Local Lidocaine–Prilocaine for Immunisation in Infants
Abstract
1. Introduction
1.1. Vaccination in Infants
1.2. Pain in Infants
1.3. Pain Perception in Infants
1.4. Factors Influencing Pain Response
1.5. Assessment of Pain in Infants
1.6. Pain-Reducing Methods
2. Materials and Methods
- Population (P): paediatric patients aged 0–12 months requiring intramuscular vaccination.
- Intervention (I): application of lidocaine–prilocaine cream to the thigh prior to vaccination.
- Comparison (C): effectiveness of the anaesthetic cream versus other interventions (placebo or no intervention).
- Outcomes (O): evaluation of whether lidocaine–prilocaine cream could effectively reduce vaccination pain, using pain scales (e.g., FLACC, NIPS), behavioural distress indicators, and physiological measures (e.g., heart rate).
- Types of studies (T): included randomised clinical trials, observational cohort studies, and relevant guidelines.
2.1. Data Sources and Search Strategies
2.2. Study Selection
- Participants aged 0–12 months requiring intramuscular vaccination.
- Studies must involve lidocaine–prilocaine cream.
- Randomised controlled trials or comparative studies.
- Full-text articles published from 2000 to 2023.
2.3. Data Extraction
2.4. Data Analysis
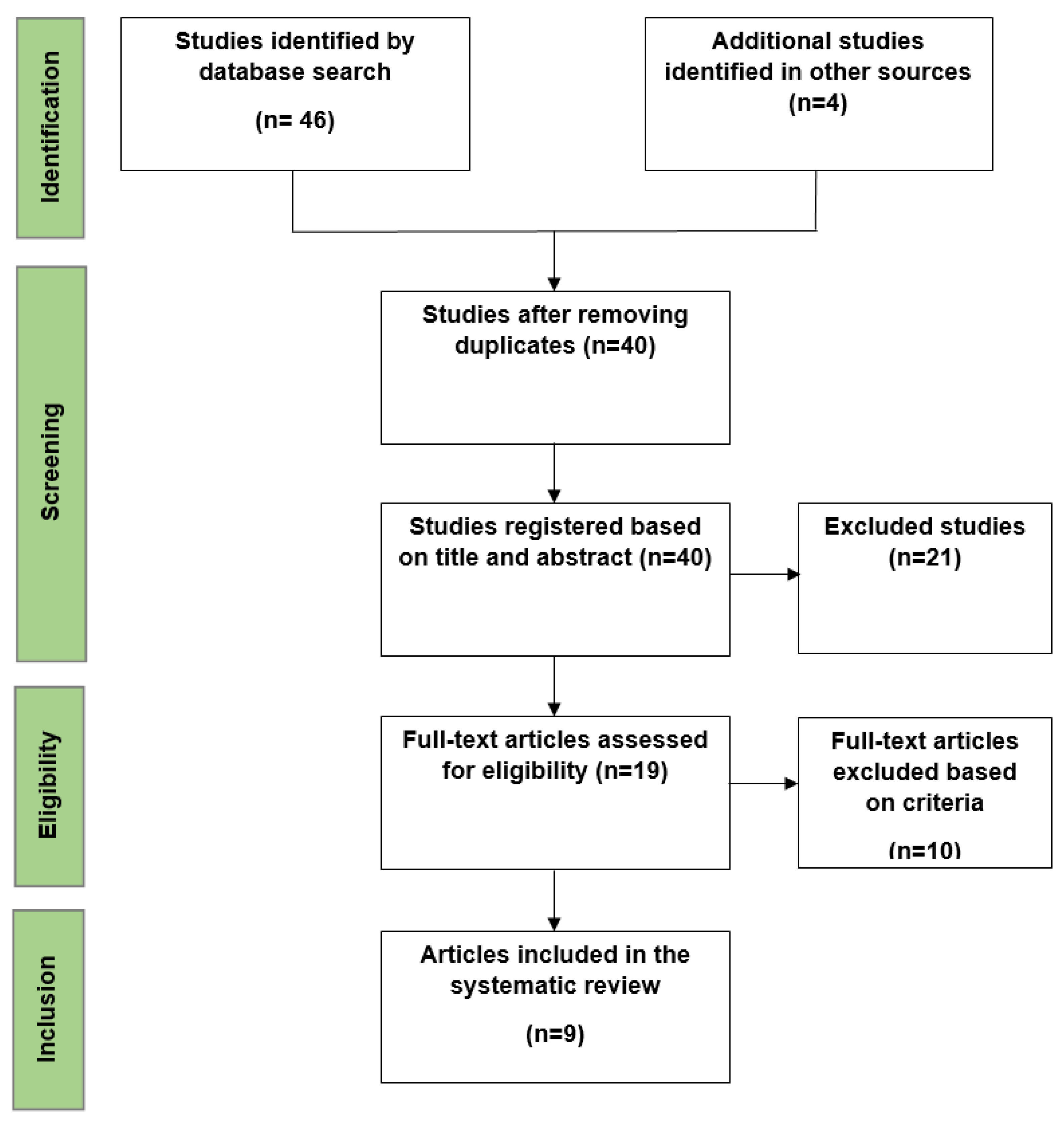
3. Results
- Selected Studies
- Study Population
- Outcomes
4. Discussion
4.1. Applicability of Evidence
4.2. Concordances and Discordances
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kassab, M.; Hamadneh, S.; Nuseir, K.; ALmomani, B.; Hamadneh, J. Factors Associated With Infant Pain Severity Undergoing Immunization Injections. J. Pediatr. Nurs. 2018, 42, e85–e90. [Google Scholar] [CrossRef] [PubMed]
- Azoicai, D.; Barbacariu, C.L.; Barbacariu, I.C.; Branza, I.L.; Chitan, L.E.; Cioc, S.M. Indreptar de Vaccinare in Cabinetul Medicului de Familie, 2023rd ed.; Amaltea: Bucharest, Romania, 2023. [Google Scholar]
- Herdea, V.; Ghionaru, R.; Lungu, C.N.; Leibovitz, E.; Diaconescu, S. Vaccine Coverage in Children Younger Than 1 Year of Age during Periods of High Epidemiological Risk: Are We Preparing for New Outbreaks? Children 2022, 9, 1334. [Google Scholar] [CrossRef] [PubMed]
- Pop, T.L.; Burlea, M.; Falup-Pecurariu, O.; Borzan, C.; Gabor-Harosa, F.; Herdea, V.; Pop, C.F.; Rajka, D.; Ognean, M.L.; Căinap, S.S. Overview of the Pediatric Healthcare System in Romania. Turk. Pediatri. Arsivi. 2020, 55, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.F.; Coblisan, P.; Capalna, L.; Panța, P.C.; Buzoianu, A.D.; Bocsan, I.C. Safety of Vaccination within First Year of Life-The Experience of One General Medicine Center. Children 2023, 10, 104. [Google Scholar] [CrossRef]
- Min, H.J.; Kim, Y.J. Analysis of Gluteal Subcutaneous and Muscle Thickness in Infants and Children for Application to Intramuscular Injection, Autologous Fat Grafting, and Gluteal Artery Perforator Flaps. Arch. Plast. Surg. 2018, 45, 550–556. [Google Scholar] [CrossRef]
- Polania Gutierrez, J.J.; Munakomi, S. Intramuscular Injection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mabbott, A.P.; Bedford, H. Pain Management in Infant Immunisation: A Cross-Sectional Survey of UK Primary Care Nurses. Prim. Health Care Res. Dev. 2023, 24, e71. [Google Scholar] [CrossRef]
- Taddio, A.; McMurtry, C.M.; Logeman, C.; Gudzak, V.; de Boer, A.; Constantin, K.; Lee, S.; Moline, R.; Uleryk, E.; Chera, T.; et al. Prevalence of Pain and Fear as Barriers to Vaccination in Children—Systematic Review and Meta-Analysis. Vaccine 2022, 40, 7526–7537. [Google Scholar] [CrossRef]
- Fahriani, M.; Anwar, S.; Yufika, A.; Bakhtiar, B.; Wardani, E.; Winardi, W.; Akel, K.B.; Wagner, A.L.; Harapan, H. Disruption of Childhood Vaccination during the COVID-19 Pandemic in Indonesia. Narra. J. 2021, 1, e7. [Google Scholar] [CrossRef]
- Taddio, A.; Shah, V.; Bucci, L.; MacDonald, N.E.; Wong, H.; Stephens, D. Effectiveness of a Hospital-Based Postnatal Parent Education Intervention about Pain Management during Infant Vaccination: A Randomized Controlled Trial. CMAJ Can. Med. Assoc. J. 2018, 190, E1245–E1252. [Google Scholar] [CrossRef]
- Eissler, A.B.; Stoffel, L.; Nelle, M.; Hahn, S.; Zwakhalen, S. Pain Responses in Preterm Infants and Parental Stress over Repeated Painful Procedures: A Randomized Pilot Trial. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2023, 36, 2183753. [Google Scholar] [CrossRef]
- The Long Life of Early Pain|Harvard Medical School. Available online: https://hms.harvard.edu/news/long-life-early-pain (accessed on 29 September 2024).
- Hatfield, L.A. Neonatal Pain: What’s Age Got to Do with It? Surg. Neurol. Int. 2014, 5, S479–S489. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Smith, E.M. Procedural Pain Management in Neonates, Infants and Children. Rev. Pain 2011, 5, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Nader, R. Age Related Differences in Infant Pain Expression and Parental Judgements of Pain Throughout the First Year of Life. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2005. [Google Scholar]
- Ullsten, A.; Campbell-Yeo, M.; Eriksson, M. Parent-Led Neonatal Pain Management-a Narrative Review and Update of Research and Practices. Front. Pain Res. 2024, 5, 1375868. [Google Scholar] [CrossRef] [PubMed]
- Piira, T.; Champion, G.D.; Bustos, T.; Donnelly, N.; Lui, K. Factors Associated with Infant Pain Response Following an Immunization Injection. Early Hum. Dev. 2007, 83, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J. Temperament and the Reactions to Unfamiliarity. Child Dev. 1997, 68, 139–143. [Google Scholar] [CrossRef]
- Kagan, J. Temperamental and Theoretical Contributions to Clinical Psychology. Annu. Rev. Clin. Psychol. 2022, 18, 1–18. [Google Scholar] [CrossRef]
- McNair, C.; Fung, M.; Taddio, A.; Ipp, M.; Moss, S.; Baker, S.; Tolkin, J.; Dave, M.; Feerasta, S.; Govan, P.; et al. Parent-Led Interventions in Reducing Infant Vaccination Pain after Participation in a Longitudinal Randomized Control Trial. Paediatr. Child Health 2017, 22, 217–219. [Google Scholar] [CrossRef]
- Crellin, D.J.; Harrison, D.; Santamaria, N.; Huque, H.; Babl, F.E. The Psychometric Properties of the FLACC Scale Used to Assess Procedural Pain. J. Pain 2018, 19, 862–872. [Google Scholar] [CrossRef]
- Kucukoglu, S.; Celebioglu, A.; Caner, I.; Ok, G.; Maden, R. The Effects of Instrumental Touching on Infant Pain Perception and the Effects of Eutectic Mixture of Local Anesthetics (EMLA) on the Reduction of Pain. Iran. J. Pediatr. 2015, 25, e532. [Google Scholar] [CrossRef]
- Gupta, N.K.; Upadhyay, A.; Dwivedi, A.K.; Agarwal, A.; Jaiswal, V.; Singh, A. Randomized Controlled Trial of Topical EMLA and Vapocoolant Spray for Reducing Pain during wDPT Vaccination. World J. Pediatr. WJP 2017, 13, 236–241. [Google Scholar] [CrossRef]
- Crellin, D.J.; Harrison, D.; Hutchinson, A.; Schuster, T.; Santamaria, N.; Babl, F.E. Procedural Pain Scale Evaluation (PROPoSE) Study: Protocol for an Evaluation of the Psychometric Properties of Behavioural Pain Scales for the Assessment of Procedural Pain in Infants and Children Aged 6-42 Months. BMJ Open 2017, 7, e016225. [Google Scholar] [CrossRef] [PubMed]
- Altay, G.; Küçükoğlu, S. Effects of the Facilitated Tucking Position in Early Period on Physiological Parameters, Comfort and Breastfeeding Performance in Late Preterm Infants: A Randomized Controlled Trial. Midwifery 2022, 115, 103492. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Engle, S.; Jorgensen, J.; Kralik, A.; Whitman, K. Effects of Facilitated Tucking during Routine Care of Infants Born Preterm. Pediatr. Phys. Ther. Off. Publ. Sect. Pediatr. Am. Phys. Ther. Assoc. 2005, 17, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Vu-Ngoc, H.; Uyen, N.C.M.; Thinh, O.P.; Don, L.D.; Danh, N.V.T.; Truc, N.T.T.; Vi, V.T.; Vuong, N.L.; Huy, N.T.; Duong, P.D.T. Analgesic Effect of Non-Nutritive Sucking in Term Neonates: A Randomized Controlled Trial. Pediatr. Neonatol. 2020, 61, 106–113. [Google Scholar] [CrossRef]
- Shah, P.S.; Herbozo, C.; Aliwalas, L.L.; Shah, V.S. Breastfeeding or Breast Milk for Procedural Pain in Neonates. Cochrane Database Syst. Rev. 2012, 12, CD004950. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivaramakrishnan, G. Pharmacological Interventions for Reducing Pain Related to Immunization or Intramuscular Injection in Children: A Mixed Treatment Comparison Network Meta-Analysis of Randomized Controlled Clinical Trials. J. Child Health Care Prof. Work. Child. Hosp. Community 2018, 22, 393–405. [Google Scholar] [CrossRef]
- Foster, J.P.; Taylor, C.; Spence, K. Topical Anaesthesia for Needle-Related Pain in Newborn Infants. Cochrane Database Syst. Rev. 2017, 2, CD010331. [Google Scholar] [CrossRef]
- Harrison, D.; Beggs, S.; Stevens, B. Sucrose for Procedural Pain Management in Infants. Pediatrics 2012, 130, 918–925. [Google Scholar] [CrossRef]
- Schweitzer-Chaput, A.; Callot, D.; Bouazza, N.; Lesage, F.; Oualha, M.; Paret, N.; Boyer-Gervoise, M.; Treluyer, J.-M.; Chouchana, L. Local Anesthetics Systemic Toxicity in Children: Analysis of the French Pharmacovigilance Database. BMC Pediatr. 2023, 23, 321. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Taddio, A.; Nulman, I.; Reid, E.; Shaw, J.; Koren, G. Effect of Lidocaine-Prilocaine Cream (EMLA®) on Pain of Intramuscular Fluzone® Injection. Can. J. Hosp. Pharm. 1992, 45. [Google Scholar] [CrossRef]
- Abuelkheir, M.; Alsourani, D.; Al-Eyadhy, A.; Temsah, M.-H.; Meo, S.A.; Alzamil, F. EMLA(R) Cream: A Pain-Relieving Strategy for Childhood Vaccination. J. Int. Med. Res. 2014, 42, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Halperin, B.A.; Halperin, S.A.; McGrath, P.; Smith, B.; Houston, T. Use of Lidocaine-Prilocaine Patch to Decrease Intramuscular Injection Pain Does Not Adversely Affect the Antibody Response to Diphtheria-Tetanus-Acellular Pertussis-Inactivated Poliovirus-Haemophilus Influenzae Type b Conjugate and Hepatitis B Vaccines in Infants from Birth to Six Months of Age. Pediatr. Infect. Dis. J. 2002, 21, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Lindh, V.; Wiklund, U.; Blomquist, H.K.; Håkansson, S. EMLA Cream and Oral Glucose for Immunization Pain in 3-Month-Old Infants. Pain 2003, 104, 381–388. [Google Scholar] [CrossRef]
- Dilli, D.; Küçük, I.G.; Dallar, Y. Interventions to Reduce Pain during Vaccination in Infancy. J. Pediatr. 2009, 154, 385–390. [Google Scholar] [CrossRef]
- Gupta, N.K.; Upadhyay, A.; Agarwal, A.; Goswami, G.; Kumar, J.; Sreenivas, V. Randomized Controlled Trial of Topical EMLA and Breastfeeding for Reducing Pain during wDPT Vaccination. Eur. J. Pediatr. 2013, 172, 1527–1533. [Google Scholar] [CrossRef]
- Basiri-Moghadam, M.; Kianmehr, M.; Pasban-Noghabi, S.; Basiri-Moghadam, K. Comparison of EMLA Cream with Rattles on Reducing Immunization Pain in Four Months Infants. JPMA J. Pak. Med. Assoc. 2014, 64, 874–878. [Google Scholar]
- Kumar, M.; Venkateshwar, V. A Study of Effect of Topical Anaesthetics on Injection Pain during Immunization in Infants. Int. J. Contemp. Pediatr. 2020, 7, 1463–1468. [Google Scholar] [CrossRef]
- Olsson Duse, B.; Sporrong, Y.; Bartocci, M.; Skoglund, K. Efficacy of Topical Lidocaine-prilocaine (EMLA®) for Management of Infant Pain during Pneumococcal Vaccination: A Randomized Controlled Trial. Paediatr. Neonatal Pain 2021, 4, 53–60. [Google Scholar] [CrossRef]
- Chapter 8: Assessing Risk of Bias in a Randomized Trial. Available online: https://training.cochrane.org/handbook/current/chapter-08 (accessed on 30 October 2024).
| Type of Vaccine | Recommended Age |
|---|---|
| Hepatitis B vaccine (HB) | the first 24 h after birth |
| Diphtheria, Tetanus, Pertussis (acellular, component), Hepatitis B (rDNA), Poliomyelitis (inactivated) and Haemophilus Influenzae type B conjugate vaccine (adsorbed) (DTaP-IPV-HB-Hib) | 2, 4, 11 months |
| Pneumococcal polysaccharide 13-valent conjugate vaccine | 2, 4, 11 months |
| Measles, Mumps, and Rubella vaccine (MMR) | 12 months |
| Author, Year, Country | Population | Intervention | Comparator | Vaccine | Scale Used | Behavioural Indicators | Conclusions |
|---|---|---|---|---|---|---|---|
| Halperin BA. et al., 2002, Canada [37] | 153 infants aged between 0 and 6 months | EMLA cream patch (1 g 60–180 min before vaccination) | Patch with placebo cream | DTaP-IPV-Hib, Hepatitis B | MBPS | - | The highest statistically significant difference between pain scores was observed in infants aged 6 months. The group of patients aged 2–4 months was too small and no statistically significant differences between pain scores were identified. No difference was seen in 2-month-old infants. |
| Lindh V. et al., 2003, Sweden [38]. | 90 patients aged under 3 months | EMLA cream patch (1 g 60 min before vaccination) and glucose solution | Patch with placebo cream and water | DTaP | MBPS; VAS. | Presence of crying, latency to first cry, and total duration of crying. | Patients in the EMLA + glucose group had a significant reduction in pain scores, with less crying than in the placebo group, and crying started later and lasted less. |
| Dilli D. et al., 2008, Turkey [39]. | 14 patients aged between 6 and 12 months | Lidocaine–prilocaine cream (1 g 60 min before vaccination) | No intervention | Hepatitis B; MMR. | NIPS | Total duration of crying | The use of anaesthetic cream was much more effective than vaccination without any pre-applied intervention. |
| Gupta NK. et al., 2013, India [40]. | 60 patients aged under 3 months | EMLA cream patch (1 g 60 min before vaccination) and distilled water. | Placebo cream (Vaseline) and distilled water | DTaP | MFCS (modified NFCS scale) | Latency to first cry and total duration of crying. | The use of EMLA cream significantly reduced the pain score and total duration of crying in the group treated with it in combination with distilled water. The EMLA + breastfeeding group was not considered. |
| Basiri-Moghadam M. et al., 2014, Iran [41]. | 32 patients aged 4 months | EMLA cream (2 g 60 min before vaccination) | Rattle toy | Unspecified | Own scale, modified. | - | In the toy group, the pain was more severe than in the EMLA group. There was also a group that did not receive any intervention, and the pain score was significantly increased compared to the intervention groups. |
| Kucukoglu S. et al., 2015, Turkey [23]. | 75 patients aged 4 months | EMLA cream (0.5 g 30 min before vaccination) | Instrumental touch (placebo) | Hepatitis B | PIPP | - | The pain score in the EMLA group was lower than in the instrumental touch group. |
| Gupta NK. et al., 2017, India [24]. | 60 infants aged 3 months | EMLA cream (1 g 60 min before vaccination) and breastfeeding | Cooling spray with breastfeeding | DTaP | MFCS; NIPS. | Latency to first cry and total duration of crying. | The use of EMLA cream or cooling spray with breastfeeding reduced the pain score, but did not reduce the duration of crying. |
| Kumar M. et al., 2020, India [42]. | 150 patients aged between 6 weeks and 6 months | Lidocaine–prilocaine cream (no data regarding the dose, 60 min before vaccination) | Without intervention | Combined vaccine DTaP–HiB–Hepatitis B | MBPS | “The group that received the lidocaine–prilocaine cream had the lowest pain scores, followed by the groups that received other types of interventions, which were not included in our study”. | |
| Duse BO. et al., 2021, Sweden [43]. | 70 patients aged 3 months | EMLA cream (1 g 60 min before vaccination) | Placebo cream | Pneumococcal | FLACC; VAS. | Latency to first cry and total duration of crying. | “The use of EMLA cream significantly reduced pain during vaccination, increasing the latency to the first cry; however, no significant reduction in the total duration of crying was observed”. |
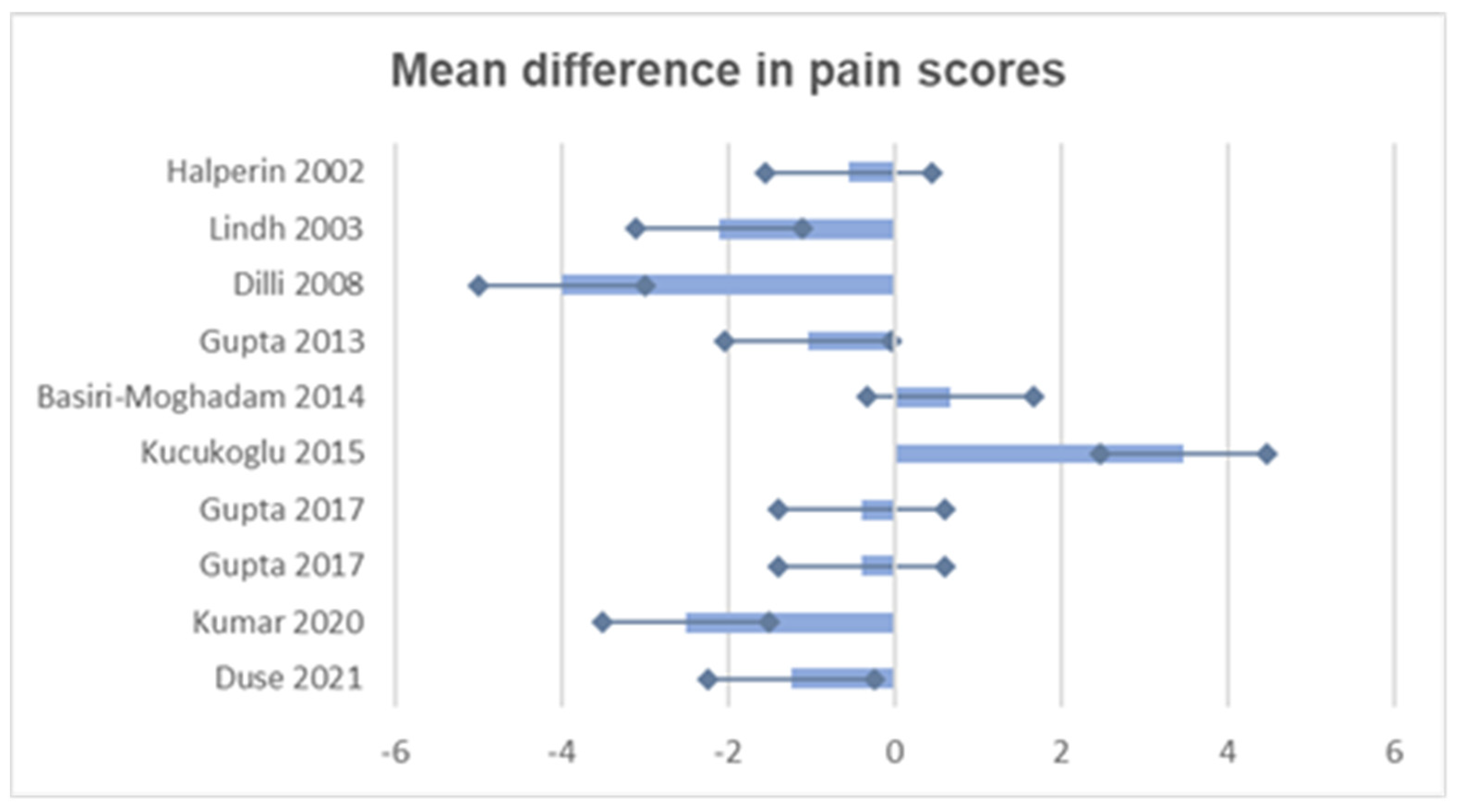
| Study | Mean Intervention (SD) | Mean Comparator (SD) | Mean Difference | Confidence Interval 95% | p Value |
|---|---|---|---|---|---|
| Halperin, 2002 [37] | 2.77 (0.335) | 3.33 (0.76) | −0.56 | (−0.88, −0.24) | 0.0029 |
| Lindh, 2003 [38] | 3.7 (1.8) | 5.8 (1.8) | −2.1 | (−3.5, −0.7) | 0.004 |
| Dilli, 2008 [39] | 2.0 (1.02) | 6.0 (0.51) | −4.0 | (−5.2, −2.8) | 0.001 |
| Gupta, 2013 [40] | 4.23 (2.66) | 5.26 (1.83) | −1.03 | (−2.08, 0.02) | 0.05 |
| Basiri-Moghadam, 2014 [41] | 4.87 (1.31) | 4.19 (1.94) | 0.68 | (−0.60, 1.96) | 0.27 |
| Kucukoglu, 2015 [23] | 9.6 (5.12) | 6.13 (5.97) | 3.47 | (1.54, 5.40) | 0.001 |
| Gupta, 2017 [24] | 1.4 (2.4) | 1.8 (2.5) | −0.4 | (−1.59, 0.79) | 0.05 |
| 1.9 (3.1) | 2.3 (3.0) | −0.4 | (−1.98, 1.18) | 0.05 | |
| Kumar, 2020 [42] | 4.1 (1.1) | 6.6 (0.9) | −2.5 | (−3.1, −1.9) | 0.0024 |
| Duse, 2021 [43] | 6.37 (2.22) | 7.62 (1.48) | −1.25 | (−2.11, −0.39) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, C.-F.; Coblișan, P.; Sas, V.; Drugă, C.; Cherecheș-Panța, P. Local Lidocaine–Prilocaine for Immunisation in Infants. Vaccines 2024, 12, 1329. https://doi.org/10.3390/vaccines12121329
Pop C-F, Coblișan P, Sas V, Drugă C, Cherecheș-Panța P. Local Lidocaine–Prilocaine for Immunisation in Infants. Vaccines. 2024; 12(12):1329. https://doi.org/10.3390/vaccines12121329
Chicago/Turabian StylePop, Claudia-Felicia, Petronela Coblișan, Valentina Sas, Cătălina Drugă, and Paraschiva Cherecheș-Panța. 2024. "Local Lidocaine–Prilocaine for Immunisation in Infants" Vaccines 12, no. 12: 1329. https://doi.org/10.3390/vaccines12121329
APA StylePop, C.-F., Coblișan, P., Sas, V., Drugă, C., & Cherecheș-Panța, P. (2024). Local Lidocaine–Prilocaine for Immunisation in Infants. Vaccines, 12(12), 1329. https://doi.org/10.3390/vaccines12121329






