liposome vaccines. J. Ophthalmol. Clin. Res. 2023, 362, 767–776. [Google Scholar] [CrossRef]
- Liu, W.; Gong, X.; Luo, J.; Jiang, L.; Lu, W.; Pan, C.; Yao, W.; Gao, X.; Tian, H. A purified acidic polysaccharide from Sarcandra glabra as vaccine adjuvant to enhance anti-tumor effect of cancer vaccine. Carbohydr. Polym. 2021, 263, 117967. [Google Scholar] [CrossRef]
- Bullón-Vela, V.; Xu, Y.; Razquin, C.; Abete, I.; Zulet, M.A.; Martínez-González, M.A.; Buil-Corsiales, P.; Vitelli-Storelli, F.; Martín Sánchez, V.; Vazquez-Ruíz, Z.; et al. Health associations of liver enzymes and inflammatory scores with urinary citrus flavonoid metabolites. Food Funct. 2023, 14, 1011–1023. [Google Scholar] [CrossRef]
- Zhou, Y.; Zong, Y.; Liu, Z.; Zhao, H.; Zhao, X.; Wang, J. Astragalus Polysaccharides Enhance the Immune Response to OVA Antigen in BALB/c Mice. BioMed Res. Int. 2021, 2021, 9976079. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Zhao, J.; Chen, H.; Si, X.; Huang, Z.; Yu, Z.; Song, W.; Tang, Z.; Chen, X. Mannan-decorated pathogen-like polymeric nanoparticles as nanovaccine carriers for eliciting superior anticancer immunity. Biomaterials 2022, 284, 121489. [Google Scholar] [CrossRef]
- Chang, W.T.; Lai, T.H.; Chyan, Y.J.; Yin, S.Y.; Chen, Y.H.; Wei, W.C.; Yang, N.S. Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PLoS ONE 2015, 10, e0122374. [Google Scholar] [CrossRef]
- Wang, S.W.; Ko, Y.A.; Chen, C.Y.; Liao, K.S.; Chang, Y.H.; Lee, H.Y.; Yu, Y.H.; Lih, Y.H.; Cheng, Y.Y.; Lin, H.H.; et al. Mechanism of Antigen Presentation and Specificity of Antibody Cross-Reactivity Elicited by an Oligosaccharide-Conjugate Cancer Vaccine. J. Am. Chem. Soc. 2023, 145, 9840–9849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasiliev, Y.M. Chitosan-based vaccine adjuvants: Incomplete characterization complicates preclinical and clinical evaluation. Expert Rev. Vaccines 2015, 14, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Ahmadi, N.; Jahantigh, H.R.; Noorbazargan, H.; Yazdi, M.H.; Mahdavi, M. Glucomannan as a Dietary Supplement for Treatment of Breast Cancer in a Mouse Model. Vaccines 2022, 10, 1746. [Google Scholar] [CrossRef]
- Lin, C.C.; Pan, I.H.; Li, Y.R.; Pan, Y.G.; Lin, M.K.; Lu, Y.H.; Wu, H.C.; Chu, C.L. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PLoS ONE 2015, 10, e0116191. [Google Scholar] [CrossRef]
- Tu, J.; He, Y.; Zhang, H.; Wang, J.; Li, Z.; Sun, H. Anti-tumor effect of Crocus sativus petals polysaccharides by reconstructing tumor microenvironment. Int. J. Biol. Macromol. 2023, 248, 125878. [Google Scholar] [CrossRef]
- Zang, X.; Zhao, X.; Hu, H.; Qiao, M.; Deng, Y.; Chen, D. Nanoparticles for tumor immunotherapy. Eur. J. Pharm. Biopharm. 2017, 115, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, Z.; Goldberg, M.S. Targeting myeloid cells using nanoparticles to improve cancer immunotherapy. Adv. Drug Deliv. Rev. 2015, 91, 38–51. [Google Scholar] [CrossRef]
- Nasirmoghadas, P.; Mousakhani, A.; Behzad, F.; Beheshtkhoo, N.; Hassanzadeh, A.; Nikoo, M.; Mehrabi, M.; Kouhbanani, M.A.J. Nanoparticles in cancer immunotherapies: An innovative strategy. Biotechnol. Prog. 2021, 37, e3070. [Google Scholar] [CrossRef]
- Wen, R.; Banik, B.; Pathak, R.K.; Kumar, A.; Kolishetti, N.; Dhar, S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv. Drug Deliv. Rev. 2016, 99, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kordalivand, N.; Fransen, M.F.; Ossendorp, F.; Raemdonck, K.; Vermonden, T.; Hennink, W.E.; Van Nostrum, C.F.J.A.F.M. Reduction-Sensitive Dextran Nanogels Aimed for Intracellular Delivery of Antigens. Adv. Funct. Mater. 2015, 25, 2993–3003. [Google Scholar]
- Jiang, P.L.; Lin, H.J.; Wang, H.W.; Tsai, W.Y.; Lin, S.F.; Chien, M.Y.; Liang, P.H.; Huang, Y.Y.; Liu, D.Z. Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomater. 2015, 11, 356–367. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hussein, W.M.; Jia, Z.; Ziora, Z.M.; McMillan, N.A.; Monteiro, M.J.; Toth, I.; Skwarczynski, M. Self-adjuvanting polymer-peptide conjugates as therapeutic vaccine candidates against cervical cancer. Biomacromolecules 2013, 14, 2798–2806. [Google Scholar] [CrossRef]
- Miyamoto, N.; Mochizuki, S.; Fujii, S.; Yoshida, K.; Sakurai, K. Adjuvant Activity Enhanced by Cross-Linked CpG-Oligonucleotides in β-Glucan Nanogel and Its Antitumor Effect. Bioconjugate Chem. 2017, 28, 565–573. [Google Scholar] [CrossRef]
- Gu, P.; Liu, Z.; Sun, Y.; Ou, N.; Hu, Y.; Liu, J.; Wu, Y.; Wang, D. Angelica sinensis polysaccharide encapsulated into PLGA nanoparticles as a vaccine delivery and adjuvant system for ovalbumin to promote immune responses. Int. J. Pharm. 2019, 554, 72–80. [Google Scholar] [CrossRef]
- Yang, J.; Dong, X.; Li, B.; Chen, T.; Yu, B.; Wang, X.; Dou, X.; Peng, B.; Hu, Q. Poria cocos polysaccharide-functionalized graphene oxide nanosheet induces efficient cancer immunotherapy in mice. Front. Bioeng. Biotechnol. 2022, 10, 1050077. [Google Scholar] [CrossRef]
- Zhao, Z.; Peng, Y.; Shi, X.; Zhao, K. Chitosan derivative composite nanoparticles as adjuvants enhance the cellular immune response via activation of the cGAS-STING pathway. Int. J. Pharm. 2023, 636, 122847. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; He, X.; Cui, X.; Liang, Z.; Wang, L.; Deng, X.; Zhang, Z.; Sheng, W.; Han, X.D. CD16 CAR-T cells enhance antitumor activity of CpG ODN-loaded nanoparticle-adjuvanted tumor antigen-derived vaccinevia ADCC approach. J. Nanobiotechnol. 2023, 21, 159. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, Y.; Wang, K.; Song, G.; Yu, Y.; Meng, T.; Yuan, H.; Hu, F. Chitosan-based nano-micelles for potential anti-tumor immunotherapy: Synergistic effect of cGAS-STING signaling pathway activation and tumor antigen absorption. Carbohydr. Polym. 2023, 321, 121346. [Google Scholar] [CrossRef] [PubMed]
- Lacaille-Dubois, M.A. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine 2019, 60, 152905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Li, Y.D.; Luo, L.L.; Liu, Y.Q.; Li, Y.; Guo, C.; Li, Z.D.; Xie, X.R.; Song, H.X.; Yang, L.P.; et al. Astragalus Saponins and Liposome Constitute an Efficacious Adjuvant Formulation for Cancer Vaccines. Cancer Biother. Radiopharm. 2018, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.L.; Guo, C.; Li, Y.D.; Luo, L.L.; Liu, Y.Q.; Duan, Y.Y.; Li, Z.D.; Xie, X.R.; Song, H.X.; et al. A vaccine targeting basic fibroblast growth factor elicits a protective immune response against murine melanoma. Cancer Biol. Ther. 2018, 19, 518–524. [Google Scholar] [CrossRef]
- Barr, A.M.; Silva, A.; Prato, S.; Belz, G.T.; Maraskovsky, E.; Baz Morelli, A. Therapeutic ISCOMATRIX™ adjuvant vaccine elicits effective anti-tumor immunity in the TRAMP-C1 mouse model of prostate cancer. Cancer Immunol. Immunother. CII 2020, 69, 1959–1972. [Google Scholar] [CrossRef]
- Cebon, J.S.; Gore, M.; Thompson, J.F.; Davis, I.D.; McArthur, G.A.; Walpole, E.; Smithers, M.; Cerundolo, V.; Dunbar, P.R.; MacGregor, D.; et al. Results of a randomized, double-blind phase II clinical trial of NY-ESO-1 vaccine with ISCOMATRIX adjuvant versus ISCOMATRIX alone in participants with high-risk resected melanoma. J. Immunother. Cancer 2020, 8, e000410. [Google Scholar] [CrossRef]
- Beck, Z.; Matyas, G.R.; Alving, C.R. Detection of liposomal cholesterol and monophosphoryl lipid A by QS-21 saponin and Limulus polyphemus amebocyte lysate. Biochim. Et Biophys. Acta BBA-Biomembr. 2015, 1848, 775–780. [Google Scholar] [CrossRef]
- Pifferi, C.; Aguinagalde, L.; Ruiz-de-Angulo, A.; Sacristán, N.; Baschirotto, P.T.; Poveda, A.; Jiménez-Barbero, J.; Anguita, J.; Fernández-Tejada, A. Development of synthetic, self-adjuvanting, and self-assembling anticancer vaccines based on a minimal saponin adjuvant and the tumor-associated MUC1 antigen. Chem. Sci. 2023, 14, 3501–3513. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, Y.; Lin, L.; Zheng, R. Ginsenoside Rg1 Activates Dendritic Cells and Acts as a Vaccine Adjuvant Inducing Protective Cellular Responses Against Lymphomas. DNA Cell Biol. 2017, 36, 1168–1177. [Google Scholar] [CrossRef]
- Luo, X.; Song, Z.; Zeng, X.; Ye, Y.; Zheng, H.; Cai, D.; Yuan, Q.; Li, H.; Tong, Y.; Lu, D.; et al. A promising self-nanoemulsifying adjuvant with plant-derived saponin D boosts immune response and exerts an anti-tumor effect. Front. Immunol. 2023, 14, 1154836. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Lu, G.; Wang, Z.; Hao, S.; Zhang, G.; Sun, H. Tubeimuside I improves the efficacy of a therapeutic Fusobacterium nucleatum dendritic cell-based vaccine against colorectal cancer. Front. Immunol. 2023, 14, 1154818. [Google Scholar] [CrossRef]
- den Brok, M.H.; Büll, C.; Wassink, M.; de Graaf, A.M.; Wagenaars, J.A.; Minderman, M.; Thakur, M.; Amigorena, S.; Rijke, E.O.; Schrier, C.C.; et al. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016, 7, 13324. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, S.; Jiang, S.; Liu, Z.; Wan, X.; Yang, C.; Zhang, L.; Zheng, Z.; Wang, B.; Li, L. Luteolin as an adjuvant effectively enhances CTL anti-tumor response in B16F10 mouse model. Int. Immunopharmacol. 2021, 94, 107441. [Google Scholar] [CrossRef]
- Lu, R.; Wang, S.; Jiang, S.; Li, C.; Wang, Y.; Li, L.; Wang, Y.; Ma, G.; Qiao, H.; Leng, Z.; et al. Chrysin enhances antitumour immunity response through the IL-12-STAT4 signal pathway in the B16F10 melanoma mouse model. Scand. J. Immunol. 2022, 96, e13177. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Liu, Z.; Zhang, L.; Wang, S.; Wang, B. Procyanidin, a kind of biological flavonoid, induces protective anti-tumor immunity and protects mice from lethal B16F10 challenge. Int. Immunopharmacol. 2017, 47, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, S.; Zhang, L.; Tian, L.; Li, L.; Liu, Z.; Dong, Q.; Lv, X.; Mu, H.; Zhang, Q.; et al. Hesperetin as an adjuvant augments protective anti-tumour immunity responses in B16F10 melanoma by stimulating cytotoxic CD8(+) T cells. Scand. J. Immunol. 2020, 91, e12867. [Google Scholar] [CrossRef]
- Mohammed, M.E.A.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Alshehri, M.A.; Alhag, S.K.; Suleiman, M.H.A.; Ghramh, H.A.; Ibrahim, A.; Alshehri, A.M.; et al. Acacia honey from different altitudes: Total phenols and flavonoids, laser-induced fluorescence (LIF) spectra, and anticancer activity. J. Int. Med. Res. 2020, 48, 300060520943451. [Google Scholar] [CrossRef]
- Kou, Y.; Li, Z.; Sun, Q.; Yang, S.; Wang, Y.; Hu, C.; Gu, H.; Wang, H.; Xu, H.; Li, Y.; et al. Prognostic value and predictive biomarkers of phenotypes of tumour-associated macrophages in colorectal cancer. Scand. J. Immunol. 2022, 95, e13137. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Wang, T.; Wang, H.D.; Wang, H.Z.; Chen, H.Y.; Zhang, S.; Guo, Y.J.; Li, H.; Hui, H. LW-213 induces immunogenic tumor cell death via ER stress mediated by lysosomal TRPML1. Cancer Lett. 2023, 577, 216435. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Fahmy, U.A.; Eldin, S.M.B.; Ahmed, O.A.A.; Aldawsari, H.M.; Okbazghi, S.Z.; Alfaleh, M.A.; Abdulaal, W.H.; Alamoudi, A.J.; Mady, F.M. Scorpion Venom-Functionalized Quercetin Phytosomes for Breast Cancer Management: In Vitro Response Surface Optimization and Anticancer Activity against MCF-7 Cells. Polymers 2021, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Jobsri, J.; Allen, A.; Rajagopal, D.; Shipton, M.; Kanyuka, K.; Lomonossoff, G.P.; Ottensmeier, C.; Diebold, S.S.; Stevenson, F.K.; Savelyeva, N. Plant virus particles carrying tumour antigen activate TLR7 and Induce high levels of protective antibody. PLoS ONE 2015, 10, e0118096. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Apka, P.; Shoeb, E.; Badar, U.; Hefferon, K. Plant Virus Nanoparticles for Anti-cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 642794. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.W. Protein-based nanoparticles in cancer vaccine development. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 164–174. [Google Scholar] [CrossRef]
- Shukla, S.; Hu, H.; Cai, H.; Chan, S.K.; Boone, C.E.; Beiss, V.; Chariou, P.L.; Steinmetz, N.F. Plant Viruses and Bacteriophage-Based Reagents for Diagnosis and Therapy. Annu. Rev. Virol. 2020, 7, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Mao, C.; Bhatia, M.; Koellhoffer, E.C.; Fiering, S.N.; Steinmetz, N.F. Inactivated Cowpea Mosaic Virus for In Situ Vaccination: Differential Efficacy of Formalin vs UV-Inactivated Formulations. Mol. Pharm. 2023, 20, 500–507. [Google Scholar] [CrossRef]
- Wang, C.; Steinmetz, N.F. CD47 Blockade and Cowpea Mosaic Virus Nanoparticle In Situ Vaccination Triggers Phagocytosis and Tumor Killing. Adv. Healthc. Mater. 2019, 8, e1801288. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Lin, R.; Steinmetz, N.F. Delivery of mitoxantrone using a plant virus-based nanoparticle for the treatment of glioblastomas. J. Mater. Chem. B 2018, 6, 5888–5895. [Google Scholar] [CrossRef]
- Lebel, M.; Chartrand, K.; Tarrab, E.; Savard, P.; Leclerc, D.; Lamarre, A. Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Lett. 2016, 16, 1826–1832. [Google Scholar] [CrossRef]
- Cai, H.; Shukla, S.; Steinmetz, N.F. The Antitumor Efficacy of CpG Oligonucleotides is Improved by Encapsulation in Plant Virus-Like Particles. Adv. Funct. Mater. 2020, 30, 1908743. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Steinmetz, N.F. Development of a Virus-Like Particle-Based Anti-HER2 Breast Cancer Vaccine. Cancers 2021, 13, 2909. [Google Scholar] [CrossRef] [PubMed]
- Finbloom, J.A.; Aanei, I.L.; Bernard, J.M.; Klass, S.H.; Elledge, S.K.; Han, K.; Ozawa, T.; Nicolaides, T.P.; Berger, M.S.; Francis, M.B. Evaluation of Three Morphologically Distinct Virus-Like Particles as Nanocarriers for Convection-Enhanced Drug Delivery to Glioblastoma. Nanomaterials 2018, 8, 1007. [Google Scholar] [CrossRef]
- Esfandiari, N.; Arzanani, M.K.; Soleimani, M.; Kohi-Habibi, M.; Svendsen, W.E. A new application of plant virus nanoparticles as drug delivery in breast cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 1229–1236. [Google Scholar] [CrossRef]
- Chung, Y.H.; Ortega-Rivera, O.A.; Volckaert, B.A.; Jung, E.; Zhao, Z.; Steinmetz, N.F. Viral nanoparticle vaccines against S100A9 reduce lung tumor seeding and metastasis. Proc. Natl. Acad. Sci. USA 2023, 120, e2221859120. [Google Scholar] [CrossRef]
- Lizotte, P.H.; Wen, A.M.; Sheen, M.R.; Fields, J.; Rojanasopondist, P.; Steinmetz, N.F.; Fiering, S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016, 11, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9733. [Google Scholar] [CrossRef] [PubMed]
- Chariou, P.L.; Beiss, V.; Ma, Y.; Steinmetz, N.F. In situ vaccine application of inactivated CPMV nanoparticles for cancer immunotherapy. Mater. Adv. 2021, 2, 1644–1656. [Google Scholar] [CrossRef]
- Wang, C.; Beiss, V.; Steinmetz, N.F. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019, 93, e00129-19. [Google Scholar] [CrossRef]
- Berzofsky, J.A.; Terabe, M.; Wood, L.V. Strategies to use immune modulators in therapeutic vaccines against cancer. Semin. Oncol. 2012, 39, 348–357. [Google Scholar] [CrossRef]
- Cai, J.; Wang, H.; Wang, D.; Li, Y. Improving Cancer Vaccine Efficiency by Nanomedicine. Adv. Biosyst. 2019, 3, e1800287. [Google Scholar] [CrossRef]
- Wu, A.; Chen, Y.; Wang, H.; Chang, Y.; Zhang, M.; Zhao, P.; Tang, Y.; Xu, Q.; Zhu, Z.; Cao, Y.; et al. Genetically-engineered all-in-one vaccine platform for cancer immunotherapy. Acta Pharm. Sin. B 2021, 11, 3622–3635. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.N.; Yang, L.Y.; Yang, Y.; Song, Z.; Peng, L.S.; Gao, J.N.; Zeng, H.; Zou, Q.M.; Sun, H.W.; Mao, X.H. An immunopotentiator, ophiopogonin D, encapsulated in a nanoemulsion as a robust adjuvant to improve vaccine efficacy. Acta Biomater. 2018, 77, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Gao, M.Z.; Gao, D.M.; Guo, Y.H.; Gao, Z.; Gao, X.J.; Wang, J.Q.; Qiao, M.Q. Tubeimoside-1: A review of its antitumor effects, pharmacokinetics, toxicity, and targeting preparations. Front. Pharmacol. 2022, 13, 941270. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Zhu, H.; Cai, X.; Sun, C.; Ye, Y.; Cai, D.; Yang, S.; Cheng, J.; Gao, J.; Yang, Y.; et al. Plant-Derived Immunomodulatory Nanoadjuvants for Cancer Vaccines: Current Status and Future Opportunities. Vaccines 2025, 13, 378. https://doi.org/10.3390/vaccines13040378
Jia Y, Zhu H, Cai X, Sun C, Ye Y, Cai D, Yang S, Cheng J, Gao J, Yang Y, et al. Plant-Derived Immunomodulatory Nanoadjuvants for Cancer Vaccines: Current Status and Future Opportunities. Vaccines. 2025; 13(4):378. https://doi.org/10.3390/vaccines13040378
Chicago/Turabian StyleJia, Yimin, Hui Zhu, Xinyu Cai, Cun Sun, Yan Ye, Dingyi Cai, Shuaifei Yang, Jingjing Cheng, Jining Gao, Yun Yang, and et al. 2025. "Plant-Derived Immunomodulatory Nanoadjuvants for Cancer Vaccines: Current Status and Future Opportunities" Vaccines 13, no. 4: 378. https://doi.org/10.3390/vaccines13040378
APA StyleJia, Y., Zhu, H., Cai, X., Sun, C., Ye, Y., Cai, D., Yang, S., Cheng, J., Gao, J., Yang, Y., Zeng, H., Zou, Q., Li, J., Sun, H., & Wang, W. (2025). Plant-Derived Immunomodulatory Nanoadjuvants for Cancer Vaccines: Current Status and Future Opportunities. Vaccines, 13(4), 378. https://doi.org/10.3390/vaccines13040378





