The Use of Residual Blood Specimens in Seroprevalence Studies for Vaccine-Preventable Diseases: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Information Sources and Literature Search
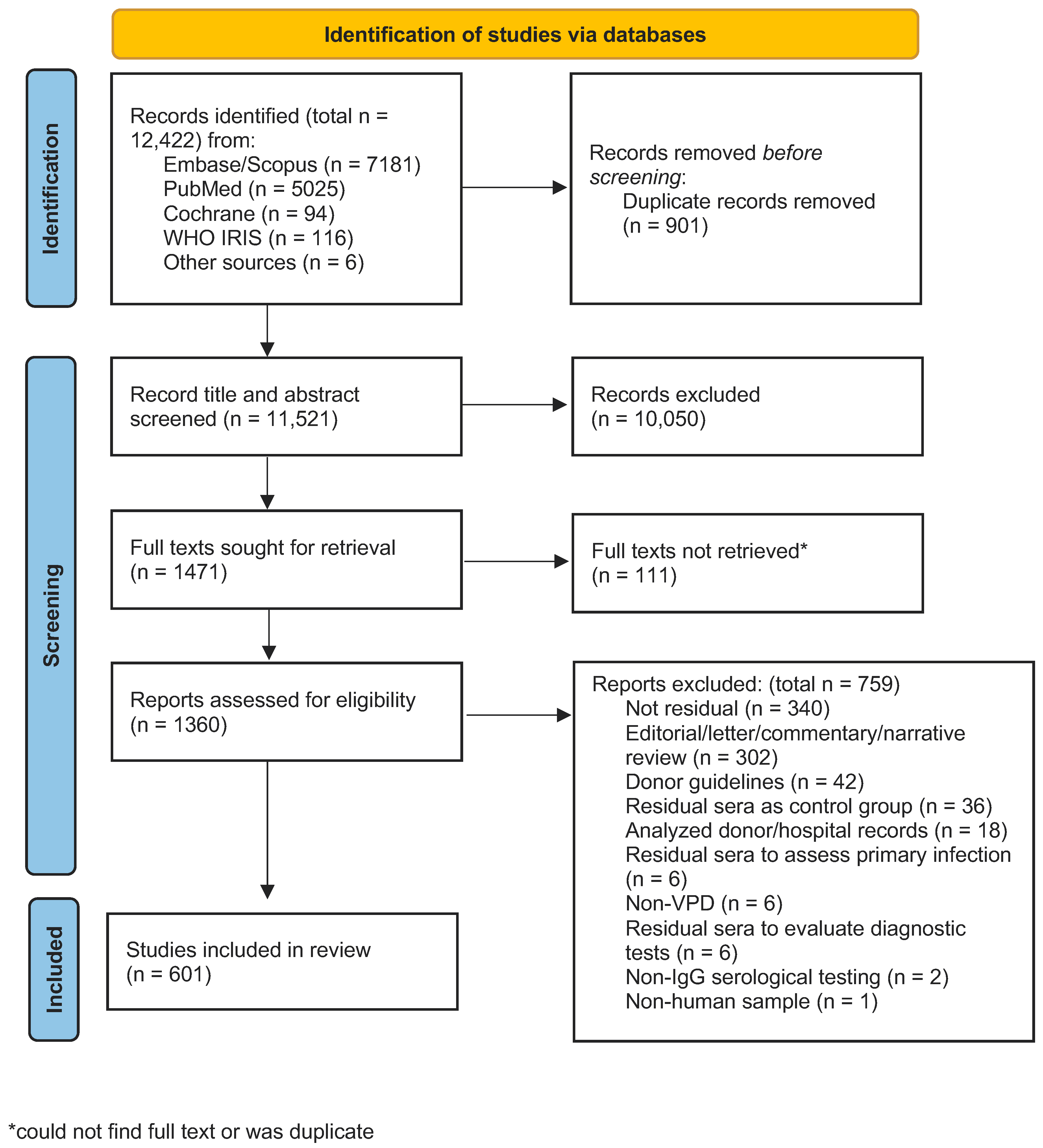
2.3. Screening Process
2.4. Data Extraction Process and Data Points
2.5. Data Synthesis
3. Results
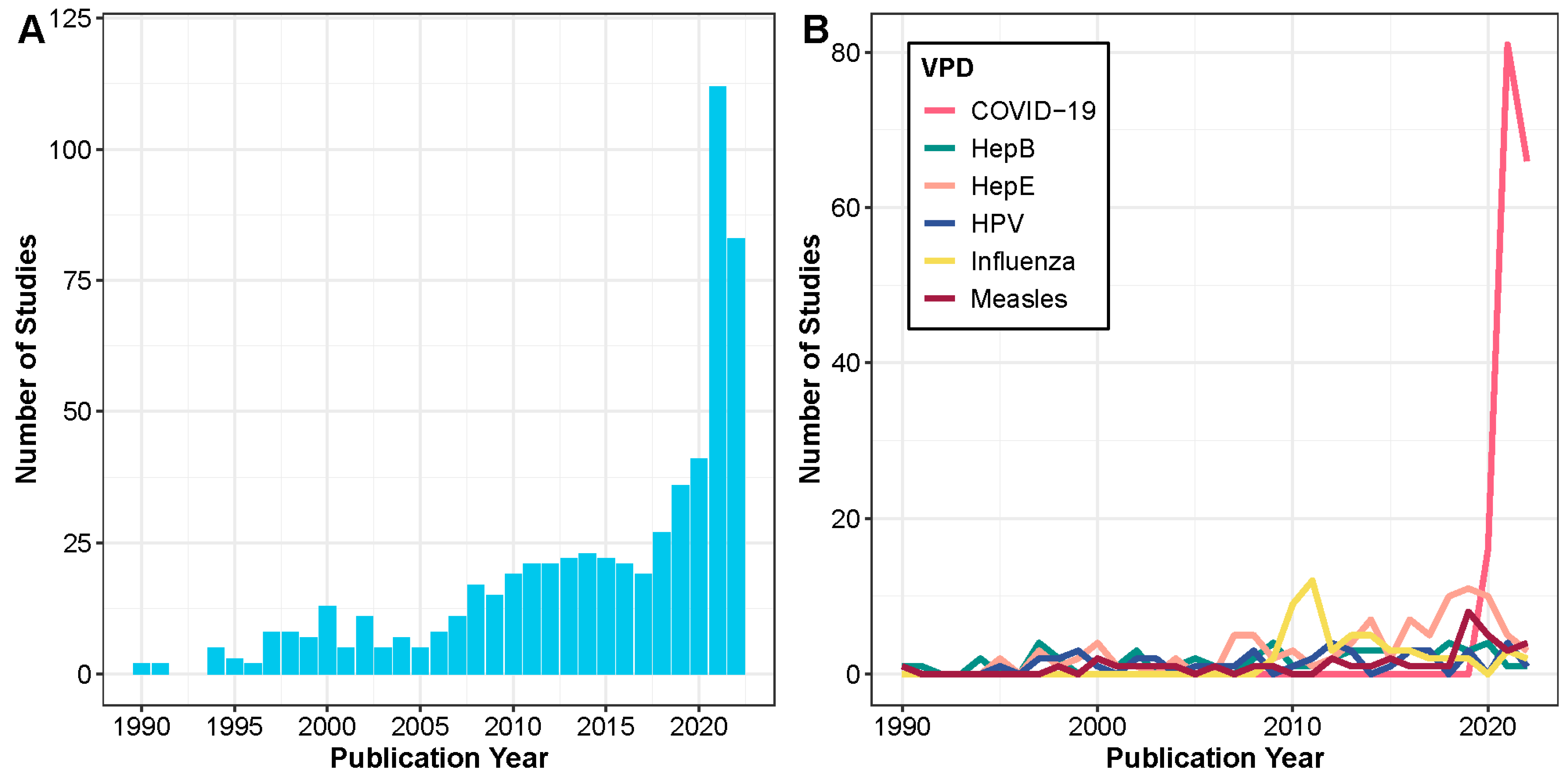
3.1. VPDs Studied, Trends, and Objectives
3.2. Original Use of Specimens
3.3. Sources of Residual Specimens
3.4. Metadata Linked to Residual Specimens
3.5. Ethical Considerations in the Use of Residual Specimens
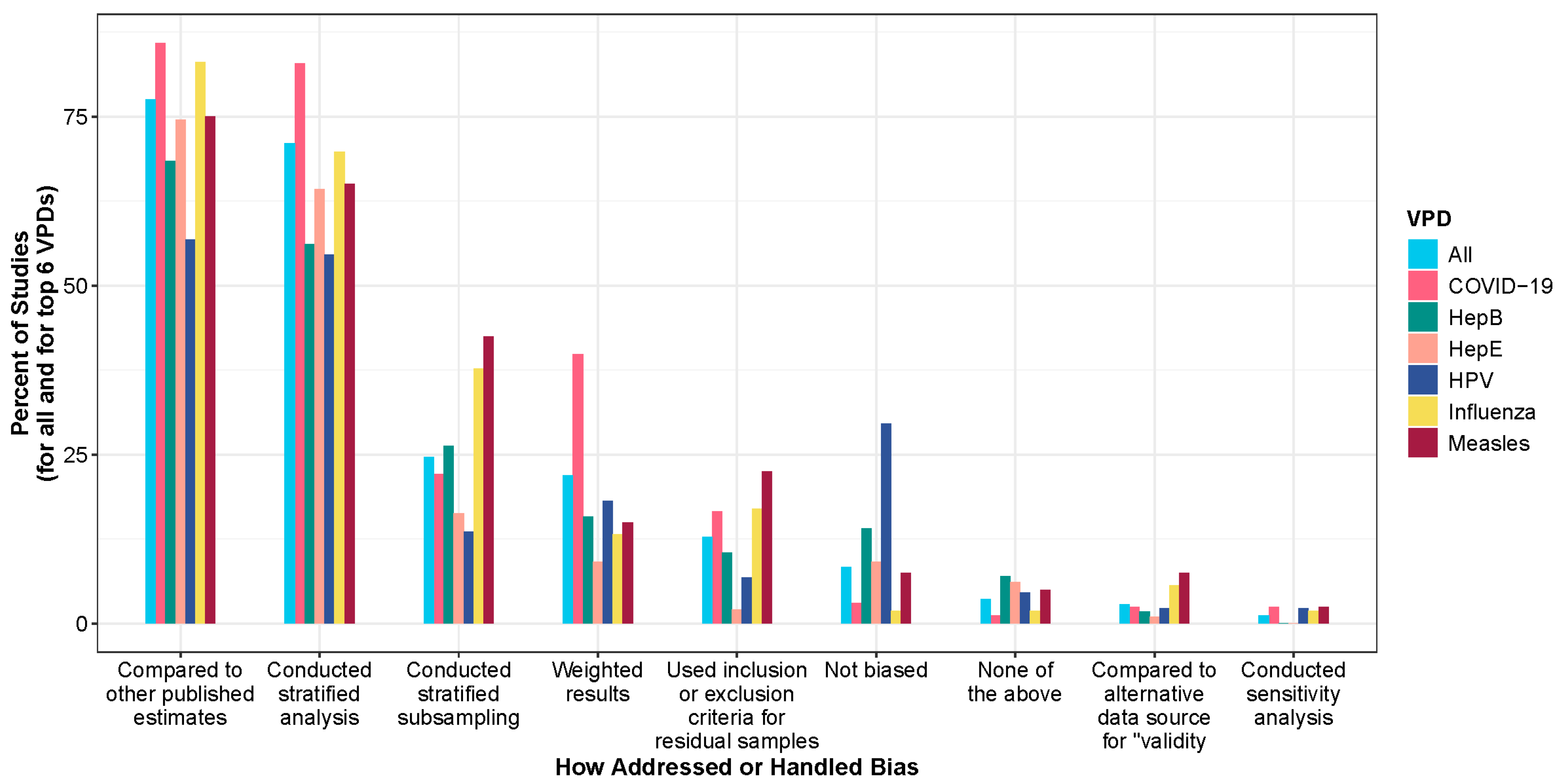
3.6. Selection Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vynnycky, E.; Adams, E.J.; Cutts, F.T.; Reef, S.E.; Navar, A.M.; Simons, E.; Yoshida, L.-M.; Brown, D.W.J.; Jackson, C.; Strebel, P.M.; et al. Using seroprevalence and immunisation coverage data to estimate the global burden of congenital rubella syndrome, 1996–2010: A systematic review. PLoS ONE 2016, 11, e0149160. [Google Scholar] [CrossRef]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M. Yellow Fever in Africa: Estimating the Burden of Disease and Impact of Mass Vaccination from Outbreak and Serological Data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Lessler, J.; Read, J.M.; Zhu, H.; Jiang, C.Q.; Guan, Y.; Cummings, D.A.T.; Riley, S. Estimating the Life Course of Influenza A(H3N2) Antibody Responses from Cross-Sectional Data. PLoS Biol. 2015, 13, e1002082. [Google Scholar] [CrossRef] [PubMed]
- Trotter, C.L.; Borrow, R.; Findlow, J.; Holland, A.; Frankland, S.; Andrews, N.J.; Miller, E. Seroprevalence of Antibodies against Serogroup C Meningococci in England in the Postvaccination Era. Clin. Vaccine Immunol. 2008, 15, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.; Ramsay, M.; Cohen, B.; Hesketh, L.; Morgan-Capner, P.; Brown, D.; Miller, E. The epidemiology of measles in England and Wales since the 1994 vaccination campaign. Commun. Dis. Rep. CDR Rev. 1997, 7, R17–R21. [Google Scholar]
- Scobie, H.M.; Mao, B.; Buth, S.; Wannemuehler, K.A.; Sørensen, C.; Kannarath, C.; Jenks, M.H.; Moss, D.M.; Priest, J.W.; Soeung, S.C.; et al. Tetanus Immunity among Women Aged 15 to 39 Years in Cambodia: A National Population-Based Serosurvey, 2012. Clin. Vaccine Immunol. 2016, 23, 546–554. [Google Scholar] [CrossRef]
- Winter, A.K.; Wesolowski, A.P.; Mensah, K.J.; Ramamonjiharisoa, M.B.; Randriamanantena, A.H.; Razafindratsimandresy, R.; Cauchemez, S.; Lessler, J.; Ferrari, M.J.; Metcalf, C.J.E.; et al. Revealing Measles Outbreak Risk with a Nested Immunoglobulin G Serosurvey in Madagascar. Am. J. Epidemiol. 2018, 187, 2219–2226. [Google Scholar] [CrossRef]
- Carcelen, A.C.; Winter, A.K.; Moss, W.J.; Chilumba, I.; Mutale, I.; Chongwe, G.; Monze, M.; Mulundu, G.; Nkamba, H.; Mwansa, F.D.; et al. Leveraging a national biorepository in Zambia to assess measles and rubella immunity gaps across age and space. Sci. Rep. 2022, 12, 10217. [Google Scholar] [CrossRef]
- Cutts, F.T.; Hanson, M. Seroepidemiology: An underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop. Med. Int. Health 2016, 21, 1086–1098. [Google Scholar] [CrossRef]
- MacNeil, A.; Lee, C.-W.; Dietz, V. Issues and considerations in the use of serologic biomarkers for classifying vaccination history in household surveys. Vaccine 2014, 32, 4893–4900. [Google Scholar] [CrossRef]
- Carcelen, A.C.; Hayford, K.; Moss, W.J.; Book, C.; Thuma, P.E.; Mwansa, F.D.; Patenaude, B. How much does it cost to measure immunity? A costing analysis of a measles and rubella serosurvey in southern Zambia. PLoS ONE 2020, 15, e0240734. [Google Scholar] [CrossRef] [PubMed]
- Cutts, F.T.; Izurieta, H.S.; Rhoda, D.A. Measuring coverage in MNCH: Design, implementation, and interpretation challenges associated with tracking vaccination coverage using household surveys. PLoS Med. 2013, 10, e1001404. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.K.; Martinez, M.E.; Cutts, F.T.; Moss, W.J.; Ferrari, M.J.; McKee, A.; Lessler, J.; Hayford, K.; Wallinga, J.; Metcalf, C.J.E. Benefits and Challenges in Using Seroprevalence Data to Inform Models for Measles and Rubella Elimination. J. Infect. Dis. 2018, 218, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Uyoga, S.; Adetifa, I.M.O.; Karanja, H.K.; Nyagwange, J.; Tuju, J.; Wanjiku, P.; Aman, R.; Mwangangi, M.; Amoth, P.; Kasera, K.; et al. Seroprevalence of anti–SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science 2021, 371, 79–82. [Google Scholar] [CrossRef]
- Saeed, S.; Uzicanin, S.; Lewin, A.; Lieshout-Krikke, R.; Faddy, H.; Erikstrup, C.; Osiowy, C.; Seed, C.R.; Steele, W.R.; Davison, K.; et al. Current challenges of severe acute respiratory syndrome coronavirus 2 seroprevalence studies among blood donors: A scoping review. Vox Sang. 2022, 117, 476–487. [Google Scholar] [CrossRef]
- Umberfield, E.E.; Kardia, S.L.R.; Jiang, Y.; Thomer, A.K.; Harris, M.R. Regulations and Norms for Reuse of Residual Clinical Biospecimens and Health Data. West. J. Nurs. Res. 2022, 44, 1068–1081. [Google Scholar] [CrossRef]
- Edwards, T.; Cadigan, R.J.; Evans, J.P.; Henderson, G.E. Biobanks containing clinical specimens: Defining characteristics, policies, and practices. Clin. Biochem. 2014, 47, 245–251. [Google Scholar] [CrossRef]
- Toolkit for Integrated Serosurveillance of Communicable Diseases in the Americas—PAHO/WHO|Pan American Health Organization. Available online: https://iris.paho.org/handle/10665.2/56364 (accessed on 15 February 2023).
- Shrestha, A.C.; Flower, R.L.P.; Seed, C.R.; Stramer, S.L.; Faddy, H.M. A Comparative Study of Assay Performance of Commercial Hepatitis E Virus Enzyme-Linked Immunosorbent Assay Kits in Australian Blood Donor Samples. J. Blood Transfus. 2016, 2016, 9647675. [Google Scholar] [CrossRef]
- Chan, Y.-J.; Lee, C.-L.; Hwang, S.-J.; Fung, C.-P.; Wang, F.-D.; Yen, D.H.; Tsai, C.-H.; Chen, Y.-M.A.; Lee, S.-D. Seroprevalence of Antibodies to Pandemic (H1N1) 2009 Influenza Virus Among Hospital Staff in a Medical Center in Taiwan. J. Chin. Med. Assoc. 2010, 73, 62–66. [Google Scholar] [CrossRef]
- Krumbholz, A.; Lange, J.; Dürrwald, R.; Hoyer, H.; Bengsch, S.; Wutzler, P.; Zell, R. Prevalence of antibodies to swine influenza viruses in humans with occupational exposure to pigs, Thuringia, Germany, 2008–2009. J. Med. Virol. 2010, 82, 1617–1625. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 22 June 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Schenk, J.; Hutse, V.; Suin, V.; Litzroth, A.; Blaizot, S.; Herzog, S.A.; Verburgh, V.; Jacques, M.; Rahman, A.; et al. Stable HEV IgG seroprevalence in Belgium between 2006 and 2014. J. Viral Hepat. 2020, 27, 1253–1260. [Google Scholar] [CrossRef]
- Quinn, H.E.; McIntyre, P.B.; Backhouse, J.L.; Gidding, H.F.; Brotherton, J.; Gilbert, G.L. The utility of seroepidemiology for tracking trends in pertussis infection. Epidemiol. Infect. 2010, 138, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Sitas, F.; Egger, S.; Urban, M.I.; Taylor, P.R.; Abnet, C.C.; Boffetta, P.; O’Connell, D.L.; Whiteman, D.C.; Brennan, P.; Malekzadeh, R.; et al. InterSCOPE Study: Associations Between Esophageal Squamous Cell Carcinoma and Human Papillomavirus Serological Markers. JNCI J. Natl. Cancer Inst. 2012, 104, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.P.; Lee, H.Y.; Khor, C.S.; Abdul-Jamil, J.; Alias, H.; Abu-Amin, N.; Mat-Radzi, M.; Rohimi, N.A.; Mokhtardin, H.N.; AbuBakar, S.; et al. The Risk of Transfusion-Transmitted Hepatitis E Virus: Evidence from Seroprevalence Screening of Blood Donations. Indian J. Hematol. Blood Transfus. 2022, 38, 145–152. [Google Scholar] [CrossRef]
- Anderson, K.S.; Wallstrom, G.; Langseth, H.; Posner, M.; Cheng, J.N.; Alam, R.; Chowell, D.; Furre, I.E.; Mork, J. Pre-diagnostic dynamic HPV16 IgG seropositivity and risk of oropharyngeal cancer. Oral Oncol. 2017, 73, 132–137. [Google Scholar] [CrossRef]
- Metcalf, C.J.E.; Farrar, J.; Cutts, F.T.; Basta, N.E.; Graham, A.L.; Lessler, J.; Ferguson, N.M.; Burke, D.S.; Grenfell, B.T. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet 2016, 388, 728–730. [Google Scholar] [CrossRef]
- Travassos, M.A.; Beyene, B.; Adam, Z.; Campbell, J.D.; Mulholland, N.; Diarra, S.S.; Kassa, T.; Oot, L.; Sequeira, J.; Reymann, M.; et al. Strategies for Coordination of a Serosurvey in Parallel with an Immunization Coverage Survey. Am. J. Trop. Med. Hyg. 2015, 93, 416–424. [Google Scholar] [CrossRef]
- Hasan, A.Z.; Kumar, M.S.; Prosperi, C.; Thangaraj, J.W.V.; Sabarinathan, R.; Saravanakumar, V.; Duraiswamy, A.; Kaduskar, O.; Bhatt, V.; Deshpande, G.R.; et al. Implementing Serosurveys in India: Experiences, Lessons Learned, and Recommendations. Am. J. Trop. Med. Hyg. 2021, 105, 1608–1617. [Google Scholar] [CrossRef]
- Bajema, K.L.; Wiegand, R.E.; Cuffe, K.; Patel, S.V.; Iachan, R.; Lim, T.; Lee, A.; Moyse, D.; Havers, F.P.; Harding, L.; et al. Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Intern. Med. 2021, 181, 450–460. [Google Scholar] [CrossRef]
- Bassal, R.; Indenbaum, V.; Pando, R.; Levin, T.; Shinar, E.; Amichay, D.; Barak, M.; Ben-Dor, A.; Bar Haim, A.; Mendelson, E.; et al. Seropositivity of measles antibodies in the Israeli population prior to the nationwide 2018–2019 outbreak. Hum. Vaccines Immunother. 2021, 17, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Routledge, I.; Takahashi, S.; Epstein, A.; Hakim, J.; Janson, O.; Turcios, K.; Vinden, J.; Risos, J.T.; Baniqued, M.R.; Pham, L.; et al. Using sero-epidemiology to monitor disparities in vaccination and infection with SARS-CoV-2. Nat. Commun. 2022, 13, 2451. [Google Scholar] [CrossRef] [PubMed]
- Adetifa, I.M.O.; Uyoga, S.; Gitonga, J.N.; Mugo, D.; Otiende, M.; Nyagwange, J.; Karanja, H.K.; Tuju, J.; Wanjiku, P.; Aman, R.; et al. Temporal trends of SARS-CoV-2 seroprevalence during the first wave of the COVID-19 epidemic in Kenya. Nat. Commun. 2021, 12, 3966. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Pupella, S.; Pisani, G.; Bruni, R.; Chionne, P.; Madonna, E.; Villano, U.; Simeoni, M.; Fabi, S.; Marano, G.; et al. A nationwide retrospective study on prevalence of hepatitis E virus infection in Italian blood donors. Blood Transfus. 2018, 16, 431. [Google Scholar] [CrossRef]
- Engle, R.E.; Bukh, J.; Alter, H.J.; Emerson, S.U.; Trenbeath, J.L.; Nguyen, H.T.; Brockington, A.; Mitra, T.; Purcell, R.H. Transfusion-associated hepatitis before the screening of blood for hepatitis risk factors. Transfusion 2014, 54, 2833–2841. [Google Scholar] [CrossRef]
- Katiyar, H.; Goel, A.; Sonker, A.; Yadav, V.; Sapun, S.; Chaudhary, R.; Aggarwal, R. Prevalence of hepatitis E virus viremia and antibodies among healthy blood donors in India. Indian J. Gastroenterol. 2018, 37, 342–346. [Google Scholar] [CrossRef]
- Bae, E.; Park, C.-H.; Ki, C.-S.; Kim, S.-J.; Huh, W.; Oh, H.-Y.; Kang, E.-S. Prevalence and clinical significance of occult hepatitis B virus infection among renal transplant recipients in Korea. Scand. J. Infect. Dis. 2012, 44, 788–792. [Google Scholar] [CrossRef]
- Izopet, J.; Labrique, A.B.; Basnyat, B.; Dalton, H.R.; Kmush, B.; Heaney, C.D.; Nelson, K.E.; Ahmed, Z.B.; Zaman, K.; Mansuy, J.-M.; et al. Hepatitis E virus seroprevalence in three hyperendemic areas: Nepal, Bangladesh and southwest France. J. Clin. Virol. 2015, 70, 39–42. [Google Scholar] [CrossRef]
- Lynch, J.A.; Lim, J.K.; Asaga, P.E.P.; Wartel, T.A.; Marti, M.; Yakubu, B.; Rees, H.; Talaat, K.; Kmush, B.; Aggarwal, R.; et al. Hepatitis E vaccine—Illuminating the barriers to use. PLoS Negl. Trop. Dis. 2023, 17, e0010969. [Google Scholar] [CrossRef]
- Sbarra, A.N.; Cutts, F.T.; Fu, H.; Poudyal, I.; Rhoda, D.A.; Mosser, J.F.; Jit, M. Evaluating Scope and Bias of Population-Level Measles Serosurveys: A Systematized Review and Bias Assessment. Vaccines 2024, 12, 585. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Kamaraj, P.; Kumar, M.S.; Khan, S.A.; Allam, R.R.; Barde, P.V.; Dwibedi, B.; Kanungo, S.; Mohan, U.; Mohanty, S.S.; et al. Immunity against diphtheria among children aged 5–17 years in India, 2017–2018: A cross-sectional, population-based serosurvey. Lancet Infect. Dis. 2021, 21, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Kugeler, K.J.; Podewils, L.J.; Alden, N.B.; Burket, T.L.; Kawasaki, B.; Biggerstaff, B.J.; Biggs, H.M.; Zacks, R.; Foster, M.A.; Lim, T.; et al. Assessment of SARS-CoV-2 Seroprevalence by Community Survey and Residual Specimens, Denver, Colorado, July–August 2020. Public Health Rep. 2022, 137, 128–136. [Google Scholar] [CrossRef]
- Bajema, K.L.; Dahlgren, F.S.; Lim, T.W.; Bestul, N.; Biggs, H.M.; Tate, J.E.; Owusu, C.; Szablewski, C.M.; Drenzek, C.; Drobeniuc, J.; et al. Comparison of Estimated Severe Acute Respiratory Syndrome Coronavirus 2 Seroprevalence through Commercial Laboratory Residual Sera Testing and a Community Survey. Clin. Infect. Dis. 2021, 73, E3120–E3123. [Google Scholar] [CrossRef]
- Kelly, H.; Riddell, M.A.; Gidding, H.F.; Nolan, T.; Gilbert, G.L. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine 2002, 20, 3130–3136. [Google Scholar] [CrossRef]
- Choisy, M.; Trinh, S.T.; Nguyen, T.N.D.; Nguyen, T.H.; Le Mai, Q.; Pham, Q.T.; Tran, N.D.; Dang, D.A.; Horby, P.W.; Boni, M.F.; et al. Sero-Prevalence Surveillance to Predict Vaccine-Preventable Disease Outbreaks; A Lesson from the 2014 Measles Epidemic in Northern Vietnam. Open Forum Infect. Dis. 2019, 6, ofz030. [Google Scholar] [CrossRef]
- Yan, B.-Y.; Lv, J.-J.; Liu, J.-Y.; Feng, Y.; Wu, W.-L.; Xu, A.-Q.; Zhang, L. Changes in seroprevalence of hepatitis A after the implementation of universal childhood vaccination in Shandong Province, China: A comparison between 2006 and 2014. Int. J. Infect. Dis. 2019, 82, 129–134. [Google Scholar] [CrossRef]
- The COVID-19 Immunity Task Force (CITF) Core Data Elements: Background (Health and Demographics). Available online: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2022/08/citf-cde-background-adult.pdf (accessed on 6 February 2025).
- Clayton, E.W.; Evans, B.J.; Hazel, J.W.; Rothstein, M.A. The law of genetic privacy: Applications, implications, and limitations. J. Law Biosci. 2019, 6, 1–36. [Google Scholar] [CrossRef]
- Molldrem, S.; Smith, A.K.J. Reassessing the Ethics of Molecular HIV Surveillance in the Era of Cluster Detection and Response: Toward HIV Data Justice. Am. J. Bioeth. 2020, 20, 10–23. [Google Scholar] [CrossRef]
- Grady, C.; Eckstein, L.; Berkman, B.; Brock, D.; Cook-Deegan, R.; Fullerton, S.M.; Greely, H.; Hansson, M.G.; Hull, S.; Kim, S.; et al. Broad Consent for Research With Biological Samples: Workshop Conclusions. Am. J. Bioeth. 2015, 15, 34–42. [Google Scholar] [CrossRef]
- Dassah, S.; Sakyi, S.A.; Frempong, M.T.; Luuse, A.T.; Ephraim, R.K.D.; Anto, E.O.; Oduro, A. Seroconversion of Hepatitis B Vaccine in Young Children in the Kassena Nankana District of Ghana: A Cross-Sectional Study. PLoS ONE 2015, 10, e0145209. [Google Scholar] [CrossRef]
- Crooke, S.N.; Haralambieva, I.H.; Grill, D.E.; Ovsyannikova, I.G.; Kennedy, R.B.; Poland, G.A. Seroprevalence and Durability of Rubella Virus Antibodies in a Highly Immunized Population. Vaccine 2019, 37, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, R.; Foufi, V.; Gaudet-Blavignac, C.; Robert, A.; Lovis, C. Use and Understanding of Anonymization and De-Identification in the Biomedical Literature: Scoping Review. J. Med. Internet Res. 2019, 21, e13484. [Google Scholar] [CrossRef] [PubMed]
- Kayaalp, M. Modes of De-identification. AMIA Annu. Symp. Proc. 2018, 2017, 1044–1050. [Google Scholar]
- Nardone, A.; de Ory, F.; Carton, M.; Cohen, D.; van Damme, P.; Davidkin, I.; Rota, M.; de Melker, H.; Mossong, J.; Slacikova, M.; et al. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine 2007, 25, 7866–7872. [Google Scholar] [CrossRef]
- Wiens, K.E.; Jauregui, B.; Arnold, B.F.; Banke, K.; Wade, D.; Hayford, K.; Denis, A.C.-S.; Hall, R.H.; Salje, H.; Rodriguez-Barraquer, I.; et al. Building an integrated serosurveillance platform to inform public health interventions: Insights from an experts’ meeting on serum biomarkers. PLoS Neglected Trop. Dis. 2022, 16, e0010657. [Google Scholar] [CrossRef]
- Arnold, B.F.; Scobie, H.M.; Priest, J.W.; Lammie, P.J. Integrated Serologic Surveillance of Population Immunity and Disease Transmission. Emerg. Infect. Dis. 2018, 24, 1188–1194. [Google Scholar] [CrossRef]
- Verberk, J.D.M.; Vos, R.A.; Mollema, L.; van Vliet, J.; van Weert, J.W.M.; de Melker, H.E.; van der Klis, F.R.M. Third national biobank for population-based seroprevalence studies in the Netherlands, including the Caribbean Netherlands. BMC Infect. Dis. 2019, 19, 470. [Google Scholar] [CrossRef]
- Tohme, R.A.; Scobie, H.M.; Okunromade, O.; Olaleye, T.; Shuaib, F.; Jegede, T.; Yahaya, R.; Nnaemeka, N.; Lawal, B.; Egwuenu, A.; et al. Tetanus and Diphtheria Seroprotection among Children Younger Than 15 Years in Nigeria, 2018: Who Are the Unprotected Children? Vaccines 2023, 11, 663. [Google Scholar] [CrossRef]
- Chan, Y.; Martin, D.; Mace, K.E.; Jean, S.E.; Stresman, G.; Drakeley, C.; Chang, M.A.; Lemoine, J.F.; Udhayakumar, V.; Lammie, P.J.; et al. Multiplex Serology for Measurement of IgG Antibodies Against Eleven Infectious Diseases in a National Serosurvey: Haiti 2014–2015. Front. Public Health 2022, 10, 897013. [Google Scholar] [CrossRef]
- Lim, T.; Delorey, M.; Bestul, N.; Johannson, M.A.; Reed, C.; Hall, A.J.; Fry, A.M.; Edens, C.; Semenova, V.; Li, H.; et al. Changes in SARS-CoV-2 Seroprevalence Over Time in Ten Sites in the United States, March–August, 2020. Clin. Infect. Dis. 2021, 73, 1831–1839. [Google Scholar] [CrossRef]
- Achonu, C.; Rosella, L.; Gubbay, J.B.; Deeks, S.; Rebbapragada, A.; Mazzulli, T.; Willison, D.; Foisy, J.; McGeer, A.; Johnson, I.; et al. Seroprevalence of pandemic influenza H1N1 in Ontario from January 2009–May 2010. PLoS ONE 2011, 6, e26427. [Google Scholar] [CrossRef]
- Fink, R.V.; Fisher, L.; Sulaeman, H.; Dave, H.; Levy, M.E.; McCann, L.; Di Germanio, C.; Notari, E.P.; Green, V.; Cyrus, S.; et al. How do we…form and coordinate a national serosurvey of SARS-CoV-2 within the blood collection industry? Transfusion 2022, 62, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Milošević, V.; Medić, S.; Malbaša, J.D.; Rajčević, S.; Boban, J.; Petrović, V. Sero-epidemiological study in prediction of the risk groups for measles outbreaks in Vojvodina, Serbia. PLoS ONE 2019, 14, e0216219. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.; Fauci, A.; Dowling, W.E.; Günther, S.; Bente, D.A.; Yadav, P.D.; Madoff, L.C.; Wang, L.; Arora, R.K.; Van Kerkhove, M.; et al. Lessons from the pandemic: Responding to emerging zoonotic viral diseases—A Keystone Symposia report. Ann. N. Y. Acad. Sci. 2022, 1518, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.A.; Agweyu, A.; Akanbi, O.A.; Alex-Wele, M.A.; Alinon, K.N.; Arora, R.K.; Balam, S.; Barekye, B.; Ben Hamida, A.; Bergeri, I.; et al. Learning from serosurveillance for SARS-CoV-2 to inform pandemic preparedness and response. Lancet 2023, 402, 356–358. [Google Scholar] [CrossRef]



| Vaccine-Preventable Disease | Total No. (%) (N = 601) |
|---|---|
| COVID-19 | 163 (27.1) |
| Hepatitis E (HepE) | 98 (16.3) |
| Hepatitis B (HepB) | 57 (9.5) |
| Influenza | 53 (8.8) |
| Human papillomavirus (HPV) | 44 (7.3) |
| Measles | 40 (6.7) |
| Hepatitis A (HepA) | 39 (6.5) |
| Rubella | 33 (5.5) |
| Dengue | 31 (5.2) |
| Varicella/Herpes zoster | 30 (5) |
| Diphtheria | 19 (3.2) |
| Mumps | 17 (2.8) |
| Pertussis | 17 (2.8) |
| Poliomyelitis | 8 (1.3) |
| Tetanus | 7 (1.2) |
| Japanese encephalitis | 4 (0.7) |
| Hemophilus influenza type b (Hib) | 3 (0.5) |
| Yellow fever | 3 (0.5) |
| Meningococcal meningitis | 2 (0.3) |
| Tick-borne encephalitis | 2 (0.3) |
| Rotavirus | 1 (0.2) |
| Typhoid fever | 1 (0.2) |
| Cholera | 0 (0.0) |
| Tuberculosis | 0 (0.0) |
| Pneumococcal | 0 (0.0) |
| Studies investigating single pathogen | 560 (93.2) |
| Studies investigating multiple pathogens | 41 (6.8) |
| Component | Considerations |
|---|---|
| Study population | Details on the source of the specimens and description of the original population or study (including sample size and sociodemographic characteristics) to assess the generalizability of findings from residual specimens |
| Original reason or objective for specimen collection prior to storage | |
| How residual specimens were sampled including inclusion and exclusion criteria (e.g., by time period and individual characteristics) | |
| Ethics | Whether the individuals whose specimens were collected were aware of potential for future research; if there were any formal consent or “opt-in/opt-out” requirements related to future research; ethical approvals or waivers obtained, whether or not specimens were identifiable |
| Metadata | How data were collected from the original population (including if part of a larger surveillance collection system) and what metadata were obtained, including the following:
|
| Serological testing | Serological testing that was performed for the original purpose prior to being stored |
| Selection bias and generalizability | How the study handled and assessed selection biases in the residual specimen source population relative to the target population. This explanation is key to providing readers with an understanding of how representative the findings may be of the target population. This could also include any potential limitations in the generalizability of the findings. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilewskie, M.; Prosperi, C.; Bernasconi, A.; Esteban, I.; Niehaus, L.; Ross, C.; Carcelen, A.C.; Moss, W.J.; Winter, A.K. The Use of Residual Blood Specimens in Seroprevalence Studies for Vaccine-Preventable Diseases: A Scoping Review. Vaccines 2025, 13, 321. https://doi.org/10.3390/vaccines13030321
Pilewskie M, Prosperi C, Bernasconi A, Esteban I, Niehaus L, Ross C, Carcelen AC, Moss WJ, Winter AK. The Use of Residual Blood Specimens in Seroprevalence Studies for Vaccine-Preventable Diseases: A Scoping Review. Vaccines. 2025; 13(3):321. https://doi.org/10.3390/vaccines13030321
Chicago/Turabian StylePilewskie, Monica, Christine Prosperi, Abigail Bernasconi, Ignacio Esteban, Lori Niehaus, Connor Ross, Andrea C. Carcelen, William J. Moss, and Amy K. Winter. 2025. "The Use of Residual Blood Specimens in Seroprevalence Studies for Vaccine-Preventable Diseases: A Scoping Review" Vaccines 13, no. 3: 321. https://doi.org/10.3390/vaccines13030321
APA StylePilewskie, M., Prosperi, C., Bernasconi, A., Esteban, I., Niehaus, L., Ross, C., Carcelen, A. C., Moss, W. J., & Winter, A. K. (2025). The Use of Residual Blood Specimens in Seroprevalence Studies for Vaccine-Preventable Diseases: A Scoping Review. Vaccines, 13(3), 321. https://doi.org/10.3390/vaccines13030321







