Exploring CD169+ Macrophages as Key Targets for Vaccination and Therapeutic Interventions
Abstract
1. General Introduction
2. Structure and Binding Function of CD169
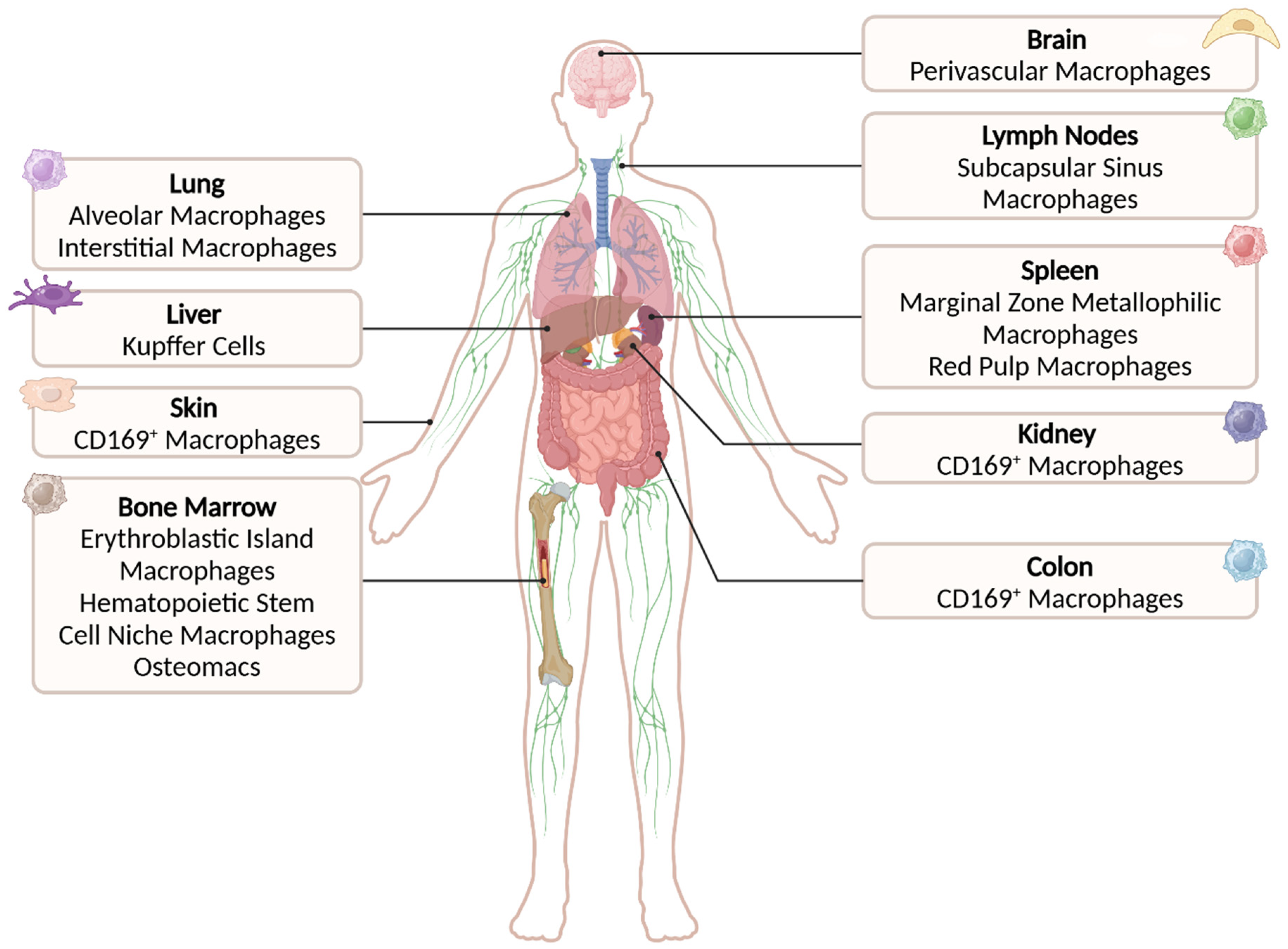
3. CD169-Expressing Macrophage Subsets Within the Tissues
3.1. CD169+ Macrophages in the Bone Marrow
3.2. CD169+ Macrophages in Spleen and Lymph Nodes
3.3. CD169+ Macrophages in the Liver
3.4. CD169+ Macrophages in the Lung
3.5. CD169+ Macrophages in the Brain, Gut, Skin, and Kidneys
| Tissue | CD169+ Macrophages | Phenotype | Function | References |
|---|---|---|---|---|
| Bone Marrow | Erythroblastic island macrophages | F4/80+ CD169+ VCAM1+ ER-HR3+ Ly6G+ Epor+ | Erythropoiesis support | [24,25] |
| Hematopoietic stem cell niche macrophages | F4/80+ CD169+ CD115+ CD68int CD11blo MHCIIint CD11cint CX3CR1− | Hematopoietic stem cell maintenance | [33] | |
| Osteomacs | F4/80+ CD169+ CD115+ CD68+ ER-HR3− | Bone homeostasis and repair | [21,35] | |
| Spleen | Marginal zone metallophilic macrophages | F4/80lo CD169++ MHCIIint CD11clo SIGNR-1− VCAM+ ICAM+ | Pathogen/antigen capture and transfer | [12,37,51] |
| Red pulp macrophages | F4/80++ CD169lo CD11blo CD163+ CD172a+ | Erythrocyte clearance and iron recycling | [37] | |
| Lymph node | Subcapsular sinus macrophages | F4/80lo CD169++ CD11b+ MHCII+ CD11clo VCAM+ ICAM+ | Pathogen/antigen capture and transfer | [45,111] |
| Medullary sinus macrophages | F4/80hi CD169+ CD11b+ MHCII+ CD11clo SIGNR-1+ | Immune surveillance | [45,111] | |
| Liver | Kupffer cells | CD11b+ CLEC4F+ CD64+ CD169+ VSIG4+ CD163+ FOLR2+ MARCO+ | Blood-borne particle clearance, lipid metabolism modulation | [4,73,75] |
| Lung | Alveolar macrophages | CD64+ CD169+ MARCO+ MerTK+ Siglec-F+ CD11c+ F4/80+ | Immune homeostasis and surveillance, surfactant level, and lipid metabolism regulation | [83] |
| Interstitial macrophages | CD169+ F4/80+ MerTK+ CD64+ MHCII+ CD11c− | Immune homeostasis | [83,90,91] | |
| Other organs | Perivascular brain macrophages | CX3CR1lo CD169+ CD11b+ IBa-1lo CD206+ CD163+ CD64+ MerTK+ | Inflammatory microenvironment regulation | [98] |
| Intestinal macrophages | F4/80+ CD169+ CD11b+ MHCII+ CD11clo CX3CR1+ CD103− CD135− CD115+ | [103,104,105] | ||
| Skin macrophages | F4/80+ CD169+ CD11b+ CD64+ MerTK+ | [107,108,112,113] | ||
| Kidney macrophages | F4/80+ CD169+ CD11bint | [110] |
4. CD169+ Macrophages in Disease
4.1. The Role of CD169+ Macrophages and the CD169 Receptor in Infectious Diseases
4.1.1. Viral Infection
4.1.2. Bacterial Infection
4.1.3. Parasitic and Fungal Infection
4.2. The Role of CD169-Expressing Cell Subsets in Autoimmunity
4.3. The Role of CD169-Expressing Cell Subsets in Cancer
5. CD169-Targeting Delivery Systems
5.1. CD169-Targeting Antibodies as a Cancer Vaccination Platform
5.2. CD169-Targeting Nanoparticles as Cancer Vaccination Strategy
5.3. CD169 Targeting in Viral Infections
6. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Crocker, P.R.; Gordon, S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J. Exp. Med. 1989, 169, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Kelm, S.; Dubois, C.; Martin, B.; McWilliam, A.S.; Shotton, D.M.; Paulson, J.C.; Gordon, S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991, 10, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.; Crocker, P.R. Sialoadhesin in recognition of self and non-self. Semin. Immunopathol. 2012, 34, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, A.; Steel, J.; Turley, H.; Jones, M.; Jackson, D.G.; Crocker, P.R. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 2001, 97, 288–296. [Google Scholar] [CrossRef]
- York, M.R.; Nagai, T.; Mangini, A.J.; Lemaire, R.; van Seventer, J.M.; Lafyatis, R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007, 56, 1010–1020. [Google Scholar] [CrossRef]
- Crocker, P.R.; Mucklow, S.; Bouckson, V.; McWilliam, A.; Willis, A.C.; Gordon, S.; Milon, G.; Kelm, S.; Bradfield, P. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 1994, 13, 4490–4503. [Google Scholar] [CrossRef]
- Crocker, P.R.; Gordon, S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J. Exp. Med. 1986, 164, 1862–1875. [Google Scholar] [CrossRef]
- Kelm, S.; Pelz, A.; Schauer, R.; Filbin, M.T.; Tang, S.; de Bellard, M.E.; Schnaar, R.L.; Mahoney, J.A.; Hartnell, A.; Bradfield, P.; et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 1994, 4, 965–972. [Google Scholar] [CrossRef]
- Collins, B.E.; Kiso, M.; Hasegawa, A.; Tropak, M.B.; Roder, J.C.; Crocker, P.R.; Schnaar, R.L. Binding specificities of the sialoadhesin family of I-type lectins. Sialic acid linkage and substructure requirements for binding of myelin-associated glycoprotein, Schwann cell myelin protein, and sialoadhesin. J. Biol. Chem. 1997, 272, 16889–16895. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Chang, Y.C.; Nizet, V. Siglecs at the Host-Pathogen Interface. Adv. Exp. Med. Biol. 2020, 1204, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, J.; Lopez-Venegas, M.A.; Affandi, A.J.; den Haan, J.M.M. CD169(+) Macrophages Capture and Dendritic Cells Instruct: The Interplay of the Gatekeeper and the General of the Immune System. Front. Immunol. 2018, 9, 2472. [Google Scholar] [CrossRef]
- Lin, S.Y.; Schmidt, E.N.; Takahashi-Yamashiro, K.; Macauley, M.S. Roles for Siglec-glycan interactions in regulating immune cells. Semin. Immunol. 2024, 77, 101925. [Google Scholar] [CrossRef]
- Raich-Regue, D.; Resa-Infante, P.; Gallemi, M.; Laguia, F.; Muniz-Trabudua, X.; Munoz-Basagoiti, J.; Perez-Zsolt, D.; Chojnacki, J.; Benet, S.; Clotet, B.; et al. Role of Siglecs in viral infections: A double-edged sword interaction. Mol. Asp. Med. 2023, 90, 101113. [Google Scholar] [CrossRef]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- van Dinther, D.; Veninga, H.; Iborra, S.; Borg, E.G.F.; Hoogterp, L.; Olesek, K.; Beijer, M.R.; Schetters, S.T.T.; Kalay, H.; Garcia-Vallejo, J.J.; et al. Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8(+) T Cell Cross-Priming. Cell Rep. 2018, 22, 1484–1495. [Google Scholar] [CrossRef]
- van den Berg, T.K.; Brevé, J.J.; Damoiseaux, J.G.; Döpp, E.A.; Kelm, S.; Crocker, P.R.; Dijkstra, C.D.; Kraal, G. Sialoadhesin on macrophages: Its identification as a lymphocyte adhesion molecule. J. Exp. Med. 1992, 176, 647–655. [Google Scholar] [CrossRef]
- Crocker, P.R.; Freeman, S.; Gordon, S.; Kelm, S. Sialoadhesin binds preferentially to cells of the granulocytic lineage. J. Clin. Investig. 1995, 95, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Rauch, U.; Korpos, E.; Song, J.; Loser, K.; Crocker, P.R.; Sorokin, L.M. Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J. Immunol. 2009, 182, 6508–6516. [Google Scholar] [CrossRef]
- Zhang, Y.; Roth, T.L.; Gray, E.E.; Chen, H.; Rodda, L.B.; Liang, Y.; Ventura, P.; Villeda, S.; Crocker, P.R.; Cyster, J.G. Migratory and adhesive cues controlling innate-like lymphocyte surveillance of the pathogen-exposed surface of the lymph node. eLife 2016, 5, e18156. [Google Scholar] [CrossRef]
- Kaur, S.; Raggatt, L.J.; Batoon, L.; Hume, D.A.; Levesque, J.P.; Pettit, A.R. Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Semin. Cell Dev. Biol. 2017, 61, 12–21. [Google Scholar] [CrossRef]
- Bai, J.; Fan, F.; Gao, C.; Li, S.; Li, W.; Wei, T.; Cheng, S.; Yu, J.; Zheng, C.; Zhao, J.; et al. CD169-CD43 interaction is involved in erythroblastic island formation and erythroid differentiation. Haematologica 2023, 108, 2205–2217. [Google Scholar] [CrossRef]
- Chow, A.; Huggins, M.; Ahmed, J.; Hashimoto, D.; Lucas, D.; Kunisaki, Y.; Pinho, S.; Leboeuf, M.; Noizat, C.; van Rooijen, N.; et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat. Med. 2013, 19, 429–436. [Google Scholar] [CrossRef]
- Jacobsen, R.N.; Forristal, C.E.; Raggatt, L.J.; Nowlan, B.; Barbier, V.; Kaur, S.; van Rooijen, N.; Winkler, I.G.; Pettit, A.R.; Levesque, J.-P. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80+VCAM1+CD169+ER-HR3+Ly6G+ erythroid island macrophages in the mouse. Exp. Hematol. 2014, 42, 547–561.e544. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhao, H.; Zhang, H.; Xu, Y.; Wang, S.; Guo, X.; Huang, Y.; Zhang, S.; Han, Y.; et al. Identification and transcriptome analysis of erythroblastic island macrophages. Blood 2019, 134, 480–491. [Google Scholar] [CrossRef]
- Seu, K.G.; Papoin, J.; Fessler, R.; Hom, J.; Huang, G.; Mohandas, N.; Blanc, L.; Kalfa, T.A. Unraveling Macrophage Heterogeneity in Erythroblastic Islands. Front. Immunol. 2017, 8, 1140. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Bisht, K.; McGirr, C.; Millard, S.M.; Pettit, A.R.; Winkler, I.G.; Levesque, J.P. Imaging flow cytometry reveals that granulocyte colony-stimulating factor treatment causes loss of erythroblastic islands in the mouse bone marrow. Exp. Hematol. 2020, 82, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Boulais, P.E.; Zhang, D.; Pinho, S.; Tanaka, M.; Frenette, P.S. Maea expressed by macrophages, but not erythroblasts, maintains postnatal murine bone marrow erythroblastic islands. Blood 2019, 133, 1222–1232. [Google Scholar] [CrossRef]
- Tumas, K.C.; Xu, F.; Wu, J.; Hernandez, M.; Pattaradilokrat, S.; Xia, L.; Peng, Y.C.; Lavali, A.M.; He, X.; Singh, B.K.; et al. Dysfunction of CD169(+) macrophages and blockage of erythrocyte maturation as a mechanism of anemia in Plasmodium yoelii infection. Proc. Natl. Acad. Sci. USA 2023, 120, e2311557120. [Google Scholar] [CrossRef]
- Spaulding, E.; Fooksman, D.; Moore, J.M.; Saidi, A.; Feintuch, C.M.; Reizis, B.; Chorro, L.; Daily, J.; Lauvau, G. STING-Licensed Macrophages Prime Type I IFN Production by Plasmacytoid Dendritic Cells in the Bone Marrow during Severe Plasmodium yoelii Malaria. PLoS Pathog. 2016, 12, e1005975. [Google Scholar] [CrossRef]
- Moore-Fried, J.; Paul, M.; Jing, Z.; Fooksman, D.; Lauvau, G. CD169(+) macrophages orchestrate plasmacytoid dendritic cell arrest and retention for optimal priming in the bone marrow of malaria-infected mice. eLife 2022, 11, e78873. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J. Mol. Med. 2014, 92, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Lucas, D.; Hidalgo, A.; Méndez-Ferrer, S.; Hashimoto, D.; Scheiermann, C.; Battista, M.; Leboeuf, M.; Prophete, C.; van Rooijen, N.; et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011, 208, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, X.; Li, H.; Borger, D.K.; Wei, Q.; Yang, E.; Xu, C.; Pinho, S.; Frenette, P.S. The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell 2022, 29, 232–247.e7. [Google Scholar] [CrossRef]
- Mohamad, S.F.; Xu, L.; Ghosh, J.; Childress, P.J.; Abeysekera, I.; Himes, E.R.; Wu, H.; Alvarez, M.B.; Davis, K.M.; Aguilar-Perez, A.; et al. Osteomacs interact with megakaryocytes and osteoblasts to regulate murine hematopoietic stem cell function. Blood Adv. 2017, 1, 2520–2528. [Google Scholar] [CrossRef]
- Batoon, L.; Millard, S.M.; Wullschleger, M.E.; Preda, C.; Wu, A.C.-K.; Kaur, S.; Tseng, H.-W.; Hume, D.A.; Levesque, J.-P.; Raggatt, L.J.; et al. CD169+ macrophages are critical for osteoblast maintenance and promote intramembranous and endochondral ossification during bone repair. Biomaterials 2019, 196, 51–66. [Google Scholar] [CrossRef]
- Borges da Silva, H.; Fonseca, R.; Pereira, R.M.; Cassado Ados, A.; Álvarez, J.M.; D’Império Lima, M.R. Splenic Macrophage Subsets and Their Function during Blood-Borne Infections. Front. Immunol. 2015, 6, 480. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Gordon, S.; Plüddemann, A.; Mukhopadhyay, S. Sinusoidal immunity: Macrophages at the lymphohematopoietic interface. Cold Spring Harb. Perspect. Biol. 2014, 7, a016378. [Google Scholar] [CrossRef]
- Saunderson, S.C.; Dunn, A.C.; Crocker, P.R.; McLellan, A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014, 123, 208–216. [Google Scholar] [CrossRef]
- Moran, I.; Grootveld, A.K.; Nguyen, A.; Phan, T.G. Subcapsular Sinus Macrophages: The Seat of Innate and Adaptive Memory in Murine Lymph Nodes. Trends Immunol. 2019, 40, 35–48. [Google Scholar] [CrossRef]
- Camara, A.; Lavanant, A.C.; Abe, J.; Desforges, H.L.; Alexandre, Y.O.; Girardi, E.; Igamberdieva, Z.; Asano, K.; Tanaka, M.; Hehlgans, T.; et al. CD169(+) macrophages in lymph node and spleen critically depend on dual RANK and LTbetaR signaling. Proc. Natl. Acad. Sci. USA 2022, 119, e2108540119. [Google Scholar] [CrossRef]
- Shou, Y.; Koroleva, E.; Spencer, C.M.; Shein, S.A.; Korchagina, A.A.; Yusoof, K.A.; Parthasarathy, R.; Leadbetter, E.A.; Akopian, A.N.; Muñoz, A.R.; et al. Redefining the Role of Lymphotoxin Beta Receptor in the Maintenance of Lymphoid Organs and Immune Cell Homeostasis in Adulthood. Front. Immunol. 2021, 12, 712632. [Google Scholar] [CrossRef]
- Habbeddine, M.; Verthuy, C.; Rastoin, O.; Chasson, L.; Bebien, M.; Bajenoff, M.; Adriouch, S.; den Haan, J.M.M.; Penninger, J.M.; Lawrence, T. Receptor Activator of NF-κB Orchestrates Activation of Antiviral Memory CD8 T Cells in the Spleen Marginal Zone. Cell Rep. 2017, 21, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.; Cordeiro, O.G.; Alloush, F.; Sponsel, J.; Chypre, M.; Onder, L.; Asano, K.; Tanaka, M.; Yagita, H.; Ludewig, B.; et al. Lymph Node Mesenchymal and Endothelial Stromal Cells Cooperate via the RANK-RANKL Cytokine Axis to Shape the Sinusoidal Macrophage Niche. Immunity 2019, 50, 1467–1481.e6. [Google Scholar] [CrossRef]
- Moseman, E.A.; Iannacone, M.; Bosurgi, L.; Tonti, E.; Chevrier, N.; Tumanov, A.; Fu, Y.X.; Hacohen, N.; von Andrian, U.H. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 2012, 36, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Xu Haifeng, C.; Huang, J.; Khairnar, V.; Duhan, V.; Pandyra Aleksandra, A.; Grusdat, M.; Shinde, P.; McIlwain David, R.; Maney Sathish, K.; Gommerman, J.; et al. Deficiency of the B Cell-Activating Factor Receptor Results in Limited CD169+ Macrophage Function during Viral Infection. J. Virol. 2015, 89, 4748–4759. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Guillen, J.A.; Gallardo, G.; Diaz, M.; de la Rosa, J.V.; Hernandez, I.H.; Casanova-Acebes, M.; Lopez, F.; Tabraue, C.; Beceiro, S.; et al. The nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat. Immunol. 2013, 14, 831–839. [Google Scholar] [CrossRef]
- Mondor, I.; Baratin, M.; Lagueyrie, M.; Saro, L.; Henri, S.; Gentek, R.; Suerinck, D.; Kastenmuller, W.; Jiang, J.X.; Bajénoff, M. Lymphatic Endothelial Cells Are Essential Components of the Subcapsular Sinus Macrophage Niche. Immunity 2019, 50, 1453–1466.e4. [Google Scholar] [CrossRef]
- D’Addio, M.; Frey, J.; Tacconi, C.; Commerford, C.D.; Halin, C.; Detmar, M.; Cummings, R.D.; Otto, V.I. Sialoglycans on lymphatic endothelial cells augment interactions with Siglec-1 (CD169) of lymph node macrophages. FASEB J. 2021, 35, e22017. [Google Scholar] [CrossRef]
- Veninga, H.; Borg, E.G.; Vreeman, K.; Taylor, P.R.; Kalay, H.; van Kooyk, Y.; Kraal, G.; Martinez-Pomares, L.; den Haan, J.M. Antigen targeting reveals splenic CD169+ macrophages as promoters of germinal center B-cell responses. Eur. J. Immunol. 2015, 45, 747–757. [Google Scholar] [CrossRef]
- Carrasco, Y.R.; Batista, F.D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 2007, 27, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Grigorova, I.; Okada, T.; Cyster, J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007, 8, 992–1000. [Google Scholar] [CrossRef]
- Junt, T.; Moseman, E.A.; Iannacone, M.; Massberg, S.; Lang, P.A.; Boes, M.; Fink, K.; Henrickson, S.E.; Shayakhmetov, D.M.; Di Paolo, N.C.; et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 2007, 450, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, R.; Mempel, T.R.; Pitcher, L.A.; Gonzalez, S.F.; Verschoor, A.; Mebius, R.E.; von Andrian, U.H.; Carroll, M.C. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 2009, 30, 264–276. [Google Scholar] [CrossRef]
- Moran, I.; Nguyen, A.; Khoo, W.H.; Butt, D.; Bourne, K.; Young, C.; Hermes, J.R.; Biro, M.; Gracie, G.; Ma, C.S.; et al. Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nat. Commun. 2018, 9, 3372. [Google Scholar] [CrossRef] [PubMed]
- Desbien, A.L.; Dubois Cauwelaert, N.; Reed, S.J.; Bailor, H.R.; Liang, H.; Carter, D.; Duthie, M.S.; Fox, C.B.; Reed, S.G.; Orr, M.T. IL-18 and Subcapsular Lymph Node Macrophages are Essential for Enhanced B Cell Responses with TLR4 Agonist Adjuvants. J. Immunol. 2016, 197, 4351–4359. [Google Scholar] [CrossRef]
- Sagoo, P.; Garcia, Z.; Breart, B.; Lemaître, F.; Michonneau, D.; Albert, M.L.; Levy, Y.; Bousso, P. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat. Med. 2016, 22, 64–71. [Google Scholar] [CrossRef]
- Kastenmüller, W.; Torabi-Parizi, P.; Subramanian, N.; Lämmermann, T.; Germain, R.N. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 2012, 150, 1235–1248. [Google Scholar] [CrossRef]
- Barral, P.; Polzella, P.; Bruckbauer, A.; van Rooijen, N.; Besra, G.S.; Cerundolo, V.; Batista, F.D. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat. Immunol. 2010, 11, 303–312. [Google Scholar] [CrossRef]
- Perez, O.A.; Yeung, S.T.; Vera-Licona, P.; Romagnoli, P.A.; Samji, T.; Ural, B.B.; Maher, L.; Tanaka, M.; Khanna, K.M. CD169(+) macrophages orchestrate innate immune responses by regulating bacterial localization in the spleen. Sci. Immunol. 2017, 2, eaah5520. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, J.; Affandi, A.J.; van Dinther, D.; Nijen Twilhaar, M.K.; Olesek, K.; Hoogterp, L.; Ambrosini, M.; Heijnen, D.A.M.; Klaase, L.; Hidalgo, A.; et al. Liposome induction of CD8(+) T cell responses depends on CD169(+) macrophages and Batf3-dependent dendritic cells and is enhanced by GM3 inclusion. J. Control. Release 2021, 331, 309–320. [Google Scholar] [CrossRef]
- Backer, R.; Schwandt, T.; Greuter, M.; Oosting, M.; Jüngerkes, F.; Tüting, T.; Boon, L.; O’Toole, T.; Kraal, G.; Limmer, A.; et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, C.A.; Ried, C.; Kochanek, S.; Brocker, T. CD169+ macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells. Proc. Natl. Acad. Sci. USA 2015, 112, 5461–5466. [Google Scholar] [CrossRef]
- Casella, V.; Domenjo-Vila, E.; Esteve-Codina, A.; Pedragosa, M.; Cebollada Rica, P.; Vidal, E.; de la Rubia, I.; Lopez-Rodriguez, C.; Bocharov, G.; Argilaguet, J.; et al. Differential kinetics of splenic CD169+ macrophage death is one underlying cause of virus infection fate regulation. Cell Death Dis. 2023, 14, 838. [Google Scholar] [CrossRef]
- Machelart, A.; Khadrawi, A.; Demars, A.; Willemart, K.; De Trez, C.; Letesson, J.J.; Muraille, E. Chronic Brucella Infection Induces Selective and Persistent Interferon Gamma-Dependent Alterations of Marginal Zone Macrophages in the Spleen. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
- Gaya, M.; Castello, A.; Montaner, B.; Rogers, N.; Reis e Sousa, C.; Bruckbauer, A.; Batista, F.D. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science 2015, 347, 667–672. [Google Scholar] [CrossRef]
- Farrell, H.E.; Bruce, K.; Lawler, C.; Cardin, R.D.; Davis-Poynter, N.J.; Stevenson, P.G. Type 1 Interferons and NK Cells Limit Murine Cytomegalovirus Escape from the Lymph Node Subcapsular Sinus. PLoS Pathog. 2016, 12, e1006069. [Google Scholar] [CrossRef] [PubMed]
- Honke, N.; Namir, S.; Giuseppe, C.; Sorg, U.; Dong-er, Z.; Trilling, M.; Klingel, K.; Sauter, M.; Kandolf, R.; Nicole, G.; et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat. Immunol. 2011, 13, 51–57. [Google Scholar] [CrossRef]
- Ravishankar, B.; Shinde, R.; Liu, H.; Chaudhary, K.; Bradley, J.; Lemos, H.P.; Chandler, P.; Tanaka, M.; Munn, D.H.; Mellor, A.L.; et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc. Natl. Acad. Sci. USA 2014, 111, 4215–4220. [Google Scholar] [CrossRef]
- Miyake, Y.; Asano, K.; Kaise, H.; Uemura, M.; Nakayama, M.; Tanaka, M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J. Clin. Investig. 2007, 117, 2268–2278. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.T.; Gordon, S.; Kubes, P. A historical perspective of Kupffer cells in the context of infection. Cell Tissue Res. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Scott, C.L. Liver macrophages in health and disease. Immunity 2022, 55, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, F.F.; Bujko, A.; Liu, Z.; Collins, P.J.; Schuermans, S.; Maueroder, C.; Amstelveen, S.; Thone, T.; Martens, L.; McKendrick, J.G.; et al. Spatially restricted and ontogenically distinct hepatic macrophages are required for tissue repair. Immunity 2025. [Google Scholar] [CrossRef]
- Guilliams, M.; Bonnardel, J.; Haest, B.; Vanderborght, B.; Wagner, C.; Remmerie, A.; Bujko, A.; Martens, L.; Thone, T.; Browaeys, R.; et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 2022, 185, 379–396.e38. [Google Scholar] [CrossRef]
- Vanderborght, B.; De Muynck, K.; Gijbels, E.; Lefere, S.; Scott, C.L.; Guilliams, M.; Beschin, A.; Vinken, M.; Verhelst, X.; Geerts, A.; et al. Transient Kupffer cell depletion and subsequent replacement by infiltrating monocyte-derived cells does not alter the induction or progression of hepatocellular carcinoma. Int. J. Cancer 2023, 152, 2615–2628. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef]
- Scott, C.L.; Zheng, F.; De Baetselier, P.; Martens, L.; Saeys, Y.; De Prijck, S.; Lippens, S.; Abels, C.; Schoonooghe, S.; Raes, G.; et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 2016, 7, 10321. [Google Scholar] [CrossRef]
- Rolot, M.; Dougall, A.M.; Javaux, J.; Lallemand, F.; Machiels, B.; Martinive, P.; Gillet, L.; Dewals, B.G. Recruitment of hepatic macrophages from monocytes is independent of IL-4Ralpha but is associated with ablation of resident macrophages in schistosomiasis. Eur. J. Immunol. 2019, 49, 1067–1081. [Google Scholar] [CrossRef]
- Xu, L.; Huang, C.; Zheng, X.; Gao, H.; Zhang, S.; Zhu, M.; Dai, X.; Wang, G.; Wang, J.; Chen, H.; et al. Elevated CD169 expressing monocyte/macrophage promotes systemic inflammation and disease progression in cirrhosis. Clin. Exp. Med. 2024, 24, 45. [Google Scholar] [CrossRef]
- Benechet, A.P.; De Simone, G.; Di Lucia, P.; Cilenti, F.; Barbiera, G.; Le Bert, N.; Fumagalli, V.; Lusito, E.; Moalli, F.; Bianchessi, V.; et al. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature 2019, 574, 200–205. [Google Scholar] [CrossRef]
- De Simone, G.; Andreata, F.; Bleriot, C.; Fumagalli, V.; Laura, C.; Garcia-Manteiga, J.M.; Di Lucia, P.; Gilotto, S.; Ficht, X.; De Ponti, F.F.; et al. Identification of a Kupffer cell subset capable of reverting the T cell dysfunction induced by hepatocellular priming. Immunity 2021, 54, 2089–2100.e8. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Neupane, A.S.; Willson, M.; Chojnacki, A.K.; Vargas, E.S.C.F.; Morehouse, C.; Carestia, A.; Keller, A.E.; Peiseler, M.; DiGiandomenico, A.; Kelly, M.M.; et al. Patrolling Alveolar Macrophages Conceal Bacteria from the Immune System to Maintain Homeostasis. Cell 2020, 183, 110–125.e11. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; MacDonald, A.S. The impact of the lung environment on macrophage development, activation and function: Diversity in the face of adversity. Mucosal Immunol. 2022, 15, 223–234. [Google Scholar] [CrossRef]
- Tanno, A.; Fujino, N.; Yamada, M.; Sugiura, H.; Hirano, T.; Tanaka, R.; Sano, H.; Suzuki, S.; Okada, Y.; Ichinose, M. Decreased expression of a phagocytic receptor Siglec-1 on alveolar macrophages in chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 30. [Google Scholar] [CrossRef]
- Gibbings, S.L.; Jakubzick, C.V. A Consistent Method to Identify and Isolate Mononuclear Phagocytes from Human Lung and Lymph Nodes. Methods Mol. Biol. 2018, 1799, 381–395. [Google Scholar] [CrossRef]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef]
- Shibata, Y.; Berclaz, P.Y.; Chroneos, Z.C.; Yoshida, M.; Whitsett, J.A.; Trapnell, B.C. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001, 15, 557–567. [Google Scholar] [CrossRef]
- Chakarov, S.; Lim, H.Y.; Tan, L.; Lim, S.Y.; See, P.; Lum, J.; Zhang, X.M.; Foo, S.; Nakamizo, S.; Duan, K.; et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019, 363, eaau0964. [Google Scholar] [CrossRef]
- Ural, B.B.; Yeung, S.T.; Damani-Yokota, P.; Devlin, J.C.; de Vries, M.; Vera-Licona, P.; Samji, T.; Sawai, C.M.; Jang, G.; Perez, O.A.; et al. Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci. Immunol. 2020, 5, eaax8756. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Travaglini, K.J.; Rustagi, A.; Xu, D.; Zhang, Y.; Andronov, L.; Jang, S.; Gillich, A.; Dehghannasiri, R.; Martinez-Colon, G.J.; et al. Interstitial macrophages are a focus of viral takeover and inflammation in COVID-19 initiation in human lung. J. Exp. Med. 2024, 221. [Google Scholar] [CrossRef] [PubMed]
- Van Breedam, W.; Verbeeck, M.; Christiaens, I.; Van Gorp, H.; Nauwynck, H.J. Porcine, murine and human sialoadhesin (Sn/Siglec-1/CD169): Portals for porcine reproductive and respiratory syndrome virus entry into target cells. J. Gen. Virol. 2013, 94 Pt 9, 1955–1960. [Google Scholar] [CrossRef]
- Lee, H.J.; You, S.H.; Lee, H.S.; Shin, Y.K.; Cho, Y.S.; Park, T.S.; Kang, S.J. Sialoadhesin-dependent susceptibility and replication of porcine reproductive and respiratory syndrome viruses in CD163-expressing cells. Front. Vet. Sci. 2024, 11, 1477540. [Google Scholar] [CrossRef]
- Vanderheijden, N.; Delputte, P.L.; Favoreel, H.W.; Vandekerckhove, J.; Van Damme, J.; van Woensel, P.A.; Nauwynck, H.J. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 2003, 77, 8207–8215. [Google Scholar] [CrossRef]
- Souza de Lima, D.; Nunes, V.C.L.; Ogusku, M.M.; Sadahiro, A.; Pontillo, A.; Alencar, B.C. Polymorphisms in SIGLEC1 contribute to susceptibility to pulmonary active tuberculosis possibly through the modulation of IL-1ss. Infect. Genet. Evol. 2017, 55, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Bukvic, B.K.; Blekic, M.; Simpson, A.; Marinho, S.; Curtin, J.A.; Hankinson, J.; Aberle, N.; Custovic, A. Asthma severity, polymorphisms in 20p13 and their interaction with tobacco smoke exposure. Pediatr. Allergy Immunol. 2013, 24, 10–18. [Google Scholar] [CrossRef]
- Wen, W.; Cheng, J.; Tang, Y. Brain perivascular macrophages: Current understanding and future prospects. Brain 2024, 147, 39–55. [Google Scholar] [CrossRef]
- Lapenna, A.; De Palma, M.; Lewis, C.E. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 2018, 18, 689–702. [Google Scholar] [CrossRef]
- Pedragosa, J.; Salas-Perdomo, A.; Gallizioli, M.; Cugota, R.; Miró-Mur, F.; Briansó, F.; Justicia, C.; Pérez-Asensio, F.; Marquez-Kisinousky, L.; Urra, X.; et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol. Commun. 2018, 6, 76. [Google Scholar] [CrossRef]
- Taylor, X.; Clark, I.M.; Fitzgerald, G.J.; Oluoch, H.; Hole, J.T.; DeMattos, R.B.; Wang, Y.; Pan, F. Amyloid-β (Aβ) immunotherapy induced microhemorrhages are associated with activated perivascular macrophages and peripheral monocyte recruitment in Alzheimer’s disease mice. Mol. Neurodegener. 2023, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Rajan, W.D.; Wojtas, B.; Gielniewski, B.; Miró-Mur, F.; Pedragosa, J.; Zawadzka, M.; Pilanc, P.; Planas, A.M.; Kaminska, B. Defining molecular identity and fates of CNS-border associated macrophages after ischemic stroke in rodents and humans. Neurobiol. Dis. 2020, 137, 104722. [Google Scholar] [CrossRef]
- Hiemstra, I.H.; Beijer, M.R.; Veninga, H.; Vrijland, K.; Borg, E.G.; Olivier, B.J.; Mebius, R.E.; Kraal, G.; den Haan, J.M. The identification and developmental requirements of colonic CD169⁺ macrophages. Immunology 2014, 142, 269–278. [Google Scholar] [CrossRef]
- Asano, K.; Takahashi, N.; Ushiki, M.; Monya, M.; Aihara, F.; Kuboki, E.; Moriyama, S.; Iida, M.; Kitamura, H.; Qiu, C.-H.; et al. Intestinal CD169+ macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat. Commun. 2015, 6, 7802. [Google Scholar] [CrossRef]
- Kikuchi, K.; Iida, M.; Ikeda, N.; Moriyama, S.; Hamada, M.; Takahashi, S.; Kitamura, H.; Watanabe, T.; Hasegawa, Y.; Hase, K.; et al. Macrophages Switch Their Phenotype by Regulating Maf Expression during Different Phases of Inflammation. J. Immunol. 2018, 201, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Q.; Yang, Y.; Hao, S.; Han, X.; Song, J.; Yin, Y.; Li, X.; Tanaka, M.; Qiu, C.H. Macrophage Subset Expressing CD169 in Peritoneal Cavity-Regulated Mucosal Inflammation Together with Lower Levels of CCL22. Inflammation 2017, 40, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kang, B.H.; Kim, H.J.; Oh, J.E.; Lee, H.K. A Microbiota-Dependent Subset of Skin Macrophages Protects Against Cutaneous Bacterial Infection. Front. Immunol. 2022, 13, 799598. [Google Scholar] [CrossRef]
- Li, M.; Yu, W.; Liu, Z.; Liu, S. CD169(+) Skin Macrophages Function as a Specialized Subpopulation in Promoting Psoriasis-like Skin Disease in Mice. Int. J. Mol. Sci. 2024, 25, 5705. [Google Scholar] [CrossRef]
- Karasawa, K.; Asano, K.; Moriyama, S.; Ushiki, M.; Monya, M.; Iida, M.; Kuboki, E.; Yagita, H.; Uchida, K.; Nitta, K.; et al. Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2015, 26, 896–906. [Google Scholar] [CrossRef]
- Teo, Y.J.; Ng, S.L.; Mak, K.W.; Setiagani, Y.A.; Chen, Q.; Nair, S.K.; Sheng, J.; Ruedl, C. Renal CD169(++) resident macrophages are crucial for protection against acute systemic candidiasis. Life Sci. Alliance 2021, 4. [Google Scholar] [CrossRef]
- Gray, E.E.; Cyster, J.G. Lymph node macrophages. J. Innate Immun. 2012, 4, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Miller, J.; Merad, M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011, 35, 323–335. [Google Scholar] [CrossRef]
- Haldar, M.; Murphy, K.M. Origin, development, and homeostasis of tissue-resident macrophages. Immunol. Rev. 2014, 262, 25–35. [Google Scholar] [CrossRef]
- Louie, D.A.P.; Liao, S. Lymph Node Subcapsular Sinus Macrophages as the Frontline of Lymphatic Immune Defense. Front. Immunol. 2019, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Rowland, R.R.R.; Yoo, D. Recent Advances in PRRS Virus Receptors and the Targeting of Receptor-Ligand for Control. Vaccines 2021, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Useros, N.; Lorizate, M.; Puertas, M.C.; Rodriguez-Plata, M.T.; Zangger, N.; Erikson, E.; Pino, M.; Erkizia, I.; Glass, B.; Clotet, B.; et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012, 10, e1001448. [Google Scholar] [CrossRef]
- Puryear, W.B.; Yu, X.; Ramirez, N.P.; Reinhard, B.M.; Gummuluru, S. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc. Natl. Acad. Sci. USA 2012, 109, 7475–7480. [Google Scholar] [CrossRef]
- Perez-Zsolt, D.; Erkizia, I.; Pino, M.; Garcia-Gallo, M.; Martin, M.T.; Benet, S.; Chojnacki, J.; Fernandez-Figueras, M.T.; Guerrero, D.; Urrea, V.; et al. Anti-Siglec-1 antibodies block Ebola viral uptake and decrease cytoplasmic viral entry. Nat. Microbiol. 2019, 4, 1558–1570. [Google Scholar] [CrossRef]
- Uchil, P.D.; Pi, R.; Haugh, K.A.; Ladinsky, M.S.; Ventura, J.D.; Barrett, B.S.; Santiago, M.L.; Bjorkman, P.J.; Kassiotis, G.; Sewald, X.; et al. A Protective Role for the Lectin CD169/Siglec-1 against a Pathogenic Murine Retrovirus. Cell Host Microbe 2019, 25, 87–100. [Google Scholar] [CrossRef]
- Lempp, F.A.; Soriaga, L.B.; Montiel-Ruiz, M.; Benigni, F.; Noack, J.; Park, Y.J.; Bianchi, S.; Walls, A.C.; Bowen, J.E.; Zhou, J.; et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature 2021, 598, 342–347. [Google Scholar] [CrossRef]
- Perez-Zsolt, D.; Munoz-Basagoiti, J.; Rodon, J.; Elosua-Bayes, M.; Raich-Regue, D.; Risco, C.; Sachse, M.; Pino, M.; Gumber, S.; Paiardini, M.; et al. SARS-CoV-2 interaction with Siglec-1 mediates trans-infection by dendritic cells. Cell. Mol. Immunol. 2021, 18, 2676–2678. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, S.; Olejnik, J.; Berrigan, J.; Nisa, A.; Suder, E.L.; Akiyama, H.; Lei, M.; Ramaswamy, S.; Tyagi, S.; Bushkin, Y.; et al. CD169-mediated restrictive SARS-CoV-2 infection of macrophages induces pro-inflammatory responses. PLoS Pathog. 2022, 18, e1010479. [Google Scholar] [CrossRef]
- Haugh, K.A.; Ladinsky, M.S.; Ullah, I.; Stone, H.M.; Pi, R.; Gilardet, A.; Grunst, M.W.; Kumar, P.; Bjorkman, P.J.; Mothes, W.; et al. In vivo imaging of retrovirus infection reveals a role for Siglec-1/CD169 in multiple routes of transmission. eLife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Perez-Zsolt, D.; Raich-Regue, D.; Munoz-Basagoiti, J.; Aguilar-Gurrieri, C.; Clotet, B.; Blanco, J.; Izquierdo-Useros, N. HIV-1 trans-Infection Mediated by DCs: The Tip of the Iceberg of Cell-to-Cell Viral Transmission. Pathogens 2021, 11, 39. [Google Scholar] [CrossRef]
- Sewald, X.; Ladinsky, M.S.; Uchil, P.D.; Beloor, J.; Pi, R.; Herrmann, C.; Motamedi, N.; Murooka, T.T.; Brehm, M.A.; Greiner, D.L.; et al. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science 2015, 350, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Martinez, E.; Benet Garrabe, S.; Mateos, N.; Erkizia, I.; Nieto-Garai, J.A.; Lorizate, M.; Borgman, K.J.E.; Manzo, C.; Campelo, F.; Izquierdo-Useros, N.; et al. Actin-regulated Siglec-1 nanoclustering influences HIV-1 capture and virus-containing compartment formation in dendritic cells. eLife 2023, 12, e78836. [Google Scholar] [CrossRef]
- Hammonds, J.E.; Beeman, N.; Ding, L.; Takushi, S.; Francis, A.C.; Wang, J.J.; Melikyan, G.B.; Spearman, P. Siglec-1 initiates formation of the virus-containing compartment and enhances macrophage-to-T cell transmission of HIV-1. PLoS Pathog. 2017, 13, e1006181. [Google Scholar] [CrossRef]
- Akiyama, H.; Ramirez, N.G.; Gudheti, M.V.; Gummuluru, S. CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog. 2015, 11, e1004751. [Google Scholar] [CrossRef]
- Carlin, A.F.; Lewis, A.L.; Varki, A.; Nizet, V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 2007, 189, 1231–1237. [Google Scholar] [CrossRef]
- Chang, Y.C.; Olson, J.; Louie, A.; Crocker, P.R.; Varki, A.; Nizet, V. Role of macrophage sialoadhesin in host defense against the sialylated pathogen group B Streptococcus. J. Mol. Med. 2014, 92, 951–959. [Google Scholar] [CrossRef]
- Lund, S.J.; Del Rosario, P.G.B.; Honda, A.; Caoili, K.J.; Hoeksema, M.A.; Nizet, V.; Patras, K.A.; Prince, L.S. Sialic Acid-Siglec-E Interactions Regulate the Response of Neonatal Macrophages to Group B Streptococcus. Immunohorizons 2024, 8, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, G.; Fernandes, V.E.; Chung, W.Y.; Wanford, J.J.; Thomson, S.; Bayliss, C.D.; Straatman, K.; Crocker, P.R.; Dennison, A.; Martinez-Pomares, L.; et al. Intracellular replication of Streptococcus pneumoniae inside splenic macrophages serves as a reservoir for septicaemia. Nat. Microbiol. 2018, 3, 600–610. [Google Scholar] [CrossRef]
- Heikema, A.P.; Bergman, M.P.; Richards, H.; Crocker, P.R.; Gilbert, M.; Samsom, J.N.; van Wamel, W.J.; Endtz, H.P.; van Belkum, A. Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect. Immun. 2010, 78, 3237–3246. [Google Scholar] [CrossRef]
- Malik, A.; Brudvig, J.M.; Gadsden, B.J.; Ethridge, A.D.; Mansfield, L.S. Campylobacter jejuni induces autoimmune peripheral neuropathy via Sialoadhesin and Interleukin-4 axes. Gut Microbes 2022, 14, 2064706. [Google Scholar] [CrossRef]
- Godschalk, P.C.; Kuijf, M.L.; Li, J.; St Michael, F.; Ang, C.W.; Jacobs, B.C.; Karwaski, M.F.; Brochu, D.; Moterassed, A.; Endtz, H.P.; et al. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barre and Miller Fisher syndromes. Infect. Immun. 2007, 75, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, S.E.; Papri, N.; Querol, L.; Rinaldi, S.; Shahrizaila, N.; Jacobs, B.C. Guillain-Barre syndrome. Nat. Rev. Dis. Primers 2024, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Mariliis, K.; Oetke, C.; Lewis, L.; Erwig, L.; Heikema, A.; Easton, A.; Willison, H.; Crocker, P. Sialoadhesin Promotes Rapid Proinflammatory and Type I IFN Responses to a Sialylated Pathogen, Campylobacter jejuni. J. Immunol. 2012, 189, 2414–2422. [Google Scholar] [CrossRef]
- Heikema, A.P.; Koning, R.I.; Duarte dos Santos Rico, S.; Rempel, H.; Jacobs, B.C.; Endtz, H.P.; van Wamel, W.J.; Samsom, J.N. Enhanced, sialoadhesin-dependent uptake of Guillain-Barre syndrome-associated Campylobacter jejuni strains by human macrophages. Infect. Immun. 2013, 81, 2095–2103. [Google Scholar] [CrossRef]
- Lisk, C.; Yuen, R.; Kuniholm, J.; Antos, D.; Reiser, M.L.; Wetzler, L.M. CD169+ Subcapsular Macrophage Role in Antigen Adjuvant Activity. Front. Immunol. 2021, 12, 624197. [Google Scholar] [CrossRef]
- Jones, C.; Virji, M.; Crocker, P.R. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 2003, 49, 1213–1225. [Google Scholar] [CrossRef]
- Han, Y.; Kelm, S.; Riesselman, M.H.; Crocker, P.R.; Cutler, J.E. Mouse sialoadhesin is not responsible for Candida albicans yeast cell binding to splenic marginal zone macrophages. Infect. Immun. 1994, 62, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lai, S.M.; Sheng, J.; Tetlak, P.; Balachander, A.; Claser, C.; Renia, L.; Karjalainen, K.; Ruedl, C. Tissue-Resident CD169(+) Macrophages Form a Crucial Front Line against Plasmodium Infection. Cell Rep. 2016, 16, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Gander-Bui, H.T.T.; Schlafli, J.; Baumgartner, J.; Walthert, S.; Genitsch, V.; van Geest, G.; Galvan, J.A.; Cardozo, C.; Graham Martinez, C.; Grans, M.; et al. Targeted removal of macrophage-secreted interleukin-1 receptor antagonist protects against lethal Candida albicans sepsis. Immunity 2023, 56, 1743–1760 e9. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, L.; Bulte, D.; Van den Kerkhof, M.; Dirkx, L.; Mabille, D.; Hendrickx, S.; Delputte, P.; Maes, L.; Caljon, G. Interferon Alpha Favors Macrophage Infection by Visceral Leishmania Species Through Upregulation of Sialoadhesin Expression. Front. Immunol. 2020, 11, 1113. [Google Scholar] [CrossRef]
- Cavalcante, T.; Medeiros, M.M.; Mule, S.N.; Palmisano, G.; Stolf, B.S. The Role of Sialic Acids in the Establishment of Infections by Pathogens, with Special Focus on Leishmania. Front. Cell Infect. Microbiol. 2021, 11, 671913. [Google Scholar] [CrossRef]
- Roy, S.; Mandal, C. Leishmania donovani Utilize Sialic Acids for Binding and Phagocytosis in the Macrophages through Selective Utilization of Siglecs and Impair the Innate Immune Arm. PLoS Negl. Trop. Dis. 2016, 10, e0004904. [Google Scholar] [CrossRef]
- Monteiro, V.G.; Lobato, C.S.; Silva, A.R.; Medina, D.V.; de Oliveira, M.A.; Seabra, S.H.; de Souza, W.; DaMatta, R.A. Increased association of Trypanosoma cruzi with sialoadhesin positive mice macrophages. Parasitol. Res. 2005, 97, 380–385. [Google Scholar] [CrossRef]
- Xiong, Y.S.; Cheng, Y.; Lin, Q.S.; Wu, A.L.; Yu, J.; Li, C.; Sun, Y.; Zhong, R.Q.; Wu, L.J. Increased expression of Siglec-1 on peripheral blood monocytes and its role in mononuclear cell reactivity to autoantigen in rheumatoid arthritis. Rheumatology 2014, 53, 250–259. [Google Scholar] [CrossRef]
- Eakin, A.J.; Ahmed, T.; McGeough, C.M.; Drain, S.; Alexander, H.D.; Wright, G.D.; Gardiner, P.V.; Small, D.; Bjourson, A.J.; Gibson, D.S. CD169+ Monocyte and Regulatory T Cell Subsets Are Associated with Disease Activity in Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 1875. [Google Scholar] [CrossRef]
- Seyhan, A.A.; Gregory, B.; Cribbs, A.P.; Bhalara, S.; Li, Y.; Loreth, C.; Zhang, Y.; Guo, Y.; Lin, L.L.; Feldmann, M.; et al. Novel biomarkers of a peripheral blood interferon signature associated with drug-naïve early arthritis patients distinguish persistent from self-limiting disease course. Sci. Rep. 2020, 10, 8830. [Google Scholar] [CrossRef]
- Zorn-Pauly, L.; von Stuckrad, A.S.L.; Klotsche, J.; Rose, T.; Kallinich, T.; Enghard, P.; Ostendorf, L.; Burns, M.; Doerner, T.; Meisel, C.; et al. Evaluation of SIGLEC1 in the diagnosis of suspected systemic lupus erythematosus. Rheumatology 2022, 61, 3396–3400. [Google Scholar] [CrossRef]
- Sakumura, N.; Yokoyama, T.; Usami, M.; Hosono, Y.; Inoue, N.; Matsuda, Y.; Tasaki, Y.; Wada, T. CD169 expression on monocytes as a marker for assessing type I interferon status in pediatric inflammatory diseases. Clin. Immunol. 2023, 250, 109329. [Google Scholar] [CrossRef]
- Oliveira, J.J.; Karrar, S.; Rainbow, D.B.; Pinder, C.L.; Clarke, P.; Rubio García, A.; Al-Assar, O.; Burling, K.; Morris, S.; Stratton, R.; et al. The plasma biomarker soluble SIGLEC-1 is associated with the type I interferon transcriptional signature, ethnic background and renal disease in systemic lupus erythematosus. Arthritis Res. Ther. 2018, 20, 152. [Google Scholar] [CrossRef]
- Rose, T.; Grützkau, A.; Hirseland, H.; Huscher, D.; Dähnrich, C.; Dzionek, A.; Ozimkowski, T.; Schlumberger, W.; Enghard, P.; Radbruch, A.; et al. IFNα and its response proteins, IP-10 and SIGLEC-1, are biomarkers of disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 2013, 72, 1639–1645. [Google Scholar] [CrossRef]
- Stuckrad, S.L.V.; Klotsche, J.; Biesen, R.; Lieber, M.; Thumfart, J.; Meisel, C.; Unterwalder, N.; Kallinich, T. SIGLEC1 (CD169) is a sensitive biomarker for the deterioration of the clinical course in childhood systemic lupus erythematosus. Lupus 2020, 29, 1914–1925. [Google Scholar] [CrossRef]
- Biesen, R.; Demir, C.; Barkhudarova, F.; Grün, J.R.; Steinbrich-Zöllner, M.; Backhaus, M.; Häupl, T.; Rudwaleit, M.; Riemekasten, G.; Radbruch, A.; et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 2008, 58, 1136–1145. [Google Scholar] [CrossRef]
- Graf, M.; von Stuckrad, S.L.; Uruha, A.; Klotsche, J.; Zorn-Pauly, L.; Unterwalder, N.; Buttgereit, T.; Krusche, M.; Meisel, C.; Burmester, G.R.; et al. SIGLEC1 enables straightforward assessment of type I interferon activity in idiopathic inflammatory myopathies. RMD Open 2022, 8, e001934. [Google Scholar] [CrossRef]
- Rose, T.; Szelinski, F.; Lisney, A.; Reiter, K.; Fleischer, S.J.; Burmester, G.R.; Radbruch, A.; Hiepe, F.; Grützkau, A.; Biesen, R.; et al. SIGLEC1 is a biomarker of disease activity and indicates extraglandular manifestation in primary Sjögren’s syndrome. RMD Open 2016, 2, e000292. [Google Scholar] [CrossRef] [PubMed]
- Bogie, J.F.J.; Boelen, E.; Louagie, E.; Delputte, P.; Elewaut, D.; van Horssen, J.; Hendriks, J.J.A.; Hellings, N. CD169 is a marker for highly pathogenic phagocytes in multiple sclerosis. Mult. Scler. J. 2017, 24, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, L.; Dittert, P.; Biesen, R.; Duchow, A.; Stiglbauer, V.; Ruprecht, K.; Bellmann-Strobl, J.; Seelow, D.; Stenzel, W.; Niesner, R.A.; et al. SIGLEC1 (CD169): A marker of active neuroinflammation in the brain but not in the blood of multiple sclerosis patients. Sci. Rep. 2021, 11, 10299. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Castilló, J.; Bustamante, M.; Vidal-Jordana, A.; Castro, Z.; Montalban, X.; Comabella, M. SIGLEC1 and SIGLEC7 expression in circulating monocytes of patients with multiple sclerosis. Mult. Scler. 2013, 19, 524–531. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Hao, S.; Han, X.; Xia, Y.; Li, X.; Chen, Y.; Tanaka, M.; Qiu, C.H. CD169 Expressing Macrophage, a Key Subset in Mesenteric Lymph Nodes Promotes Mucosal Inflammation in Dextran Sulfate Sodium-Induced Colitis. Front. Immunol. 2017, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ohnishi, K.; Miyashita, A.; Nakahara, S.; Fujiwara, Y.; Horlad, H.; Motoshima, T.; Fukushima, S.; Jinnin, M.; Ihn, H.; et al. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol. Res. 2015, 3, 1356–1363. [Google Scholar] [CrossRef]
- Pucci, F.; Garris, C.; Lai, C.P.; Newton, A.; Pfirschke, C.; Engblom, C.; Alvarez, D.; Sprachman, M.; Evavold, C.; Magnuson, A.; et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016, 352, 242–246. [Google Scholar] [CrossRef]
- Shiota, T.; Miyasato, Y.; Ohnishi, K.; Yamamoto-Ibusuki, M.; Yamamoto, Y.; Iwase, H.; Takeya, M.; Komohara, Y. The Clinical Significance of CD169-Positive Lymph Node Macrophage in Patients with Breast Cancer. PLoS ONE 2016, 11, e0166680. [Google Scholar] [CrossRef] [PubMed]
- Bjork Gunnarsdottir, F.; Auoja, N.; Bendahl, P.O.; Ryden, L.; Ferno, M.; Leandersson, K. Co-localization of CD169(+) macrophages and cancer cells in lymph node metastases of breast cancer patients is linked to improved prognosis and PDL1 expression. Oncoimmunology 2020, 9, 1848067. [Google Scholar] [CrossRef]
- Tacconi, C.; Commerford, C.D.; Dieterich, L.C.; Schwager, S.; He, Y.; Ikenberg, K.; Friebel, E.; Becher, B.; Tugues, S.; Detmar, M. CD169(+) lymph node macrophages have protective functions in mouse breast cancer metastasis. Cell Rep. 2021, 35, 108993. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Shiota, T.; Yagi, T.; Ohnishi, K.; Baba, Y.; Miyasato, Y.; Kiyozumi, Y.; Yoshida, N.; Takeya, M.; Baba, H.; et al. High CD169 expression in lymph node macrophages predicts a favorable clinical course in patients with esophageal cancer. Pathol. Int. 2018, 68, 685–693. [Google Scholar] [CrossRef]
- Kumamoto, K.; Tasaki, T.; Ohnishi, K.; Shibata, M.; Shimajiri, S.; Harada, M.; Komohara, Y.; Nakayama, T. CD169 Expression on Lymph Node Macrophages Predicts in Patients with Gastric Cancer. Front. Oncol. 2021, 11, 636751. [Google Scholar] [CrossRef]
- Saito, Y.; Fujiwara, Y.; Miyamoto, Y.; Ohnishi, K.; Nakashima, Y.; Tabata, Y.; Baba, H.; Komohara, Y. CD169(+) sinus macrophages in regional lymph nodes do not predict mismatch-repair status of patients with colorectal cancer. Cancer Med. 2023, 12, 10199–10211. [Google Scholar] [CrossRef]
- Ohnishi, K.; Komohara, Y.; Saito, Y.; Miyamoto, Y.; Watanabe, M.; Baba, H.; Takeya, M. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013, 104, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Ishizaka, K.; Asano, T. CD169(+) Macrophages Residing in the Draining Lymph Nodes and Infiltrating the Tumor Play Opposite Roles in the Pathogenesis of Bladder Cancer. Res. Rep. Urol. 2023, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ohnishi, K.; Shiota, T.; Motoshima, T.; Sugiyama, Y.; Yatsuda, J.; Kamba, T.; Ishizaka, K.; Komohara, Y. CD169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018, 109, 1723–1730. [Google Scholar] [CrossRef]
- Stromvall, K.; Sundkvist, K.; Ljungberg, B.; Halin Bergstrom, S.; Bergh, A. Reduced number of CD169(+) macrophages in pre-metastatic regional lymph nodes is associated with subsequent metastatic disease in an animal model and with poor outcome in prostate cancer patients. Prostate 2017, 77, 1468–1477. [Google Scholar] [CrossRef]
- Anami, T.; Pan, C.; Fujiwara, Y.; Komohara, Y.; Yano, H.; Saito, Y.; Sugimoto, M.; Wakita, D.; Motoshima, T.; Murakami, Y.; et al. Dysfunction of sinus macrophages in tumor-bearing host induces resistance to immunotherapy. Cancer Sci. 2024, 115, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, D.; Zhu, B.; Hua, Z. Single-cell transcriptome analysis identifies a novel tumor-associated macrophage subtype predicting better prognosis in pancreatic ductal adenocarcinoma. Front. Cell Dev. Biol. 2024, 12, 1466767. [Google Scholar] [CrossRef]
- Zwart, E.S.; van Ee, T.; Affandi, A.J.; Boyd, L.N.C.; Rodriguez, E.; den Haan, J.M.M.; Farina, A.; van Grieken, N.C.T.; Meijer, L.L.; van Kooyk, Y.; et al. Spatial immune composition of tumor microenvironment in patients with pancreatic cancer. Cancer Immunol. Immunother. 2023, 72, 4385–4397. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.H.; Kim, H.C.; Kim, C.W.; Kang, I.; Lee, H.K. Blood monocyte-derived CD169(+) macrophages contribute to antitumor immunity against glioblastoma. Nat. Commun. 2022, 13, 6211. [Google Scholar] [CrossRef]
- Li, J.Q.; Yu, X.J.; Wang, Y.C.; Huang, L.Y.; Liu, C.Q.; Zheng, L.; Fang, Y.J.; Xu, J. Distinct patterns and prognostic values of tumor-infiltrating macrophages in hepatocellular carcinoma and gastric cancer. J. Transl. Med. 2017, 15, 37. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.Q.; Jiang, Z.Z.; Li, L.; Wu, Y.; Zheng, L. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J. Pathol. 2016, 239, 231–241. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Xiong, F.; Chen, Y.J. The correlation between tumor-associated macrophages and the prognosis of east Asian hepatocellular carcinoma patients: A systematic review and meta-analysis. Pathol. Res. Pract. 2023, 252, 154919. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zeng, D.N.; Li, J.Z.; Hua, Q.M.; Huang, C.X.; Xu, J.; Wu, C.; Zheng, L.; Wen, W.P.; Wu, Y. Type I IFNs repolarized a CD169(+) macrophage population with anti-tumor potentials in hepatocellular carcinoma. Mol. Ther. 2022, 30, 632–643. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602. [Google Scholar] [CrossRef]
- Briem, O.; Kallberg, E.; Kimbung, S.; Veerla, S.; Stenstrom, J.; Hatschek, T.; Hagerling, C.; Hedenfalk, I.; Leandersson, K. CD169(+) Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, T(regs) and a Worse Prognosis for Patients with Advanced Breast Cancer. Cancers 2023, 15, 1262. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Guo, X.; Wang, G.; Bi, Y.; Han, L.; Zhu, Q.; Qiu, C.; Tanaka, M.; Zhao, Y. Breast cancer cells promote CD169(+) macrophage-associated immunosuppression through JAK2-mediated PD-L1 upregulation on macrophages. Int. Immunopharmacol. 2020, 78, 106012. [Google Scholar] [CrossRef]
- Gunnarsdottir, F.B.; Briem, O.; Lindgren, A.Y.; Kallberg, E.; Andersen, C.; Grenthe, R.; Rosenqvist, C.; Millrud, C.R.; Wallgren, M.; Viklund, H.; et al. Breast cancer associated CD169(+) macrophages possess broad immunosuppressive functions but enhance antibody secretion by activated B cells. Front. Immunol. 2023, 14, 1180209. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, X.; Lin, Y.; Tang, X.; Ling, L.; Wang, L.; Jiang, Y. A Higher Frequency of CD14+ CD169+ Monocytes/Macrophages in Patients with Colorectal Cancer. PLoS ONE 2015, 10, e0141817. [Google Scholar] [CrossRef]
- van Dinther, D.; Veninga, H.; Revet, M.; Hoogterp, L.; Olesek, K.; Grabowska, J.; Borg, E.G.F.; Kalay, H.; van Kooyk, Y.; den Haan, J.M.M. Comparison of Protein and Peptide Targeting for the Development of a CD169-Based Vaccination Strategy Against Melanoma. Front. Immunol. 2018, 9, 1997. [Google Scholar] [CrossRef]
- van Dinther, D.; Lopez Venegas, M.; Veninga, H.; Olesek, K.; Hoogterp, L.; Revet, M.; Ambrosini, M.; Kalay, H.; Stockl, J.; van Kooyk, Y.; et al. Activation of CD8(+) T Cell Responses after Melanoma Antigen Targeting to CD169(+) Antigen Presenting Cells in Mice and Humans. Cancers 2019, 11, 183. [Google Scholar] [CrossRef]
- Affandi, A.J.; Olesek, K.; Grabowska, J.; Nijen Twilhaar, M.K.; Rodríguez, E.; Saris, A.; Zwart, E.S.; Nossent, E.J.; Kalay, H.; de Kok, M.; et al. CD169 Defines Activated CD14(+) Monocytes with Enhanced CD8(+) T Cell Activation Capacity. Front. Immunol. 2021, 12, 697840. [Google Scholar] [CrossRef]
- Wang, A.Z.; Brink, H.J.; Bouma, R.G.; Affandi, A.J.; Nijen Twilhaar, M.K.; Heijnen, D.A.M.; van Elk, J.; Maaskant, J.J.; Konijn, V.A.L.; Stolwijk, J.G.C.; et al. Development of a Versatile Cancer Vaccine Format Targeting Antigen-Presenting Cells Using Proximity-Based Sortase A-Mediated Ligation of T-Cell Epitopes. Bioconjug. Chem. 2024, 35, 1805–1814. [Google Scholar] [CrossRef]
- Nycholat, C.M.; Rademacher, C.; Kawasaki, N.; Paulson, J.C. In silico-aided design of a glycan ligand of sialoadhesin for in vivo targeting of macrophages. J. Am. Chem. Soc. 2012, 134, 15696–15699. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Kawasaki, N.; Nycholat, C.M.; Han, S.; Pilotte, J.; Crocker, P.R.; Paulson, J.C. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS ONE 2012, 7, e39039. [Google Scholar] [CrossRef]
- Edgar, L.J.; Kawasaki, N.; Nycholat, C.M.; Paulson, J.C. Targeted Delivery of Antigen to Activated CD169(+) Macrophages Induces Bias for Expansion of CD8(+) T Cells. Cell Chem. Biol. 2019, 26, 131–136.e4. [Google Scholar] [CrossRef] [PubMed]
- Nijen Twilhaar, M.K.; Czentner, L.; Grabowska, J.; Affandi, A.J.; Lau, C.Y.J.; Olesek, K.; Kalay, H.; van Nostrum, C.F.; van Kooyk, Y.; Storm, G.; et al. Optimization of Liposomes for Antigen Targeting to Splenic CD169(+) Macrophages. Pharmaceutics 2020, 12, 1138. [Google Scholar] [CrossRef]
- Affandi, A.J.; Grabowska, J.; Olesek, K.; Lopez Venegas, M.; Barbaria, A.; Rodríguez, E.; Mulder, P.P.G.; Pijffers, H.J.; Ambrosini, M.; Kalay, H.; et al. Selective tumor antigen vaccine delivery to human CD169(+) antigen-presenting cells using ganglioside-liposomes. Proc. Natl. Acad. Sci. USA 2020, 117, 27528–27539. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, J.; Léopold, V.; Olesek, K.; Nijen Twilhaar, M.K.; Affandi, A.J.; Brouwer, M.C.; Jongerius, I.; Verschoor, A.; van Kooten, C.; van Kooyk, Y.; et al. Platelets interact with CD169(+) macrophages and cDC1 and enhance liposome-induced CD8(+) T cell responses. Front. Immunol. 2023, 14, 1290272. [Google Scholar] [CrossRef]
- Kawasaki, N.; Vela, J.L.; Nycholat, C.M.; Rademacher, C.; Khurana, A.; van Rooijen, N.; Crocker, P.R.; Kronenberg, M.; Paulson, J.C. Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proc. Natl. Acad. Sci. USA 2013, 110, 7826–7831. [Google Scholar] [CrossRef]
- Grabowska, J.; Stolk, D.A.; Nijen Twilhaar, M.K.; Ambrosini, M.; Storm, G.; van der Vliet, H.J.; de Gruijl, T.D.; van Kooyk, Y.; den Haan, J.M.M. Liposomal Nanovaccine Containing α-Galactosylceramide and Ganglioside GM3 Stimulates Robust CD8+ T Cell Responses via CD169+ Macrophages and cDC1. Vaccines 2021, 9, 56. [Google Scholar] [CrossRef]
- Barral, P.; Sánchez-Niño, M.D.; van Rooijen, N.; Cerundolo, V.; Batista, F.D. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 2012, 31, 2378–2390. [Google Scholar] [CrossRef]
- Nijen Twilhaar, M.K.; Czentner, L.; Bouma, R.G.; Olesek, K.; Grabowska, J.; Wang, A.Z.; Affandi, A.J.; Belt, S.C.; Kalay, H.; van Nostrum, C.F.; et al. Incorporation of Toll-Like Receptor Ligands and Inflammasome Stimuli in GM3 Liposomes to Induce Dendritic Cell Maturation and T Cell Responses. Front. Immunol. 2022, 13, 842241. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, T.; Zhang, H.; Saeed, M.; Liu, X.; Huang, L.; Wang, X.; Gao, J.; Hou, B.; Lai, Y.; et al. Acid-Ionizable Iron Nanoadjuvant Augments STING Activation for Personalized Vaccination Immunotherapy of Cancer. Adv. Mater. 2023, 35, e2209910. [Google Scholar] [CrossRef] [PubMed]
- Detienne, S.; Welsby, I.; Collignon, C.; Wouters, S.; Coccia, M.; Delhaye, S.; Van Maele, L.; Thomas, S.; Swertvaegher, M.; Detavernier, A.; et al. Central Role of CD169(+) Lymph Node Resident Macrophages in the Adjuvanticity of the QS-21 Component of AS01. Sci. Rep. 2016, 6, 39475. [Google Scholar] [CrossRef]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody fragments as nanoparticle targeting ligands: A step in the right direction. Chem. Sci. 2017, 8, 63–77. [Google Scholar] [CrossRef] [PubMed]
- van der Meel, R.; Oliveira, S.; Altintas, I.; Haselberg, R.; van der Veeken, J.; Roovers, R.C.; van Bergen en Henegouwen, P.M.; Storm, G.; Hennink, W.E.; Schiffelers, R.M.; et al. Tumor-targeted Nanobullets: Anti-EGFR nanobody-liposomes loaded with anti-IGF-1R kinase inhibitor for cancer treatment. J. Control. Release 2012, 159, 281–289. [Google Scholar] [CrossRef]
- Bian, X.; Wu, P.; Sha, H.; Qian, H.; Wang, Q.; Cheng, L.; Yang, Y.; Yang, M.; Liu, B. Anti-EGFR-iRGD recombinant protein conjugated silk fibroin nanoparticles for enhanced tumor targeting and antitumor efficiency. Onco Targets Ther. 2016, 9, 3153–3162. [Google Scholar] [CrossRef]
- D’Hollander, A.; Jans, H.; Velde, G.V.; Verstraete, C.; Massa, S.; Devoogdt, N.; Stakenborg, T.; Muyldermans, S.; Lagae, L.; Himmelreich, U. Limiting the protein corona: A successful strategy for in vivo active targeting of anti-HER2 nanobody-functionalized nanostars. Biomaterials 2017, 123, 15–23. [Google Scholar] [CrossRef]
- Bouma, R.G.; Nijen Twilhaar, M.K.; Brink, H.J.; Affandi, A.J.; Mesquita, B.S.; Olesek, K.; van Dommelen, J.M.A.; Heukers, R.; de Haas, A.M.; Kalay, H.; et al. Nanobody-liposomes as novel cancer vaccine platform to efficiently stimulate T cell immunity. Int. J. Pharm. 2024, 660, 124254. [Google Scholar] [CrossRef]
- Yu, X.; Xu, F.; Ramirez, N.G.; Kijewski, S.D.; Akiyama, H.; Gummuluru, S.; Reinhard, B.M. Dressing up Nanoparticles: A Membrane Wrap to Induce Formation of the Virological Synapse. ACS Nano 2015, 9, 4182–4192. [Google Scholar] [CrossRef]
- Xu, F.; Reiser, M.; Yu, X.; Gummuluru, S.; Wetzler, L.; Reinhard, B.M. Lipid-Mediated Targeting with Membrane-Wrapped Nanoparticles in the Presence of Corona Formation. ACS Nano 2016, 10, 1189–1200. [Google Scholar] [CrossRef]
- Yu, X.; Feizpour, A.; Ramirez, N.G.; Wu, L.; Akiyama, H.; Xu, F.; Gummuluru, S.; Reinhard, B.M. Glycosphingolipid-functionalized nanoparticles recapitulate CD169-dependent HIV-1 uptake and trafficking in dendritic cells. Nat. Commun. 2014, 5, 4136. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Bandara, A.; Akiyama, H.; Eshaghi, B.; Stelter, D.; Keyes, T.; Straub, J.E.; Gummuluru, S.; Reinhard, B.M. Membrane-wrapped nanoparticles probe divergent roles of GM3 and phosphatidylserine in lipid-mediated viral entry pathways. Proc. Natl. Acad. Sci. USA 2018, 115, E9041–E9050. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, B.; Alsharif, N.; An, X.; Akiyama, H.; Brown, K.A.; Gummuluru, S.; Reinhard, B.M. Stiffness of HIV-1 Mimicking Polymer Nanoparticles Modulates Ganglioside-Mediated Cellular Uptake and Trafficking. Adv. Sci 2020, 7, 2000649. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Siddiqui, M.; Gummuluru, S.; Wong, W.W.; Reinhard, B.M. Ganglioside-Functionalized Nanoparticles for Chimeric Antigen Receptor T-Cell Activation at the Immunological Synapse. ACS Nano 2022, 16, 18408–18420. [Google Scholar] [CrossRef]
- Zang, H.; Fofana, J.; Xu, F.; Nodder, S.B.; Gummuluru, S.; Reinhard, B.M. Characterizing Lipid-Coated Mesoporous Silica Nanoparticles as CD169-Binding Delivery System for Rilpivirine and Cabotegravir. Adv. Nanobiomed Res. 2022, 2, 2100157. [Google Scholar] [CrossRef]
- Eshaghi, B.; Fofana, J.; Nodder, S.B.; Gummuluru, S.; Reinhard, B.M. Virus-Mimicking Polymer Nanoparticles Targeting CD169(+) Macrophages as Long-Acting Nanocarriers for Combination Antiretrovirals. ACS Appl. Mater. Interfaces 2022, 14, 2488–2500. [Google Scholar] [CrossRef]
- Resa-Infante, P.; Erkizia, I.; Muñiz-Trabudua, X.; Linty, F.; Bentlage, A.E.H.; Perez-Zsolt, D.; Muñoz-Basagoiti, J.; Raïch-Regué, D.; Izquierdo-Useros, N.; Rispens, T.; et al. Preclinical development of humanized monoclonal antibodies against CD169 as a broad antiviral therapeutic strategy. Biomed. Pharmacother. 2024, 175, 116726. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouma, R.G.; Wang, A.Z.; den Haan, J.M.M. Exploring CD169+ Macrophages as Key Targets for Vaccination and Therapeutic Interventions. Vaccines 2025, 13, 330. https://doi.org/10.3390/vaccines13030330
Bouma RG, Wang AZ, den Haan JMM. Exploring CD169+ Macrophages as Key Targets for Vaccination and Therapeutic Interventions. Vaccines. 2025; 13(3):330. https://doi.org/10.3390/vaccines13030330
Chicago/Turabian StyleBouma, Rianne G., Aru Z. Wang, and Joke M. M. den Haan. 2025. "Exploring CD169+ Macrophages as Key Targets for Vaccination and Therapeutic Interventions" Vaccines 13, no. 3: 330. https://doi.org/10.3390/vaccines13030330
APA StyleBouma, R. G., Wang, A. Z., & den Haan, J. M. M. (2025). Exploring CD169+ Macrophages as Key Targets for Vaccination and Therapeutic Interventions. Vaccines, 13(3), 330. https://doi.org/10.3390/vaccines13030330






