Safety and Cross-Neutralizing Immunity Against SARS-CoV-2 Omicron Sub-Variant After a Booster Dose with SOBERANA® Plus in Children and Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Approvals
2.2. Subjects and Ethics
2.3. Safety Assessment
2.4. Immunogenicity Assessment
2.5. Data Management and Statistical Analysis
3. Results
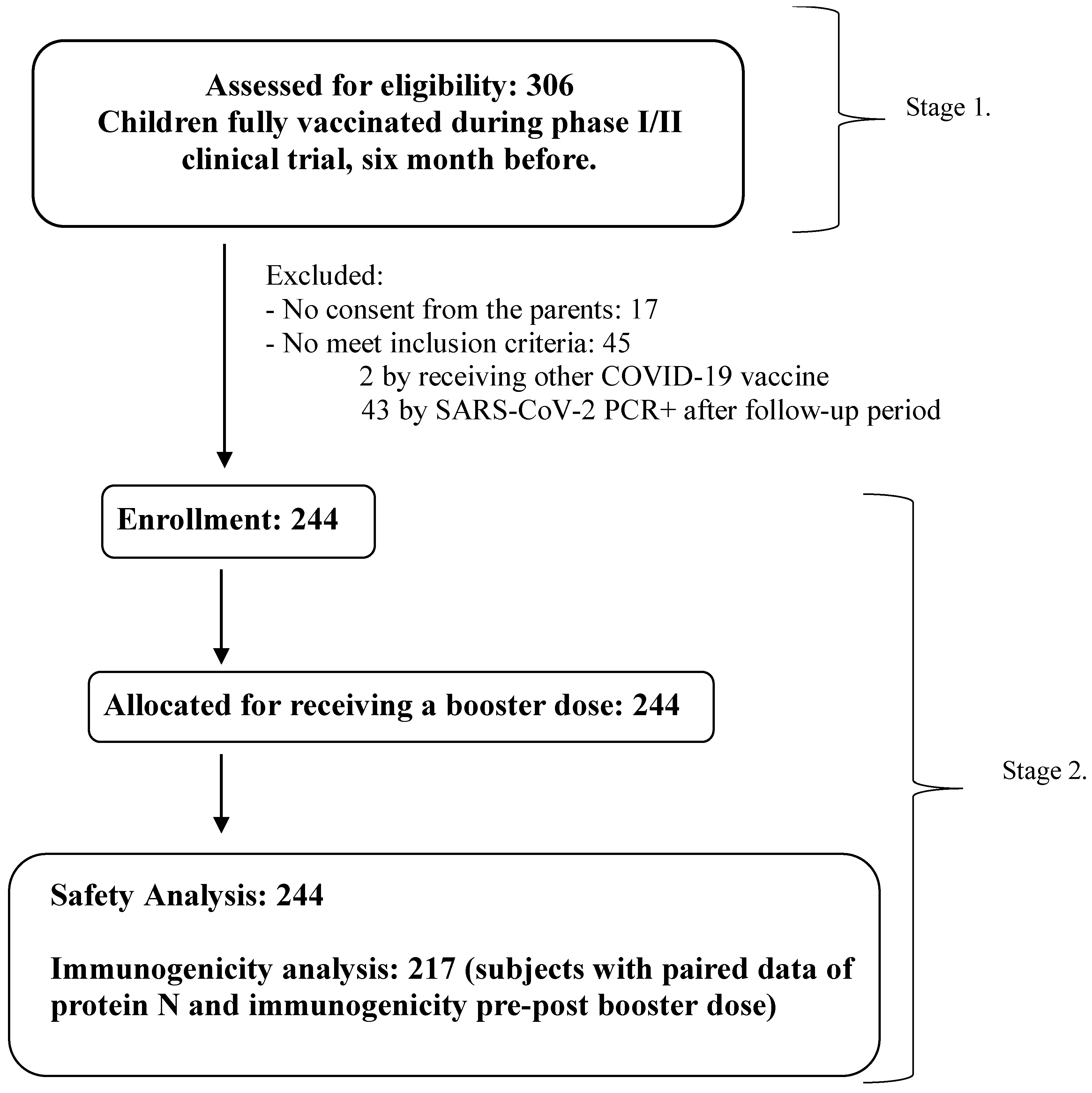
3.1. Demographic Characteristic of Subjects and Flow Chart
3.2. Safety After Applying the Booster Dose with SOBERANA® Plus
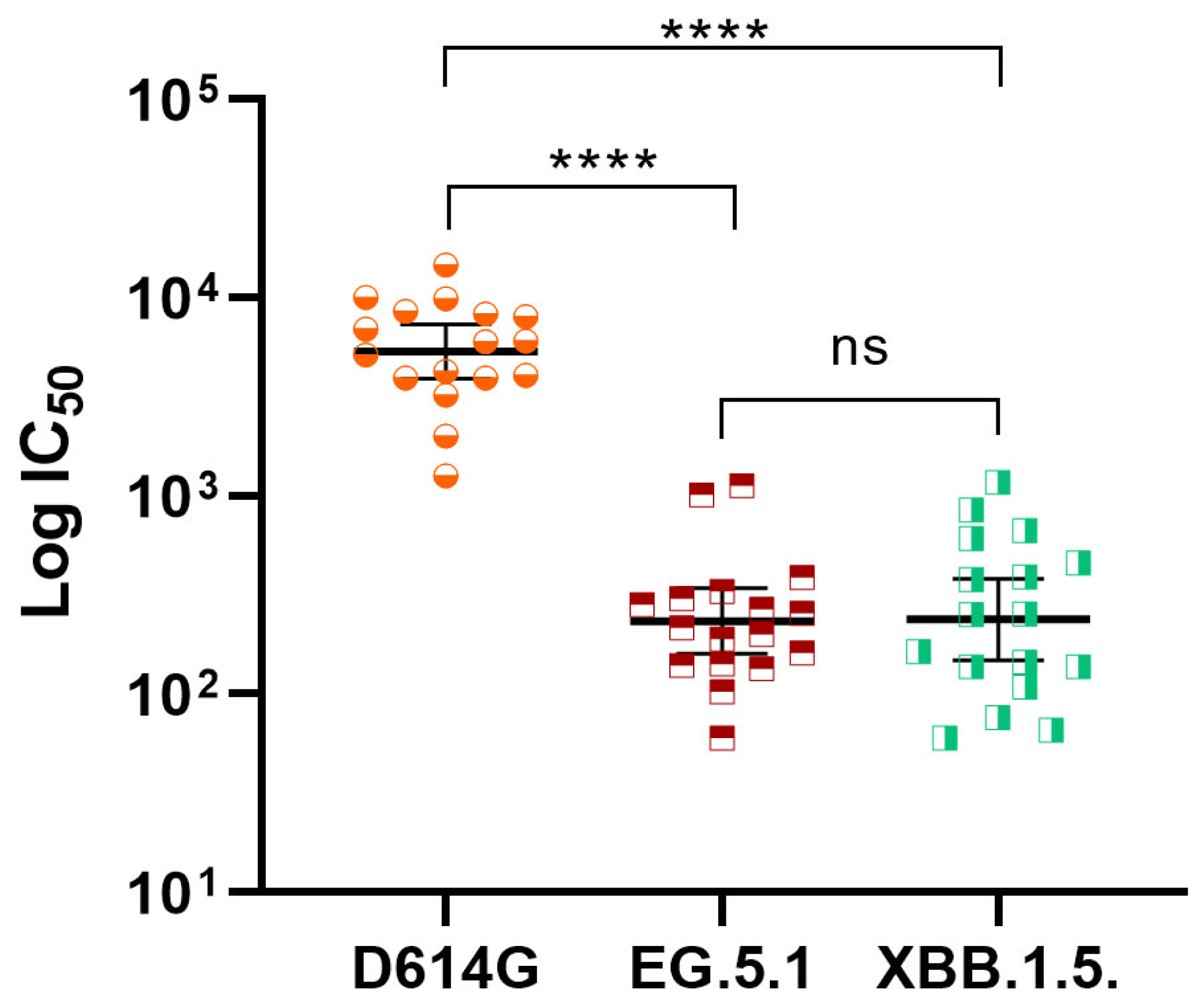
3.3. Immune Response After Applying the Booster Dose with SOBERANA® Plus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID-19 Weekly Epidemiological Update-12 March 2025. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update-edition-177 (accessed on 14 March 2025).
- WHO. COVID-19 Advice for the Public: Getting Vaccinated. 8 October 2024. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 14 March 2025).
- Paul, L.A.; Daneman, N.; Schwartz, K.L.; Science, M.; Brown, K.A.; Whelan, M.; Chan, E.; Buchan, S.A. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr. 2021, 175, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Zhu, F.C. The era of SARS-CoV-2 variants calls for an urgent strategy for COVID-19 vaccination in children. Lancet Infect. Dis. 2024, 24, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Romani, M.E.; García-Carmenate, M.; Verdecia-Sánchez, L.; Pérez-Rodríguez, S.; Rodriguez-González, M.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sanchez-Ramirez, B.; González-Mugica, R.; Hernández-Garcia, T.; et al. Safety and immunogenicity of anti-SARS-CoV-2 heterologous scheme with SOBERANA 02 and SOBERANA Plus vaccines: Phase IIb clinical trial in adults. Med 2022, 3, 760–773.e5. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Eybpoosh, S.; Karamouzian, M.; Khalili, M.; Haji-Maghsoudi, S.; Salehi-Vaziri, M.; Khamesipour, A.; Jalali, T.; Nakhaeizadeh, M.; Sharifi, H.; et al. Efficacy and Safety of a protein-based SARS-CoV-2 vaccine. A randomized clinical trial. JAMA Netw. Open 2023, 6, e2310302. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Romaní, M.E.; García-Carmenate, M.; Valenzuela-Silva, C.; Baldoquín-Rodríguez, W.; Martínez-Pérez, M.; Rodríguez-González, M.; Paredes-Moreno, B.; Mendoza-Hernández, I.; Romero, R.G.-M.; Samón-Tabio, O.; et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA PLUS: Double-blind, randomized, placebo-controlled phase 3 clinical trial. Lancet Reg. Health—Am. 2023, 18, 100423. [Google Scholar] [CrossRef] [PubMed]
- Puga-Gómez, R.; Ricardo-Delgado, Y.; Rojas-Iriarte, C.; Céspedes-Henriquez, L.; Piedra-Bello, M.; Vega-Mendoza, D.; Pérez, N.P.; Paredes-Moreno, B.; Rodríguez-González, M.; Valenzuela-Silva, C.; et al. Open label phase I/II clinical trial of SARS-CoV-2 receptor binding domain-tetanus toxoid conjugate vaccine (FINLAY-FR-2) in combination with receptor binding domain-protein vaccine (FINLAY-FR-1A) in children. Int. J. Infect. Dis. 2023, 126, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Nicado, R.; Massa, C.; Rodríguez-Noda, L.M.; Müller, A.; Puga-Gómez, R.; Ricardo-Delgado, Y.; Paredes-Moreno, B.; Rodríguez-González, M.; García-Ferrer, M.; Palmero-Álvarez, I.; et al. Comparative Immune Response after Vaccination with SOBERANA® 02 and SOBERANA® plus Heterologous Scheme and Natural Infection in Young Children. Vaccines 2023, 11, 1636. [Google Scholar] [CrossRef] [PubMed]
- García-Rivera, D.; Puga-Gómez, R.; Fernández-Castillo, S.; Paredes-Moreno, B.; Ricardo-Delgado, Y.; Rodríguez-González, M.; Silva, C.V.; Pérez-Nicado, R.; Rodríguez-Noda, L.; Santana-Mederos, D.; et al. Safety and durability of the immune response after vaccination with the heterologous schedule of anti-COVID-19 vaccines SOBERANA®02 and SOBERANA® Plus in children 3–18 years old. Vaccine X 2025, 22, 100595. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Romaní, M.E.; Valenzuela-Silva, C.; Montero-Díaz, M.; Iñiguez-Rojas, L.; Rodríguez-González, M.; Martínez-Cabrera, M.; Puga-Gómez, R.; German-Almeida, A.; Fernández-Castillo, S.; Climent-Ruiz, Y.; et al. Real-world effectiveness of the heterologous SOBERANA-02 and SOBERANA-Plus vaccine scheme in 2–11 years-old children during the SARS-CoV-2 Omicron wave in Cuba: A longitudinal case-population study. Lancet Reg. Health—Am. 2024, 34, 100750. [Google Scholar] [CrossRef] [PubMed]
- Link-Gelles, R.; Ciesla, A.A.; Mak, J.; Miller, J.D.; Silk, B.J.; Lambrou, A.S.; Paden, C.R.; Shirk, P.; Britton, A.; Smith, Z.R.; et al. Early estimates of updated 2023–2024 (monovalent XBB.1.5) COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection attributable to co-circulating Omicron variants among immunocompetent adults—Increasing Community Access to Testing Program, United States, September 2023–January 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C. Why COVID Vaccines for Young Children Have Been Hard to Get. 21 November 2023. Available online: https://www.scientificamerican.com/article/why-covid-vaccines-for-young-children-have-been-hard-to-get/ (accessed on 14 March 2025).
- Pradenas, E.; Trinité, B.; Urrea, V.; Marfil, S.; Ávila-Nieto, C.; de la Concepción, M.L.R.; Tarrés-Freixas, F.; Pérez-Yanes, S.; Rovirosa, C.; Ainsua-Enrich, E.; et al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Med 2021, 2, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Fadlyana, E.; Rusmil, K.; Dwi Putra, M.G.; Fulendry, F.P.; Somantri, N.K.; Putri, A.D.; Sari, R.M.; Puspita, M.; Dewi, G.P. Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVacR) as a Heterologous Booster Dose against COVID-19 in Indonesian Adolescents. Vaccines 2024, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Summary of Product Characteristics. Nuvaxovid. 20 December 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/nuvaxovid-epar-product-information_en.pdf (accessed on 14 March 2025).
- COVID-19 Update. Booster Dose of the Pfizer Vaccine for Children 5-11 Years Old. Med. Lett. Drugs Ther. 2022, 64, 94.
- Wang, L.; Wu, Z.; Ying, Z.; Li, M.; Hu, Y.; Shu, Q.; Li, J.; Wang, H.; Zhang, H.; Jiao, W.; et al. Safety and immunogenicity following a homologous booster dose of CoronaVac in children and adolescents. Nat. Commun. 2022, 13, 6952. [Google Scholar] [CrossRef] [PubMed]
- Thuluva, S.; Paradkar, V.; Gunneri, S.; Mogulla, R.R.; Yerroju, V.; Dhar, C.; Ningaiah, S.; Panchakshari, M.; Gillurkar, C.S.; Narang, M.; et al. Safety and immunogenicity of booster doses of an XBB.1.5 RBD subunit COVID-19 vaccine among individuals aged 5–80 years in India: A phase 3, single-blind, randomised controlled trial. Lancet Reg. Health—Southeast Asia 2025, 4, 100642. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Staropoli, I.; Michel, V.; Lemoine, F.; Donati, F.; Prot, M.; Porrot, F.; Guivel-Benhassine, F.; Jeyarajah, B.; Brisebarre, A.; et al. Distinct evolution of SARSCoV-2 Omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat. Commun. 2024, 15, 2254. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, Y. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023, 29, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, J.; Bai, S.; Lv, M.; Li, J.; Chen, W.; Suo, L.; Chen, M.; Zhao, W.; Zhou, S.; et al. The contributions of vaccination and natural infection to the production of neutralizing antibodies against the SARS-CoV-2 prototype strain and variants. Int. J. Infect. Dis. 2024, 144, 107060. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Sorouri, R.; Maghsoudi, S.H.; Dahmardeh, S.; Doroud, D.; Larijani, M.S.; Eybpoosh, S.; Mostafavi, E.; Olyaeemanesh, A.; Salehi-Vaziri, M.; et al. Pasto-Covac and PastoCovac Plus as protein subunit COVID-19 vaccines led to great humoral immune responses in BBIP-CorV immunized individuals. Sci. Rep. 2023, 13, 8065. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO SAGE Roadmap on Uses of COVID-19 Vaccines in the Context of Omicron and High Population Immunity. 10 November 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2023.1 (accessed on 16 October 2025).
- International Register Clinical Trials. Identifier RPCEC00000374. Phase I–II Study, Sequential During Phase I, Open-Label, Adaptive and Multicenter to Evaluate the Safety, Reactogenicity and Immunogenicity of a Heterologous Two-Dose Schedule of the Prophylactic Anti-SARS-CoV-2 Vaccine Candidate, FINLAY-FR-2 and a Dose of FINLAY-FR-1A. 2021. Available online: https://rpcec.sld.cu/trials/RPCEC00000374-En (accessed on 14 March 2025).

| Age Groups | |||
|---|---|---|---|
| 3–11 Years | 12–18 Years | Total | |
| n | 129 | 115 | 244 |
| Sex | |||
| Female | 58 (45.0%) | 52 (45.2%) | 110 (45.1%) |
| Male | 71 (55.0%) | 63 (54.8%) | 134 (54.9%) |
| Skin color | |||
| White | 93 (72.1%) | 73 (63.5%) | 166 (68.0%) |
| Black | 8 (6.2%) | 10 (8.7%) | 18 (7.4%) |
| Multiracial | 28 (21.7%) | 32 (27.8%) | 60 (24.6%) |
| Age (years) | |||
| Mean (SD) | 7.5 (2.5) | 14.8 (2.1) | 10.9 (4.4) |
| Median (IQR) | 8.0 (5.0) | 15.0 (4.0) | 11.0 (6.0) |
| Range | 3–11 | 12–18 | 3–18 |
| Weight (kg) | |||
| Mean (SD) | 29.2 (9.9) | 54.1 (9.0) | 40.9 (15.6) |
| Median (IQR) | 27.5 (14.0) | 55.0 (12.0) | 42.0 (27.0) |
| Range | 14.0–55.0 | 32.0–73.0 | 14.0–73.0 |
| Height (cm) | |||
| Mean (SD) | 128.9 (17.2) | 164.2 (9.8) | 145.5 (22.6) |
| Median (IQR) | 131.0 (26.0) | 163.0 (14.0) | 150.0 (33.0) |
| Range | 94–169 | 142–190 | 94–190 |
| BMI (kg/m2) | |||
| Mean (SD) | 17.0 (2.0) | 19.9 (2.3) | 18.4 (2.6) |
| Median (IQR) | 16.7 (2.7) | 19.8 (3.9) | 18.1 (3.7) |
| Range | 13.6–22.8 | 14.6–24.6 | 13.6–24.6 |
| Age Group | |||
|---|---|---|---|
| 3–11 y/o | 12–18 y/o | Total | |
| n | 129 | 115 | 244 |
| Subjects with some AE | 22 (17.1%) | 22 (19.1%) | 44 (18.0%) |
| Subjects with some vaccine-related AE | 20 (15.5%) | 21 (18.3%) | 41 (16.8%) |
| Subjects with some serious AE | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Subjects with some vaccine-related serious AE | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Subjects with some severe AE | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Subjects with some vaccine-related severe AE | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Total of AE | 50 | 38 | 88 |
| Mild | 49 (98.0%) | 37 (97.4%) | 86 (97.7%) |
| Moderate | 1 (2.0%) | 1 (2.6%) | 2 (2.3%) |
| Serious AE | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Local | 42 (84.0%) | 33 (86.8%) | 75 (85.2%) |
| Systemic | 8 (16.0%) | 5 (13.2%) | 13 (14.8%) |
| AE consistent with vaccination | 46 (92.0%) | 36 (94.7) | 82 (93.2%) |
| Serious AE consistent | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Severe AE consistent | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rivera, D.; Rodríguez-González, M.; Paredes-Moreno, B.; Puga-Gomez, R.; Ricardo-Delgado, Y.; Silva, C.V.; Fernández-Castillo, S.; Pérez-Nicado, R.; Rodríguez-Noda, L.; Santana-Mederos, D.; et al. Safety and Cross-Neutralizing Immunity Against SARS-CoV-2 Omicron Sub-Variant After a Booster Dose with SOBERANA® Plus in Children and Adolescents. Vaccines 2025, 13, 1198. https://doi.org/10.3390/vaccines13121198
García-Rivera D, Rodríguez-González M, Paredes-Moreno B, Puga-Gomez R, Ricardo-Delgado Y, Silva CV, Fernández-Castillo S, Pérez-Nicado R, Rodríguez-Noda L, Santana-Mederos D, et al. Safety and Cross-Neutralizing Immunity Against SARS-CoV-2 Omicron Sub-Variant After a Booster Dose with SOBERANA® Plus in Children and Adolescents. Vaccines. 2025; 13(12):1198. https://doi.org/10.3390/vaccines13121198
Chicago/Turabian StyleGarcía-Rivera, Dagmar, Meiby Rodríguez-González, Beatriz Paredes-Moreno, Rinaldo Puga-Gomez, Yariset Ricardo-Delgado, Carmen Valenzuela Silva, Sonsire Fernández-Castillo, Rocmira Pérez-Nicado, Laura Rodríguez-Noda, Darielys Santana-Mederos, and et al. 2025. "Safety and Cross-Neutralizing Immunity Against SARS-CoV-2 Omicron Sub-Variant After a Booster Dose with SOBERANA® Plus in Children and Adolescents" Vaccines 13, no. 12: 1198. https://doi.org/10.3390/vaccines13121198
APA StyleGarcía-Rivera, D., Rodríguez-González, M., Paredes-Moreno, B., Puga-Gomez, R., Ricardo-Delgado, Y., Silva, C. V., Fernández-Castillo, S., Pérez-Nicado, R., Rodríguez-Noda, L., Santana-Mederos, D., Climent-Ruiz, Y., Noa-Romero, E., Cruz-Sui, O., Sánchez-Ramírez, B., Hernández-García, T., Palenzuela-Diaz, A., Valdés-Balbín, Y., & Vérez-Bencomo, V. G. (2025). Safety and Cross-Neutralizing Immunity Against SARS-CoV-2 Omicron Sub-Variant After a Booster Dose with SOBERANA® Plus in Children and Adolescents. Vaccines, 13(12), 1198. https://doi.org/10.3390/vaccines13121198






