Abstract
Background/Objectives: Aeromonas hydrophila is a significant opportunistic pathogen with a broad host range. It produces a catecholate siderophore, amonabactin, during iron starvation, but the in vivo infection mechanism that involves amonabactin is unclear. This study aims to elucidate the role of amonabactin synthetase G (AmoG) in the pathogenicity of A. hydrophila and its impact on gut barrier function. Methods: ΔAmoG was generated by deleting the AMP-binding domain of AmoG in A. hydrophila CCL1. In vivo infection experiments were conducted to assess the mutant’s iron-chelating ability and pathogenicity. Complementation of ΔAmoG with AmoG (ΔAmoG-C) was performed to confirm the observed phenotypes. Transcriptomic and qRT-PCR analyses were used to investigate gene expression changes in infected fish. Goblet cell counts, tight junction expression, and D-lactic acid and LPS levels were measured to evaluate gut barrier function. Results: ΔAmoG exhibited impaired iron-chelating ability and reduced pathogenicity compared to wild-type CCL1. Complementation with AmoG restored virulence in ΔAmoG-C. Transcriptomic and qRT-PCR analyses revealed an elevated expression of Wnt/β-catenin pathway components and antimicrobial genes in ΔAmoG-infected fish. Further investigation indicated increased goblet cells and an enhanced expression of tight junctions, as well as lower D-lactic acid and LPS levels, in ΔAmoG-infected fish. However, gut permeability, bacterial load, and lethality did not significantly differ between CCL1, ΔAmoG, and ΔAmoG-C infections when the Wnt/β-catenin pathway was activated. Conclusions: AmoG plays a crucial role in A. hydrophila pathogenicity by modulating host Wnt/β-catenin signaling and gut mucosal barrier function. This study provides insights into the pathogenesis of A. hydrophila and potential therapeutic targets.
1. Introduction
Aeromonas hydrophila is a ubiquitous pathogen within aquatic ecosystems and has led to substantial economic burdens for aquaculture [1,2,3]. Several prominent virulence factors have been identified, such as Type Three Secretion Systems (T3SSs) and T6SSs; toxins like Alt (heat-labile cytotonic enterotoxin), Act (cytotoxic enterotoxin), Ahp (serine protease); and various hemolysins [4]. In addition, siderophores are crucial for A. hydrophila to override the iron limitations imposed by the host or the environment [5]. Amonabactins, siderophores produced by the Aeromonas genus, can be used for iron acquisition to support a bacterium’s growth [6,7]. A previous study showed that A. hydrophila without the amonabactin biosynthesis gene AmoG was unable to synthesize any amonabactin or grow under iron stress conditions [8]. Nonetheless, the direct impact of A. hydrophila-produced amonabactin on host infection dynamics and its potential roles in modulating the fish epithelial barrier remain unexplored.
The integrity of the gut epithelial barrier is critical for preserving gut functionality and systemic immunity [9]. Pathogens employ different virulence mechanisms to disrupt epithelial junctions, thereby undermining gut barrier integrity [10,11,12]. A. hydrophila, notorious for its ability to severely compromise gut integrity and trigger septicemia, has been observed to cause gut lesions and induce gut inflammation in fish [13,14,15]. In a previous study, we identified a secretory serine protease (Ssp1) from A. hydrophila that plays a critical role in disrupting the gut’s tight junction barrier [16]. In addition, our earlier work pinpointed WbuB, a GT4 galactosaminogalactan synthase from A. hydrophila, as vital for inflammasome activation and gut tight junction disruption [17]. Nevertheless, the specifics of how A. hydrophila exploits nutrient acquisition, notably through amonabactin, to impact the gut mucosa barriers remain unclear.
In this study, we identified an amonabactin cluster with a potential iron uptake ability in A. hydrophila CCL1 (GenBank Accession No. CP092356). To elucidate this pathogenic mechanism, we selected the amonabactin biosynthesis gene AmoG for further study to determine whether it is a virulence factor in vivo. Through engineering ΔAmoG and its complement strain, comparative analyses encompassing physiological attributes and virulence capacities were conducted for these strains. Moreover, in vivo studies suggested that A. hydrophila may use amonabactin as one of its virulence factors to inhibit the Wnt/β-catenin pathway, consequently impairing the structure and function of the gut mucosal barrier. Our findings suggested that, unlike Ssp1 and WbuB, which predominantly influence the tight junction and inflammasome pathway, AmoG seems to manipulate the host Wnt/β-catenin signaling pathway through nutrient acquisition. These results provide key insights into gut mucosal dysfunction during A. hydrophila infection.
2. Materials and Methods
2.1. Fish
Crucian carps (~14.2 g) were obtained from Hunan Yuelu Mountain Science and Technology Co., Ltd.: Changsha, China, which carries out aquatic breeding in Changsha. During the experiment, fish were housed pre-experimentally under controlled conditions (25.25 ± 0.58 °C, pH 7.05 ± 0.12, >7.0 mg/L O2). Pre-experimental screening confirmed that the fish were disease-free [18]. Tissues were acquired following ethical guidelines (GB/T 35892-2018 [19]). All animal experiments were approved by the Hunan Normal University’s Animal Care Committee (No. 2023109).
2.2. Generation of A. hydrophila ΔAmoG and ΔAmoG-C
To generate the A. hydrophila ΔAmoG mutant, a 300 bp in-frame deletion of AmoG (aa 661–760) was engineered via an overlap extension PCR. The primers AmoG-F1/AmoG-R1 and AmoG-F2/AmoG-R2 were used sequentially for overlap PCRs, followed by a fusion PCR using AmoG-F1/AmoG-R2 (Table S1). The resultant amplicon was ligated into pDM4 at the BglII site, yielding pDMAmoG. Escherichia coli S17-1 λpir harboring pDMAmoG was conjugated with A. hydrophila CCL1. Following coculture and selection on polymyxin B/chloramphenicol plates, sucrose-resistant yet chloramphenicol-sensitive colonies were isolated. Confirmation of in-frame deletion in these isolates was achieved through PCR and sequencing, leading to the identification of the ΔAmoG mutant.
For the creation of the AmoG complement strain, designated ΔAmoG-C, the gene encoding AmoG, along with its native promoter, was PCR-amplified using the primers AmoG-F3 and AmoG-R3 (Table S1). This amplicon was then cloned into the pACYC184 plasmid at its EcoRV restriction site, forming pACYCAmoG-C. Subsequently, this construct was introduced into E. coli S17-1 λpir, which was subsequently mated with the ΔAmoG mutant. Transconjugants were identified on selective LB agar plates containing polymyxin B and chloramphenicol. One selected colony, designated ΔAmoG-C, was further validated by PCR and sequence confirmation.
2.3. Characterization of ΔAmoG and ΔAmoG-C
The strains CCL1, ΔAmoG, and ΔAmoG-C were cultured in LB broth at 28 °C for 24 h. Scanning electron microscopy was employed to assess cell morphology. Growth dynamics were monitored by cultivating the strains in LB with or without 100 μM 2, 2′-dipyridyl (Dp), starting at an A600 of 0.01; samples were taken at various intervals to measure A600. Motility assays and biofilm formation tests were conducted according to Reference [16]. The Chrome Azurol S (CAS) plate assay and Arnow’s test were used to analyze siderophore production according to Reference [20]. Each assay was replicated three times.
2.4. Cellular Adherence and Cytotoxicity Analyses
Caco-2 cells, obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), were maintained in DMEM enriched with 20% FBS and antibiotics (penicillin/streptomycin) at 37 °C in 5% CO2. Approximately 1 × 105 Caco-2 cells were seeded into 96-well plates and infected with CCL1, ΔAmoG, ΔAmoG-C, or PBS (control) at an MOI of 10:1. Cells were incubated for 1 h before washing away unbound bacteria using PBS. After infection, cells were lysed with Triton X-100, and the lysates were spread on LB agar to determine the bacterial counts. The adhesion index was calculated as the ratio of associated bacteria to Caco-2 cells. Each assay was replicated three times. Cytotoxicity was measured by the lactate dehydrogenase (LDH) method. Briefly, after 1 h of infection as above, LDH leakage was assessed using a Cytotoxicity Detection Kit (Roche Diagnostics, Mannheim, Germany). Cells treated with Triton X-100 represented the maximum LDH release (100%). Each assay was replicated three times.
2.5. In Vivo Infection
For in vivo infection, fish were injected intramuscularly (i.m.) with 100 µL suspensions containing 1 × 106 CFU/mL of CCL1, ΔAmoG, ΔAmoG-C, or an equivalent volume of PBS (control). At 24 h post infection (hpi), distal gut, kidney, spleen, and blood from CCL1-, ΔAmoG-, ΔAmoG-C-, or PBS (control)-treated fish were carefully sampled under sterile conditions for subsequent experimental procedures. (i) Bacterial dissemination in the kidney and spleen and AB-PAS staining were carried out as reported previously [21]. (ii) A qRT-PCR was performed to analyze the genes’ expression in the distal gut. The genes and PCR primers used are listed in Table S1. Expression levels were measured through the 2−ΔΔCT method, referencing beta-actin, with PCR efficiency (E) and correlation (R2) checked as previously detailed [22]. (iii) Plasma was derived from the blood of each group. D-lactic acid concentrations were measured using a D-lactic acid ELISA assay kit procured from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Additionally, Limulus Amebocyte Lysate (LAL) QCL-1000 kits from Lonza were employed to quantify lipopolysaccharide (LPS) levels within the plasma. (iv) An immunohistochemistry (IHC) assay was used to analyze the expression of Occludin in the distal gut as reported previously [16]. Mouse anti-rOccludin antibody (prepared in [16]) and Cy3-conjugated goat anti-mouse secondary antibody (Servicebio, Wuhan, China) were used as the primary and secondary antibodies, respectively. (v) Iron concentrations in the serum and distal gut were determined using an iron-unsaturated iron binding capacity kit (Nanjing Jiancheng) according to the manufacturer’s instruction. The DAB-enhanced Prussian blue iron staining kit (Servicebio, Wuhan, China) was utilized to detect iron deposits within liver samples according to the manufacturer’s instructions. (vi) To conduct an apoptosis assessment targeting the distal guts, an apoptosis assay was carried out using the TUNEL Apoptosis Detection Kit (Servicebio). (vii) For assessing survival rates, twenty crucian carps per group were challenged with CCL1, ΔAmoG, ΔAmoG-C, or received a PBS control, following the same inoculation procedure as described above. Over a period of two weeks, fish mortality was meticulously documented post infection as reported previously [21]. Each assay was replicated three times, with three fish used each time.
2.6. Gut Transcriptome and Microbiome Analyses
Transcriptome analysis was performed as outlined in previous work [16]. At 24 hpi, as above, distal guts from fish infected with CCL1, ΔAmoG, ΔAmoG-C, or exposed to PBS (serving as control) were collected under sterile conditions (with four fish in each group). Gut specimens from each group were frozen at −80 °C prior to RNA extraction for subsequent RNA sequencing (RNA-Seq). RNA extraction, library construction, and data analysis were performed by Novogene (Beijing, China).
For a comprehensive analysis of the microbiome shifts in the guts of fish exposed to CCL1, ΔAmoG, ΔAmoG-C, or treated with PBS (control), as above, distal guts from each group were taken and forwarded to Novogene for expert processing and sequencing (with four fish in each group). Following CTAB/SDS DNA extraction, libraries were constructed and sequenced using the 515F and 806R primer pair on an Illumina NovaSeq6000. Rigorous data analysis was performed through QIIME2 as reported previously [16].
2.7. Effect of the Activation of the Wnt/β-Catenin Pathway on Infection
Fish (twenty fish in each group) were injected i.m. with 100 µL of the Wnt/β-catenin agonist BIO (0.5 mg/mL, Sigma, St. Louis, MO, USA). Two hours following the initial procedure, each fish received an intramuscular injection comprising 100 µL of suspension, with concentrations of 1 × 106 CFU/mL for either CCL1, ΔAmoG, ΔAmoG-C, or PBS. At 24 hpi, the kidney, spleen, and blood from CCL1-, ΔAmoG-, ΔAmoG-C-, or PBS (control)-injected fish were carefully sampled as above. Plasma LPS levels, bacterial dissemination, and survival rate assays were determined as reported above. Each assay was replicated three times.
2.8. Statistical Analysis
The sample size was calculated using PASS software (PASS 11. NCSS, LLC. Kaysville, UT, USA). Data were statistically analyzed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) and the Unpaired Mann–Whitney test or the Log-Rank test.
3. Results
3.1. Phenotypic Characterization of ΔAmoG
A. hydrophila produces a catecholate siderophore named amonabactin in response to iron starvation. A thorough examination of the genomic sequence of the fish pathogen A. hydrophila CCL1 (GenBank Accession No. CP092356) unveiled a gene cluster likely implicated in amonabactin production (Figure S1). This gene cluster possesses AmoC, AmoE, AmoB, AmoF, AmoA, AmoG, and AmoH (Figure S1). We focused on the potential amonabactin synthetase G (AmoG) from A. hydrophila CCL1 for further analysis. The deduced amino acid sequence of AmoG is composed of 2089 residues (GenBank Accession No. XIH98845.1). Sequence alignment shows that AmoG shares 94.5–26.4% overall sequence identities with homologs from A. veronii, A. salmonicida, Vibrio parahaemolyticus, V. alginolyticus, V. harveyi, E. coli, Campylobacter jejuni, and Yersinia enterocolitica (Figure S2). SMART could predict an AMP-binding domain (from 537 to 923 aa) and three Pfam condensations in AmoG (Figure 1A).
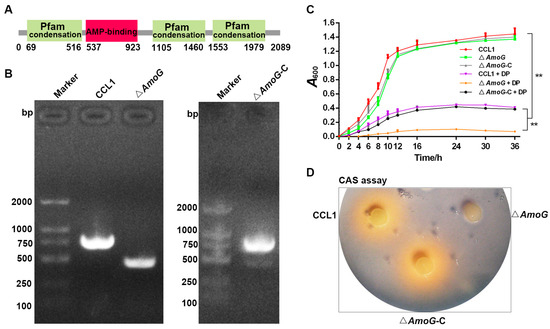
Figure 1.
Generation of Aeromonas hydrophila ΔAmoG mutant and its complement strain ΔAmoG-C. (A) Depiction of AmoG’s domain. (B) PCR confirmation of the construction of ΔAmoG mutant and ΔAmoG-C by agarose gel electrophoresis analysis. (C) Growth levels of A. hydrophila CCL1, ΔAmoG, and ΔAmoG-C with or without 100 µM 2, 2′-dipyridyl (Dp). Values are Means ± SEM (N = 3). Mann–Whitney test. ** p < 0.01. (D) Representative photos of CCL1, ΔAmoG, and ΔAmoG-C and their siderophore production activity. CCL1, ΔAmoG, and ΔAmoG-C were spotted onto a filter disk in a CAS plate. One representative image from triplicate trials.
To probe AmoG’s role in pathogenicity, we engineered a variant of A. hydrophila CCL1, termed ΔAmoG, featuring an in-frame deletion of the AmoG segment (residues 661–760, Figure 1B). We also generated a complemented strain, ΔAmoG-C, reintroducing a functional AmoG to ΔAmoG (Figure 1B). Both ΔAmoG and ΔAmoG-C exhibited similar morphology and growth to their parental CCL1 in LB media (Table 1, Figure 1C), without noticeable changes in biofilm formation or swimming motility (Table 1). While CCL1 had an LD50 of 9.26 × 104 CFU/fish, ΔAmoG displayed a reduced virulence, with an LD50 of 8.58 × 105 CFU/fish, indicating a roughly ten-fold decrease (Table 1). In addition, under the iron-depleted condition imposed by adding 100 µM Dp, both CCL1 and ΔAmoG-C demonstrated a suppressed growth level; however, ΔAmoG was virtually incapable of growing in such an environment (Figure 1C). Furthermore, ΔAmoG failed to produce the expected yellow hue in CAS medium, indicating its role in iron–siderophore interactions (Figure 1D). This observation underscores AmoG’s critical role in facilitating metabolic adaptations for iron uptake, revealing its importance in enabling survival under conditions of iron scarcity.

Table 1.
Characteristics of the Aeromonas hydrophila AmoG mutant.
3.2. AmoG-Mediated Iron Chelation and Bacterial Infection
Following the above findings, which suggested a deficiency in the iron-chelating process of the ΔAmoG strain, we proceeded to investigate whether there was an alteration in its capability to produce siderophore. The Arnow assay confirmed that ΔAmoG exhibited a significantly diminished ability to synthesize the iron-chelating siderophore amonabactin (Figure 2A). When incubated with Caco-2 cells, an intestinal epithelial cell line, notably reduced adherence and cytotoxicity were found in the ∆AmoG-treated group compared to the CCL1- and ΔAmoG-C-treated groups (Figure 2B,C). Moreover, iron levels in the serum, gut, and liver of fish infected with CCL1 and ∆AmoG-C were substantially lower than those seen after infection with ∆AmoG (Figure 2D–F). This finding suggested that the deletion of AmoG in A. hydrophila led to an accumulation of iron in these tissues, potentially due to the impaired iron-chelating abilities of the bacteria. Following up on these observations of the effects of AmoG on the iron-chelating process, a TUNEL analysis was used to investigate cell apoptosis under iron deficiency conditions. The result showed that distal gut apoptosis was significantly lower in ∆AmoG-infected fish compared to those with CCL1 or ∆AmoG-C infections (Figure 2G,H). Upon analyzing the bacterial loads within the distal guts, kidneys, and spleens of infected fish, we observed considerably lower numbers of A. hydrophila in those infected with the ∆AmoG strain (Figure 2I–K). Correspondingly, there was a notable decline in mortality among ∆AmoG-infected fish, indicating that the virulence of ∆AmoG had been significantly dampened (Figure 2L). These results suggest that A. hydrophila may employ AmoG in the synthesis of amonabactin to facilitate iron chelation, consequently impairing the structure and function of the gut mucosal barrier.
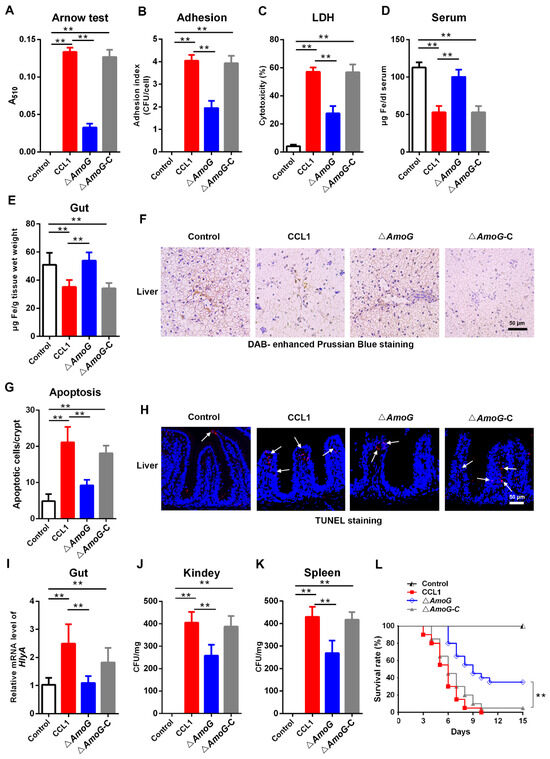
Figure 2.
Effect of AmoG on bacterial infection. (A) An Arnow assay was used to analyze the production of amonabactin in Aeromonas hydrophila CCL1, ΔAmoG, and ΔAmoG-C. Caco-2 cells were challenged with CCL1, ΔAmoG, ΔAmoG-C, and PBS (control) for 1 h, and their (B) adhesion index and (C) cytotoxicity were determined. Crucian carps were challenged with CCL1, ΔAmoG, ΔAmoG-C, or PBS (control) for 24 h. Iron contents in the (D) serum, (E) distal gut, and (F) liver were analyzed. (G,H) The apoptotic cells in the distal guts of infected fish were examined using a TUNEL analysis. White arrow indicates apoptotic cells. The bacterial burdens in the distal guts (I), kidneys (J), and spleens (K) were measured. Values are Means ± SEM (N = 3). Mann–Whitney test. ** p < 0.01. (L) Survival curves. Fish infected with CCL1, ΔAmoG, ΔAmoG-C, and PBS (control) were monitored over two weeks. Log-rank test (p < 0.01).
3.3. Transcriptomic Analysis
To further investigate the pathogenic mechanism orchestrated by AmoG, we conducted transcriptomic analyses on the distal guts of A. hydrophila-infected crucian carps. The results revealed that compared to ΔAmoG infection, CCL1 and ΔAmoG-C infection notably repressed the expression of several key genes, including wnt10b, axin2, ccnd1, and ctnnb1, whereas genes like IL-8, ptx3a, and hsp90b1 experienced pronounced upregulation (Figure 3A,B). A further KEGG pathway analysis highlighted that the Wnt/β-catenin signaling pathway (a key regulator of cell proliferation, differentiation, and homeostasis) was significantly impacted during ΔAmoG infection, underscoring its pivotal role in mediating disease progression and the host’s response (Figure 3C–F). This finding also suggested a possible connection between the AmoG-mediated iron-chelating process and the Wnt/β-catenin signaling pathway.
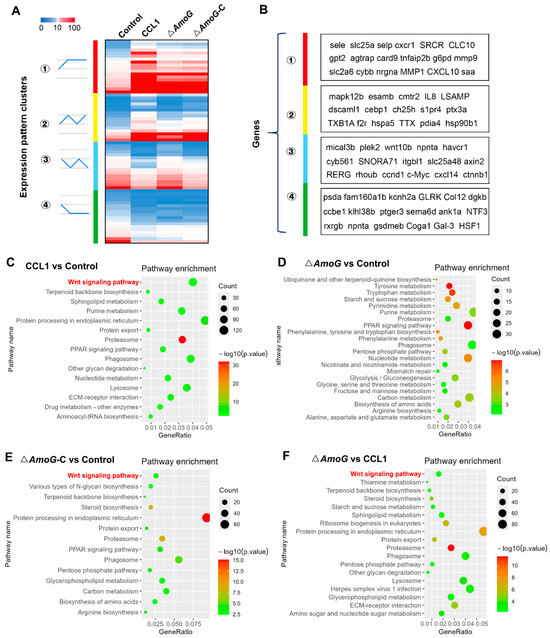
Figure 3.
Transcriptomic impact of AmoG on the distal gut during Aeromonas hydrophila infection. Crucian carps were challenged with A. hydrophila CCL1, ΔAmoG, ΔAmoG-C, or PBS (control) for 24 h, and a transcriptomic analysis of their distal gut was performed. (A) Heatmap representations of differentially expressed genes upon infection with CCL1, ΔAmoG, ΔAmoG-C, or PBS. Clustering reveals the genes sharing the same expression trends. Mann–Whitney test, p < 0.01. (B) Highlighted genes exhibiting notable expression changes within identified clusters. (C–F) KEGG pathway enrichment analysis identifying the Wnt signaling pathway as being significantly affected during A. hydrophila infection.
3.4. Impact of AmoG on the Wnt/β-Catenin Pathway
The Wnt/β-catenin signaling pathway is fundamental for the regeneration of the gut mucosal barrier. Given that transcriptomic data suggested a disruption of the Wnt/β-catenin pathway during A. hydrophila infection, we proceeded to examine the effect of the Wnt/β-catenin pathway on the gut mucosal barrier. Initially, consistent with the transcriptomic data, key components of the Wnt/β-catenin pathway, such as wnt3a, wnt10b, ctnnb1, and Lgr6, displayed a significantly upregulated expression in the fish treated with ΔAmoG relative to those treated with CCL1 and ΔAmoG-C (Figure 4A–D). Subsequently, AB-PAS staining indicated that the counts of distal gut goblet cells in ΔAmoG-infected fish notably surpassed those seen in CCL1- and ΔAmoG-C-infected fish (Figure 4E,F). IL-22, known to foster gut epithelial cell proliferation and antimicrobial peptide synthesis [23], also exhibited heightened expression in the distal guts following ΔAmoG administration (Figure 4G). Moreover, a qRT-PCR revealed an upsurged expression of MUC2, LEAP-2, and Hepcidin-1 in ΔAmoG-treated fish compared to CCL1- or ΔAmoG-C-treated fish (Figure 4H–J). Conversely, fish treated with CCL1 or ΔAmoG-C demonstrated a suppressed expression of these genes. These results indicated that without AmoG as a key virulence factor to synthesize amonabactin, the ∆AmoG mutant’s capacity to inhibit the Wnt/β-catenin pathway was markedly reduced.
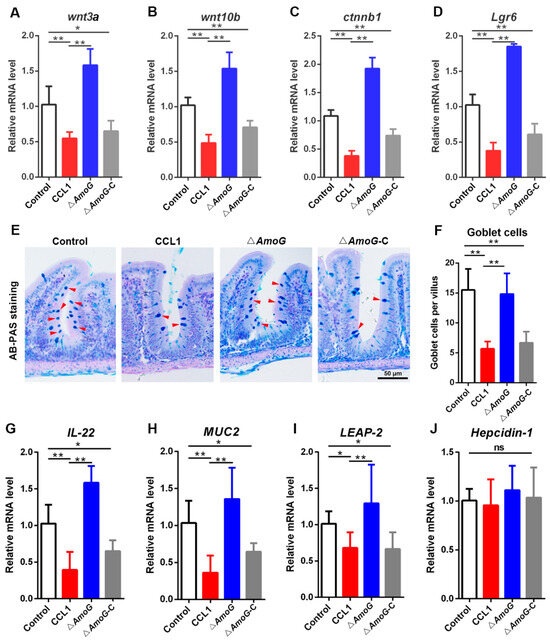
Figure 4.
Effect of AmoG on goblet cells and antimicrobial molecules in the distal gut. Crucian carps were challenged with Aeromonas hydrophila CCL1, ΔAmoG, ΔAmoG-C, or PBS (control) for 24 h. (A–D) The expression of wnt3a, wnt10b, ctnnb1, and Lgr6 in the distal guts was analyzed by qRT-PCR. (E,F) AB-PAS staining was used to show the goblet cells in the distal guts. Red arrow indicates goblet cells. Bar: 50 μm. (G–J) The expression of antimicrobial genes in the distal guts was analyzed by qRT-PCR. Values are Means ± SEM (N = 3). Mann–Whitney test. * p < 0.05; ** p < 0.01; ns: no significance.
3.5. Impact of AmoG on Gut Mucosal Barrier
Given the marked reduction in ΔAmoG’s capacity to inhibit the Wnt/β-catenin pathway, we proceeded to investigate its influence on the gut mucosal barrier. THe qRT-PCR showed that a heightened expression of Occludin was observed in the distal gut of ΔAmoG-exposed fish, compared to that in both the CCL1 and ΔAmoG-C groups (Figure 5A,B). The IHC analysis also demonstrated that, unlike in CCL1- or ΔAmoG-C-treated fish, Occludin, a vital tight junction constituent, was markedly upregulated in the distal guts of ΔAmoG-treated fish (Figure 5E). Additionally, plasma D-lactic acid and LPS, markers of gut lining integrity [24], were notably lower in ΔAmoG-infected fish compared to those with CCL1 and ΔAmoG-C infections (Figure 5H,I). Since disrupted gut barrier function can lead to alterations in the gut microbiota, we also investigated the gut microbiome differences among fish infected with CCL1, ΔAmoG, or ΔAmoG-C (Figure S3). The results showed that CCL1 infection led to discernible alterations in the gut microbiota compared to ΔAmoG infection, indicating that the ∆AmoG mutant exhibited a diminished ability to compromise the gut mucosal barrier.
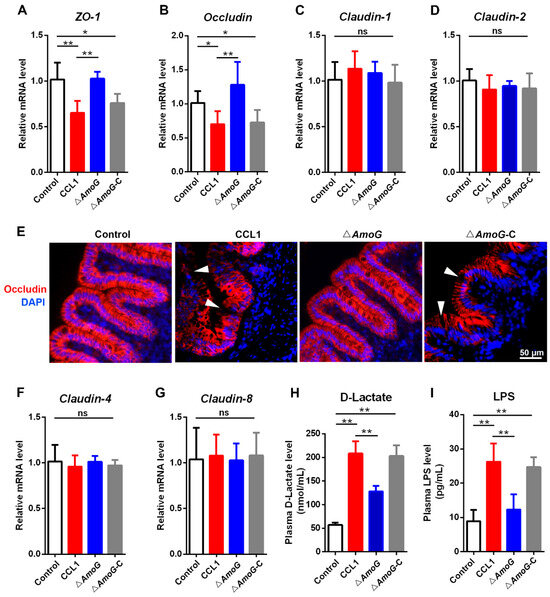
Figure 5.
Effect of AmoG on the gut mucosa barrier. Crucian carps were challenged with Aeromonas hydrophila CCL1, ΔAmoG, ΔAmoG-C, or PBS (control) for 24 h. The expression of (A) ZO-1, (B) Occludin, (C) Claudin-1, (D) Claudin-2, (F) Claudin-4, and (G) Claudin-8 in the distal guts was determined by qRT-PCR. (E) Immunohistochemistry demonstrates Occludin’s distribution in the distal guts. White arrow indicates the location of Occludin. Gut permeability was measured by (H) plasma D-lactic acid and (I) LPS levels. Values are Means ± SEM (N = 3). Mann–Whitney test. * p < 0.05, ** p < 0.01. ns: no significance.
3.6. Impact of the Wnt/β-Catenin Pathway on Bacterial Infection
To further explore the impact of Wnt/β-catenin signaling pathway activation on A. hydrophila infection, we pre-treated fish with the Wnt/β-catenin pathway activator BIO before infecting them with either CCL1, ΔAmoG, or ΔAmoG-C. Our results revealed that upon activation of the Wnt/β-catenin signaling pathway, the gut permeability of these fish, as well as the bacterial burdens in their kidneys and spleens, showed no significant differences among the infections caused by CCL1, ΔAmoG, or ΔAmoG-C (Figure 6A–C). Similarly, the lethal effects attributed to each bacterial strain remained statistically indistinguishable following the activation of the Wnt/β-catenin pathway (Figure 6D). In summary, these findings suggest that once the Wnt/β-catenin pathway is activated, the presence or absence of amonabactin does not appear to significantly influence the bacterium’s infectivity. Additionally, these results suggest that A. hydrophila may employ amonabactin as a strategic tool to dampen the Wnt/β-catenin signaling pathway, which in turn facilitates the bacterium’s ability to be invasive, making this a probable mechanism underlying its pathogenicity (Figure S4).
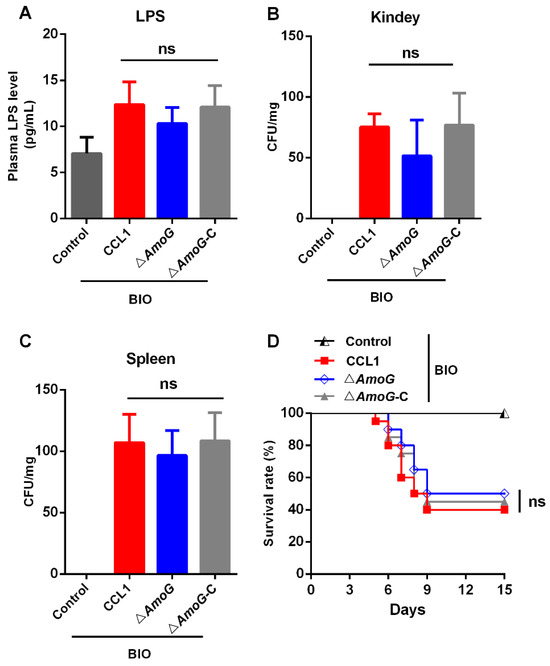
Figure 6.
Effect of the Wnt signaling pathway on antibacterial immunity. Crucian carps were pre-treated with the Wnt/β-catenin pathway activator BIO (0.5 mg/mL) and were subsequently challenged with Aeromonas hydrophila CCL1, ΔAmoG, ΔAmoG-C, or PBS (control) for 24 h. (A) Gut permeability was analyzed using plasma LPS levels. The bacterial burdens in the (B) kidneys and (C) spleens were determined. Values are Means ± SEM (N = 3). Mann–Whitney test, ns: no significance. (D) Survival curves. Crucian carps were pre-treated with the Wnt/β-catenin pathway activator BIO as above and then were challenged with CCL1, ΔAmoG, ΔAmoG-C, or PBS (control). Fish were monitored over two weeks. Log-rank test (p < 0.01), ns: no significance.
4. Discussion
Iron is an essential trace element for most organisms [25,26,27]. In response to iron starvation, A. hydrophila has been shown to possess multiple systems for the sequestration of host iron, including heme-bound iron transport [28], the utilization of enterobactin siderophores produced by Enterobacteriaceae [6,7], and the secretion of amonabactin [29]. Among these virulence factors, amonabactin represents a family of catechol peptidic siderophores that is composed of seven genes named AmoCEBFAGH [8]. A previous study indicated that a mutant of AmoG was unable to synthesize any amonabactin or to grow in iron-stress conditions [8]. Consistent with this study, our results showed that under a treatment with 100 µM Dp, ΔAmoG was almost unable to grow, indicating that the AmoG of A. hydrophila CCL1 may be involved in bacterial iron acquisition and survival under iron stress.
Until now, AmoG’s in vivo function has remained unexplored experimentally. To explore this, firstly, we embarked on a comprehensive transcriptomic analysis targeting the guts of crucian carps infected with A. hydrophila. Our findings unveiled that in comparison to ΔAmoG infections, CCL1 and ΔAmoG-C infections markedly suppressed the expression of pivotal genes in the Wnt/β-catenin signaling pathway including wnt10b, axin2, ccnd1, and ctnnb1. The Wnt/β-catenin pathway is integral to the structural integrity of the gut barrier and is essential for the proper functioning of gut epithelial cells, such as goblet cells [30]. In line with the above observations, we observed a significant decline in goblet cells and MUC2, IL-22, Hepcidin-1, and LEAP-2 expression in fish subjected to CCL1 or ΔAmoG-C infection. These results indicated that AmoG may be involved in the pathogenicity of A. hydrophila infection through the inhibition of the Wnt/β-catenin pathway. Secondly, the Wnt/β-catenin pathway promotes the assembly and stability of gut tight junctions, ensuring minimal paracellular leakage [31,32]. Previous investigations have documented the capacity of specific pathogens, notably Helicobacter pylori and Campylobacter jejuni, to inhibit the Wnt/β-catenin pathway and compromise the integrity of tight junctions during the course of their infection [33]. For this purpose, we analyzed the impact of AmoG on gut permeability in an in vivo setting. Our findings revealed a significant upregulation of occludin in fish infected with ΔAmoG. Consistently, the decreased concentrations of plasma D-lactic acid and LPS indicated a reduced gut mucosal permeability following ΔAmoG infection. Moreover, our data demonstrated a slight but notable enhancement in alpha diversity within the gut flora of fish infected with ΔAmoG relative to those harboring CCL1, hinting at potential disturbances in bacterial colonization elicited by AmoG [34,35]. Collectively, the above results suggested that AmoG might be involved in the infection process, particularly under conditions of an inhibited Wnt/β-catenin pathway and disrupted tight junction barriers. However, additional studies are warranted to validate whether AmoG’s effects are direct or mediated indirectly through other pathways.
Iron is an indispensable micronutrient for bacterial metabolism, proliferation, and infection. Bacterial pathogens have developed intricate systems for iron acquisition, employing iron carriers known as siderophores to chelate and sequester iron from the host environment, influencing their infection capacities [36,37]. Similarly, our results demonstrated that ΔAmoG infection leads to elevated iron levels in the serum, gut, and liver of infected fish, possibly due to its impaired iron-chelating efficiency, which may subsequently affect the infection dynamics of A. hydrophila. Indeed, upon infecting fish with ΔAmoG, we observed notably smaller bacterial counts in their tissues compared to those in fish infected with CCL1. The reason may be that the iron-chelating process used by A. hydrophila enables the bacterium to disrupt the gut barrier and simultaneously enhance its invasive capabilities. Research suggests that the inhibition of the Wnt/β-catenin signaling pathway by pathogens through iron chelation provides the bacteria with an advantage in disrupting the gut barrier, thereby enhancing their invasiveness [38]. Studies have reported that a shortage of iron, brought about by siderophores, can lead to the suppression of the Wnt/β-catenin signaling pathway, favoring bacterial infection [39,40]. Consistent with these findings, our findings revealed that once the Wnt/β-catenin pathway is activated, the presence or absence of amonabactin does not seem to significantly alter the bacterium’s infectivity. This observation further points to the possibility that A. hydrophila may use amonabactin as one of its virulence factors to inhibit the Wnt/β-catenin pathway and consequently impair the structure and function of the gut mucosal barrier. In addition, a study on Edwardsiella piscicida highlights the role of Fur in iron regulation and virulence, similar to the role of AmoG in A. hydrophila. In the future, using siderophore-deficient strains as vaccines may be an effective prevention and control strategy in aquaculture [41].
Interestingly, our previous studies have identified Ssp1 and WbuB as essential components for disrupting the gut’s tight junction barrier [16,17]. In contrast to Ssp1 and WbuB, which primarily influence the tight junction and inflammasome pathway, AmoG uniquely targets the Wnt/β-catenin signaling pathway. Through iron acquisition, AmoG appears to manipulate the host’s Wnt/β-catenin signaling pathway, thereby facilitating both pathogenesis and nutrient acquisition. Further exploration is needed to understand the precise mechanisms linking iron chelation to the mediation of the Wnt/β-catenin signaling pathway.
5. Conclusions
In conclusion, our findings indicate that AmoG is a previously unrecognized determinant in the pathogenicity of A. hydrophila, essential for successful host invasion. Our observations highlight AmoG’s substantial disruption of the Wnt/β-catenin signaling pathway and gut barrier integrity. Moreover, the diminished expression of AmoG correlates with reduced A. hydrophila colonization in key immune tissues, significantly attenuating its virulence. These discoveries offer new perspectives on A. hydrophila’s pathogenesis, contributing valuable knowledge to the prevention and treatment of this aquatic pathogen.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13020195/s1, Figure S1: Genomic organization of the amonabactin synthesis gene cluster in Aeromonas hydrophila CCL1 (GenBank Accession No. CP092356); Figure S2: Alignment of AmoG; Figure S3: Effect of AmoG on gut microbiota in vivo; Figure S4: Summary depiction suggesting a possible mechanism by which Aeromonas hydrophila AmoG modulates the infectivity through the manipulation of the Wnt/β-catenin pathway; Table S1: PCR primers used in this study.
Author Contributions
Y.T.: Writing—original draft, investigation. X.L.: Writing—original draft, investigation. C.Z.: Investigation. Y.L.: Investigation, funding acquisition. Y.Y.: Investigation, funding acquisition. J.H.: Investigation. P.L.: Investigation. Z.Z.: Writing—review and editing, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Plan Program (2024YFD2401104), National Natural Science Foundation of China (32102847), Natural Science Foundation of Hunan Province (2024JJ5261), Natural Science Foundation of Hunan Province for Young Student (2024JJ10032), and Undergraduate Research Project of Shi-Cheng College of Hunan Normal University (Y.Y. and Y.L.).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Hunan Normal University’s Animal Care Committee (no. 2023109, Approval Date 7 March 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data from the RNA-Seq and 16S rDNA Amplicon Sequencing have been uploaded to the NCBI Sequence Read Archive under accession numbers PRJNA1192974 and PRJNA807750.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stratev, D.; Odeyemi, O.A. Antimicrobial Resistance of Aeromonas hydrophila Isolated from Different Food Sources: A mini-Review. J. Infect. Public Health 2016, 9, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Awan, F.; Dong, Y.; Wang, N.; Liu, J.; Ma, K.; Liu, Y. The Fight for Invincibility: Environmental Stress Response Mechanisms and Aeromonas hydrophila. Microb. Pathog. 2018, 116, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen-Ivey, C.R.; Figueras, M.J.; McGarey, D.; Liles, M.R. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front. Microbiol. 2016, 7, 1337. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Sureda, J.; Rey-Varela, D.; Rodríguez, J.; Balado, M.; Lemos, M.L.; Jiménez, C. Selective Detection of Aeromonas spp. by a Fluorescent Probe Based on the Siderophore Amonabactin. J. Inorg. Biochem. 2022, 230, 111743. [Google Scholar] [CrossRef]
- Funahashi, T.; Tanabe, T.; Miyamoto, K.; Tsujibo, H.; Maki, J.; Yamamoto, S. Characterization of A Gene Encoding the Outer Membrane Receptor for Ferric Enterobactin in Aeromonas hydrophila ATCC 7966(T). Biosci. Biotechnol. Biochem. 2013, 77, 353–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balado, M.; Souto, A.; Vences, A.; Careaga, V.P.; Valderrama, K.; Segade, Y.; Rodríguez, J.; Osorio, C.R.; Jiménez, C.; Lemos, M.L. Two Catechol Siderophores, Acinetobactin and Amonabactin, Are Simultaneously Produced by Aeromonas salmonicida subsp. salmonicida Sharing Part of the Biosynthetic Pathway. ACS Chem. Biol. 2015, 10, 2850–2860. [Google Scholar] [PubMed]
- Esmaeel, Q.; Chevalier, M.; Chataigné, G.; Subashkumar, R.; Jacques, P.; Leclère, V. Nonribosomal Peptide Synthetase with A Unique Iterative-Alternative-Optional Mechanism Catalyzes Amonabactin Synthesis in Aeromonas. Appl. Microbiol. Biotechnol. 2016, 100, 8453–8463. [Google Scholar] [CrossRef]
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Piazuelo, M.B.; Chaturvedi, R.; Schumacher, M.; Aihara, E.; Feng, R.; Noto, J.M.; Delgado, A.; Israel, D.A.; Zavros, Y.; et al. Helicobacter pylori Targets Cancer-Associated Apical-Junctional Constituents in Gastroids and Gastric Epithelial Cells. Gut 2015, 64, 720–730. [Google Scholar] [CrossRef]
- Harrer, A.; Bücker, R.; Boehm, M.; Zarzecka, U.; Tegtmeyer, N.; Sticht, H.; Schulzke, J.D.; Backert, S. Campylobacter jejuni Enters Gut Epithelial Cells and Impairs Intestinal Barrier Function through Cleavage of Occludin by Serine Protease HtrA. Gut Pathog. 2019, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Köhler, H.; Gu, X.; McCormick, B.A.; Reinecker, H.C. Shigella flexneri Regulates Tight Junction-Associated Proteins in Human Intestinal Epithelial Cells. Cell. Microbiol. 2002, 4, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Syakuri, H.; Jung-Schroers, V.; Adamek, M.; Brogden, G.; Irnazarow, I.; Steinhagen, D. Beta-Glucan Feeding Differentiated the Regulation of Mrna Expression of Claudin Genes and Prevented an Intestinal Inflammatory Response Post Aeromonas Hydrophila Intubation in Common Carp, Cyprinus carpio L. J. Fish Dis. 2014, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.G.; Li, S.S.; Chen, X.X.; Huang, Y.Q.; Tang, Y.; Wu, Z.X. A Study of the Damage of the Intestinal Mucosa Barrier Structure and Function of Ctenopharyngodon idella with Aeromonas hydrophila. Fish Physiol. Biochem. 2017, 43, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, Y.; Liu, J.; Awan, F.; Lu, C.; Liu, Y. Inhibition of Aeromonas hydrophila-Induced Intestinal Inflammation and Mucosal Barrier Function Damage in Crucian Carp by Oral Administration of Lactococcus lactis. Fish Shellfish Immunol. 2018, 83, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Liu, X.; Hu, N.; Tang, Y.; Feng, M.; Zhou, Z. Aeromonas hydrophila Ssp1: A Secretory Serine Protease that Disrupts Tight Junction Integrity and is essential for Host Infection. Fish Shellfish Immunol. 2022, 127, 530–541. [Google Scholar] [CrossRef]
- Zeng, Q.; Qiang, J.; Yang, Y.; Li, Z.; Li, P.; Hu, N.; Zhou, Z. WbuB, a Glycosyltransferase Family 4 Protein, Regulates the Activation of NLRP3 Inflammasome and Contributes to the Virulence of Aeromonas hydrophila CCL1. Rep. Bre. 2023, 4, 38–45. [Google Scholar] [CrossRef]
- Taha, M.D.; Didinen, B.I.; Emek Onuk, E.; Metin, S.; Yilmaz, S.; Mohamed, A.A.; Pakır, S.; Gülşen, O.; Abdel-Latif, H.M.R. Identification of Four Autochthonous Yeasts from the Intestines of Goldfish, Carassius auratus with Potential Probiotic Properties and their Effects on the Most Common Fish Bacterial Pathogens. Microb. Pathog. 2023, 184, 106381. [Google Scholar] [CrossRef] [PubMed]
- GB/T 35892-2018; Laboratory Animal-Guideline for Ethical Review of Animal Welfare. Standards Press of China: Beijing, China, 2018.
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of Five Bacterial Strains Producing Siderophores with Ability to Chelate Iron Under Alkaline Conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef]
- Metin, S.; Yigit, N.O.; Didinen, B.I.; Koca, S.B.; Ozmen, O.; Aslankoc, R.; Kara, N. Effects of Sage (Salvia officinalis) Essential Oil on Growth, Health and Antioxidant Capacity of Common Carp (Cyprinus carpio). Vet. Res. Commun. 2024, 48, 911–921. [Google Scholar] [CrossRef]
- Szczygieł, J.; Kamińska-Gibas, T.; Petit, J.; Jurecka, P.; Wiegertjes, G.; Irnazarow, I. Re-evaluation of Common Carp (Cyprinus carpio L.) Housekeeping Genes for Gene Expression Studies—Considering Duplicated Genes. Fish Shellfish Immunol. 2021, 115, 58–69. [Google Scholar] [CrossRef]
- Watanabe, M.; Okamura, Y.; Kono, T.; Sakai, M.; Hikima, J.I. Interleukin-22 Induces Immune-Related Gene Expression in the Gills of Japanese medaka Oryzias latipes. Dev. Comp. Immunol. 2023, 148, 104916. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Barger, P.C.; Liles, M.R.; Newton, J.C. Type II Secretion is Essential for Virulence of the Emerging Fish Pathogen, Hypervirulent Aeromonas hydrophila. Front. Vet. Sci. 2020, 7, 574113. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Chifman, J.; Laubenbacher, R.; Torti, S.V. A Systems Biology Approach to Iron Metabolism. Adv. Exp. Med. Biol. 2014, 844, 201–225. [Google Scholar] [PubMed]
- Maltz, M.; LeVarge, B.L.; Graf, J. Identification of Iron and Heme Utilization Genes in Aeromonas and their Role in the Colonization of the Leech Digestive Tract. Front. Microbiol. 2015, 6, 763. [Google Scholar] [CrossRef]
- Barghouthi, S.; Young, R.; Olson, M.O.; Arceneaux, J.E.; Clem, L.W.; Byers, B.R. Amonabactin, a Novel Tryptophan- or Phenylalanine-Containing Phenolate Siderophore in Aeromonas hydrophila. J. Bacteriol. 1989, 171, 1811–1816. [Google Scholar] [CrossRef]
- Xie, L.W.; Cai, S.; Lu, H.Y.; Tang, F.L.; Zhu, R.Q.; Tian, Y.; Li, M. Microbiota-Derived I3A Protects the Intestine against Radiation Injury by Activating AhR/IL-10/Wnt Signaling and Enhancing the Abundance of Probiotics. Gut Microbes 2024, 16, 2347722. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-Driven Gut Vascular Barrier Disruption is a Prerequisite for Non-Alcoholic Steatohepatitis Development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Jauregi-Miguel, A. The Tight Junction and the Epithelial Barrier in Coeliac Disease. Int. Rev. Cell Mol. Biol. 2021, 358, 105–132. [Google Scholar]
- Backert, S.; Bernegger, S.; Skórko-Glonek, J.; Wessler, S. Extracellular HtrA Serine Proteases: An Emerging New Strategy in Bacterial Pathogenesis. Cell. Microbiol. 2018, 20, e12845. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Jia, A.; Wang, Y.; Bi, Y.; Liu, G. The Crosstalk Between Gut Bacteria and Host Immunity in Intestinal Inflammation. J. Cell. Physiol. 2021, 236, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Xiao, X.; Yeoh, B.S.; Chen, Q.; Katkere, B.; Kirimanjeswara, G.S.; Vijay-Kumar, M. The Bacterial Siderophore Enterobactin Confers Survival Advantage to Salmonella in Macrophages. Gut Microbes 2019, 10, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.D.; Abernathy, J.; Shoemaker, C.A.; Zhang, D.; Kirby, A.; Peatman, E.; Beck, B.H. Proteome Analysis of Virulent Aeromonas hydrophila Reveals the Upregulation of Iron Acquisition Systems in the Presence of a Xenosiderophore. FEMS Microbiol. Lett. 2020, 367, fnaa169. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.L.; Zhou, J.Y.; Liang, S.J.; Wang, X.Q. Impaired Intestinal Stem Cell Activity in ETEC Infection: Enterotoxins, Cyclic Nucleotides, and Wnt Signaling. Arch. Toxicol. 2022, 96, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, A.; Ardakani, M.R.; Hashemi, M.S.; Peymani, M.; Ghaedi, K.; Baharvand, H.; Nasr-Esfahani, M.H. Acute Course of Deferoxamine Promoted Neuronal Differentiation of Neural Progenitor Cells Through Suppression of Wnt/Β-Catenin Pathway: A Novel Efficient Protocol for Neuronal Differentiation. Neurosci. Lett. 2015, 590, 138–144. [Google Scholar] [CrossRef]
- Gerner, R.R.; Hossain, S.; Sargun, A.; Siada, K.; Norton, G.J.; Zheng, T.; Neumann, W.; Nuccio, S.P.; Nolan, E.M.; Raffatellu, M. Siderophore Immunization Restricted Colonization of Adherent-Invasive Escherichia coli and Ameliorated Experimental Colitis. mBio 2022, 13, e0218422. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Powell, C.T.; Curtiss, R., III. Pathogenicity and Immunogenicity of Edwardsiella piscicida Ferric Uptake Regulator (Fur) Mutations in Zebrafish. Fish Shellfish Immunol. 2020, 107 Pt B, 497–510. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).