Efficacy of Inactivated Bivalent SARS-CoV-2 Vaccines Targeting Ancestral Strain (ERAGEM), Delta, and Omicron Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Viruses
2.2. Facility and Ethics Statement
2.3. Animals
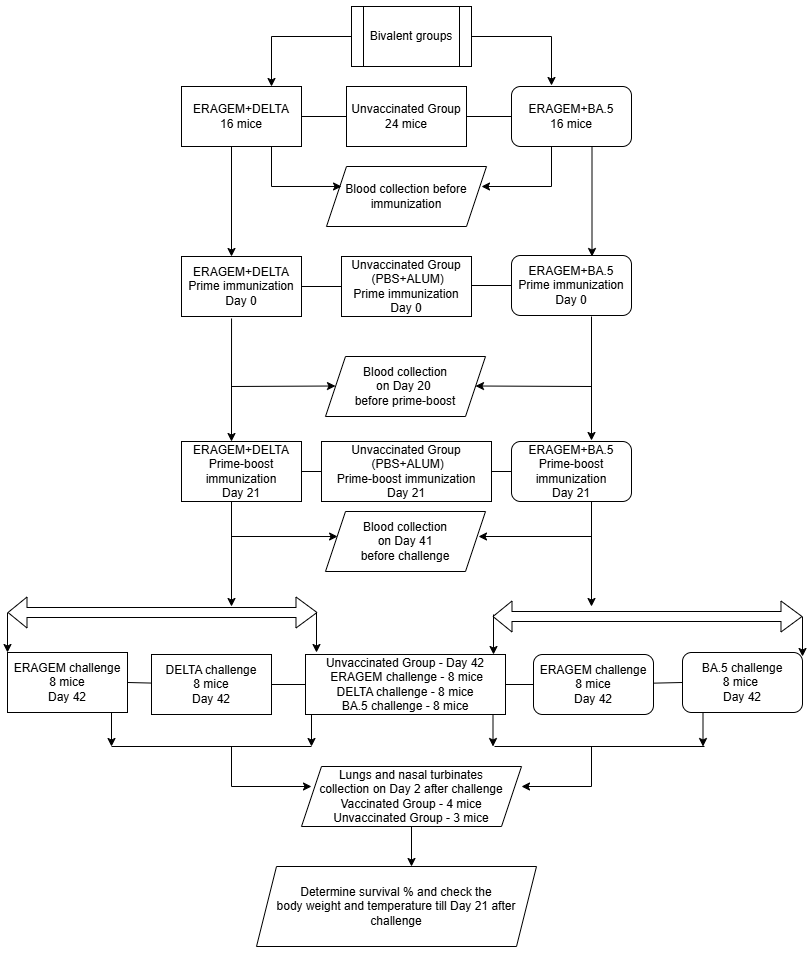
2.4. Vaccine Antigens and Vaccinations of Mice
2.5. Virus Titer and Fluorescent Focus Assay
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Microneutralization Test (MNT)
2.9. Statistical Analysis
3. Results
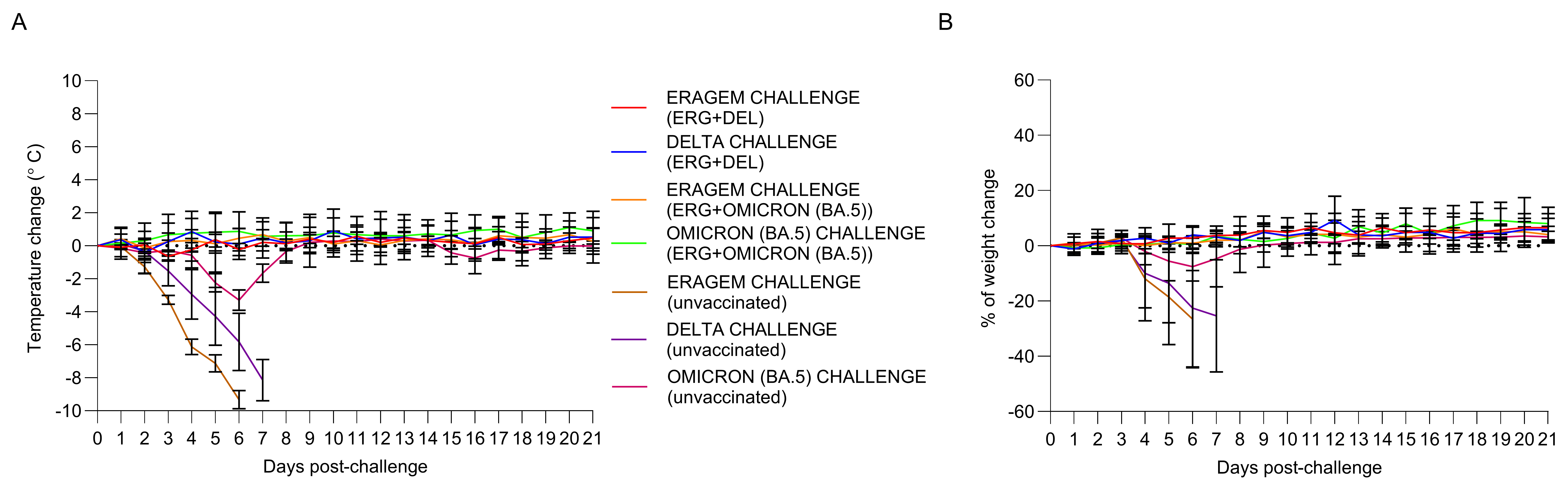
3.1. Bivalent Vaccines Protect K18-hACE2 Transgenic Mice Against the Lethal SARS-CoV-2 Variants Challenge
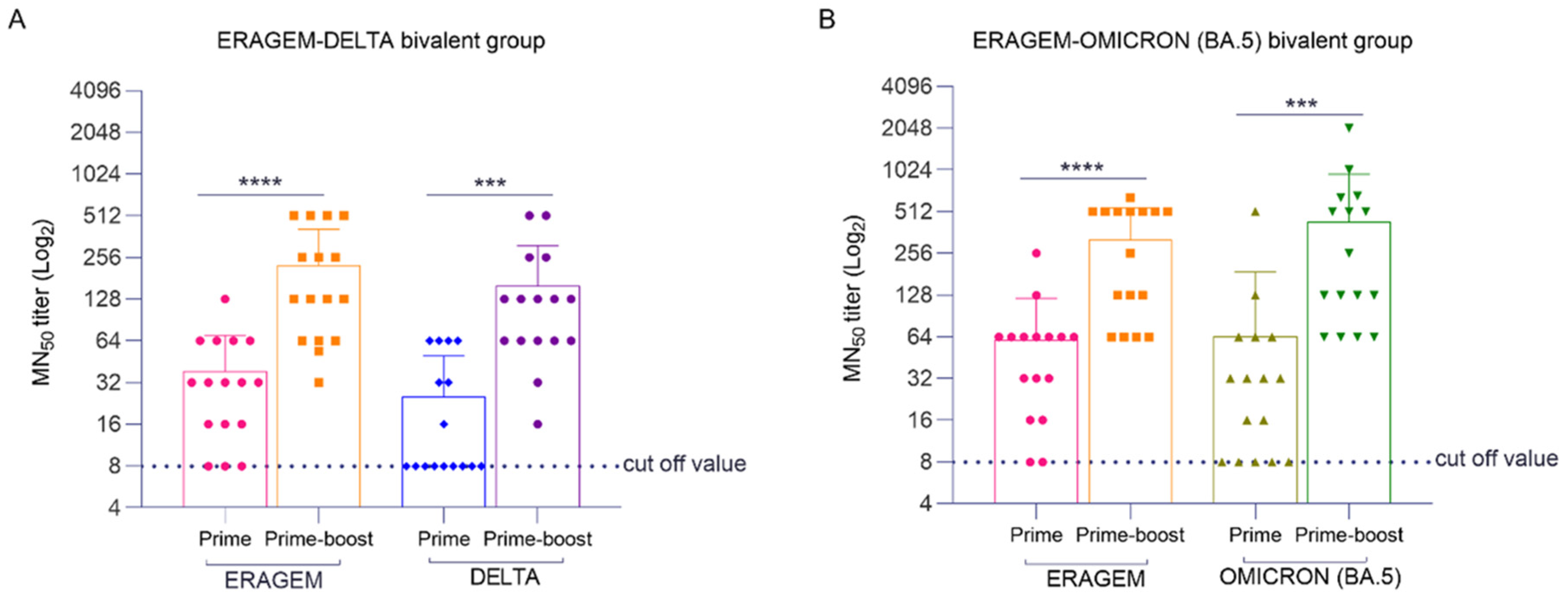
3.2. Bivalent Vaccines Induce Humoral Immune Responses in K18-hACE2 Transgenic Mice
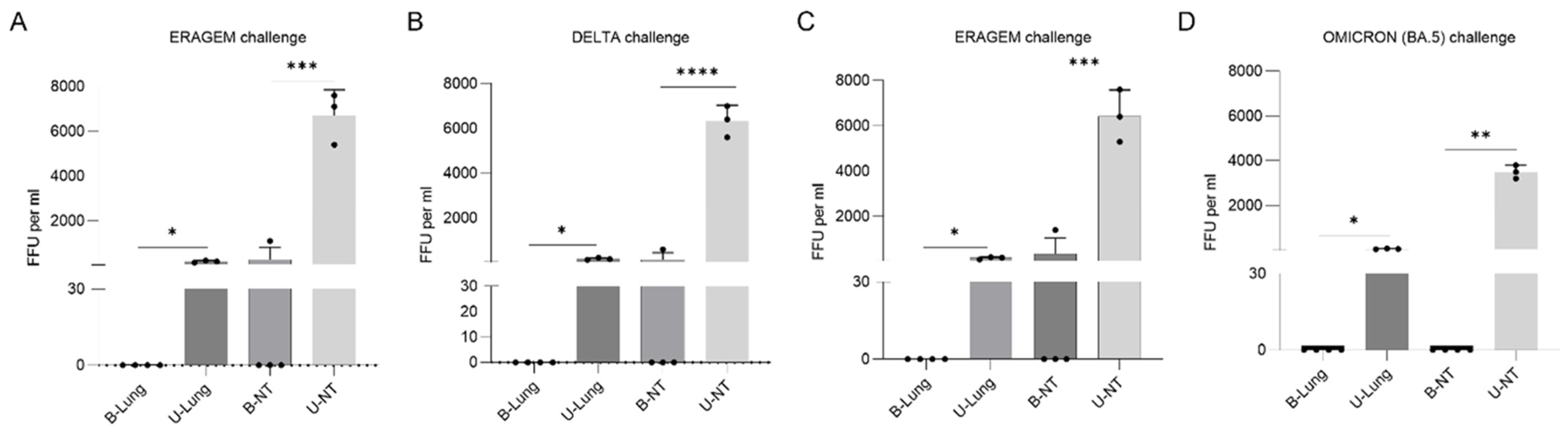
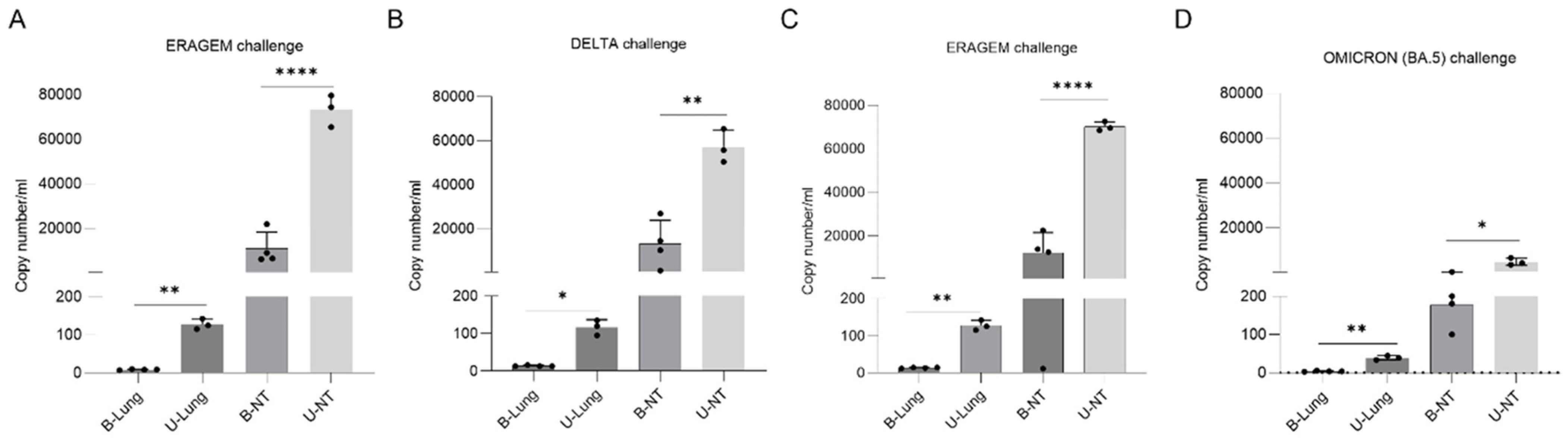
3.3. Bivalent Vaccines Provide Complete Inhibition of Viral Replication in the Lungs and a Significant Reduction in Nasal Turbinates
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brian, D.A.; Baric, R.S. Coronavirus genome structure and replication. In Coronavirus Replication and Reverse Genetics; Springer: Berlin/Heidelberg, Germany, 2005; Volume 287, pp. 1–30, Current Topics in Microbiology and Immunology. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Garg, S.; Joshi, H.; Ayaz, S.; Sharma, S.; Bhandari, M. A review: Epidemics and pandemics in human history. Int. J. Pharma Res. Health Sci. 2020, 8, 3139–3142. [Google Scholar] [CrossRef]
- Flint, J.; Skalka, A.M.; Rall, G.F.; Racaniello, V.R. Principles of Virology, Vol. I: Molecular Biology; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Domańska-Blicharz, K.; Woźniakowski, G.; Michalczyk, M.; Wróbel, A.; Szymańska-Czerwińska, M.; Smreczak, M.; Śmietanka, K. Animal coronaviruses in the light of COVID-19. J. Vet. Res. 2020, 64, 439–450. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Z.L.; Zhou, P. Bat coronaviruses in China. Viruses 2019, 11, 210. [Google Scholar] [CrossRef]
- Chen, J. Pathogenicity and transmissibility of 2019-nCoV—A quick overview and comparison with other emerging viruses. Microbes Infect. 2020, 22, 69–71. [Google Scholar] [CrossRef]
- Plante, J.A.; Mitchell, B.M.; Plante, K.S.; Debbink, K.; Weaver, S.C.; Menachery, V.D. The variant gambit: COVID-19’s next move. Cell Host Microbe 2021, 29, 508–515. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 Variants; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/emergencies/overview/tracking-SARS-CoV-2-variants (accessed on 15 March 2023).
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Crandon, S.; Boardman, K.C.; Jackson, R.M. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; van Damme, W.; Leroux-Roels, I.; et al. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Palacios, R.; Batista, A.P.; Albuquerque, C.S.N.; Patiño, E.G.; Santos, J.D.P.; Conde, M.T.R.P.; Zeng, G.; Xin, Q.; Kallas, E.G.; Flores, J.; et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: The PROFISCOV study. Lancet 2021, 398, 2173–2181. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Aydin, O.A.; Guner, R.; Yildiz, O.; Celik, I.; Doganay, H.L.; Kose, S.; Akhan, S.; Akalin, E.H.; Sezer, Z.; et al. Efficacy, immunogenicity, and safety of the two-dose schedules of TURKOVAC versus CoronaVac in healthy subjects: A randomized, observer-blinded, non-inferiority phase III trial. Vaccines 2022, 10, 1865. [Google Scholar] [CrossRef]
- Ozdarendeli, A.; Sezer, Z.; Pavel, S.T.I.; Inal, A.; Yetiskin, H.; Kaplan, B.; Uygut, M.A.; Bayram, A.; Mazicioglu, M.; Unuvar, G.K.; et al. Safety and immunogenicity of an inactivated whole virion SARS-CoV-2 vaccine, TURKOVAC, in healthy adults: Interim results from randomised, double-blind, placebo-controlled phase 1 and 2 trials. Vaccine 2023, 41, 380–390. [Google Scholar] [CrossRef]
- Aggarwal, A.; Stella, A.; Walker, G.; Akerman, A.; Milogiannakis, V.; Brilot, F.; Amatayakul-Chantler, S.; Roth, N.; Coppola, G.; Schofield, P.; et al. SARS-CoV-2 Omicron: Evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. medRxiv 2021. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; Wang, Y.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Arabi, M.; Paul, P.; Khanjar, B.; Burney, Z.; D’Souza, A.; Sinha, P.; Bhatti, M.; Pillai, K.V.; Homssi, M.; Bshesh, K.; et al. Severity of the Omicron SARS-CoV-2 variant compared with the previous lineages: A systematic review. J. Cell. Mol. Med. 2023, 27, 1443–1464. [Google Scholar] [CrossRef]
- Iketani, S.; Guo, Y.; Chan, J.F.W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Chik, K.K.H.; Yuen, T.T.T.; Yoon, C.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Rophina, M.; Pandhare, K.; Shamnath, A.; Imran, M.; Jolly, B.; Scaria, V. ESC: A comprehensive resource for SARS-CoV-2 immune escape variants. Nucleic Acids Res. 2022, 50, D771–D776. [Google Scholar] [CrossRef]
- Davies, N.G.; Jarvis, C.I.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, J.E.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef]
- Sabino, E.C.; Buss, L.F.; Carvalho, M.P.S.; Prete, C.A.; Crispim, M.A.E.; Fraiji, N.A.; Pereira, R.H.M.; Parag, K.V.; da Silva Peixoto, P.; Kraemer, M.U.G.; et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet 2021, 397, 452–455. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Guo, Y.; Han, J.; Zhang, Y.; He, J.; Yu, W.; Zhang, X.; Wu, J.; Zhang, S.; Kong, Y.; Guo, Y.; et al. SARS-CoV-2 Omicron Variant: Epidemiological Features, Biological Characteristics, and Clinical Significance. Front. Immunol. 2022, 13, 877101. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, Z.; Xiao, J.; Wu, Y.; Xia, N.; Yuan, Q. Evolution of the SARS-CoV-2 Omicron variants: Genetic impact on viral fitness. Viruses 2024, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Pavel, S.T.I.; Yetiskin, H.; Aydin, G.; Holyavkin, C.; Uygut, M.A.; Dursun, Z.B.; Celik, İ.; Cevik, C.; Ozdarendeli, A. Isolation and characterization of severe acute respiratory syndrome coronavirus 2 in Turkey. PLoS ONE 2020, 15, e0238614. [Google Scholar] [CrossRef] [PubMed]
- Pavel, S.T.I.; Yetiskin, H.; Uygut, M.A.; Aslan, A.F.; Aydın, G.; İnan, Ö.; Kaplan, B.; Ozdarendeli, A. Development of an inactivated vaccine against SARS-CoV-2. Vaccines 2021, 9, 1266. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Epidemiological Update; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Wang, Z.; Li, W.; Wang, C.; Li, J.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- McMenamin, M.E.; Nealon, J.; Lin, Y.; Wong, J.Y.; Cheung, J.K.; Lau, E.H.Y.; Wu, P.; Leung, G.M.; Cowling, B.J. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: A population-based observational study. Lancet Infect. Dis. 2022, 22, 1435–1443. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Qing, E.; Kicmal, T.; Kumar, B.; Hawkins, G.M.; Timm, E.; Perlman, S.; Gallagher, T. Dynamics of SARS-CoV-2 spike proteins in cell entry: Control elements in the amino-terminal domains. mBio 2021, 12, e0159021. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Sakr, M.M.; Elsayed, N.S.; El-Housseiny, G.S. Latest updates on SARS-CoV-2 genomic characterization, drug, and vaccine development; a comprehensive bioinformatics review. Microb. Pathog. 2021, 154, 104809. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Edwards, D.K.; Sablerolles, R.S.G.; Maaske, J.; et al. Safety, immunogenicity and antibody persistence of a bivalent Beta-containing booster vaccine against COVID-19: A phase 2/3 trial. Nat. Med. 2022, 28, 2388–2397. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhuang, X.; Jiang, H.; Wang, X.; He, Z.; Li, Y.; Li, S.; Xu, W.; Li, X.; et al. Inactivated vaccine-elicited potent antibodies can broadly neutralize SARS-CoV-2 circulating variants. Nat. Commun. 2023, 14, 2179. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Tungatt, K.; Aggarwal, A.; Stubis, A.; Fewings, N.L.; Fichter, C.; Akerman, A.; Rodrigo, C.; Tedla, N.; Lee, S.; et al. Bivalent Omicron BA.1 vaccine booster increases memory B cell breadth and neutralising antibodies against emerging SARS-CoV-2 variants. eBioMedicine 2024, 110, 105461. [Google Scholar] [CrossRef]
- Ying, B.; Darling, T.L.; Desai, P.; Liang, C.-Y.; Dmitriev, I.P.; Soudani, N.; Bricker, T.; Kashentseva, E.A.; Harastani, H.; Schmidt, A.G.; et al. A bivalent ChAd nasal vaccine protects against SARS-CoV-2 BQ.1.1 and XBB.1.5 infection and disease in mice and hamsters. bioRxiv 2023. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, B.; Pavel, S.T.I.; Uygut, M.A.; Tunc, M.; Eroksuz, Y.; Celik, I.; Eren, E.E.; Korukluoglu, G.; Kara, A.; Ozdarendeli, A.; et al. Efficacy of Inactivated Bivalent SARS-CoV-2 Vaccines Targeting Ancestral Strain (ERAGEM), Delta, and Omicron Variants. Vaccines 2025, 13, 169. https://doi.org/10.3390/vaccines13020169
Kaplan B, Pavel STI, Uygut MA, Tunc M, Eroksuz Y, Celik I, Eren EE, Korukluoglu G, Kara A, Ozdarendeli A, et al. Efficacy of Inactivated Bivalent SARS-CoV-2 Vaccines Targeting Ancestral Strain (ERAGEM), Delta, and Omicron Variants. Vaccines. 2025; 13(2):169. https://doi.org/10.3390/vaccines13020169
Chicago/Turabian StyleKaplan, Busra, Shaikh Terkis Islam Pavel, Muhammet Ali Uygut, Merve Tunc, Yesari Eroksuz, Ilhami Celik, Esma Eryilmaz Eren, Gulay Korukluoglu, Ates Kara, Aykut Ozdarendeli, and et al. 2025. "Efficacy of Inactivated Bivalent SARS-CoV-2 Vaccines Targeting Ancestral Strain (ERAGEM), Delta, and Omicron Variants" Vaccines 13, no. 2: 169. https://doi.org/10.3390/vaccines13020169
APA StyleKaplan, B., Pavel, S. T. I., Uygut, M. A., Tunc, M., Eroksuz, Y., Celik, I., Eren, E. E., Korukluoglu, G., Kara, A., Ozdarendeli, A., & Yetiskin, H. (2025). Efficacy of Inactivated Bivalent SARS-CoV-2 Vaccines Targeting Ancestral Strain (ERAGEM), Delta, and Omicron Variants. Vaccines, 13(2), 169. https://doi.org/10.3390/vaccines13020169







