Disease and Economic Burden Averted by Hib Vaccination in 160 Countries: A Machine-Learning Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Sources
2.2. Counterfactual Simulation Using Machine Learning
2.3. Health Benefits Analysis of Hib Immunization
2.4. Economic Benefits Analysis of Hib Immunization
2.5. Cost-Effectiveness Analysis of Hib Immunization
2.6. China-Specific Scenario Projection
2.7. Uncertainty Analysis
3. Results
3.1. Health Benefits Analysis of Hib Vaccination
3.2. Health Equity Assessment
3.3. Economic Benefits and Cost-Effectiveness Analysis of Hib Vaccination
3.4. Results of China-Specific Scenario Projection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, E.C.; Begg, N.T.; Crawshaw, S.C.; Hargreaves, R.M.; Howard, A.J.; Slack, M.P.E. Epidemiology of invasive Haemophilus influenzae infections in England and Wales in the pre-vaccination era (1990–2). Epidemiol. Infect. 1995, 115, 89–100. [Google Scholar] [CrossRef]
- Watt, J.P.; Wolfson, L.J.; O’Brien, K.L.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Levine, O.S.; Hajjeh, R.; Mulholland, K.; et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: Global estimates. Lancet 2009, 374, 903–911. [Google Scholar] [CrossRef]
- Peltola, H. Worldwide Haemophilus influenzae Type b Disease at the Beginning of the 21st Century: Global Analysis of the Disease Burden 25 Years after the Use of the Polysaccharide Vaccine and a Decade after the Advent of Conjugates. Clin. Microbiol. Rev. 2000, 13, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.K.; Moss, W.J.; Halsey, N. Haemophilus influenzae type b conjugate vaccine use and effectiveness. Lancet Infect. Dis. 2008, 8, 435–443. [Google Scholar] [CrossRef]
- Ning, G.; Yin, Z.; Li, Y.; Wang, H.; Yang, W. Cost-effectiveness of the Haemophilus influenzae type b vaccine for infants in mainland China. Hum. Vaccines Immunother. 2018, 14, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. DTPa-HBV-IPV/Hib Vaccine (Infanrix hexaTM): A Review of its Use as Primary and Booster Vaccination. Drugs 2010, 70, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, L.; Spanjaard, L.; Van Der Ende, A.; Schuurman, I.; Dankert, J. Effect of nationwide vaccination of 3-month-old infants in The Netherlands with conjugate Haemophilus influenzae type b vaccine: High efficacy and lack of herd immunity. J. Pediatr. 1997, 131, 869–873. [Google Scholar] [CrossRef]
- Perdue, D.G. Invasive Haemophilus influenzae Disease in Alaskan Residents Aged 10 Years and Older Before and After Infant Vaccination Programs. JAMA 2000, 283, 3089. [Google Scholar] [CrossRef]
- Garpenholt, Ö.; Hugosson, S.; Fredlund, H.; Bodin, L.; Olcén, P. Epiglottitis in Sweden before and after introduction of vaccination against Haemophilus influenzae type b. Pediatr. Infect. Dis. J. 1999, 18, 490–493. [Google Scholar] [CrossRef]
- Ojo, L.R.; O’Loughlin, R.E.; Cohen, A.L.; Loo, J.D.; Edmond, K.M.; Shetty, S.S.; Bear, A.P.; Privor-Dumm, L.; Griffiths, U.K.; Hajjeh, R. Global use of Haemophilus influenzae type b conjugate vaccine. Vaccine 2010, 28, 7117–7122. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. GBD Results. 2025. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 25 June 2025).
- Gosnell, G.; Braid, E.; Basnak, M. Vaccine Delivery: Timelines and Drivers of Delay in Low- and Middle-Income Countries; Rethink Priorities: San Francisco, CA, USA, 2023. [Google Scholar]
- Lewis, R. Action for child survival: Elimination of Haemophilus influenzae type b meningitis in Uganda. Bull. World Health Organ. 2008, 86, 292–301. [Google Scholar] [CrossRef]
- Cowgill, K.D.; Ndiritu, M.; Nyiro, J.; Slack, M.P.E.; Chiphatsi, S.; Ismail, A.; Kamau, T.; Mwangi, I.; English, M.; Newton, C.R.J.C.; et al. Effectiveness of Haemophilus influenzae type b Conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA 2006, 296, 671–678. [Google Scholar] [CrossRef]
- Sultana, N.K.; Saha, S.K.; Al-Emran, H.M.; Modak, J.K.; Sharker, M.A.Y.; El-Arifeen, S.; Cohen, A.L.; Baqui, A.H.; Luby, S.P. Impact of Introduction of the Haemophilus influenzae Type b Conjugate Vaccine into Childhood Immunization on Meningitis in Bangladeshi Infants. J. Pediatr. 2013, 163, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Puliyel, J.M. Making a case for universal Hib immunization in India: Over interpreting the data. Indian. J. Med. Res. 2013, 137, 639–641. [Google Scholar] [PubMed]
- Agampodi, S.; Tadesse, B.T.; Sahastrabuddhe, S.; Excler, J.-L.; Kim, J.H. Biases in COVID-19 vaccine effectiveness studies using cohort design. Front. Med. 2024, 11, 1474045. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, X.; Mak, J.; Sriudomporn, S.; Zhang, H.; Fang, H.; Patenaude, B. Coverage and Equity of Childhood Vaccines in China. JAMA Netw. Open 2022, 5, e2246005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Garcia, C.; Yu, W.; Knoll, M.D.; Lai, X.; Xu, T.; Jing, R.; Qin, Y.; Yin, Z.; Wahl, B.; et al. National and provincial impact and cost-effectiveness of Haemophilus influenzae type b conjugate vaccine in China: A modeling analysis. BMC Med. 2021, 19, 181. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, X.; Patenaude, B.N.; Jit, M.; Fang, H. Adding new childhood vaccines to China’s National Immunization Program: Evidence, benefits, and priorities. Lancet Public. Health 2023, 8, e1016–e1024. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Asturias, E.; Tang, S.; Cui, F. Promoting higher-valent pediatric combination vaccines in China: Challenges and recommendations for action. Infect. Dis. Poverty 2024, 13, 12. [Google Scholar] [CrossRef]
- World Bank DataBank. Health Nutrition and Population Statistics. 2025. Available online: https://databank.worldbank.org/source/health-nutrition-and-population-statistics# (accessed on 25 June 2025).
- UNICEF Vaccines Pricing Data. 2025. Available online: https://www.unicef.org/supply/vaccines-pricing-data (accessed on 25 June 2025).
- International Labour Organization. Data Tools to Find and Download Labour Statistics—ILOSTAT 2025. Available online: https://ilostat.ilo.org/data/ (accessed on 26 June 2025).
- Feenstra, R.C.; Inklaar, R.; Timmer, M.P. The Next Generation of the Penn World Table. Am. Econ. Rev. 2015, 105, 3150–3182. [Google Scholar] [CrossRef]
- World Bank Open Data. GDP (Current US$). 2025. Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD (accessed on 26 June 2025).
- World Bank. World Bank Country and Lending Groups—World Bank Data Help Desk. 2025. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 26 June 2025).
- Our World in Data. Gavi Supported Countries. 2025. Available online: https://ourworldindata.org/grapher/gavi-supported-countries (accessed on 26 June 2025).
- Athey, S.; Wager, S. Estimating Treatment Effects with Causal Forests: An Application. Obs. Stud. 2019, 5, 37–51. [Google Scholar] [CrossRef]
- Miller, S. Causal forest estimation of heterogeneous and time-varying environmental policy effects. J. Environ. Econ. Manag. 2020, 103, 102337. [Google Scholar] [CrossRef]
- Pandey, A.; Brauer, M.; Cropper, M.L.; Balakrishnan, K.; Mathur, P.; Dey, S.; Turkgulu, B.; Kumar, G.A.; Khare, M.; Beig, G.; et al. Health and economic impact of air pollution in the states of India: The Global Burden of Disease Study 2019. Lancet Planet. Health 2021, 5, e25–e38. [Google Scholar] [CrossRef]
- Evaluation U of WI for HM and, Weltbank. In The Cost of Air Pollution: Strengthening the Economic Case For Action; World Bank Group: Washington, DC, USA, 2016.
- Haacker, M.; Hallett, T.B.; Atun, R. On discount rates for economic evaluations in global health. Health Policy Plan. 2019, 35, czz127. [Google Scholar] [CrossRef]
- Edejer, T.T.-T.; Edejer, T.T.-T. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis; World Health Organization: Geneva, Switzerland, 2003; Volume 1. [Google Scholar]
- Schaffer, A.L.; Dobbins, T.A.; Pearson, S.-A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: A guide for evaluating large-scale health interventions. BMC Med. Res. Methodol. 2021, 21, 58. [Google Scholar] [CrossRef]
- Doubilet, P.; Begg, C.B.; Weinstein, M.C.; Braun, P.; McNeil, B.J. Probabilistic Sensitivity Analysis Using Monte Carlo Simulation: A Practical Approach. Med. Decis. Mak. 1985, 5, 157–177. [Google Scholar] [CrossRef]
- Hodson, T.O. Root mean square error (RMSE) or mean absolute error (MAE): When to use them or not. Geosci. Model. Dev. Discuss. 2022, 15, 5481–5487. [Google Scholar] [CrossRef]
- Adegbola, R.A.; Secka, O.; Lahai, G.; Lloyd-Evans, N.; Usen, S.; Oluwalana, C.; Obaro, S.; Weber, M.; Corrah, T.; Nije, A.; et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: A prospective study. Lancet 2005, 366, 144–150. [Google Scholar] [CrossRef]
- Flatt, A.; Vivancos, R.; French, N.; Quinn, S.; Ashton, M.; Decraene, V.; Hungerford, D.; Taylor-Robinson, D. Inequalities in uptake of childhood vaccination in England, 2019–2023: Longitudinal study. BMJ 2024, 387, e079550. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.N.; Costa, F.S.; Cata-Preta, B.O.; Lanata, C.F.; Araujo, M.A.M.; Ochoa, J.; Mengistu, T.; Hogan, D.; Barros, A.J.D.; Victora, C.G.; et al. Analysis of national health surveys for equity of vaccine introductions, Peru, 2004–2022. Bull. World Health Organ. Available online: https://cdn.who.int/media/docs/default-source/bulletin/online-first/blt.24.292434.pdf?sfvrsn=6db09fe3 (accessed on 24 November 2025).
- Ozawa, S.; Clark, S.; Portnoy, A.; Grewal, S.; Stack, M.L.; Sinha, A.; Mirelman, A.; Franklin, H.; Friberg, I.K.; Tam, Y.; et al. Estimated economic impact of vaccinations in 73 low- and middle-income countries, 2001–2020. Bull. World Health Organ. 2017, 95, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mukandavire, C.; Cucunubá, Z.M.; Echeverria Londono, S.; Abbas, K.; Clapham, H.E.; Jit, M.; Johnson, H.L.; Papadopoulos, T.; Vynnycky, E.; et al. Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: A modelling study. Lancet 2021, 397, 398–408. [Google Scholar] [CrossRef]
- Deogaonkar, R.; Hutubessy, R.; Van Der Putten, I.; Evers, S.; Jit, M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public. Health 2012, 12, 878. [Google Scholar] [CrossRef]
- Saxenian, H.; Alkenbrack, S.; Freitas Attaran, M.; Barcarolo, J.; Brenzel, L.; Brooks, A.; Ekeman, E.; Griffiths, U.; Rozario, S.; Maele, N.V.; et al. Sustainable financing for Immunization Agenda 2030. Vaccine 2024, 42, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Yim, V.; Cabrera, M.; Moore, M.; Lomazzi, M. Sustainable financing of immunization programs: A narrative review of the literature. Popul. Med. 2024, 6, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Leung, K.; Jit, M.; Wu, J.T.; Lin, L. Global socioeconomic inequalities in vaccination coverage, supply, and confidence. Npj Vaccines 2025, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhang, H.; Pouwels, K.B.; Patenaude, B.; Jit, M.; Fang, H. Estimating global and regional between-country inequality in routine childhood vaccine coverage in 195 countries and territories from 2019 to 2021: A longitudinal study. eClinicalMedicine 2023, 60, 102042. [Google Scholar] [CrossRef] [PubMed]
- Luthra, K.; Zimmermann Jin, A.; Vasudevan, P.; Kirk, K.; Marzetta, C.; Privor-Dumm, L. Assessing vaccine introduction and uptake timelines in Gavi-supported countries: Are introduction timelines accelerating across vaccine delivery platforms? BMJ Glob. Health 2021, 6, e005032. [Google Scholar] [CrossRef]
- Espinal, C.; Becerra-Posada, F.; Torres, J.R. Improving Middle-Income Countries Access to Vaccines. A Blueprint to Overcome Current Challenges. Ann. Glob. Health 2023, 89, 80. [Google Scholar] [CrossRef]
- Pang, T. Vaccination in Developing Countries: Problems, Challenges and Opportunities. Available online: https://www.eolss.net/sample-chapters/c03/e1-14-05-06.pdf (accessed on 28 October 2025).
- Jamison, D.T.; Summers, L.H.; Alleyne, G.; Arrow, K.J.; Berkley, S.; Binagwaho, A.; Bustreo, F.; Evans, D.; A Feachem, R.G.; Frenk, J.; et al. Global health 2035: A world converging within a generation. Lancet 2013, 382, 1898–1955. [Google Scholar] [CrossRef]
- Zhou, F.; Jatlaoui, T.C.; Leidner, A.J.; Carter, R.J.; Dong, X.; Santoli, J.M.; Stokley, S.; Daskalakis, D.C.; Peacock, G. Health and Economic Benefits of Routine Childhood Immunizations in the Era of the Vaccines for Children Program—United States, 1994–2023. Morb. Mortal. Wkly. Rep. 2024, 73, 682–685. [Google Scholar] [CrossRef]
- Abou-Nader, A.; Heffelfinger, J.D.; Amarasinghe, A.; Nelson, E.A.S. Assessing perceptions of establishing a vaccine pooled procurement mechanism for the Western Pacific Region. PLoS Glob. Public Health 2022, 2, e0000801. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, K.; Pisani, E.; Bal, R.; Kok, M.O. A systematic review of pooled procurement of medicines and vaccines: Identifying elements of success. Glob. Health 2022, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- He, A.J. Scaling-up through piloting: Dual-track provider payment reforms in China’s health system. Health Policy Plan. 2023, 38, 218–227. [Google Scholar] [CrossRef] [PubMed]
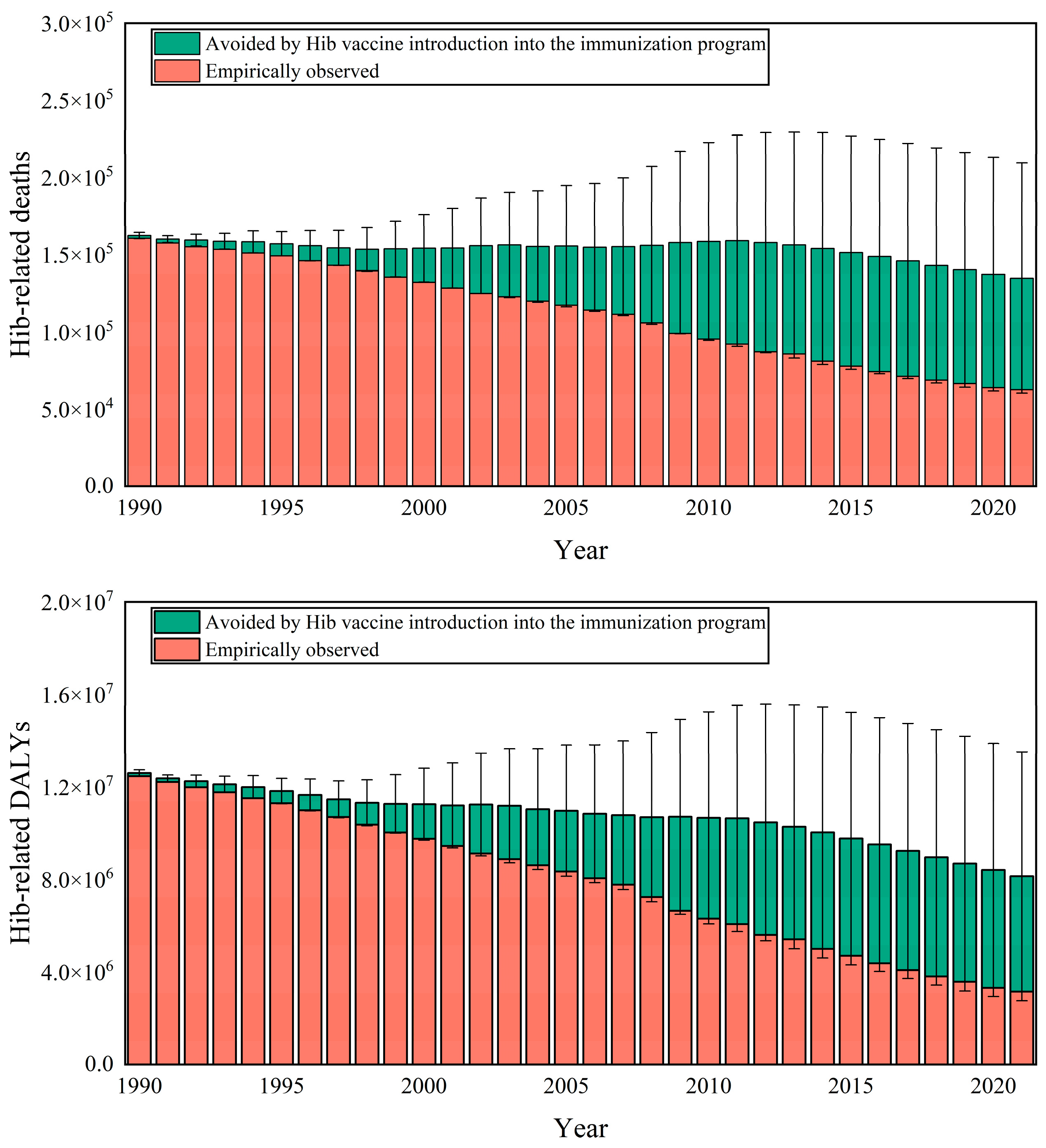
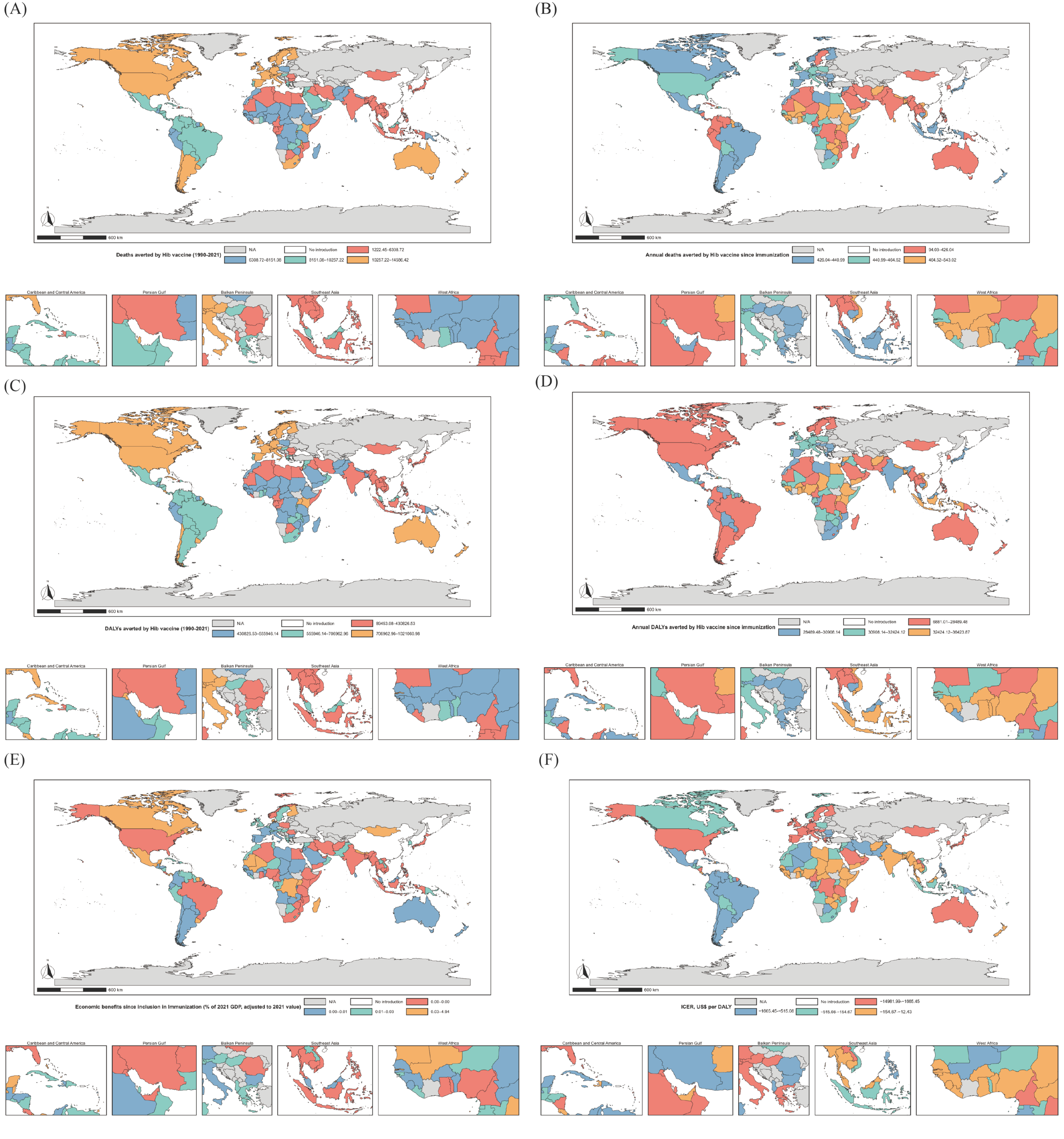
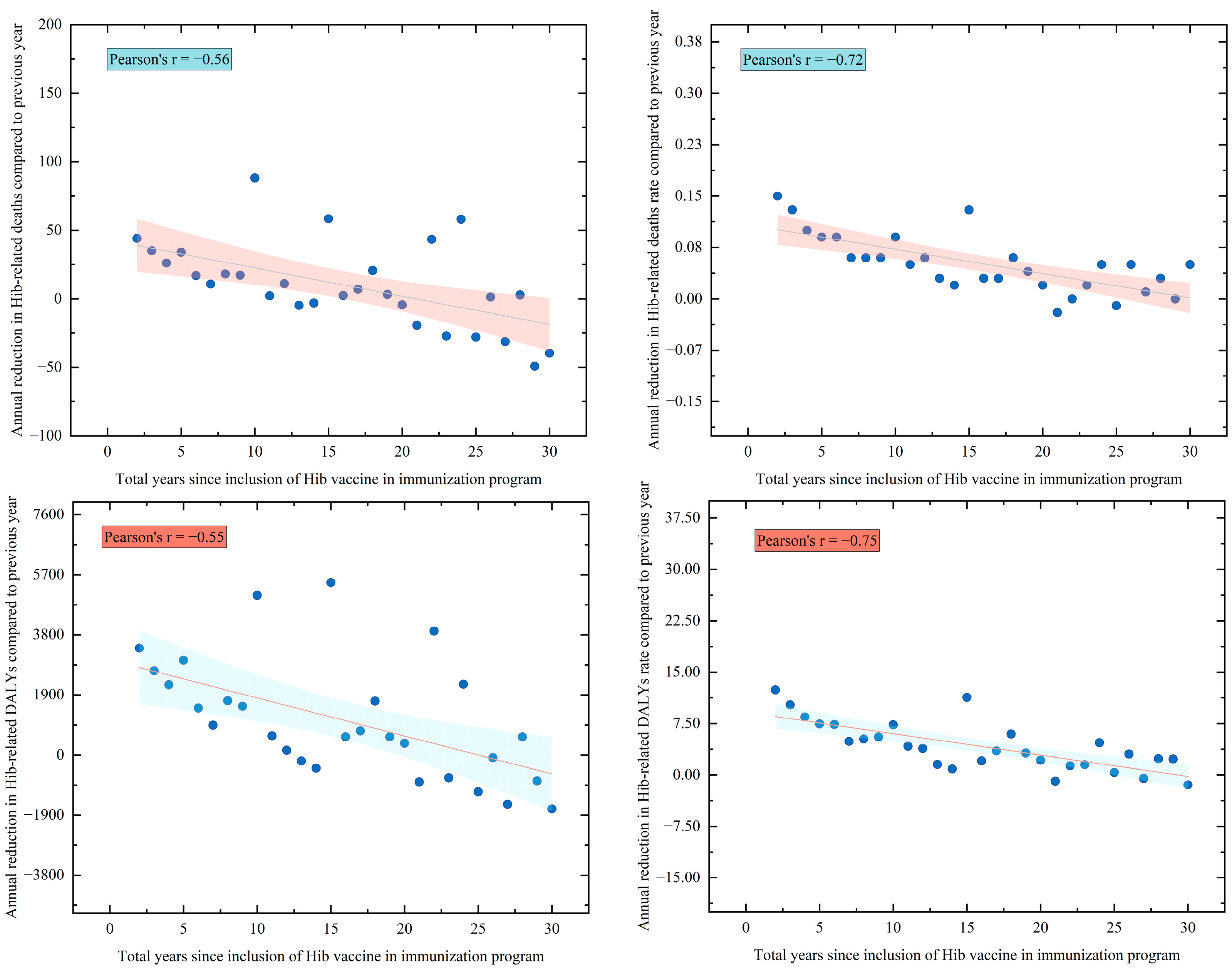

| Groups | Average Introduce Years | 50% Inclusion | Health Benefits of Disease Burden Averted (95% CI) | |||
|---|---|---|---|---|---|---|
| Deaths, Thousand | Death Rate | DALYs, Million | DALY Rate, Thousand | |||
| Region | ||||||
| Africa | 15.90 | 2008 | 351.77 (8.99–749.33) | 954.52 (699.20–1233.56) | 24.35 (1.10–53.05) | 78.00 (55.13–105.70) |
| America | 20.91 | 2000 | 325.97 (8.94–667.68) | 366.61 (318.25–496.74) | 22.19 (0.99–48.2) | 17.61 (17.21–25.28) |
| Asia | 16.19 | 2009 | 234.28 (10.31–478.38) | 320.21 (244.36–427.61) | 16.54 (0.56–36.26) | 18.29 (15.38–26.45) |
| Europe | 23.00 | 1994 | 287.96 (3.34–572.01) | 306.84 (216.89–463.70) | 19.66 (0.61–41.59) | 7.72 (10.34–12.81) |
| Oceania | 17.14 | 2005 | 121.14 (0.45–255.91) | 178.54 (149.44–235.35) | 8.22 (0.31–17.99) | 12.45 (9.45–18.38) |
| Income | ||||||
| Low income | 15.91 | 2008 | 542.07 (9.97–1092.96) | 584.44 (438.19–853.09) | 36.96 (1.2–78.72) | 18.62 (20.95–29.18) |
| Lower-middle income | 16.82 | 2008 | 174.90 (2.97–382.35) | 559.20 (398.89–711.62) | 12.10 (0.22–26.88) | 47.6 (32.61–64.35) |
| Upper-middle income | 16.83 | 2003 | 300.02 (12.51–626.89) | 603.75 (473.36–789.94) | 20.88 (1.38–45.82) | 46.79 (35.43–64.92) |
| High income | 21.75 | 1998 | 304.14 (6.59–621.11) | 379.32 (317.71–502.32) | 21.03 (0.77–45.68) | 21.07 (18.53–30.16) |
| Gavi | ||||||
| Non-Gavi | 18.98 | 2002 | 978.19 (23.33–1981.71) | 1160.54 (920.15–1615.50) | 67.14 (2.47–144.58) | 52.73 (50.32–78.48) |
| Gavi | 16.69 | 2007 | 342.93 (8.71–741.6) | 966.17 (708.00–1241.46) | 23.83 (1.10–52.52) | 81.35 (57.19–110.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Chan, S.; Zhong, Y.; Xu, Z.; Wang, J.; Wang, Y.; Gao, Y.; Xia, Y.; Zhang, D.; Tang, W. Disease and Economic Burden Averted by Hib Vaccination in 160 Countries: A Machine-Learning Analysis. Vaccines 2025, 13, 1197. https://doi.org/10.3390/vaccines13121197
Zhou D, Chan S, Zhong Y, Xu Z, Wang J, Wang Y, Gao Y, Xia Y, Zhang D, Tang W. Disease and Economic Burden Averted by Hib Vaccination in 160 Countries: A Machine-Learning Analysis. Vaccines. 2025; 13(12):1197. https://doi.org/10.3390/vaccines13121197
Chicago/Turabian StyleZhou, Dachuang, Siyang Chan, Yimei Zhong, Zhehong Xu, Jun Wang, Yuntian Wang, Yiyang Gao, Yuting Xia, Di Zhang, and Wenxi Tang. 2025. "Disease and Economic Burden Averted by Hib Vaccination in 160 Countries: A Machine-Learning Analysis" Vaccines 13, no. 12: 1197. https://doi.org/10.3390/vaccines13121197
APA StyleZhou, D., Chan, S., Zhong, Y., Xu, Z., Wang, J., Wang, Y., Gao, Y., Xia, Y., Zhang, D., & Tang, W. (2025). Disease and Economic Burden Averted by Hib Vaccination in 160 Countries: A Machine-Learning Analysis. Vaccines, 13(12), 1197. https://doi.org/10.3390/vaccines13121197






