Maternal Vaccination and Neonatal Feeding Strategies Among Polish Women
Abstract
1. Introduction
2. Materials and Methods
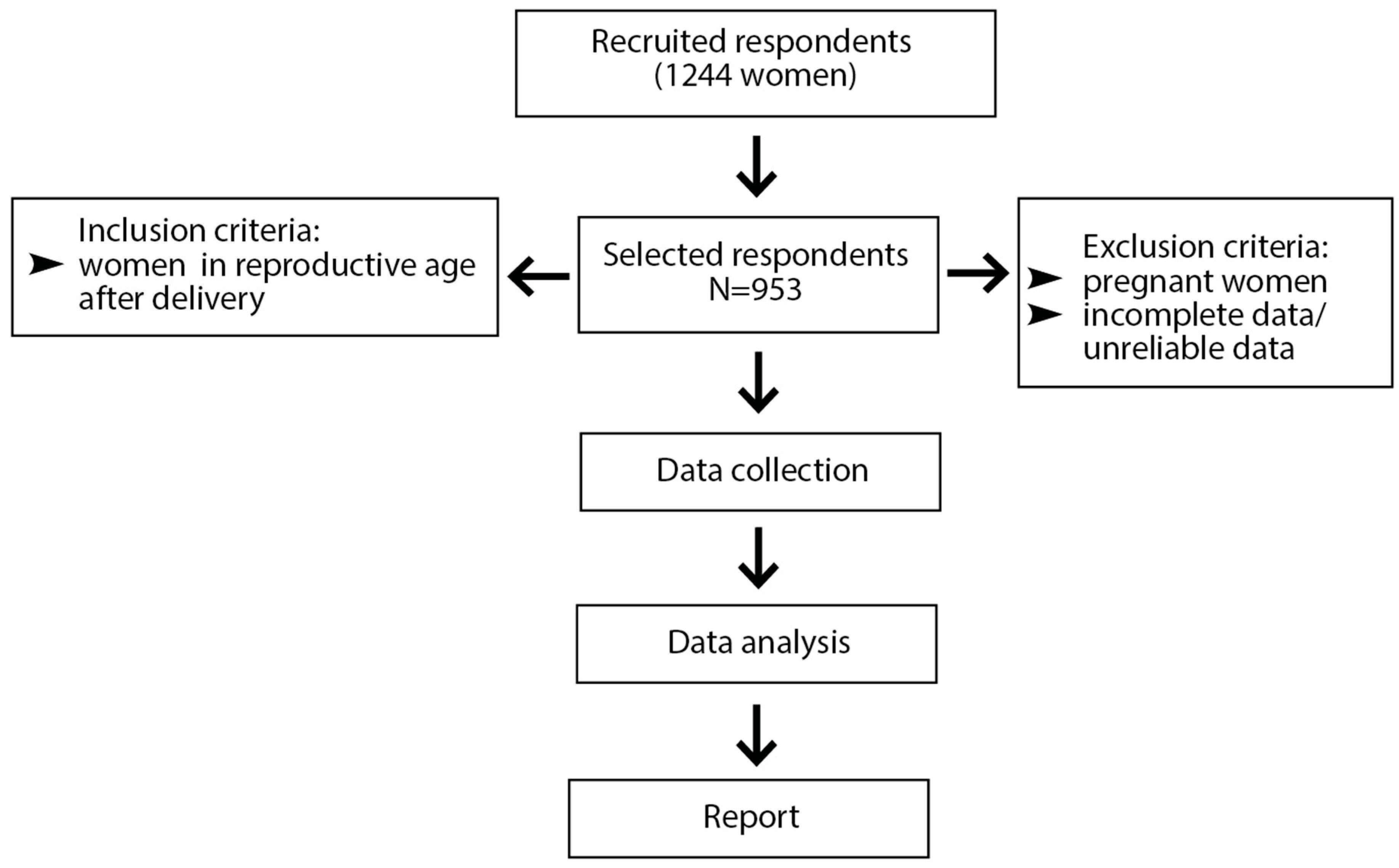
2.1. Study Design and Participants
2.2. Data Source and Study Size
2.3. Quantitative and Qualitative Variables
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic and Obstetric Data of Respondents
3.2. Sociodemographic and Obstetric Data of Respondents in Relation to Vaccination Status
3.3. Sociodemographic and Obstetric Data of Respondents in Relation to Neonatal Feeding Patterns
3.4. Respondents’ Sociodemographics and Obstetric Data in Relation to Maternal Experience
3.5. Associations Between Sociodemographic Variables and Dual Vaccination Among Mothers
3.6. Associations Between Sociodemographic Variables, Health-Enhancing Behaviors, and Neonatal Feeding Strategy
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellwanger, J.H.; Veiga, A.B.G.; Kaminski, V.L.; Valverde-Villegas, J.M.; Freitas, A.W.Q.; Chies, J.A.B. Control and prevention of infectious diseases from a One Health perspective. Genet. Mol. Biol. 2021, 44, e20200256. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.P.; Westerbeek, E.A.; van der Klis, F.R.; Berbers, G.A.; van Elburg, R.M. Transplacental transport of IgG antibodies to preterm infants: A review of the literature. Early Hum. Dev. 2011, 87, 67–72. [Google Scholar] [CrossRef]
- Lozano, N.A.; Lozano, A.; Marini, V.; Saranz, R.J.; Blumberg, R.S.; Baker, K.; Agresta, M.F.; Ponzio, M.F. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Reprod. Immunol. 2018, 80, e12972. [Google Scholar] [CrossRef]
- Coler, C.; King-Nakaoka, E.; Every, E.; Chima, S.; Vong, A.; Del Rosario, B.; VanAbel, R.; Adams Waldorf, K.M. Impact of Infections During Pregnancy on Transplacental Antibody Transfer. Vaccines 2024, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, J.P. Fetal and maternal immunoglobulin levels during pregnancy. Am. J. Obstet. Gynecol. 1969, 103, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lacy, J.B. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst. Rev. 2020, 1, CD001239. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Hoyte, T.; Davis, N.; Deonarinesingh, C.; De Silva, A.; Dhanpaul, D.; Dookhoo, C.; Doorpat, J.; Dopson, A.; Durgapersad, J.; et al. A Systematic Review of the Clinical Diagnosis of Transient Hypogammaglobulinemia of Infancy. Children 2023, 10, 1358. [Google Scholar] [CrossRef]
- Maródi, L. Neonatal innate immunity to infectious agents. Infect. Immun. 2006, 74, 1999–2006. [Google Scholar] [CrossRef]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar]
- Demers-Mathieu, V.; Underwood, M.A.; Beverly, R.L.; Nielsen, S.D.; Dallas, D.C. Comparison of Human Milk Immunoglobulin Survival during Gastric Digestion between Preterm and Term Infants. Nutrients 2018, 10, 631. [Google Scholar] [CrossRef]
- Atyeo, C.; Alter, G. The multifaceted roles of breast milk antibodies. Cell 2021, 184, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Semmes, E.C.; Chen, J.L.; Goswami, R.; Burt, T.D.; Permar, S.R.; Fouda, G.G. Understanding Early-Life Adaptive Immunity to Guide Interventions for Pediatric Health. Front. Immunol. 2021, 11, 595297. [Google Scholar]
- Stafford, L.; Valcarce, V.; Henry, M.; Neu, J.; Parker, L.; Martina, M.; Vicuna, V.; Gowen, T.; Cato, E.; Kosik, I.; et al. Detection of SARS-CoV-2 IgA and IgG in human milk and breastfeeding infant stool 6 months after maternal COVID-19 vaccination. J. Perinatol. 2023, 43, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cano, F.J.; Demers-Mathieu, V.; Billeaud, C. Editorial: Human milk, nutrition and infant development. Front. Nutr. 2024, 11, 1525112. [Google Scholar]
- Branche, A.R.; Falsey, A.R. Parainfluenza Virus Infection. Semin. Respir. Crit. Care Med. 2016, 37, 538–554. [Google Scholar]
- Wildenbeest, J.G.; van der Schee, M.P.; Hashimoto, S.; Benschop, K.S.; Minnaar, R.P.; Sprikkelman, A.B.; Haarman, E.G.; van Aalderen, W.M.; Sterk, P.J.; Pajkrt, D.; et al. Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin. Microbiol. Infect. 2016, 22, 736.e9–736.e15. [Google Scholar]
- Ljubin-Sternak, S.; Meštrović, T.; Ivković-Jureković, I.; Kolarić, B.; Slović, A.; Forčić, D.; Tot, T.; Mijač, M.; Vraneš, J. The Emerging Role of Rhinoviruses in Lower Respiratory Tract Infections in Children—Clinical and Molecular Epidemiological Study From Croatia, 2017–2019. Front. Microbiol. 2019, 10, 2737. [Google Scholar]
- Chaiut, W.; Sapbamrer, R.; Dacha, S.; Sudjaritruk, T.; Malasao, R. Epidemiology and associated factors for hospitalization related respiratory syncytial virus infection among children less than 5 years of age in Northern Thailand. J. Infect. Public Health 2023, 16, 1659–1665. [Google Scholar]
- Munro, A.P.S.; Martinón-Torres, F.; Drysdale, S.B.; Faust, S.N. The disease burden of respiratory syncytial virus in Infants. Curr. Opin. Infect. Dis. 2023, 36, 379–384. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Yan, Y.; Shi, Y.; Huang, J.; Du, H.; Du, Q.; Li, Y.; Lin, Y.; Liu, D.; et al. Prevalence of respiratory viruses among hospitalized children with lower respiratory tract infections during the COVID-19 pandemic in Wuhan, China. Int. J. Infect. Dis. 2024, 139, 6–12. [Google Scholar]
- Argun, M.; İnan, D.B.; Hörmet ÖZ, H.T.; Duyar, M.O.; Başargan, G.; Elmalı, F.; Çelik, İ. Lymphocyte Subsets in Mild COVID-19 Pediatric Patients. Turk. Arch. Pediatr. 2022, 57, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Chen, W.; Cai, J.; He, Y. The mechanisms of milder clinical symptoms of COVID-19 in children compared to adults. Ital. J. Pediatr. 2024, 50, 28. [Google Scholar] [CrossRef]
- Polish Society of Gynecologists and Obstetricians Position on Vaccination of Pregnant Women Against COVID-19. Available online: https://www.ptgin.pl/artykul/stanowisko-ptgip-dotyczace-szczepien-kobiet-ciezarnych-przeciwko-covid19 (accessed on 15 January 2025). (In Polish).
- Antczak, A.; Kucha, E.; Nitsch-Osuch, A.; Sieroszewski, P.; Wielgoś, M.; Zimmer, M. Stanowisko Ekspertów OPZG i PTGiP Dotyczące Szczepienia Przeciw Grypie Kobiet w Ciąży. 2020. Available online: https://www.ptgin.pl/sites/scm/files/2022-01/09.2020%20Stanowisko%20ekspert%C3%B3w%20OPZG%20i%20PTGiP%20dotycz%C4%85ce%20szczepienia%20przeciw%20grypie%20kobiet%20w%20ci%C4%85%C5%BCy.pdf (accessed on 15 January 2025). (In Polish).
- Szeląg, J.; Mastalerz-Migas, A. Lekarze rodzinni w Polsce wobec szczepienia kobiet w ciąży—Postawy i praktyka. 2019. Available online: https://www.mp.pl/szczepienia/artykuly (accessed on 15 January 2025). (In Polish).
- Seremak-Mrozikiewicz, A.; Nitsch-Osuch, A.; Czajkowski, K.; Drews, K.; Huras, H.; Kalinka, J.; Kuchar, E.; Leszczynska-Gorzelak, B.; Mastalerz-Migas, A.; Swiatkowska-Freund, M.; et al. Guidelines of the Polish Society of Gynecologists and Obstetricians, the Polish Society for Vaccinology, and the Polish Society for Family Medicine on vaccinating women with reproductive plans and pregnant or breastfeeding women. Ginekol. Pol. 2023, 94, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Jurga, J.; Mierzwa, G.; Kuciel, J.A.; Kołak, M.; Jaworowski, A.; Huras, H. Maternal Vaccination in Pregnancy: An Assessment of Influenza, Pertussis, and COVID-19 Vaccination Rates in Cracow, Poland. Med. Sci. Monit. 2024, 30, e943304. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.H.; Hobeika, E.; Chamsy, D.; El-Kak, F.; Usta, I.M. Vaccination in pregnancy. Int. J. Gynaecol. Obstet. 2023, 162, 18–23. [Google Scholar] [CrossRef]
- Ciapponi, A.; Berrueta, M.; P K Parker, E.; Bardach, A.; Mazzoni, A.; Anderson, S.A.; Argento, F.J.; Ballivian, J.; Bok, K.; Comandé, D.; et al. Safety of COVID-19 vaccines during pregnancy: A systematic review and meta-analysis. Vaccine 2023, 41, 3688–3700. [Google Scholar] [CrossRef]
- Favilli, A.; Mattei Gentili, M.; De Paola, F.; Laganà, A.S.; Vitagliano, A.; Bosco, M.; Cicinelli, E.; Chiantera, V.; Uccella, S.; Parazzini, F.; et al. COVID-19 and Pregnancy: An Updated Review about Evidence-Based Therapeutic Strategies. J. Pers. Med. 2023, 13, 1035. [Google Scholar] [CrossRef]
- Norman, M.; Magnus, M.C.; Söderling, J.; Juliusson, P.B.; Navér, L.; Örtqvist, A.K.; Håberg, S.; Stephansson, O. Neonatal Outcomes After COVID-19 Vaccination in Pregnancy. JAMA. 2024, 331, 396–407. [Google Scholar] [CrossRef]
- Munoz, F.M.; Jamieson, D.J. Maternal Immunization. Obstet. Gynecol. 2019, 133, 739–753. [Google Scholar] [CrossRef]
- Röbl-Mathieu, M.; Kunstein, A.; Liese, J.; Mertens, T.; Wojcinski, M. Vaccination in Pregnancy. Dtsch. Arztebl. Int. 2021, 118, 262–268. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; DaPra, C.; Medo, E. Influenza Vaccine Associated with the Gene Expression of T Cell Surface Markers in Human Milk. Breastfeed. Med. 2022, 17, 218–225. [Google Scholar] [PubMed]
- Demers-Mathieu, V. Editorial: Breast milk and passive immunity during the COVID-19 pandemic. Front. Nutr. 2023, 10, 1155901. [Google Scholar]
- Suteerojntrakool, O.; Mekangkul, E.; Ananta, P.; Maitreechit, D.; Khabuan, S.; Sodsai, P.; Hirankarn, N.; Thumbovorn, R.; Chomtho, S. The Persistence of Specific Immunoglobulin A Against SARS-CoV-2 in Human Milk After Maternal COVID-19 Vaccination. Breastfeed. Med. 2023, 18, 943–950. [Google Scholar]
- Quincer, E.M.; Cranmer, L.M.; Kamidani, S. Prenatal Maternal Immunization for Infant Protection: A Review of the Vaccines Recommended, Infant Immunity and Future Research Directions. Pathogens 2024, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Rodolakis, A. Vaccination of pregnant women against influenza: What is the optimal timing? Hum. Vaccin. Immunother. 2021, 17, 2723–2727. [Google Scholar]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar]
- Lopez, P.A.; Nziza, N.; Chen, T.; Shook, L.L.; Burns, M.D.; Demidkin, S.; Jasset, O.; Akinwunmi, B.; Yonker, L.M.; Gray, K.J.; et al. Placental transfer dynamics and durability of maternal COVID-19 vaccine-induced antibodies in infants. iScience 2024, 27, 109273. [Google Scholar]
- Tannis, A.; Englund, J.A.; Perez, A.; Harker, E.J.; Staat, M.A.; Schlaudecker, E.P.; Halasa, N.B.; Stewart, L.S.; Williams, J.V.; Michaels, M.G.; et al. SARS-CoV-2 Epidemiology and COVID-19 mRNA Vaccine Effectiveness Among Infants and Children Aged 6 Months–4 Years—New Vaccine Surveillance Network, United States, July 2022–September 2023. MMWR Morb. Mortal Wkly Rep. 2023, 72, 1300–1306. [Google Scholar]
- Jorgensen, S.C.J.; Drover, S.S.M.; Fell, D.B.; Austin, P.C.; D’Souza, R.; Guttmann, A.; Buchan, S.A.; Wilson, S.E.; Nasreen, S.; Schwartz, K.L.; et al. Newborn and Early Infant Outcomes Following Maternal COVID-19 Vaccination During Pregnancy. JAMA Pediatr. 2023, 177, 1314–1323. [Google Scholar]
- Atyeo, C.; DeRiso, E.A.; Davis, C.; Bordt, E.A.; De Guzman, R.M.; Shook, L.L.; Yonker, L.M.; Fasano, A.; Akinwunmi, B.; Lauffenburger, D.A.; et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2021, 13, eabi8631. [Google Scholar]
- Collier, A.-R.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar]
- Proto, A.; Agliardi, S.; Pani, A.; Renica, S.; Gazzaniga, G.; Giossi, R.; Senatore, M.; Di Ruscio, F.; Campisi, D.; Vismara, C.; et al. COVID-Vaccines in Pregnancy: Maternal and Neonatal Response over the First 9 Months after Delivery. Biomolecules 2024, 14, 435. [Google Scholar] [CrossRef]
- Lauritsen, C.J.; Trinh, I.V.; Desai, S.P.; Clancey, E.; Murrell, A.E.; Rambaran, S.; Chandra, S.; Elliott, D.H.; Smira, A.R.; Mo, Z.; et al. Passive antibody transfer from pregnant women to their fetus are maximized after SARS-CoV-2 vaccination irrespective of prior infection. J. Allergy Clin. Immunol. Glob. 2023, 3, 100189. [Google Scholar]
- Schlaudecker, E.P.; Steinhoff, M.C.; Omer, S.B.; McNeal, M.M.; Roy, E.; Arifeen, S.E.; Dodd, C.N.; Raqib, R.; Breiman, R.F.; Zaman, K. IgA and neutralizing antibodies to influenza a virus in human milk: A randomized trial of antenatal influenza immunization. PLoS ONE 2013, 8, e70867. [Google Scholar]
- Faucette, A.N.; Pawlitz, M.D.; Pei, B.; Yao, F.; Chen, K. Immunization of pregnant women: Future of early infant protection. Hum. Vaccin. Immunother. 2015, 11, 2549–2555. [Google Scholar] [PubMed]
- Suragh, T.A.; Hibbs, B.; Marquez, P.; McNeil, M.M. Age inappropriate influenza vaccination in infants less than 6 months old, 2010-2018. Vaccine 2020, 38, 3747–3751. [Google Scholar]
- Fayad, D.; Frenck, R.W., Jr. COVID-19 Vaccines in Children. J. Clin. Med. 2023, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.J.; Enriquez, L.F.; Elmi, A.F.; Zavadoski, J.V.; Bielak, L.G.; Baker, C.D.; Mansbach, J.M. Influenza and COVID-19 Vaccination Rates Among Children Receiving Long-Term Ventilation. JAMA Netw. Open. 2024, 7, e2430989. [Google Scholar]
- Artzi-Medvedik, R.; Haile, Z.T.; Chertok, I.R.A. Association Between Influenza Vaccination During Pregnancy and Breastfeeding Duration. Breastfeed. Med. 2022, 17, 484–492. [Google Scholar]
- Weston, K.; Bullock, L.; Hsu, A.L.; Wan, X.H.; Burnam-Cole, M.; Everett, K.D.; McElroy, J.A. Maternal COVID vaccination and breastfeeding during a pandemic: Habitus and health behavior decision making. Public Health Nurs. 2023, 40, 750–757. [Google Scholar]
- Ladomenou, F.; Moschandreas, J.; Kafatos, A.; Tselentis, Y.; Galanakis, E. Protective effect of exclusive breastfeeding against infections during infancy: A prospective study. Arch. Dis. Child. 2010, 95, 1004–1008. [Google Scholar] [PubMed]
- World Health Organization (WHO). Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 4 March 2025).
- Mulatu, T.; Yimer, N.B.; Alemnew, B.; Linger, M.; Liben, M.L. Exclusive breastfeeding lowers the odds of childhood diarrhea and other medical conditions: Evidence from the 2016 Ethiopian demographic and health survey. Ital. J. Pediatr. 2021, 47, 166. [Google Scholar]
- WHO Exclusive Breastfeeding for Six Months Best for Babies Everywhere. Available online: https://www.who.int/news/item/15-01-2011-exclusive-breastfeeding-for-six-months-best-for-babies-everywhere (accessed on 17 January 2025).
- Królak-Olejnik, B.; Błasiak, I.; Szczygieł, A. Promotion of breastfeeding in Poland: The current situation. J. Int. Med. Res. 2017, 45, 1976–1984. [Google Scholar]
- Gajewska, D.; Gudej, S. The dietary habits and nutritional status of women and the length of exclusive breastfeeding. Assessment of women’s awareness of the importance of breastfeeding. Med. Stand. Pediatr. 2018, 15, 869–877. [Google Scholar]
- Grzyb, J.; Grzyb, Ł.; Wilińska, M. Perception and practice of breastfeeding in public in Poland. J. Mother Child 2021, 25, 277–284. [Google Scholar] [PubMed]
- Karcz, K.; Lehman, I.; Królak-Olejnik, B. The link between knowledge of the maternal diet and breastfeeding practices in mothers and health workers in Poland. Int. Breastfeed. J. 2021, 16, 58. [Google Scholar]
- Kolmaga, A.; Dems-Rudnicka, K.; Garus-Pakowska, A. Attitudes and Barriers of Polish Women towards Breastfeeding—Descriptive Cross-Sectional On-Line Survey. Healthcare 2024, 12, 1744. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Polish Women Have Moderate Knowledge of Gestational Diabetes Mellitus and Breastfeeding Benefits. Int. J. Environ. Res. Public Health 2021, 18, 10409. [Google Scholar]
- Nancy, S.; Sindhuri, R.; Arunagirinathan, A.; Dongre, A.R. Breastfeeding Positioning and Attachment among Postnatal Mothers: A Mixed Methods Study in a Tertiary Care Hospital in Puducherry, South India. Indian J. Community Med. 2022, 47, 120–124. [Google Scholar]
- Sultana, M.; Dhar, S.; Hasan, T.; Shill, L.C.; Purba, N.H.; Chowdhury, A.I.; Shuvo, S.D. Knowledge, attitudes, and predictors of exclusive breastfeeding practice among lactating mothers in Noakhali, Bangladesh. Heliyon 2022, 8, e11069. [Google Scholar]
- Afreen, M.S.; Majumder, B.; Mazumder, M.; Arju, F.; Islam, S.; Majumder, B.K. Maternal Knowledge and Practice during Postnatal Period Regarding Newborn Care at Hospital Setting. Mymensingh Med. J. 2025, 34, 213–219. [Google Scholar] [PubMed]
- Lis-Kuberka, J.; Berghausen-Mazur, M.; Orczyk-Pawiłowicz, M. Attitude and Level of COVID-19 Vaccination among Women in Reproductive Age during the Fourth Pandemic Wave: A Cross-Sectional Study in Poland. Int. J. Environ. Res. Public Health 2022, 19, 6872. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Body Mass Index. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 4 March 2025).
- Ramli, N.; Rahman, N.A.A.; Haque, M. Knowledge, attitude, and practice regarding osteoporosis among allied health sciences students in a public University in Malaysia. Erciyes Med. J. 2018, 40, 210–217. [Google Scholar] [CrossRef]
- The Position of the Ministry of Health and the National Health Fund Regarding Vaccination of Women During Pregnancy. Available online: https://pacjent.gov.pl/aktualnosc/czy-w-czasie-ciazy-mozna-sie-szczepic (accessed on 15 January 2025). (In Polish)
- World Health Organization (WHO). Updates on Monitoring Safety During Pregnancy and Breastfeeding Projects: PERLA and COVID-19 Pregnancy Cohort Study. Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/pregnancy-and-lactation/vaccines (accessed on 15 January 2025).
- Fallucca, A.; Immordino, P.; Ferro, P.; Mazzeo, L.; Petta, S.; Maiorana, A.; Maranto, M.; Casuccio, A.; Restivo, V. Attitude to Co-Administration of Influenza and COVID-19 Vaccines among Pregnant Women Exploring the Health Action Process Approach Model. Vaccines 2024, 12, 470. [Google Scholar] [CrossRef]
- Brydak, L.B.; Nitsch-Osuch, A. Vaccination against influenza in pregnant women. Acta Biochim. Pol. 2014, 61, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Samel-Kowalik, P.; Jankowski, M.; Lisiecka-Biełanowicz, M.; Ostrowska, A.; Gujski, M.; Kobuszewski, B.; Pinkas, J.; Raciborski, F. Factors Associated with Attitudes towards Seasonal Influenza Vaccination in Poland: A Nationwide Cross-Sectional Survey in 2020. Vaccines 2021, 9, 1336. [Google Scholar] [CrossRef]
- Pisula, A.; Sienicka, A.; Pawlik, K.K.; Dobrowolska-Redo, A.; Kacperczyk-Bartnik, J.; Romejko-Wolniewicz, E. Pregnant Women’s Knowledge of and Attitudes towards Influenza Vaccination during the COVID-19 Pandemic in Poland. Int. J. Environ. Res. Public Health 2022, 19, 4504. [Google Scholar] [CrossRef]
- Gu, X.; Agrawal, U.; Midgley, W.; Bedston, S.; Anand, S.N.; Goudie, R.; Byford, R.; Joy, M.; Jamie, G.; Hoang, U.; et al. COVID-19 and influenza vaccine uptake among pregnant women in national cohorts of England and Wales. NPJ Vaccines 2024, 9, 147. [Google Scholar] [CrossRef]
- Steinmetz, L. Sociodemographic predictors of and main reasons for COVID-19 vaccine hesitancy in eastern Oslo: A cross-sectional study. BMC Public Health 2022, 22, 1878. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, J.; Zhao, Q.; Lv, M.; Wu, J.; Nicholas, S.; Maitland, E.; He, P.; Zhu, D. Predicting future vaccination habits: The link between influenza vaccination patterns and future vaccination decisions among old aged adults in China. J. Infect. Public Health 2024, 17, 1079–1085. [Google Scholar] [CrossRef]
- Kessy, S.J.; Wei, T.; Zhou, Y.; Zhang, W.X.; Alwy Al-Beity, F.M.; Zhang, S.S.; Du, J.; Cui, F.; Lu, Q.B. Vaccination willingness, vaccine hesitancy, and estimated coverage of SARS-CoV-2 vaccine among healthcare workers in Tanzania: A call for action. Immun. Inflamm. Dis. 2023, 11, e1126. [Google Scholar] [PubMed]
- Townsend, M.J.; Kyle, T.K.; Stanford, F.C. COVID-19 Vaccination and Obesity: Optimism and Challenges. Obesity (Silver Spring) 2021, 29, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Frahsa, A. Impact of BMI on COVID-19 vaccine effectiveness. Lancet Diabetes Endocrinol. 2022, 10, 551–552. [Google Scholar] [CrossRef]
- Piernas, C.; Patone, M.; Astbury, N.M.; Gao, M.; Sheikh, A.; Khunti, K.; Shankar-Hari, M.; Dixon, S.; Coupland, C.; Aveyard, P.; et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2022, 10, 571–580. [Google Scholar] [PubMed]
- Ballesta-Castillejos, A.; Gómez-Salgado, J.; Rodríguez-Almagro, J.; Ortiz-Esquinas, I.; Hernández-Martínez, A. Factors that influence mothers’ prenatal decision to breastfeed in Spain. Int. Breastfeed. J. 2020, 15, 97. [Google Scholar]
- Wojcicki, J.M. Maternal prepregnancy body mass index and initiation and duration of breastfeeding: A review of the literature. J. Womens Health 2011, 20, 341–347. [Google Scholar]
- Mohamed, M.J.; Ochola, S.; Owino, V.O. Comparison of knowledge, attitudes and practices on exclusive breastfeeding between primiparous and multiparous mothers attending Wajir District hospital, Wajir County, Kenya: A cross-sectional analytical study. Int. Breastfeed. J. 2018, 13, 11. [Google Scholar]
- Flacking, R.; Tandberg, B.S.; Niela-Vilén, H.; Jónsdóttir, R.B.; Jonas, W.; Ewald, U.; Thomson, G. Positive breastfeeding experiences and facilitators in mothers of preterm and low birthweight infants: A meta-ethnographic review. Int. Breastfeed. J. 2021, 16, 88. [Google Scholar] [CrossRef]
- Wesołowska, A.; Walczak, B.; Kalita-Kurzyńska, K.; Mołas, A.; Bzikowska-Jura, A. Feeding Strategies in Newborns and Infants During the COVID-19 Pandemic-Polish Cross-Sectional Study. Int. J. Public Health 2023, 68, 1605590. [Google Scholar]
- Wako, W.G.; Wayessa, Z.; Fikrie, A. Effects of maternal education on early initiation and exclusive breastfeeding practices in sub-Saharan Africa: A secondary analysis of Demographic and Health Surveys from 2015 to 2019. BMJ Open 2022, 12, e054302. [Google Scholar]
- Koziol-Kozakowska, A.; Stochel-Gaudyn, A.; Łuszczki, E. Evaluation of complementary feeding practices and mothers’ nutritional knowledge in reference to current Polish recommendations. J. Health Inequalities 2022, 8, 145–154. [Google Scholar]
- Titaley, C.R.; Dibley, M.J.; Ariawan, I.; Mu’asyaroh, A.; Paramashanti, B.A.; Alam, A.; Damayanti, R.; Do, T.T.; Ferguson, E.; Htet, M.K.; et al. The impact of a package of behaviour change interventions on breastfeeding practices in East Java Province, Indonesia. Matern. Child Nutr. 2022, 18, e13362. [Google Scholar] [PubMed]
- Magnano San Lio, R.; Maugeri, A.; La Rosa, M.C.; Cianci, A.; Panella, M.; Giunta, G.; Agodi, A.; Barchitta, M. The Impact of Socio-Demographic Factors on Breastfeeding: Findings from the “Mamma & Bambino” Cohort. Medicina 2021, 57, 103. [Google Scholar] [CrossRef]
- Lindsey, B.; Kampmann, B.; Jones, C.E. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr. Opin. Infect. Dis. 2013, 26, 248–253. [Google Scholar] [PubMed]
- Kachikis, A.; Englund, J.A. Maternal immunisation: Optimising protection for the mother and infant. J. Infect. 2016, 72, S83–S90. [Google Scholar] [PubMed]
- Bergin, N.; Murtagh, J.; Philip, R.K. Maternal Vaccination as an Essential Component of Life-Course Immunization and Its Contribution to Preventive Neonatology. Int. J. Environ. Res. Public Health 2018, 15, 847. [Google Scholar] [CrossRef]
- Payakachat, N.; Hadden, K.B.; Ragland, D. Promoting Tdap immunization in pregnancy: Associations between maternal perceptions and vaccination rates. Vaccine 2016, 34, 179–186. [Google Scholar]
- Moschese, V.; De Angelis, L.; Capogna, M.V.; Graziani, S.; Baglivo, F.; Pietropolli, A.; Miraglia Del Giudice, M.; Rizzo, C. Italian Society of Pediatric Allergology and Immunology (SIAIP) Vaccine Committee. Vaccine hesitancy and knowledge regarding maternal immunization among reproductive age women in central Italy: A cross sectional study. Front. Glob. Womens Health 2023, 4, 1237064. [Google Scholar]
- Mose, A. Willingness to Receive COVID-19 Vaccine and Its Determinant Factors Among Lactating Mothers in Ethiopia: A Cross-Sectional Study. Infect. Drug Resist. 2021, 14, 4249–4259. [Google Scholar]
- Maisonneuve, E.; Gerbier, E.; Tauqeer, F.; Pomar, L.; Favre, G.; Winterfeld, U.; Passier, A.; Oliver, A.; Baud, D.; Nordeng, H.; et al. Determinants of Vaccination and Willingness to Vaccinate against COVID-19 among Pregnant and Postpartum Women during the Third Wave of the Pandemic: A European Multinational Cross-Sectional Survey. Viruses 2023, 15, 1090. [Google Scholar] [CrossRef]
- Comparcini, D.; Cicolini, G.; Totaro, M.; Governatori, L.; Pastore, F.; Miniscalco, D.; Flacco, M.E.; Cuscianna, E.; Tafuri, S.; Simonetti, V. Influenza vaccination hesitancy and related factors among pregnant and breastfeeding women: A cross-sectional study. Hum. Vaccin. Immunother. 2025, 21, 2450858. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.C.; Banginwar, A.S.; Ziyo, F.; Toweir, A.A. Breastfeeding practices: Positioning, attachment (latch-on) and effective suckling—A hospital-based study in Libya. J. Family Community Med. 2011, 18, 74–79. [Google Scholar] [PubMed]
- Brown, A.; Jordan, S. Impact of birth complications on breastfeeding duration: An internet survey. J. Adv. Nurs. 2013, 69, 828–839. [Google Scholar] [CrossRef]
- Oakley, L.; Benova, L.; Macleod, D.; Lynch, C.A.; Campbell, O.M.R. Early breastfeeding practices: Descriptive analysis of recent Demographic and Health Surveys. Matern. Child Nutr. 2018, 14, e12535. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.B.; Sclafani, V. Birth Experiences, Breastfeeding, and the Mother-Child Relationship: Evidence from a Large Sample of Mothers. Can. J. Nurs. Res. 2022, 54, 518–529. [Google Scholar] [CrossRef]
- Etti, M.; Calvert, A.; Galiza, E.; Lim, S.; Khalil, A.; Le Doare, K.; Heath, P.T. Maternal vaccination: A review of current evidence and recommendations. Am. J. Obstet. Gynecol. 2022, 226, 459–474. [Google Scholar] [CrossRef]
- Lagousi, T.; Gkentzi, D.; Geropeppa, M.; Tsagkli, P.; Spoulou, V. Protecting the offspring, the gift of maternal immunization: Current status and future perspectives. Vaccines 2022, 10, 1953. [Google Scholar] [CrossRef]
| Data | n/N | % | |
|---|---|---|---|
| Age (years) | Mean ± SD Median (interquartile range 25–75%) | 31.0 ± 4.4 31.0 (28.0–34.0) | |
| 18–25 | 92/953 | 9.7 | |
| 26–34 | 649/953 | 68.1 | |
| ≥35 | 212/953 | 22.2 | |
| Pre-pregnancy BMI, kg/m2 | Mean ± SD Median (interquartile range 25–75%) | 23.5 ± 4.3 22.5 (20.4–25.5) | |
| Underweight (<18.5) | 61/953 | 6.4 | |
| Normal weight (18.5–24.9) | 623/953 | 65.4 | |
| Overweight (25–29.9) | 177/953 | 18.6 | |
| Obesity (≥30) | 92/953 | 9.7 | |
| Residence | Urban, >100,000 residents | 460/953 | 48.3 |
| Urban, 10,000–100,000 residents | 196/953 | 20.6 | |
| Urban, <10,000 residents | 66/953 | 6.9 | |
| Rural | 231/953 | 24.2 | |
| Education | Vocational and primary | 23/953 | 2.4 |
| High school | 175/953 | 18.4 | |
| University | 755/953 | 79.2 | |
| Marital status | Married | 782/953 | 82.1 |
| Cohabiting | 153/953 | 16.1 | |
| Single parent and divorced | 18/953 | 1.9 | |
| Mode of delivery | Vaginal birth | 532/953 | 55.8 |
| Elective caesarean section | 219/953 | 23.0 | |
| Emergency caesarean section | 202/953 | 21.2 | |
| Maternal experience | 0 | 92/953 | 9.7 |
| 1 | 483/953 | 50.7 | |
| 2 | 268/953 | 28.1 | |
| ≥3 | 110/953 | 11.5 | |
| Neonatal feeding strategy | Breastfeeding | 764/953 | 80.2 |
| Mixed feeding | 103/953 | 10.8 | |
| Formula feeding | 86/953 | 9.0 | |
| COVID-19 vaccination | Yes | 629/953 | 66.0 |
| No | 324/953 | 34.0 | |
| Influenza vaccination | Yes | 173/953 | 18.2 |
| No | 780/953 | 81.8 | |
| Dual vaccination | Yes | 149/953 | 15.6 |
| No | 804/953 | 84.4 | |
| Data | Dual Vaccination | Chi2 p-Value | COVID-19 Vaccination | Chi2 p-Value | Influenza Vaccination | Chi2 p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes N = 149 | No N = 804 | Yes N = 629 | No N = 324 | Yes N = 173 | No N = 780 | |||||
| Age (years) | Mean ± SD Median (interquartile range 25–75%) | 32.01 ± 3.94 32.0 (29.0–34.0) | 30.8 ± 4.4 30.0 (28.0–34.0) | 31.5 ± 4.2 31.0 (29.0–34.0) | 30.0 ± 4.6 29.0 (26.0–33.0) | 31.6 ± 4.2 31.0 (29.0–34.0) | 30.8 ± 4.4 30.0 (28.0–34.0) | |||
| 18–25 | 6.3% (6/953) | 9.1% (87/953) | Chi2 = 6.7 p < 0.04 | 4.3% (41/953) | 5.5% (52/953) | Chi2 = 23.1 p < 0.00009 | 1.2% (11/953) | 8.6% (82/953) | Chi2 = 3.0 p < 0.3 | |
| 26–34 | 11.1% (106/953) | 56.9% (542/953) | 45.9% (437/953) | 22.1% (211/953) | 12.6% (120/953) | 55.4% (528/953) | ||||
| ≥35 | 3.9% (37/953) | 18.4% (175/953) | 15.8% (151/953) | 6.4% (61/953) | 4.4% (42/953) | 17.8% (170/953) | ||||
| Pre-pregnancy BMI, kg/m2 | Mean ± SD Median (interquartile range25–75%) | 22.59 ± 3.75 21.8 (20.2–24.1) | 23.6 ± 4.3 22.7 (20.5–25.7) | 23.7 ± 4.5 22.7 (20.5–25.7) | 23.1 ± 3.9 22.5 (20.3–25.1) | 22.6 ± 3.7 22.0 (20.2–24.2) | 23.6 ± 4.4 22.8 (20.5–25.7) | |||
| Underweight (<18.5) | 1.2% (11/953) | 5.6% (53/953) | Chi2 = 8.0 p < 0.05 | 4.4% (42/953) | 2.3% (22/953) | Chi2 = 0.2 p < 0.9 | 1.4% (13/953) | 5.4% (51/953) | Chi2 = 8.6 p < 0.04 | |
| Normal weight (18.5–24.9) | 11.5% (110/953) | 53.9% (514/953) | 42.6% (406/953) | 22.9% (218/953) | 13.3% (127/953) | 52.2% (497/953) | ||||
| Overweight (25–29.9) | 2.2% (21/953) | 16.1% (153/953) | 11.9% (113/953) | 6.4% (61/953) | 2.5% (24/953) | 15.7% (150/953) | ||||
| Obesity (≥30) | 0.7% (7/953) | 8.8% (84/953) | 9.3% (89/953) | 2.4% (23/953) | 0.9% (9/953) | 8.6% (82/953) | ||||
| Residence | Urban, above 100,000 residents | 10.3% (98/953) | 40.0% (362/953) | Chi2 = 21.9 p < 0.00007 | 36.9% (352/953) | 11.3% (108/953) | Chi2 = 21.5 p < 0.0003 | 10.7% (102/953) | 37.6% (358/953) | Chi2 = 10.3 p < 0.02 |
| Urban, 10,000–100,000 residents | 2.3% (22/953) | 18.3% (174/953) | 13.2% (126/953) | 7.3% (70/953) | 3.1% (30/953) | 17.4% (166/953) | ||||
| Urban, <10,000 residents | 7.3% (7/953) | 6.2% (59/953) | 3.7% (35/953) | 3.2% (31/953) | 1.2% (11/953) | 5.8% (55/953) | ||||
| Rural | 2.3% (22/953) | 21.9% (209/953) | 12.2% (116/953) | 12.1% (115/953) | 3.1% (30/953) | 21.1% (201/953) | ||||
| Education | Vocational and primary | 4.2% (4/953) | 2.0% (19/953) | Chi2 = 9.5 p < 0.009 | 1.0% (10/953) | 1.4% (13/953) | Chi2 = 36.4 p < 0.00002 | 0.5% (5/953) | 1.9% (18/9530 | Chi2 = 3.7 p < 0.2 |
| High school | 1.5% (14/953) | 16.9% (161/953) | 8.9% (85/953) | 9.4% (90/953) | 2.4% (23/953) | 15.9% (152/953) | ||||
| University | 13.7% (131/953) | 65.5% (624/953) | 56.0% (534/953) | 23.2% (221/953) | 15.2% (145/953) | 64.0% (610/953) | ||||
| Marital status | Married | 13.6% (130/953) | 68.4% (652/953) | Chi2 = 3.2 p < 0.2 | 56.5% (538/953) | 25.6% (244/953) | Chi2 = 15.9 p < 0.0004 | 14.9% (142/953) | 67.2% (640/953) | Chi2 = 3.1 p < 0.2 |
| Cohabiting | 1.8% (17/953) | 14.3% (136/953) | 8.7% (83/953) | 7.3% (70/953) | 2.6% (25/953) | 13.4% (128/953) | ||||
| Single parent & divorced | 0.2% (2/953) | 1.7% (16/953) | 0.8% (8/953) | 1.0% (10/953) | 0.6% (6/953) | 1.3% (12/953) | ||||
| Mode of delivery | Vaginal birth | 8.8% (84/953) | 47.1% (449/953) | Chi2 = 0.3 p < 0.9 | 35.3% (336/953) | 20.7% (197/953) | Chi2 = 5.7 p < 0.06 | 9.9% (95/953) | 46.0% (438/953) | Chi2 = 0.3 p < 0.9 |
| Elective c-section | 3.8% (36/953) | 19.2% (183/953) | 15.2% (145/953) | 5.9% (56/953) | 4.1% (39/953) | 17.0% (162/953) | ||||
| Emergency c-section | 3.0% (29/953) | 18.0% (172/953) | 15.5% (148/953) | 7.5% (71/953) | 4.1% (39/953) | 18.9% (180/953) | ||||
| Maternal experience | 0 | 1.9% (18/953) | 7.8% (74/953) | Chi2 = 7.1 p < 0.2 | 7.0% (67/953) | 2.6% (25/953) | Chi2 = 4.8 p < 0.4 | 2.1% (20/953) | 7.6% (72/953) | Chi2 = 5.6 p < 0.3 |
| 1 | 7.2% (69/953) | 43.5% (415/953) | 33.2% (316/953) | 17.6% (168/953) | 8.4% (80/953) | 42.4% (404/953) | ||||
| 2 | 5.4% (51/953) | 22,7% (216/953) | 19.0% (181/953) | 9.0% (86/953) | 6.1% (58/953) | 21.9% (209/953) | ||||
| 3 | 1.1% (11/953) | 10.4% (99/953) | 6.8% (65/953) | 4.8% (45/953) | 1.5% (15/953) | 9.9% (95/953) | ||||
| Knowledge level regarding perinatal vaccination | Poor | 3.4% (32/953) | 40.9% (390/953) | Chi2 = 37.3 p < 0.0008 | 17.7% (169/953) | 28.0% (267/953) | Chi2 = 266.7 p < 0.0002 | 5.4% (51/953) | 40.4% (385/953) | Chi2 = 22.8 p < 0.00002 |
| Moderate | 3.3% (31/953) | 11.4% (109/953) | 11.9% (113/0953) | 2.0% (19/953) | 3.5% (33/953) | 10.4% (99/953) | ||||
| Detailed | 9.0% (86/953) | 32.0% (305/953) | 36.4% (347/953) | 4.0% (38/953) | 9.3% (89/953) | 31.1% (296/953) | ||||
| Data | Breastfeeding % (n/N) | Mixed Feeding % (n/N) | Formula Feeding % (n/N) | Chi2 Test p-Value | |
|---|---|---|---|---|---|
| Age (years) | Mean ± SD Median (interquartile range 25–75%) | 31.0 ± 4.4 31.0 (28.0–34.0) | 31.1 ± 4.4 30.0 (28.0–34.0) | 30.23 ± 4.6 31.00 (27.0–33.0) | Chi2 = 4.9 p < 0.29 |
| 18–25 | 10.0% (76/764) | 6.8% (7/103) | 11.6% (10/86) | ||
| 26–34 | 67.0% (512/764) | 70.0% (72/103) | 74.4% (64/86) | ||
| ≥35 | 23.0% (176/764) | 23.3% (24/103) | 14.0% (12/86) | ||
| Pre-pregnancy BMI, kg/m2 | Mean ± SD Median (interquartile range 25–75%) | 23.3 ± 4.1 22.5 (20.3–25.3) | 23.7 ± 4.2 22.8 (20.8–26.0) | 24.7 ± 5.6 22.7 (20.5–27.3) | Chi2 = 23.3 p < 0.0006 |
| Underweight (<18.5) | 6.5% (50/764) | 8.7% (9/103) | 5.8% (5/86) | ||
| Normal weight (18.5–24.9) | 67.1% (513/764) | 60.2% (62/103) | 57.0% (49/86) | ||
| Overweight (25–29.9) | 7.8% (60/764) | 20.4% (21/103) | 14.0% (12/86) | ||
| Obesity (≥30) | 18.5% (141/764) | 10.7% (11/103) | 23.3% (20/86) | ||
| Residence | Urban, above 100,000 residents | 48.2% (368/764) | 54.4% (56/103) | 41.9% (36/86) | Chi2 = 4.2 p < 0.65 |
| Urban, 10,000–100,000 residents | 20.5% (157/764) | 18.4% (19/103) | 23.3% (20/86) | ||
| Urban, <10,000 residents | 6.5% (50/764) | 7.8% (8/103) | 9.3% (8/86) | ||
| Rural | 24.7% (189/764) | 19.4% (20/103) | 25.6% (22/86) | ||
| Education | Vocational and primary | 2.5% (19/764) | 1.0% (1/103) | 3.5% (3/86) | Chi2 = 6.4 p < 0.17 |
| High school | 17.0% (130/764) | 22.3% (23/103) | 25.6% (22/86) | ||
| University | 80.5% (615/764) | 76.7% (79/103) | 70.9% (61/86) | ||
| Marital status | Married | 82.9% (633/764) | 80.6% (83/103) | 76.7% (66/86) | Chi2 = 7.3 p < 0.12 |
| Cohabiting | 15.3% (117/764) | 15.5% (16/103) | 23.3% (20/86) | ||
| Single parent & divorced | 1.8% (14/764) | 3.9% (4/103) | 0% (0/86) | ||
| Mode of delivery | Vaginal birth | 57.6% (440/764) | 50.0% (51/103) | 48.8% (42/86) | Chi2 = 4. 6 p < 0.34 |
| Elective c-section | 21.9% (167/764) | 27.2% (28/103) | 27.9% (24/86) | ||
| Emergency c-section | 20.5% (157/764) | 23.3% (24/103) | 23.3% (20/86) | ||
| Maternal experience | 0 | 7.7% (59/764) | 22.3% (23/103) | 11.6% (10/86) | Chi2 = 30.1 p < 0.0001 |
| 1 | 51.2% (391/764) | 40.8% (42/103) | 59.3% (51/86) | ||
| 2 | 28.5% (218/764) | 32.0% (33/103) | 18.6% (16/86) | ||
| ≥3 | 12.6% (96/764) | 4.8% (5/103) | 10.5% (9/86) | ||
| Knowledge level regarding maternal vaccination | Poor | 42.0% (321/764) | 49.5% (51/103) | 58.1% (50/86) | Chi2 = 11.0 p < 0.03 |
| Moderate | 15.6% (119/764) | 9.7% (10/103) | 12.8% (11/86) | ||
| Detailed | 42.4% (324/764) | 40.8% (42/103) | 29.1% (25/86) | ||
| COVID-19 vaccination | Yes | 66.5% (508/764) | 67.0% (69/103) | 60.5% (52/86) | Chi2 = 1.3 p < 0.53 |
| No | 33.5% (256/764) | 33.0% (34/103) | 39.5% (34/86) | ||
| Influenza vaccination | Yes | 19.2% (147/764) | 14.6% (15/103) | 12.8% (11/86) | Chi2 = 3.2 p < 0.21 |
| No | 80.8% (617/764) | 85.4% (88/103) | 87.2% (75/86) | ||
| Dual vaccination | Yes | 16.6% (127/764) | 12.6% (13/103) | 10.5% (9/86) | Chi2 = 3.0 p < 0.22 |
| No | 83.4% (637/764) | 87.4% (90/103) | 89.5% (77/86) | ||
| Data | No Maternal Experience % (n/N) | 1 Child % (n/N) | 2 Children % (n/N) | ≥3 Children % (n/N) | Chi2 Test p-Value | |
|---|---|---|---|---|---|---|
| Age (years) | Mean ± SD Median (interquartile range 25–75%) | 30.6 ± 3.7 30.0 (28.0–32.0) | 29.6 ± 4.1 29.0 (27.0–32.0) | 32.1 ± 3.8 32.0 (29.0–35.0) | 34.8 ± 4.5 36.0 (32.0–38.0) | |
| 18–25 | 5.4% (5/92) | 15.1% (73/483) | 4.1% (11/268) | 3.6% (4/110) | Chi2 = 119.02 p < 0.0001 | |
| 26–34 | 80.4% (74/92) | 71.8% (347/483) | 67.5% (181/268) | 41.8% (46/110) | ||
| ≥35 | 14.1% (13/92) | 13.3% (64/483) | 28.0% (75/268) | 54.5% (60/110) | ||
| Pre-pregnancy BMI, kg/m2 | Mean ± SD Median (interquartile range 25–75%) | 23.8 ± 4.3 23.0 (20.7–25.7) | 23.2 ± 4.2 22.2 (20.3–25.3) | 23.6 ± 4.3 22.7 (20.6–25.3) | 24.0 ± 4.8 23.5 (20.5–27.1) | |
| Underweight (<18.5) | 5.4% (5/92) | 7.7% (37/483) | 4.9% (13/268) | 8.2% (9/110) | Chi2 = 6.25 p < 0.72 | |
| Normal Weight (18.5–24.9) | 64.1% (59/92) | 66.3% (320/483) | 67.5% (181/268) | 58.2% (64/110) | ||
| Overweight (25–29.9) | 19.6% (18/92) | 17.2% (83/483) | 17.5% (47/268) | 23.6% (26/110) | ||
| Obesity (≥30) | 10.9% (10/92) | 9.1% (44/483) | 9.7% (26/268) | 10.0% (11/110) | ||
| Residence | Urban, Above 100,000 Residents | 62.0% (57/92) | 50.9% (246/483) | 44.4% (119/268) | 34.5% (38/110) | Chi2 = 20.33 p < 0.006 |
| Urban, 10,000–100,000 Residents | 15.2% (14/92) | 18.6% (90/483) | 21.2% (57/268) | 31.8% (35/110) | ||
| Urban, <10,000 Residents | 8.7% (8/92) | 6.6% (32/483) | 7.1% (19/268) | 6.4% (7/110) | ||
| Rural | 14.1% (13/92) | 24.0% (116/483) | 26.9% (72/268) | 27.3% (30/110) | ||
| Education | Vocational and Primary | 0% (0/92) | 1.9% (9/483) | 3.0% (8/268) | 5.5% (6/110) | Chi2 = 32.42 p < 0.0001 |
| High School | 7.6% (7/92) | 18.2% (88/483) | 16.4% (44/268) | 32.7% (36/110) | ||
| University | 92.4% (85/92) | 80.1% (387/483) | 80.2% (215/268) | 61.8% (68/110) | ||
| Marital status | Married | 82.6% (76/92) | 78.1% (377/483) | 86.6% (232/268) | 88.2% (97/110) | Chi2 = 18.11 p < 0.008 |
| Cohabiting | 17.4% (16/92) | 19.0% (92/483) | 12.7% (34/268) | 10.0% (11/110) | ||
| Single Parent & Divorced | 0% (0/92) | 3.1% (15/483) | 0.4% (1/268) | 1.8% (2/110) | ||
| Mode of delivery | Vaginal Birth | 52.2% (48/92) | 56.1% (271/483) | 56.3% (151/268) | 57.3% (63/110) | Chi2 = 53.04 p < 0.0001 |
| Elective C-Section | 14.1% (13/92) | 17.8% (86/483) | 30.6% (82/268) | 34.5% (38/110) | ||
| Emergency C-Section | 33.7% (31/92) | 26.3% (127/483) | 12.7% (34/268) | 8.2% (9/110) | ||
| Knowledge level regarding maternal vaccination | Poor | 42.4% (39/92) | 43.3% (209/483) | 43/7% (117/268) | 51.8% (57/110) | Chi2 = 3.97 p < 0.68 |
| Moderate | 15.2% (14/92) | 15.7% (76/483) | 13.1% (35/268) | 13.6% (15/110) | ||
| Detailed | 42.4% (39/92) | 41.2% (199/483) | 42.9% (115/268) | 34.5% (38/110) | ||
| COVID-19 vaccination | Yes | 72.8% (67/92) | 65.4% (316/483) | 67.5% (181/268) | 69.1% (65/110) | Chi2 = 4.75 p < 0.19 |
| No | 27.2% (25/92) | 34.8% (168/483) | 32.1% (86/268) | 40.9% (45/110) | ||
| Influenza vaccination | Yes | 21.7% (20/92) | 16.6% (80/483) | 21.6% (58/268) | 13.6% (15/110) | Chi2 = 7.00 p < 0.07 |
| No | 78.3% (72/92) | 83.6% (404/483) | 78.0% (209/268) | 86.4% (95/110) | ||
| Dual vaccination | Yes | 19.6% (18/92) | 14.3% (69/483) | 19.0% (51/268) | 10.0% (11/110) | Chi2 = 6.86 p < 0.08 |
| No | 80.4% (74/92) | 85.9% (415/483) | 80.6% (216/268) | 90.0% (99/110) | ||
| Data | Odds Ratio (OR) | 95% Lower—Upper Confidence Interval (CI) | p-Value |
|---|---|---|---|
| Age | 1.07 | 1.02–1.11 | 0.005 |
| BMI | |||
| Normal Weight (18.5–24.9) (ref) | |||
| Underweight (<18.5) | 0.99 | 0.49–2.02 | 0.98 |
| Overweight (25–29.9) | 0.65 | 0.39–1.10 | 0.11 |
| Obese (≥30) | 0.34 | 0.15–0.77 | 0.009 |
| Residence | |||
| Urban, >100,000 Residents (ref) | |||
| Urban, 10,000–100,000 Residents | 0.52 | 0.31–0.87 | 0.02 |
| Urban, <10,000 Residents | 0.49 | 0.21–1.14 | 0.097 |
| Rural | 0.52 | 0.31–0.88 | 0.01 |
| Education | |||
| University (ref) | |||
| High School | 0.66 | 0.36–1.21 | 0.18 |
| Vocational and Primary | 1.77 | 0.56–5.73 | 0.33 |
| Knowledge Level Regarding Maternal Vaccination | |||
| High (ref) | |||
| Moderate | 1.05 | 0.64–1.70 | 0.86 |
| Low | 0.31 | 0.19–0.45 | <0.001 |
| Feeding Strategy | |||
| Breastfeeding (ref) | |||
| Mixed Feeding | 0.878 | 0.41–1.48 | 0.45 |
| Formula Feeding | 0.87 | 0.41–1.84 | 0.72 |
| Data | Odds Ratio (OR) | 95% Lower—Upper Confidence Interval (CI) | p-Value |
|---|---|---|---|
| BMI | |||
| Normal Weight (18.5–24.9) (ref) | |||
| Underweight (<18.5) | 0.77 | 0.41–1.45 | 0.42 |
| Overweight (25–29.9) | 0.94 | 0.61–1.46 | 0.80 |
| Obese (≥30) | 0.40 | 0.24–0.65 | <0.001 |
| Maternal experience | |||
| 1 child (ref) | |||
| no maternal experience | 0.41 | 0.25–0.67 | <0.0001 |
| 2nd child | 1.06 | 0.71–1.56 | 0.78 |
| 3rd and more child | 1.76 | 0.96–3.26 | 0.07 |
| Level of Knowledge Regarding Perinatal Vaccination | |||
| High (ref) | |||
| Moderate | 1.15 | 0.67–1.97 | 0.62 |
| Low | 0.63 | 0.44–0.90 | 0.02 |
| Influenza Vaccination | |||
| Yes (ref) | |||
| No | 0.69 | 0.23–2.08 | 0.52 |
| Dual Vaccination | |||
| Yes (ref) | |||
| No | 1.15 | 0.35–3.77 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Maternal Vaccination and Neonatal Feeding Strategies Among Polish Women. Vaccines 2025, 13, 376. https://doi.org/10.3390/vaccines13040376
Lis-Kuberka J, Orczyk-Pawiłowicz M. Maternal Vaccination and Neonatal Feeding Strategies Among Polish Women. Vaccines. 2025; 13(4):376. https://doi.org/10.3390/vaccines13040376
Chicago/Turabian StyleLis-Kuberka, Jolanta, and Magdalena Orczyk-Pawiłowicz. 2025. "Maternal Vaccination and Neonatal Feeding Strategies Among Polish Women" Vaccines 13, no. 4: 376. https://doi.org/10.3390/vaccines13040376
APA StyleLis-Kuberka, J., & Orczyk-Pawiłowicz, M. (2025). Maternal Vaccination and Neonatal Feeding Strategies Among Polish Women. Vaccines, 13(4), 376. https://doi.org/10.3390/vaccines13040376






