Inflammatory and Humoral Immune Responses to Commercial Autogenous Salmonella Bacterin Vaccines in Light-Brown Leghorn Pullets: Primary and Secondary Vaccine Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design
2.3. Sample Collection
2.4. Quantification of S. Enteritidis-Specific Immunoglobulins in Blood Plasma
2.5. Assessing Leukocyte Recruitment and Cytokine mRNA Expression in GF Pulps
2.5.1. Preparation of Cell Suspensions and Cell Population Analysis by Flow Cytometry
2.5.2. Total RNA Isolation, cDNA Synthesis, and Relative Gene Expression Analysis
2.6. Statistical Analysis
3. Results
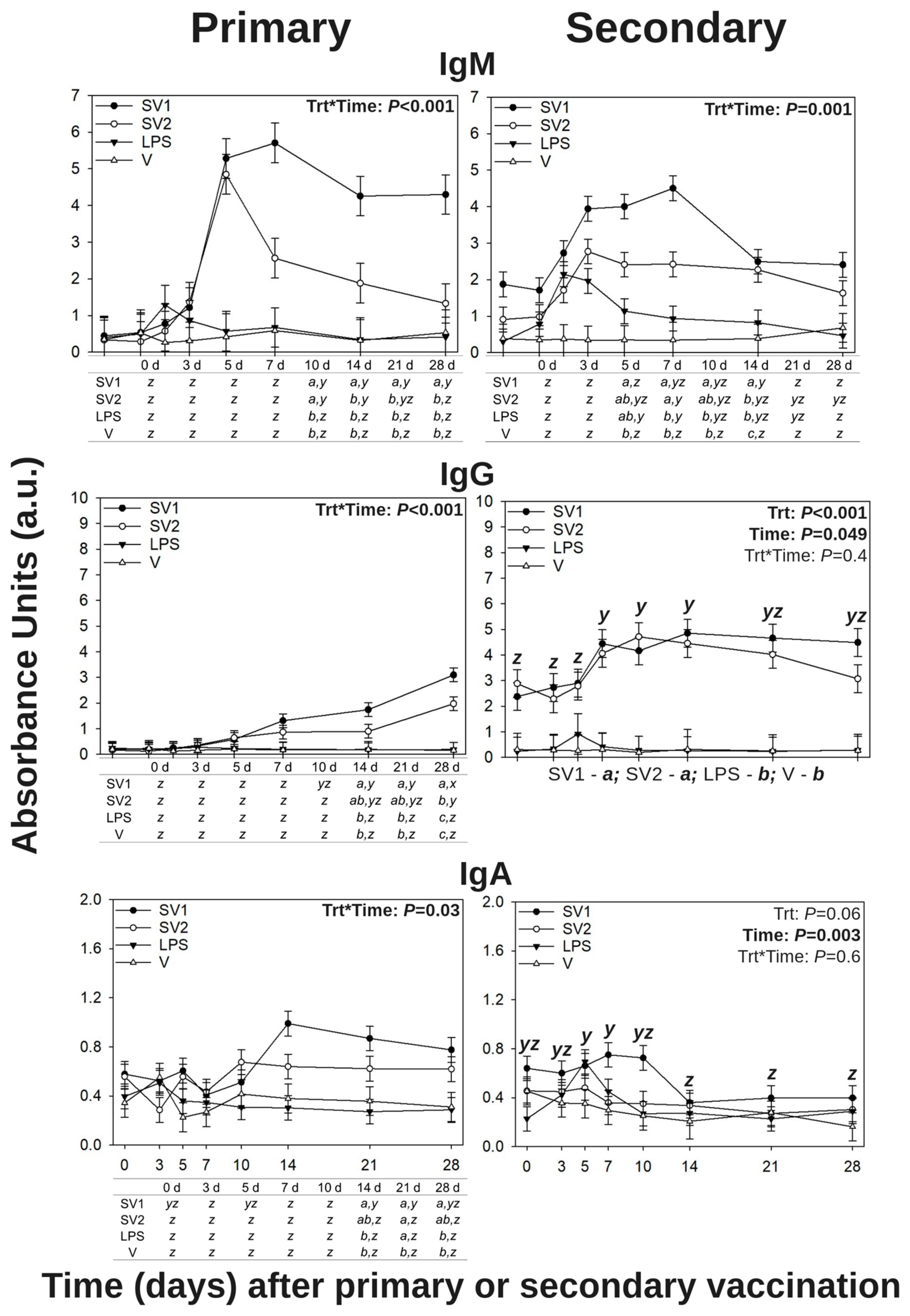
3.1. Salmonella Enteritidis-Specific Antibody Levels in Peripheral Blood Plasma
3.1.1. Salmonella Enteritidis-Specific IgM
3.1.2. Salmonella Enteritidis-Specific IgY (Avian IgG)
3.1.3. Salmonella Enteritidis-Specific IgA
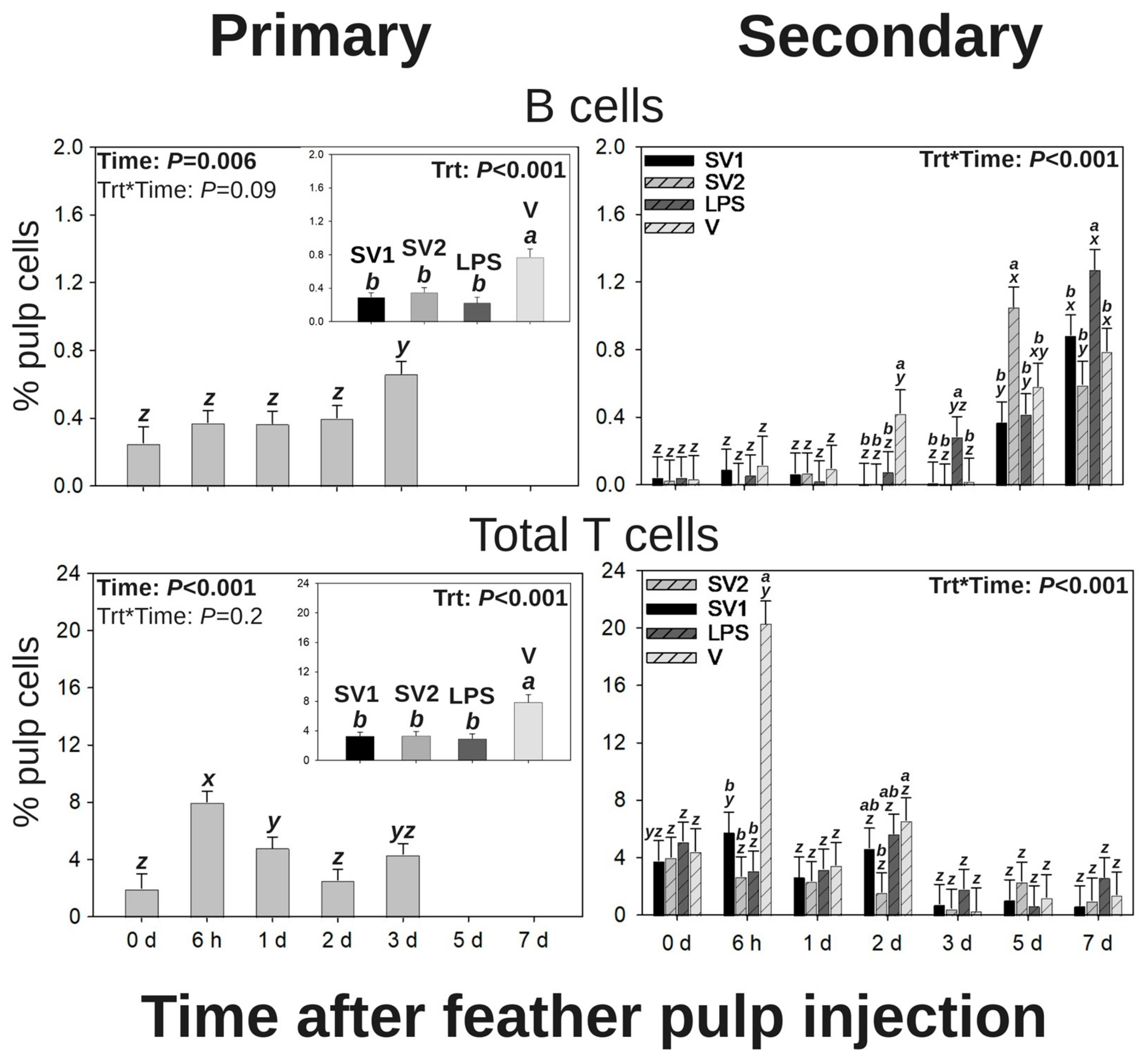
3.2. Leukocyte Profiles in GF Pulps Following Salmonella Bacterin Vaccine Administration
3.2.1. Heterophils
3.2.2. Macrophages
3.2.3. Total Lymphocytes
3.2.4. B Cells
3.2.5. Total T Cells
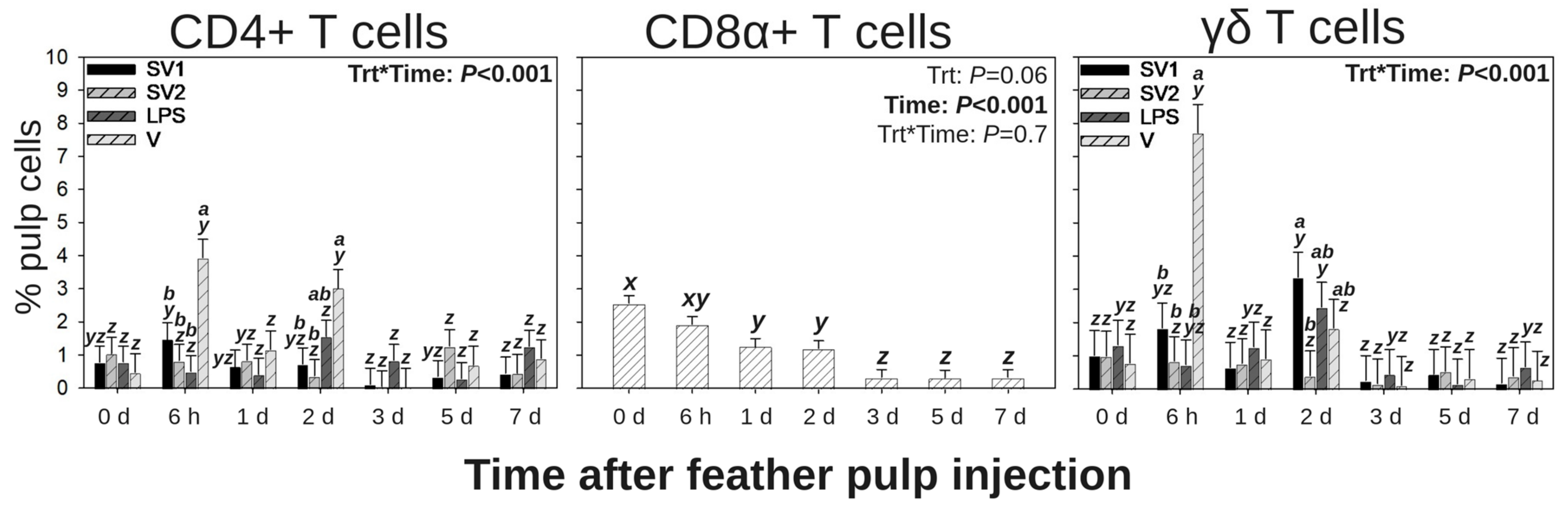
3.3. T Cell Subpopulations in GF Pulps Following Secondary Administration of Salmonella Bacterin Vaccines
3.3.1. CD4+ T Cells
3.3.2. CD8α+ T Cells
3.3.3. γδ T Cells
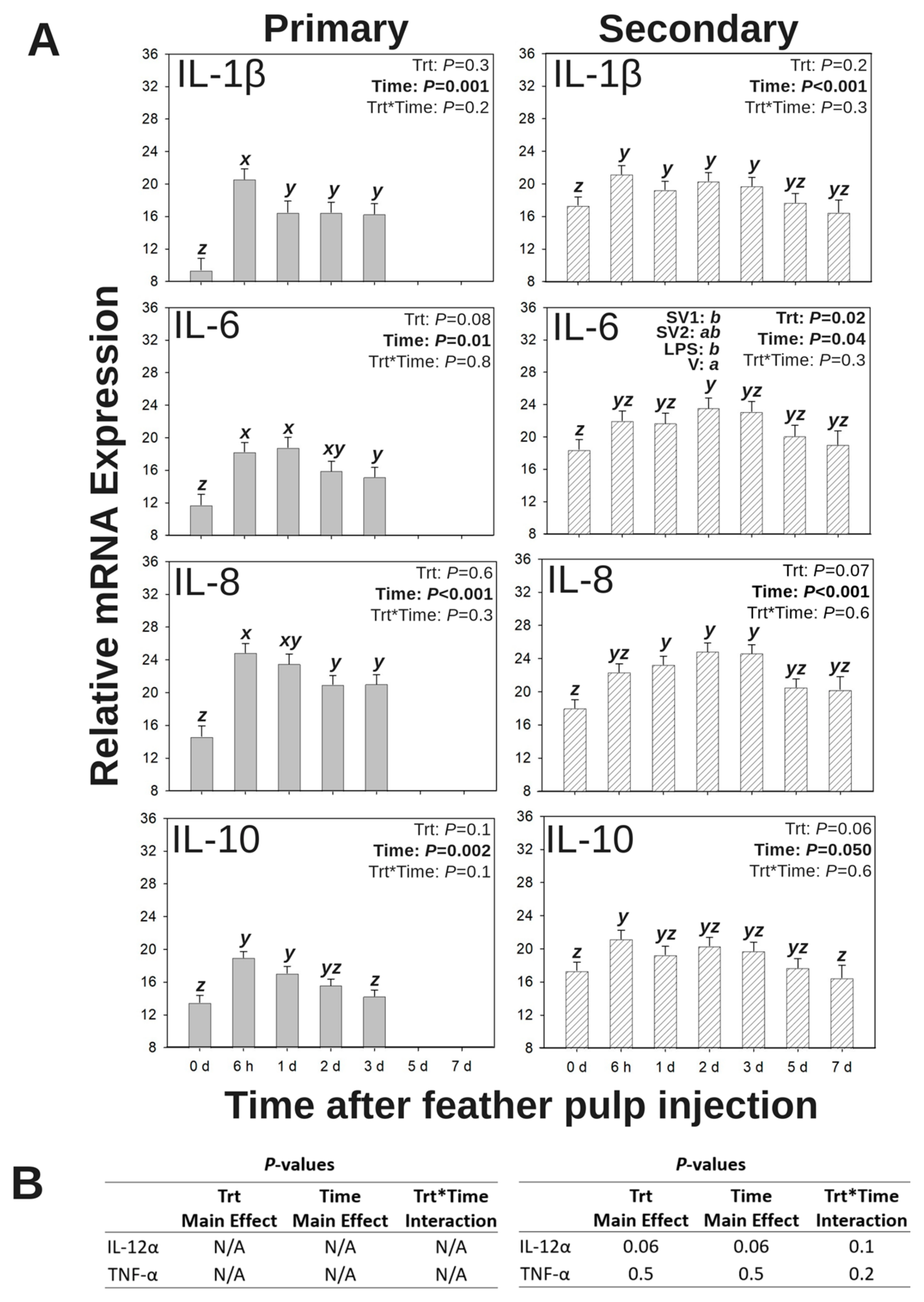
3.4. Relative mRNA Expression of Inflammatory Cytokines in GF Pulps Following Salmonella Bacterin Vaccine Administration
3.4.1. IL-1β mRNA Expression
3.4.2. IL-6 mRNA Expression
3.4.3. IL-8 (CXCL8) mRNA Expression
3.4.4. IL-10 mRNA Expression
3.5. Relative mRNA Expression of Effector T Cell Cytokines in GF Pulps Following Salmonella Bacterin Vaccine Administration
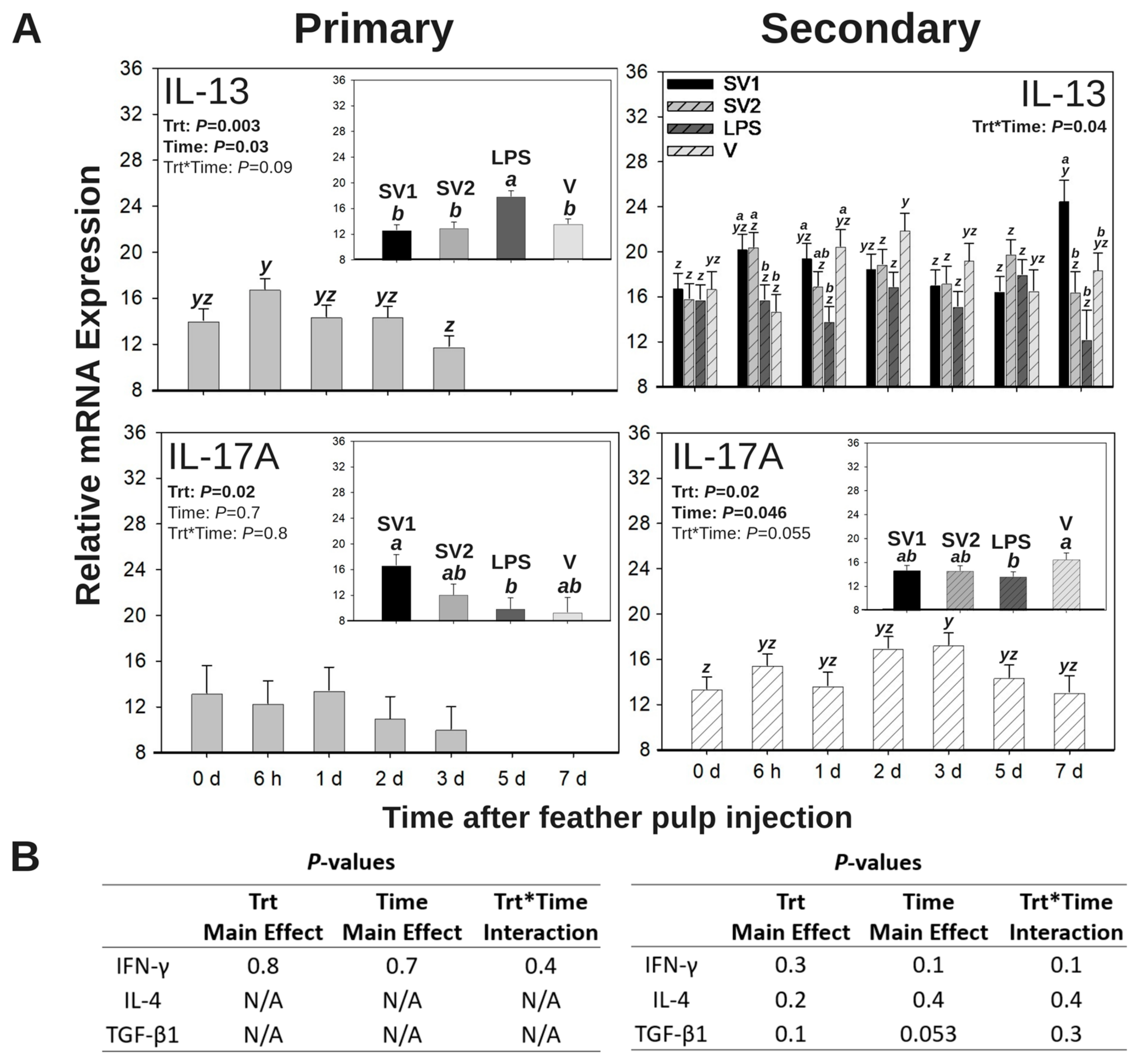
3.5.1. IL-13 mRNA Expression
3.5.2. IL-17A mRNA Expression
4. Discussion
4.1. Vaccination with Autogenous Salmonella Bacterin Induced a Humoral Response with Evidence of Immunological Memory
4.2. Secondary Intradermal Injection of Autogenous Salmonella Bacterin Stimulated Prolonged Heterophil and Macrophage Activity at the Site of Injection
4.3. A Secondary Intradermal Vaccination of Autogenous Salmonella Bacterin Stimulated Cell-Mediated Activities at the Site of Injection
4.4. Water–Oil–Water Emulsion Alone Recruited High Levels of CD4+ and γδ T Cells at 6 h Post-Intradermal Injection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hy-Line International. Vaccination Recommendations. Available online: https://www.hyline.com/ViewFile?id=7c275f35-64c0-466a-a40f-eb24d1fe0d9f (accessed on 4 May 2021).
- Armwood, B.T.; Rieth, A.; Baldwin, L.; Stephen Roney, C.; Barbieri, N.L.; Logue, C.M. Assessing the Ability of Maternal Antibodies to Protect Broiler Chicks Against Colonization by Salmonella Heidelberg. Avian Dis. 2019, 63, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Crouch, C.F.; Nell, T.; Reijnders, M.; Donkers, T.; Pugh, C.; Patel, A.; Davis, P.; van Hulten, M.C.W.; de Vries, S.P.W. Safety and Efficacy of a Novel Inactivated Trivalent Salmonella enterica Vaccine in Chickens. Vaccine 2020, 38, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.J.; Sharpe, S.M.; Muir, W.I.; Pavic, A.; Cox, J.M. Live and Inactivated Vaccine Regimens against Caecal Salmonella Typhimurium Colonisation in Laying Hens. Aust. Vet. J. 2016, 94, 387–393. [Google Scholar] [CrossRef]
- Kaiser, M.G.; Lakshmanan, N. Lamont Salmonella enterica Serovar Enteritidis Burden in Broiler Breeder Chicks Genetically Associated with Vaccine Antibody Response. Avian Dis. 2002, 46, 25–31. [Google Scholar] [CrossRef]
- Penha Filho, R.A.C.; Moura, B.S.; de Almeida, A.M.; Montassier, H.J.; Barrow, P.A.; Berchieri Junior, A. Humoral and Cellular Immune Response Generated by Different Vaccine Programs before and after Salmonella Enteritidis Challenge in Chickens. Vaccine 2012, 30, 7637–7643. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Lindner, T.; Ahrens, M.; Woitow, G.; Prandini, F.; Selbitz, H.-J. Duration of Immunity Induced in Chickens by an Attenuated Live Salmonella Vaccine and an Inactivated Enteritidis/Typhimurium. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 89–93. [Google Scholar] [PubMed]
- Methner, U. Immunisation of Chickens with Live Salmonella Vaccines—Role of Booster Vaccination. Vaccine 2018, 36, 2973–2977. [Google Scholar] [CrossRef]
- Cawthraw, S.A.; Goddard, A.; Huby, T.; Ring, I.; Chiverton, L.; Mueller-Doblies, D. Early Vaccination of Laying Hens with the Live Bivalent Salmonella Vaccine AviProTM Salmonella DUO Results in Successful Vaccine Uptake and Increased Gut Colonization. Front. Microbiol. 2023, 14, 1327739. [Google Scholar] [CrossRef]
- Sáenz, L.; Guzmán, M.; Vidal, S.; Caruffo, M.; Siel, D.; Zayas, C.; Paredes, R.; Valenzuela, C.; Hidalgo, H.; Pérez, O.; et al. Efficacy of Multivalent, Cochleate-Based Vaccine against Salmonella Infantis, S. enteritidis and S. typhimurium in Laying Hens. Vaccines 2022, 10, 226. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Oral Vaccination with a Live Salmonella Enteritidis/Typhimurium Bivalent Vaccine in Layers Induces Cross-Protection against Caecal and Internal Organ Colonization by a Salmonella Infantis Strain. Vet. Microbiol. 2018, 218, 7–12. [Google Scholar] [CrossRef]
- Lin, T.L.; Fan, Y.H.; Chang, Y.L.; Ho, H.J.; Liang, L.L.; Chen, Y.J.; Wu, C.Y. Early-Life Antibiotic Exposure Associated With Varicella Occurrence and Breakthrough Infections: Evidence From Nationwide Pre-Vaccination and Post-Vaccination Cohorts. Front. Immunol. 2022, 13, 848835. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Yokoyama, E.; Honda, T.; Mizuno, K. Efficacy of a Novel Trivalent Inactivated Vaccine against the Shedding of Salmonella in a Chicken Challenge Model. Avian Dis. 2009, 53, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Young, S.D.; Olusanya, O.; Jones, K.H.; Liu, T.; Liljebjelke, K.A.; Hofacre, C.L. Salmonella Incidence in Broilers from Breeders Vaccinated with Live and Killed Salmonella. J. Appl. Poult. Res. 2007, 16, 521–528. [Google Scholar] [CrossRef]
- Foss, D.L.; Agin, T.S.; Bade, D.; Dearwester, D.A.; Jolie, R.; Keich, R.L.; Lohse, R.M.; Reed, M.; Rosey, E.L.; Schneider, P.A.; et al. Protective Immunity to Salmonella enterica Is Partially Serogroup Specific. Vet. Immunol. Immunopathol. 2013, 155, 76–86. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, A.; Zhao, R.; Yin, L.; Yin, D.; Dai, Y.; Hou, H.; Wang, J.; Hu, X.; Pan, X.; et al. Effects of Adding Antibiotics to an Inactivated Oil-Adjuvant Avian Influenza Vaccine on Vaccine Characteristics and Chick Health. Poult. Sci. 2024, 103, 104135. [Google Scholar] [CrossRef]
- Droual, R.; Bickford, A.A.; Cutler, G.J. Local Reaction and Serological Response in Commercial Layer Chickens Injected Intramuscularly in the Leg with Oil-Adjuvanted Mycoplasma Gallisepticum Bacterin. Avian Dis. 1993, 37, 1001–1008. [Google Scholar] [CrossRef]
- Yamanaka, M.; Okabe, T.; Nakai, M.; Goto, N. Local Pathological Reactions and Immune Response of Chickens to ISA-70 and Other Adjuvants Containing Newcastle Disease Virus Antigen. Avian Dis. 1993, 37, 459–466. [Google Scholar] [CrossRef]
- Droual, R.; Bickford, A.A.; Charlton, B.R.; Kuney, D.R. Investigation of Problems Associated with Intramuscular Breast Injection of Oil-Adjuvanted Killed Vaccines in Chickens. Avian Dis. 1990, 34, 473–478. [Google Scholar] [CrossRef]
- Wettere, A.J. Van Injection Site Injury in Poultry. Available online: https://www.merckvetmanual.com/poultry/myopathies/injection-site-injury-in-poultry (accessed on 12 December 2024).
- Tracy, L.M.; Shepherd, E.; França, M.; Williams, S.M.; Grogan, K.B.; Nicholds, J.A.; Shamoun, K.; Lee, C. Hemorrhagic Hepatopathy in Broiler Breeder Pullets: Gross and Microscopic Pathology and Factors Associated with Incidence. Avian Dis. 2021, 65, 269–280. [Google Scholar] [CrossRef]
- Erf, G.F. In Vivo System to Monitor Tissue Responses in Birds 2012. US Patent #US8216551B2. Available online: https://patentcenter.uspto.gov/applications/12467727 (accessed on 3 March 2025).
- Erf, G.F.; Ramachandran, I.R. The Growing Feather as a Dermal Test Site: Comparison of Leukocyte Profiles during the Response to Mycobacterium Butyricum in Growing Feathers, Wattles, and Wing Webs. Poult. Sci. 2016, 95, 2011–2022. [Google Scholar] [CrossRef]
- Sullivan, K.S.; Erf, G.F. CD4+ T Cells Dominate the Leukocyte Infiltration Response Initiated by Intra-Dermal Injection of Phytohemagglutinin into Growing Feathers in Chickens. Poult. Sci. 2017, 96, 3574–3580. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, J.M.; Beck, C.N.; Erf, G.F. Local Inflammatory and Systemic Antibody Responses Initiated by a First Intradermal Administration of Autogenous Salmonella-Killed Vaccines and Their Components in Pullets. Vaccines 2024, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Erf, G.F.; Falcon, D.M.; Sullivan, K.S.; Bourdo, S.E. T Lymphocytes Dominate Local Leukocyte Infiltration in Response to Intradermal Injection of Functionalized Graphene-Based Nanomaterial. J. Appl. Toxicol. 2017, 37, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Erf, G.F.; Kong, H.R.; Falcon, D.M.; Byrne, K.A. Two-Window Approach to Monitor and Assess Cellular and Humoral Immune Responses in Poultry. Poultry 2023, 2, 82–97. [Google Scholar] [CrossRef]
- French, C.E.; Sales, M.A.; Rochell, S.J.; Rodriguez, A.; Erf, G.F. Local and Systemic Inflammatory Responses to Lipopolysaccharide in Broilers: New Insights Using a Two-Window Approach. Poult. Sci. 2020, 99, 6593–6605. [Google Scholar] [CrossRef]
- Rocchi, A.J.; Santamaria, J.M.; Beck, C.N.; Sales, M.A.; Hargis, B.M.; Tellez-Isaias, G.; Erf, G.F. The Immuno-Suppressive Effects of Cyclic, Environmental Heat Stress in Broiler Chickens: Local and Systemic Inflammatory Responses to an Intradermal Injection of Lipopolysaccharide. Vet. Sci. 2024, 11, 16. [Google Scholar] [CrossRef]
- Berthelot-Hérault, F.; Mompart, F.; Zygmunt, M.S.; Gérard, D.; Duchet-Suchaux, M. Antibody Responses in the Serum and Gut of Chicken Lines Differing in Cecal Carriage of Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2003, 96, 43–52. [Google Scholar] [CrossRef]
- Holt, P.S.; Porter, R.E. Effect of Induced Molting on the Recurrence of a Previous Salmonella Enteritidis Infection. Poult. Sci. 1993, 72, 2069–2078. [Google Scholar] [CrossRef]
- O’Meara, K.M.; Kremer, C.J.; Layton, S.L.; Berghman, L.R.; Hargis, B.M.; Cole, K. Evaluation of Recombinant Salmonella Expressing Cd154 for Persistence and Enhanced Antibody Response in Commercial Turkeys. Poult. Sci. 2010, 89, 1399–1405. [Google Scholar] [CrossRef]
- Seliger, C.; Schaerer, B.; Kohn, M.; Pendl, H.; Weigend, S.; Kaspers, B.; Härtle, S. A Rapid High-Precision Flow Cytometry Based Technique for Total White Blood Cell Counting in Chickens. Vet. Immunol. Immunopathol. 2012, 145, 86–99. [Google Scholar] [CrossRef]
- Borowska, D.; Rothwell, L.; Bailey, R.A.; Watson, K.; Kaiser, P. Identification of Stable Reference Genes for Quantitative PCR in Cells Derived from Chicken Lymphoid Organs. Vet. Immunol. Immunopathol. 2016, 170, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kothlow, S.; Schenk-Weibhauser, K.; Ratcliffe, M.J.H.; Kaspers, B. Prolonged Effect of BAFF on Chicken B Cell Development Revealed by RCAS Retroviral Gene Transfer in Vivo. Mol. Immunol. 2010, 47, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, B.G.; Methner, U.; Pieper, J.; Berndt, A. Effects of Salmonella enterica Serovar Enteritidis on Cellular Recruitment and Cytokine Gene Expression in Caecum of Vaccinated Chickens. Vaccine 2008, 26, 5423–5433. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Urwat, U.; Ahmad, S.M.; Ahmad, R.; Kashoo, Z.A.; Dar, T.A.; Bhat, S.A.; Mumtaz, P.T.; Shabir, N.; Shah, R.A.; et al. Gene Expression and Antibody Response in Chicken against Salmonella Typhimurium Challenge. Poult. Sci. 2019, 98, 2008–2013. [Google Scholar] [CrossRef]
- Kaiser, M.G.; Wing, T.; Lamont, S.J. Effect of Genetics, Vaccine Dosage, and Postvaccination Sampling Interval on Early Antibody Response to Salmonella Enteritidis Vaccine in Broiler Breeder Chicks. Poult. Sci. 1997, 77, 271–275. [Google Scholar] [CrossRef]
- Härtle, S.; Magor, K.E.; Göbel, T.W.; Davison, F.; Kaspers, B. Structure and Evolution of Avian Immunoglobulins. In Avian Immunology; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 103–120. [Google Scholar]
- Selander, B.; Weintraub, A.; Holmström, E.; Sturfelt, G.; Truedsson, L.; Mårtensson, U.; Jensenius, J.C.; Sjöholm, A.G. Low Concentrations of Immunoglobulin G Antibodies to Salmonella Serogroup C in C2 Deficiency: Suggestion of a Mannan-Binding Lectin Pathway- Dependent Mechanism. Scand. J. Immunol. 1999, 50, 555–561. [Google Scholar] [CrossRef]
- Marouf, S.; Ibrahim, H.M.; El-Naggar, M.S.; Swelum, A.A.; Alqhtani, A.H.; El-Saadony, M.T.; El-Tarabily, K.A.; Salem, H.M. Inactivated Pentavalent Vaccine against Mycoplasmosis and Salmonellosis for Chickens. Poult. Sci. 2022, 101, 102139. [Google Scholar] [CrossRef]
- Beal, R.K.; Powers, C.; Wigley, P.; Barrow, P.A.; Smith, A.L. Temporal Dynamics of the Cellular, Humoral and Cytokine Responses in Chickens during Primary and Secondary Infection with Salmonella enterica Serovar Typhimurium. Avian Pathol. 2004, 33, 25–33. [Google Scholar] [CrossRef]
- Withanage, G.S.K.; Wigley, P.; Kaiser, P.; Mastroeni, P.; Brooks, H.; Powers, C.; Beal, R.; Barrow, P.; Maskell, D.; McConnell, I. Cytokine and Chemokine Responses Associated with Clearance of a Primary Salmonella enterica Serovar Typhimurium Infection in the Chicken and in Protective Immunity to Rechallenge. Infect. Immun. 2005, 73, 5173–5182. [Google Scholar] [CrossRef]
- Maiti, S.; Halder, P.; Banerjee, S.; Dutta, M.; Kumar Mukhopadhyay, A.; Dutta, S.; Koley, H. Development of a Novel Trivalent Invasive Non-Typhoidal Salmonella Outer Membrane Vesicles Based Vaccine against Salmonellosis and Fowl Typhoid in Chickens. Immunobiology 2022, 227, 152183. [Google Scholar] [CrossRef]
- Jung, B.; Park, S.; Kim, E.; Yoon, H.; Hahn, T.W. Salmonella Typhimurium Lacking PhoBR as a Live Vaccine Candidate against Poultry Infection. Vet. Microbiol. 2022, 266, 109342. [Google Scholar] [CrossRef]
- Huberman, Y.D.; Caballero-García, M.; Rojas, R.; Ascanio, S.; Olmos, L.H.; Malena, R.; Lomónaco, J.; Nievas, P.; Chero, P.; Lévano-Gracía, J.; et al. The Efficacy of a Trivalent Inactivated Salmonella Vaccine Combined with the Live S. Gallinarum 9R Vaccine in Young Layers after Experimental Infections with S. enteritidis, S. typhimurium, and S. infantis. Vaccines 2022, 10, 1113. [Google Scholar] [CrossRef]
- Shin, H.; La, T.-M.; Lee, H.-J.; Kim, T.; Song, S.; Park, E.; Park, G.-H.; Choi, I.-S.; Park, S.-Y.; Lee, J.-B.; et al. Evaluation of Immune Responses and Protective Efficacy of a Novel Live Attenuated Salmonella Enteritidis Vaccine Candidate in Chickens. Vaccines 2022, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B. Avian Heterophils in Inflammation and Disease Resistance. Poult. Sci. 1998, 77, 972–977. [Google Scholar] [CrossRef]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, M.H. The Avian Heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [CrossRef]
- Pieper, J.; Locke, M.; Ruzaike, G.; Voigt, S.; Methner, U.; Berndt, A. In Vitro and in Vivo Generation of Heterophil Extracellular Traps after Salmonella Exposure. Vet. Immunol. Immunopathol. 2017, 188, 1–11. [Google Scholar] [CrossRef]
- Abbas, A.; Lichtman, A.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2017; ISBN 9780323479783. [Google Scholar]
- Kogut, M.H.; Swaggerty, C.; He, H.; Pevzner, I.; Kaiser, P. Toll-like Receptor Agonists Stimulate Differential Functional Activation and Cytokine and Chemokine Gene Expression in Heterophils Isolated from Chickens with Differential Innate Responses. Microbes Infect. 2006, 8, 1866–1874. [Google Scholar] [CrossRef]
- Singer, M.; Sansonetti, P.J. IL-8 Is a Key Chemokine Regulating Neutrophil Recruitment in a New Mouse Model of Shigella- Induced Colitis. J. Immunol. 2004, 173, 4197–4206. [Google Scholar] [CrossRef]
- Kogut, M.H. Dynamics of a Protective Avian Inflammatory Response: The Role of an IL-8-like Cytokine in the Recruitment of Heterophils to the Site of Organ Invasion by Salmonella Enteritidis. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 159–172. [Google Scholar] [CrossRef]
- Kogut, M.H.; Genovese, K.J.; He, H.; Kaiser, P. Flagellin and Lipopolysaccharide Up-Regulation of IL-6 and CXCLi2 Gene Expression in Chicken Heterophils Is Mediated by ERK1/2-Dependent Activation of AP-1 and NF-ΚB Signaling Pathways. Innate. Immun. 2008, 14, 213–222. [Google Scholar] [CrossRef]
- Cui, H.; Sun, Y.; Lin, H.; Zhao, Y.; Zhao, X. The Outer Membrane Vesicles of Salmonella enterica Serovar Typhimurium Activate Chicken Immune Cells through Lipopolysaccharides and Membrane Proteins. Pathogens 2022, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P.; Kaiser, P. Avian Cytokines in Health and Disease. Rev. Bras. Cienc. Avic. 2003, 5, 1–14. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a Cross-Talk between Human Neutrophils and Th17 Cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Verwoolde, M.B.; van den Biggelaar, R.H.G.A.; van Baal, J.; Jansen, C.A.; Lammers, A. Training of Primary Chicken Monocytes Results in Enhanced Pro-Inflammatory Responses. Vet. Sci. 2020, 7, 115. [Google Scholar] [CrossRef]
- Subhasinghe, I.; Ahmed, K.A.; Ayalew, L.E.; Gautam, H.; Popowich, S.; Matsuyama-Kato, A.; Chow-Lockerbie, B.; Tikoo, S.K.; Griebel, P.; Gomis, S. Induction of Trained Immunity in Broiler Chickens Following Delivery of Oligodeoxynucleotide Containing CpG Motifs to Protect against Escherichia Coli Septicemia. Sci. Rep. 2024, 14, 18882. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Nii, T.; Isobe, N. Innate Immune Training in Chickens for Improved Defense against Pathogens: A Review. J. Poult. Sci. 2024, 61, 2024008. [Google Scholar] [CrossRef]
- Härtle, S.; Sutton, K.; Vervelde, L.; Dalgaard, T.S. Delineation of Chicken Immune Markers in the Era of Omics and Multicolor Flow Cytometry. Front. Vet. Sci. 2024, 11, 1385400. [Google Scholar] [CrossRef]
- Tang, Y.; Foster, N.; Jones, M.A.; Barrow, P.A. Model of Persistent Salmonella Infection: Salmonella enterica Serovar Pullorum Modulates the Immune Response of the Chicken from a Th17-Type Response towards a Th2-Type Response. Infect. Immun. 2018, 86, e00307-18. [Google Scholar] [CrossRef]
- Setta, A.M.; Barrow, P.A.; Kaiser, P.; Jones, M.A. Early Immune Dynamics Following Infection with Salmonella enterica Serovars Enteritidis, Infantis, Pullorum and Gallinarum: Cytokine and Chemokine Gene Expression Profile and Cellular Changes of Chicken Cecal Tonsils. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Skin as a Peripheral Lymphoid Organ: Revisiting the Concept of Skin-Associated Lymphoid Tissues. J. Investig. Dermatol. 2011, 131, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Erf, G.F.; Trejo-Skalli, A.V.; Poulin, M.; Smyth, J.R. Lymphocyte Populations in Dermal Lymphoid Aggregates of Vitiliginous Smyth Line and Normally Pigmented Light Brown Leghorn Chickens. Vet. Immunol. Immunopathol. 1997, 58, 335–343. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Pastore, G.; Andersen, P.; Pozzi, G.; Medaglini, D. Modulation of Primary Immune Response by Different Vaccine Adjuvants. Front. Immunol. 2016, 7, 427. [Google Scholar] [CrossRef]
- Knudsen, N.P.H.; Olsen, A.; Buonsanti, C.; Follmann, F.; Zhang, Y.; Coler, R.N.; Fox, C.B.; Meinke, A.; D’Oro, U.; Casini, D.; et al. Different Human Vaccine Adjuvants Promote Distinct Antigen-Independent Immunological Signatures Tailored to Different Pathogens. Sci. Rep. 2016, 6, 19570. [Google Scholar] [CrossRef]
- Heegaard, P.M.H.; Dedieu, L.; Johnson, N.; Le Potier, M.F.; Mockey, M.; Mutinelli, F.; Vahlenkamp, T.; Vascellari, M.; Sørensen, N.S. Adjuvants and Delivery Systems in Veterinary Vaccinology: Current State and Future Developments. Arch. Virol. 2011, 156, 183–202. [Google Scholar] [CrossRef]
- Jawale, C.V.; Lee, J.H. Evaluation of Immunogenicity and Protective Efficacy of Adjuvanted Salmonella Typhimurium Ghost Vaccine against Salmonellosis in Chickens. Vet. Q. 2016, 36, 130–136. [Google Scholar] [CrossRef]
- Kogut, M.; He, H.; Kaiser, P. Lipopolysaccharide Binding Protein/CD14/TLR4-Dependent Recognition of Salmonella LPS Induces the Functional Activation of Chicken Heterophils and Up-Regulation of Pro-Inflammatory Cytokine and Chemokine Gene Expression in These Cells. Anim. Biotechnol. 2005, 16, 165–181. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Swaggerty, C.L.; MacKinnon, K.M.; Kogut, M.H. Co-Stimulation with TLR3 and TLR21 Ligands Synergistically up-Regulates Th1-Cytokine IFN-γ and Regulatory Cytokine IL-10 Expression in Chicken Monocytes. Dev. Comp. Immunol. 2012, 36, 756–760. [Google Scholar] [CrossRef]
- Smith, C.K.; Kaiser, P.; Rothwell, L.; Humphrey, T.; Barrow, P.A.; Jones, M.A. Campylobacter jejuni-Induced Cytokine Responses in Avian Cells. Infect. Immun. 2005, 73, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.; Rothwell, L.; Degen, W.D.J.; Schijns, V.E.J.C.; Young, J.; Kaufman, J.; Kaiser, P. Characterization of the First Nonmammalian T2 Cytokine Gene Cluster: The Cluster Contains Functional Single-Copy Genes for IL-3, IL-4, IL-13, and GM-CSF, a Gene for IL-5 That Appears to Be a Pseudogene, and a Gene Encoding Another Cytokinelike Transcript, KK34. J. Interferon Cytokine Res. 2004, 24, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Falcon, D.M. Assessment of Melanocyte-Specific Primary and Memory Autoimmune Responses in Vitiligo-Prone Smyth and Vitiligo-Susceptible, Non-Expressing Brown Line Chickens. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2018. [Google Scholar]
- Rohde, F.; Schusser, B.; Hron, T.; Farkašová, H.; Plachý, J.; Härtle, S.; Hejnar, J.; Elleder, D.; Kaspers, B. Characterization of Chicken Tumor Necrosis Factor-α, a Long Missed Cytokine in Birds. Front. Immunol. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]

| Primary Vaccination | Secondary Vaccination | |||||
|---|---|---|---|---|---|---|
| Group 1: 1 | I.d. GF-pulp injection 3 | |||||
| Trt 5 | Age | Injection Vol. per Bird | ||||
| SV1 | 14 wk | 120 μL | ||||
| SV2 | ||||||
| LPS | ||||||
| V | ||||||
| Group 2: 2 | S.c. vaccination 4 | I.d. GF-pulp injection | ||||
| Trt | Age | Injection Vol. per Bird | Trt | Age | Injection Vol. per Bird | |
| SV1 | 14 wk | 500 μL | SV1 | 19 wk | 210 μL | |
| SV2 | SV2 | |||||
| LPS | LPS | |||||
| V | V | |||||
| Stain Combinations | Antibody 1 | Label 2 | Detected Leukocyte |
|---|---|---|---|
| A | mac-MHCII | FITC | Antigen-presenting cells |
| mac-KUL01 | PE | Monocytes/Macrophages | |
| mac-CD45 | SPRD | All leukocytes | |
| B | mac-CD45 | FITC | All leukocytes |
| mac-Bu-1 | PE | B lymphocytes | |
| mac-CD3 | SPRD | All T lymphocytes | |
| C | mac-TCRgd | FITC | γδ T lymphocytes |
| mac-CD4 | PE | CD4+ T lymphocytes | |
| mac-CD8 | SPRD | CD8α+ T lymphocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, C.N.; Santamaria, J.M.; Erf, G.F. Inflammatory and Humoral Immune Responses to Commercial Autogenous Salmonella Bacterin Vaccines in Light-Brown Leghorn Pullets: Primary and Secondary Vaccine Responses. Vaccines 2025, 13, 311. https://doi.org/10.3390/vaccines13030311
Beck CN, Santamaria JM, Erf GF. Inflammatory and Humoral Immune Responses to Commercial Autogenous Salmonella Bacterin Vaccines in Light-Brown Leghorn Pullets: Primary and Secondary Vaccine Responses. Vaccines. 2025; 13(3):311. https://doi.org/10.3390/vaccines13030311
Chicago/Turabian StyleBeck, Chrysta N., Jossie M. Santamaria, and Gisela F. Erf. 2025. "Inflammatory and Humoral Immune Responses to Commercial Autogenous Salmonella Bacterin Vaccines in Light-Brown Leghorn Pullets: Primary and Secondary Vaccine Responses" Vaccines 13, no. 3: 311. https://doi.org/10.3390/vaccines13030311
APA StyleBeck, C. N., Santamaria, J. M., & Erf, G. F. (2025). Inflammatory and Humoral Immune Responses to Commercial Autogenous Salmonella Bacterin Vaccines in Light-Brown Leghorn Pullets: Primary and Secondary Vaccine Responses. Vaccines, 13(3), 311. https://doi.org/10.3390/vaccines13030311







