1. Introduction
Adenovirus, a non-enveloped double-stranded DNA virus [
1], is a common pathogen whose infection can lead to a wide range of clinical manifestations, from mild colds to severe pneumonia [
2]. It poses a heightened health risk to children and immunocompromised individuals [
3], making it a significant public health concern. The virus exhibits distinct “population-specific” and “environment-dependent clustering” transmission patterns, with crowded settings or poorly ventilated enclosed environments being particularly conducive to clustered outbreaks. Among the numerous adenovirus serotypes, types 4 and 7 are the primary serotypes responsible for outbreaks of acute respiratory disease [
4]. Specifically, HAdV-4 is classified within species Human mastadenovirus E, the currently only known member, characterized by a relatively unique genetic structure [
5,
6]. HAdV-4 infection typically presents as classic pharyngoconjunctival fever, characterized clinically by the triad of fever, pharyngitis, and acute conjunctivitis. Regarding prevention and control strategies, the primary measure relies on oral enteric vaccines [
7]. However, there is still a lack of specific antiviral drugs for clinical treatment of adenovirus infection. Current management primarily involves supportive care to alleviate symptoms and cannot directly target the virus itself. This therapeutic gap not only affects patient recovery but also places an additional burden on healthcare systems [
8]. Consequently, the development of safe and effective specific anti-adenovirus therapeutics has become an urgent priority in both research and clinical practice.
We had previously reported the development of a human monoclonal antibody (mAb) directed against human adenovirus type 4 (HAdV-4) and validated its potent neutralizing efficacy [
9]. In parallel, nanobodies (Nbs)—a unique class of single-domain antibodies—have attracted increasing attention as promising anti-infective biologics, owing to their compact architecture and modular nature [
10,
11,
12]. In contrast to conventional mAbs, nanobodies exhibit enhanced tissue permeability, facilitating efficient access to confined infection sites such as the respiratory mucosa [
10]. By targeting cryptic and conserved viral epitopes, single-domain antibodies effectively counteract highly mutable pathogens, making nanobody screening a more suitable strategy [
13]. Additionally, nanobodies demonstrate exceptional structural and thermal stability, favorable pharmacokinetics, and ease of engineering [
10], rendering them compelling candidates for novel antiviral strategies.
Antibody-based therapeutics have demonstrated significant and unique advantages in the treatment of viral infections [
14,
15]. Antibody-based therapies play a central role in the treatment of viral infections by leveraging their high specificity for target engagement. The primary mechanism of action involves the precise recognition of key viral surface antigens by exogenously administered neutralizing antibodies. This binding directly blocks viral attachment to host cell receptors, thereby neutralizing viral infectivity [
16]. Certainly, the therapeutic effect extends beyond direct viral neutralization to actively mobilizing and reprogramming immune cells within the body. Upon binding to viruses or infected cells via their Fab regions, therapeutic antibodies employ their Fc segment as a potent “recruitment signal,” rapidly activating natural killer (NK) cells of the innate immune system. This engagement significantly enhances the clearance of antibody-tagged viral particles or damaged cells. Notably, this process also shapes adaptive immunity through enhanced antigen presentation—viral particles opsonized by antibodies or debris from cleared infected cells can be more efficiently captured, processed, and presented by antigen-presenting cells such as dendritic cells. This process amplifies the activation and expansion of virus-specific CD8
+ T lymphocytes, which in turn execute targeted elimination of infected host cells, thereby resolving established infections and establishing durable antiviral immunity [
17].
TRIM21 (tripartite motif-containing protein 21) is a multifunctional intracellular protein that exhibits both cytosolic Fc receptor and E3 ubiquitin ligase activities, playing a central role in innate immune responses [
18]. Conventionally, antibodies are thought to neutralize viruses extracellularly via their variable regions, thereby preventing viral entry into host cells [
16]. Recent studies have revealed that antibodies against non-enveloped viruses can mediate a process known as antibody-dependent intracellular neutralization (ADIN): following the internalization of virus–antibody complexes into the cell, TRIM21 recognizes and binds to the complex, leading to viral degradation via the ubiquitin–proteasome pathway and activation of signaling cascades such as NF-κB and AP-1, thereby achieving efficient clearance of intracellular viruses [
19]. As a typical non-enveloped virus, HAdV-4 has a cell membrane receptor that remains incompletely characterized [
20]. Our previous work suggested that TRIM21 may serve as a potential intracellular receptor for the human monoclonal antibody 2CF4 against HAdV-4, although the underlying molecular mechanism requires further validation. In this study, we identified an engineered nanobody, NVA17, which is hypothesized to exert antiviral effects by activating TRIM21 and its downstream ubiquitin–proteasome pathway [
21]. The present work aims to further elucidate the detailed molecular mechanism by which NVA17 mediates antiviral activity via the TRIM21-dependent ADIN pathway.
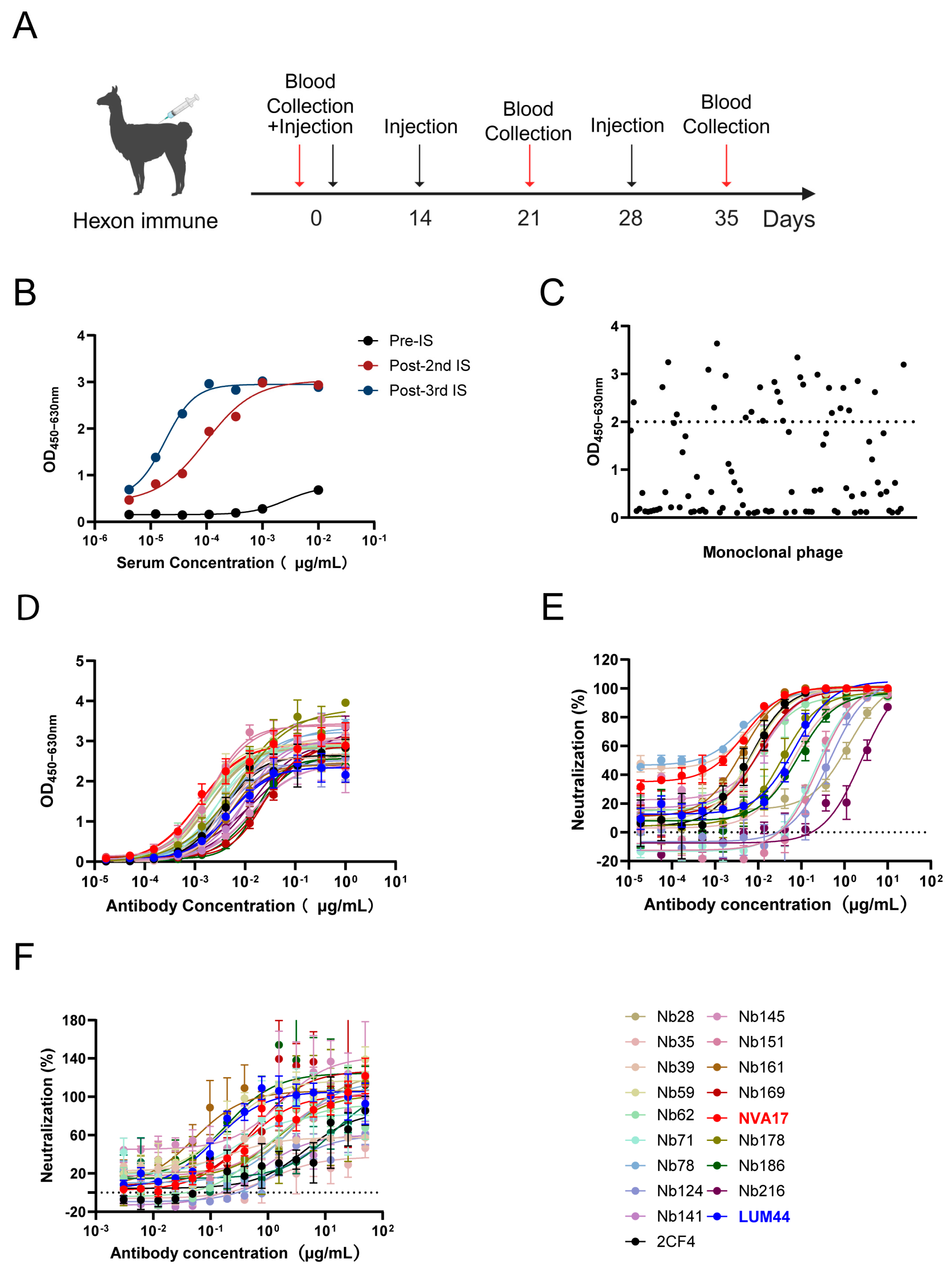
Therefore, this study employed an alpaca immunization strategy. Following three rounds of immunization with the hexon antigen protein, we successfully isolated multiple neutralizing nanobodies targeting HAdV-4 from the immune serum. To enhance its immune effector functions and drug-like properties [
22], we further engineered it by fusion with a human Fc fragment. In in vitro assays, the lead candidate, designated NVA17, exhibited excellent neutralizing activity. In a STAT1-deficient mouse model of lethal HAdV-4 infection [
9], this antibody provided complete protection and effectively modulated systemic immune responses.
2. Materials and Methods
2.1. Viruses and Cell Lines
The human embryonic kidney cell line (HEK293T) and the human lung adenocarcinoma epithelial cell line (A549) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NE, USA, Cat. No. C11995500BT) supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin, and 100 IU/mL penicillin (Gibco, Cat. No. 15140122). All cells were maintained at 37 °C in a humidified incubator with 5% CO2. Expi293F human cells (derived from the 293F cell line) were grown in suspension using Expi293 Expression Medium at 37 °C, 5% CO2, and 110 rpm on an orbital shaker. The recombinant adenoviruses Ad4-RI67 and Ad4-Luc used in this study were either maintained or generated in our laboratory.
2.2. Expression and Purification of the Hexon Protein
To obtain the HAdV-4 viral protein, infected 293F cells were cultured for 72 h and then subjected to three freeze–thaw cycles for complete lysis. The lysate was centrifuged at 4 °C and 4500 rpm for 15 min. The supernatant was collected and filtered through a 0.22 μm filter (PALL, Cat. No. 4612). The filtrate was first purified using a Core 700 size-exclusion column (Cytiva, Uppsala, Sweden, Cat. No. 17548115) equilibrated with a buffer containing 20 mM Tris and 150 mM NaCl (pH 7.5). The elution peak corresponding to the target protein, monitored by UV absorbance, was collected. This fraction was concentrated using a 30 kDa ultrafiltration tube (Merck Millipore, Carrigtwohill, Co Cork, Ireland, Cat. No. UFC903096) and then further purified by a Superdex 200 size-exclusion column (Cytiva) using PBS as the mobile phase. Again, the elution peak of the target protein was collected based on UV monitoring. The purified fractions were pooled, and the protein concentration was determined using a BCA assay kit (Merck Millipore, Darmstadt, Germany, Cat. No. 23225). The final protein samples were aliquoted and stored at −80 °C. The purity and immunoreactivity of the protein samples were verified by SDS-PAGE and Western blotting, respectively.
2.3. Alpaca Immunization
To isolate neutralizing Nbs against HAdV-4, an alpaca was subcutaneously immunized with 1 mg of recombinant HAdV-4 hexon protein formulated in Freund’s adjuvant on day 0. This was followed by two booster immunizations administered at two-week intervals, each with 0.7 mg of hexon protein in Freund’s adjuvant, resulting in a total of three immunizations. Beginning after the second immunization, blood was collected 7 days after each booster, and serum antibody titers were measured by ELISA. Whole RNA was extracted from peripheral blood mononuclear cells and reverse-transcribed into cDNA after quality verification by electrophoresis. The VHH-encoding sequences were amplified by two rounds of PCR, purified, digested with SfiI, and cloned into the phagemid vector pComb3X. The ligated product was electroporated into XL1-Blue competent cells, yielding an initial library with a capacity of 1.2 × 109 CFU. Random clone sequencing confirmed 100% correctness. Finally, the library was infected with VCSM13 helper phage to generate the nanobody phage display library.
2.4. VHH Library Generation
To screen for specific nanobodies targeting human adenovirus type 4 (HAdV-4), two rounds of biopanning were performed. The high-binding plates were coated with HAdV-4 antigen (5 μg/mL) and incubated overnight at 4 °C, followed by blocking with 3% skim milk powder to reduce non-specific binding. The phage display library, with an initial capacity of 1.2 × 109 CFU (approximately 5 × 1012 phage particles), was added to the antigen-coated wells and incubated at 37 °C for 2 h to facilitate binding. Unbound and weakly bound phages were removed by washing with PBS containing 0.1% Tween 20 (PBST). To increase selection stringency, the number of washing steps was incrementally raised—10 times in the first round and 15 times in the second round. Specifically bound phages were eluted using 0.1 M glycine-HCl (pH 2.2) and immediately neutralized with 1 M Tris-HCl (pH 7.4). The eluted phages were used to infect E. coliXL1-Blue cells, rescued with VCSM13 helper phage, amplified, and purified by PEG 8000/NaCl precipitation for the subsequent round of panning. Enrichment efficiency was evaluated after each round by phage titer determination. Following the second round of panning, individual clones were selected for phage ELISA screening. An HRP-conjugated anti-M13 antibody was used as the secondary antibody. Clones exhibiting an OD450 value ≥ 0.5 and a positive-to-negative (P/N) ratio greater than 3 were identified as positive binders. Positive candidates were sent to Sangon Biotech (Shanghai, China) for sequencing and sequence alignment. In all ELISA, 5% bovine serum albumin (BSA) was used as the negative control.
2.5. Protein Purification
To obtain VHH-Fc fusion proteins, the VHH coding sequences from selected phage clones were amplified and cloned into the pcDNA3.4 eukaryotic expression vector. The vector contains a CMV promoter, a tPA signal peptide, and the human IgG1 Fc gene frame, allowing in-frame fusion expression under the control of the SV40 poly(A) signal when the gene of interest is inserted into the multiple cloning site. The recombinant plasmids were transfected into Expi293F cells using ExpiFectamine 293 Transfection Kit (Thermo Fisher Scientific, Carlsbad, CA, USA, Cat. No. A14526), followed by 4 days of culture after which the supernatant was collected. The collected supernatant was pre-treated by two-step centrifugation (800× g for 10 min and 4000× g for 15 min) and filtration through a 0.22 μm membrane (PALL, Bristol, UK, Ca. No. 4612). The target protein was then purified using an ÄKTA pure 150 system with a 5 mL Protein A affinity chromatography column, equilibrated with PBS (pH 7.5) and eluted with 0.1 M glycine (pH 2.7). Protein purity was analyzed by SDS-PAGE, and concentration was determined by either the BCA method or UV absorbance measurement.
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
To evaluate the antibody binding activity to hexon protein, an indirect ELISA was performed. Purified hexon protein was coated onto 96-well plates at 2 μg/mL (100 μL/well) and incubated overnight at 4 °C. After discarding the coating solution, the plates were washed three times with PBS containing 0.02% Tween 20 (PBST). Each well was then blocked with 100 μL of PBS containing 2% BSA at 37 °C for 1 h. Subsequently, purified antibodies were subjected to threefold serial dilution starting from 1 μg/mL (with three replicates per concentration). A volume of 100 μL of diluted antibody was added to each well and incubated at 37 °C for 1 h, followed by three washes with PBST. Then, HRP-conjugated goat anti-human IgG Fc secondary antibody (Abcam97225, Cambridge, UK, 1:10,000 dilution) diluted at 1:10,000 was added and incubated at 37 °C for 1 h in the dark. After washing, 100 μL of TMB substrate (Solarbio, Beijing, China, Cat. No. PR1200) was added to each well and incubated at room temperature in the dark for 5–6 min. The reaction was terminated by adding 50 μL of stop solution. The absorbance was measured at 450 nm with a reference wavelength of 630 nm using a microplate reader, and the specific binding signal was calculated after background correction.
2.7. Nanobodies Neutralization Experiment Against HAdV-4
The Nbs were serially diluted threefold in DMEM supplemented with 10% FBS, starting from an initial concentration of 50 μg/mL. An equal volume (50 μL) of the diluted Nbs was mixed with 50 μL of the Ad4-Luc recombinant virus solution and incubated (37 °C, 5% CO2) for 1 h. Subsequently, 100 μL of an A549 cell suspension (2 × 105 cells/mL) was added to each well. (final volume: 200 μL/well), with appropriate controls included (positive control: virus plus cells; negative control: cells only). After 24 h of culture at 37 °C under 5% CO2, the medium was aspirated, and cells were lysed with luciferase cell culture lysis reagent for 10 min. Luciferase activity was measured using a 20 μL aliquot of the lysate. The neutralization efficiency was calculated based on the signal reduction in test groups relative to the virus control.
To investigate the role of TRIM21 in antibody-mediated intracellular neutralization, the same assay was performed using TRIM21-knockdown, TRIM21-overexpressing A549 cells, and their corresponding empty vector controls instead of wild-type cells. The neutralizing efficiencies across these genetically modified cell lines were compared.
To examine the dependency of the neutralization process on the proteasomal pathway, the proteasome inhibitor MG132 (1 μM) was added to the culture wells at the time of seeding with either wild-type or TRIM21-overexpressing A549 cells. Following a 24 h incubation at 37 °C, luciferase activity was measured and compared with that of untreated control groups.
2.8. Authentic Virus Neutralization Assay
The neutralizing activity against wild-type HAdV-4 (strain RI67) was determined using a microneutralization assay under Biosafety Level 2 (BSL-2) conditions. Briefly, A549 cells were seeded in 96-well plates at a density of 5 × 104 cells/well in 100 μL of DMEM supplemented with 10% FBS and cultured overnight at 37 °C under 5% CO2. Antibodies were subjected to threefold serial dilution starting from 50 μg/mL and mixed with an equal volume of HAdV-4 containing 100 TCID50, followed by incubation at 37 °C for 1 h. After removing the culture medium, 100 μL of the virus-antibody mixture was added to the cells and incubated for 3 days at 37 °C with 5% CO2. Cytopathic effects were examined by microscopy, and cell viability was quantified using a CCK-8 assay. For the CCK-8 assay, the supernatant was discarded, and 100 μL of DMEM containing 2% FBS and 4% CCK-8 reagent was added to each well, followed by incubation at 37 °C for 40 min in the dark. Absorbance was measured at 450 nm using a microplate reader. The half-maximal inhibitory concentration (IC50) was calculated based on antibody dilution concentrations and corresponding cell viability rates.
2.9. Competition-Binding ELISA
A competitive ELISA was performed to analyze the binding competition of antibodies to the hexon protein. Antibodies (100 μg) were biotinylated using EZ-Link™ Sulfo-NHS-Biotin at a 1:20 molar ratio (antibody:biotin), purified using a desalting column, and stored at 4 °C in the dark after concentration measurement. Ninety-six-well plates were coated with 1 μg/mL hexon protein (100 μL/well) and incubated overnight at 4 °C. After washing with PBST, the plates were blocked with 2% BSA for 1 h at 37 °C. A total of 50 μL of unlabeled competing antibody (100 × EC50) was added to each well and incubated for 30 min at 37 °C, followed by the addition of 50 μL of biotinylated detection antibody (1 × EC50) and another 30 min incubation at 37 °C. After washing, a 1:10,000 dilution of streptavidin-HRP was added and incubated for 1 h at 37 °C. The reaction was developed with TMB substrate for 6 min, stopped, and the absorbance was measured. The competition rate was calculated as (OD of competition well/OD of control well) × 100%. A competition rate of <33.3% was considered strong competition, 33.3–66.7% as weak competition, and >66.7% as no competition.
2.10. Surface Plasmon Resonance (SPR) Assay
Antibody–antigen binding kinetics were determined by surface plasmon resonance (SPR) using a Biacore T200 instrument (Cytiva). The antibody was diluted in HBS-EP+ buffer (Cytiva) to a concentration of 0.5 μg/mL and captured on a Protein A sensor chip at a flow rate of 10 μL/min for 60 s. The purified antigen was tested at serially diluted concentrations (100, 50, 25, 12.5, and 6.25 nM) at a flow rate of 30 μL/min. The association and dissociation phases were monitored for 120 s and 900 s, respectively.
2.11. In Vivo Animal Challenge Experiment
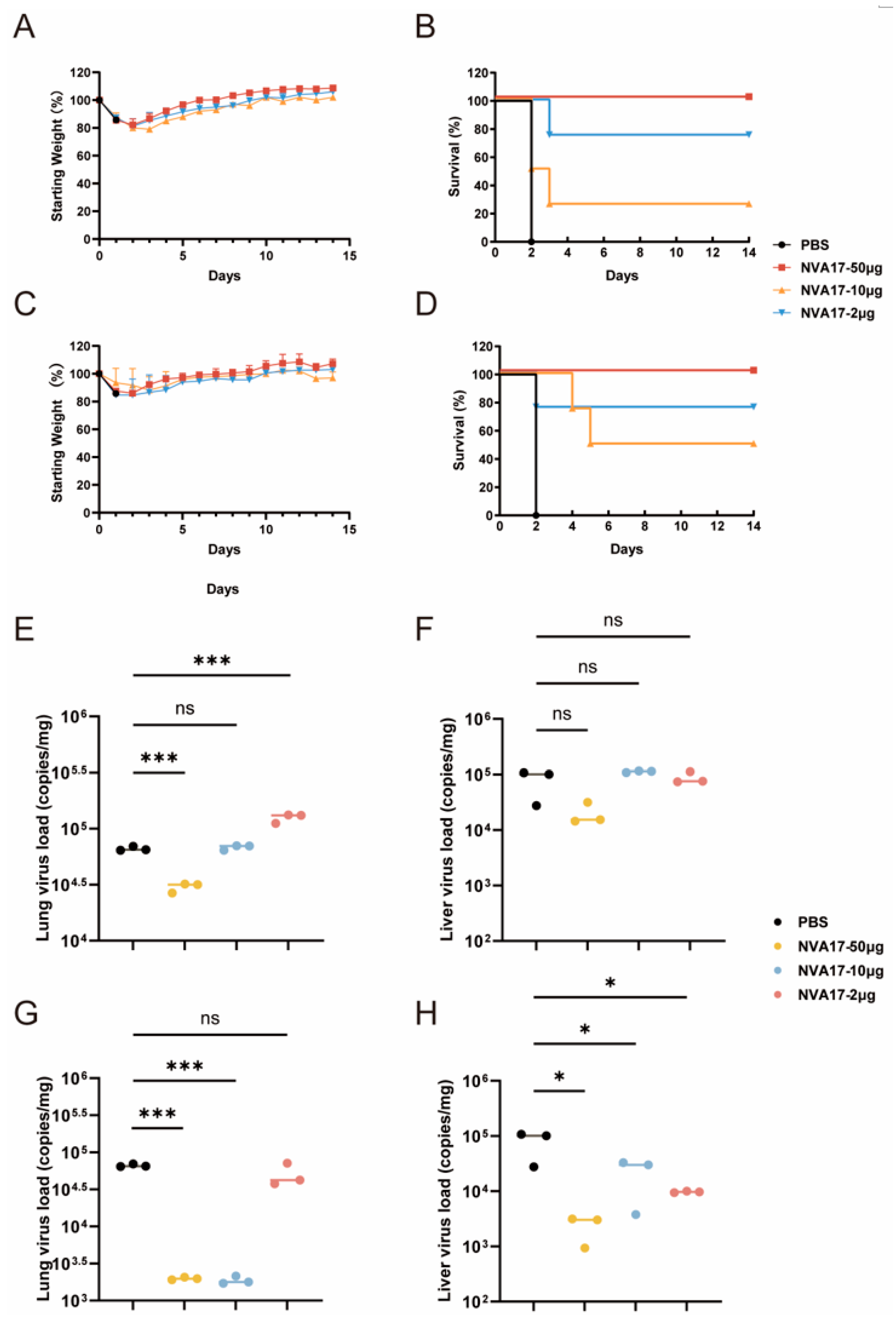
All procedures involving 6- to 8-week-old male STAT1+/− transgenic mice (Shanghai Model Organisms Center, Beijing, China), which were housed under BSL-2 conditions with ad libitum access to food and water, were approved by the Animal Ethics Committee of the Laboratory Animal Center, Academy of Military Medical Sciences. In the prophylactic model, mice were intraperitoneally administered 100 μL of PBS containing graded doses (50, 10, or 2 μg) of nanobody NVA17. 24 h later, they were challenged intraperitoneally with 6 × 1010 PFU of authentic HAdV-4. In the therapeutic model, mice were first infected with an equivalent viral dose and then received the same nanobody treatments at 3 h post-infection. Survival rates and body weight changes were monitored daily for 14 days post-infection (n = 4). For the viral load experiment, on day 3 post-infection, euthanasia was performed via cervical dislocation (n = 4). Heart, liver, spleen, lung, and kidney tissues were collected for viral load quantification by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Histopathological examination was conducted after 48 h of fixation in 10% formalin, paraffin embedding, sectioning, and hematoxylin-eosin (H&E) staining.
2.12. Viral Load Quantification by RT-qPCR
Viral load was quantified by quantitative real-time PCR (RT-qPCR), using DNA from viral stocks as a standard to calculate genomic DNA copy numbers. Genomic DNA was extracted from tissue samples using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany, Cat. No. 57704). Amplification was performed using SYBR Green Supermix with the following primers: HAdV-4-F (5′-CAAGGACTACCAGGCCGTCA-3′) and HAdV-4-R (5′-GTTAGCATAGAGCATGTTCT-3′). The thermal cycling protocol included an initial step at 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. A melting curve analysis was performed by heating from 60 °C to 95 °C at a rate of 0.15 °C/s with continuous fluorescence acquisition to verify amplification specificity. Viral genome copies were quantified using a standard curve generated from serially diluted DNA standards.
2.13. Flow Cytometry
Spleens were aseptically harvested from euthanized mice and placed in ice-cold PBS containing 2% FBS. A single-cell suspension was obtained by gently homogenizing the spleen through a 70 μm cell strainer. The filtrate was centrifuged at 600× g for 5 min at 4 °C, and the supernatant was discarded. The cell pellet was treated with red blood cell lysis buffer for 1–2 min at room temperature protected from light. After stopping the reaction, the sample was centrifuged again under the same conditions. Cell viability and concentration were assessed using an automated cell counter. Cells were adjusted to a concentration of 1 × 107 cells/mL and aliquoted into a U-bottom 96-well plate. Sequential incubations were performed as follows: Fc receptor blocking with anti-mouse CD16/CD32 antibody (20 min, 4 °C, dark); staining with a fixable viability dye (eFluor™ 506; 20 min, 4 °C, dark); and incubation with a pre-titrated cocktail of fluorochrome-conjugated antibodies (30 min, 4 °C, dark). Cells were then washed three times with PBS containing 2% FBS by centrifugation. Finally, the cell suspension was filtered through a 40 μm cell strainer prior to flow cytometry acquisition.
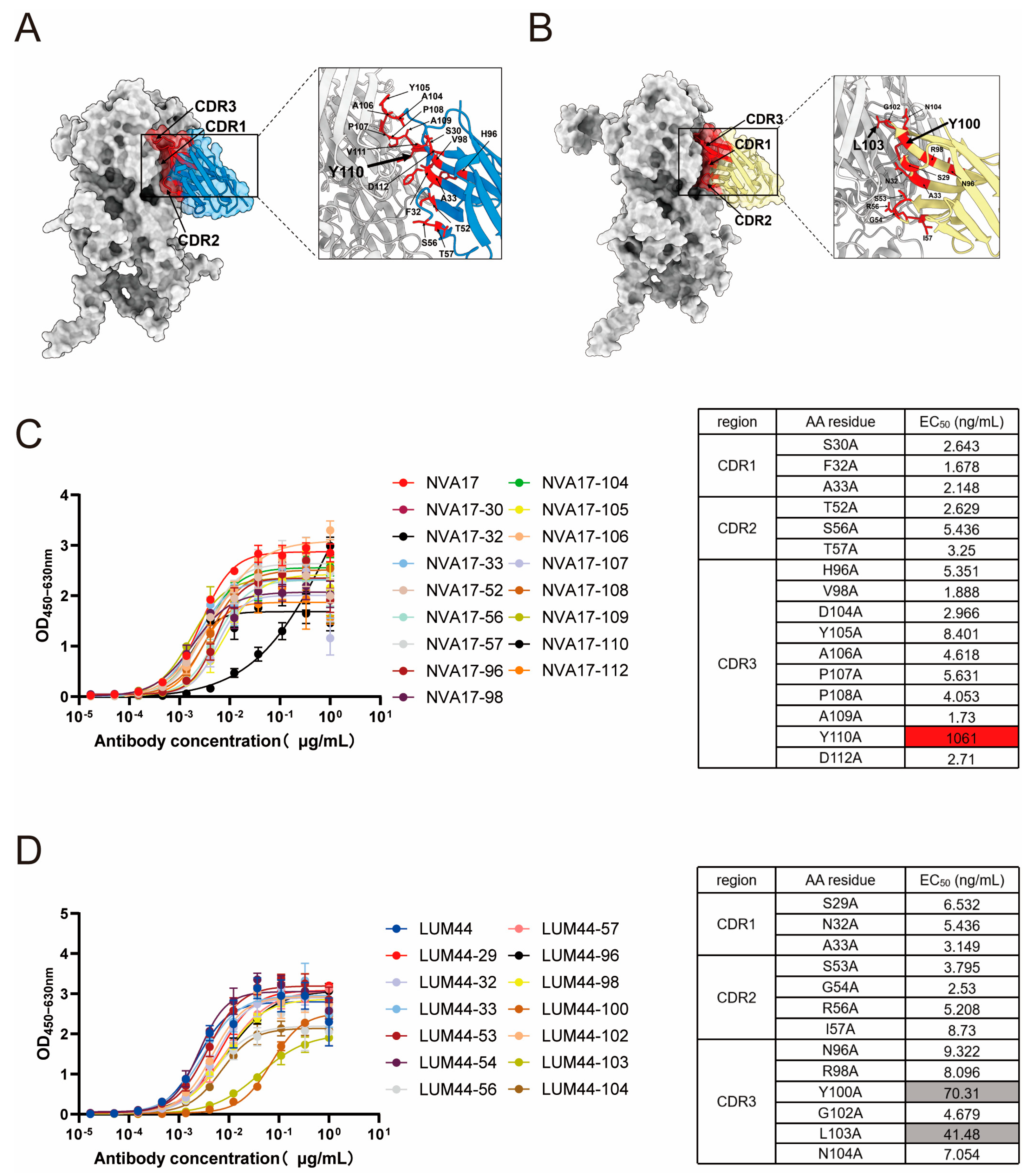
2.14. Simulation of Structure of Antigen–Antibody Complexes Through Alphafold3
The amino acid sequences of the adenovirus hexon protein (obtained from the GenBank database) and the nanobody heavy chain were input into a locally deployed AlphaFold3 (version 3.0.0) platform for complex structure prediction. Simulations were performed using default parameters. The generated models were evaluated based on multiple confidence metrics, including the predicted local distance difference test (pLDDT), predicted aligned error (PAE), predicted template modeling (pTM) score, and interface pTM (ipTM) score. The highest-ranking model was selected and visualized using UCSF ChimeraX (version 1.8) to analyze key interacting residues at the antigen–antibody interface.
2.15. Immunofluorescence Assay
Wild-type, TRIM21-overexpressing, and TRIM21-knockdown A549 cells were seeded on coverslips in 24-well plates at a density of 5 × 104 cells/well and cultured overnight at 37 °C for adhesion. Prior to infection, cells were gently washed twice with DMEM. Ad4-RI67 virus (1 × 108 PFU) was mixed with 10 μg of nanobody NVA17 (negative control: non-neutralizing nanobody 127) in 500 μL of DMEM and pre-incubated at room temperature for 30 min. After removing the culture medium, 500 μL of the virus-antibody mixture was added to each well and incubated at 37 °C for 6 h. Post-infection, cells were washed three times with PBS, fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized with 0.5% Triton X-100 for 15 min, and blocked with PBS containing 5% BSA and 0.1% Tween-20 for 1 h. For immunostaining, cells were incubated with a rabbit anti-TRIM21 primary antibody (1:200 dilution) at 4 °C for 1 h, followed by three washes with PBST. Subsequently, cells were incubated with Alexa Fluor 594-conjugated goat anti-rabbit secondary antibody (1:400) and DyLight 488-conjugated goat anti-human secondary antibody (1:1000) for 30 min each at room temperature in the dark, with three PBST washes after each secondary antibody incubation. Nuclei were stained with DAPI (1:2000 dilution) for 10–15 min. All fluorescence images were acquired using a Zeiss Axio Observer microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) equipped with a 63× objective.
2.16. Differential Scanning Calorimetry (DSC) Assay
The thermodynamic stability of the antibody was assessed by differential scanning calorimetry (DSC) using a Nano DSC instrument. The system was equilibrated for 1 h using the DSCRun™ software (version 3.11.0) prior to measurement. The antibody sample was diluted to 1 mg/mL in PBS, and the reference (PBS) was degassed for 10 min using a degassing station. After pressurizing the system to 45 psi, the temperature program was initiated with a heating rate of 1 °C/min from 25 °C to 100 °C, with an initial equilibration time of 600 s. Multiple replicate scans were performed to verify the reproducibility of the results and the stability of the system.
2.17. Cytotoxicity Assay of MG132
A549 cells were seeded in 96-well plates at a density of 8 × 104 cells/well in 100 μL of DMEM supplemented with 10% FBS and cultured overnight at 37 °C under 5% CO2. After removing the medium, cells were treated with 100 μL of serially diluted MG132 in DMEM containing 10% FBS and incubated for 24 h. Following microscopic examination for cytopathic effect, cell viability was assessed using a CCK-8 assay. Briefly, the supernatant was discarded, and cells in each well were incubated with 100 μL of DMEM containing 2% FBS and 4% CCK-8 reagent at 37 °C in the dark for 40 min. Absorbance was measured at 450 nm using a microplate reader.
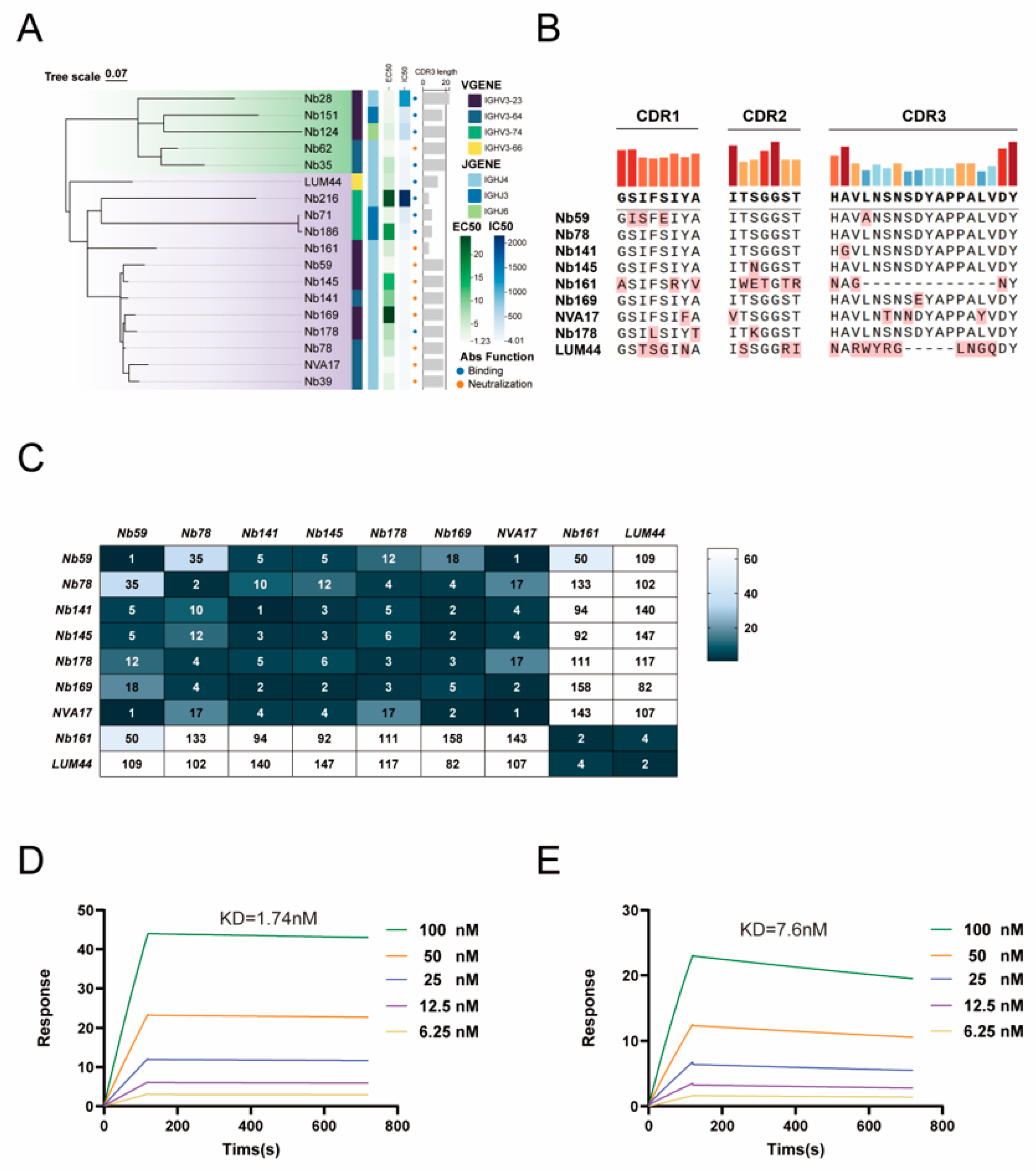
2.18. Quantification and Statistical Analysis
The half-maximal effective concentration (EC50) of sera and neutralizing antibodies in the ELISA was determined by four-parameter nonlinear regression analysis using GraphPad Prism software (version 10.2.1). The neutralization percentage in the viral neutralization assay was calculated as follows: (sample signal − blank control signal)/(virus control signal − blank control signal) × 100%, and the data were fitted using a three-parameter nonlinear regression model in GraphPad Prism (v10.2.1). A phylogenetic tree was constructed with ChiPlot, and the complementarity-determining regions (CDR1, CDR2, and CDR3) of nanobodies were aligned using Abalign (version 1.2.9). Affinity parameters derived from surface plasmon resonance (SPR) experiments—including the association rate (Ka), dissociation rate (Kd), and equilibrium dissociation constant (KD)—were calculated using a 1:1 binding model in the Biacore T200 Evaluation Software (version 3.2). For statistical analysis, a two-tailed Student’s t-test was used for comparisons between two groups, and one-way analysis of variance (ANOVA) followed by Dunnett’s test was applied for comparisons among multiple groups. Data are expressed as the mean ± standard deviation, with significance levels set at * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Discussion
Human adenovirus type 4 (HAdV-4) is a significant pathogen responsible for acute respiratory infections, capable of causing sporadic cases as well as outbreaks, thereby posing a threat to public health security [
24]. Although vaccines against HAdV-4 are available, their use has long been restricted to military populations in the United States, effectively reducing the infection burden among recruits but remaining unavailable to civilian groups. In most regions globally, particularly in China, there is still a lack of preventive vaccines suitable for broad populations or safe and effective antiviral drugs, indicating a significant gap in control measures. Against this background, the development of novel antibody-based therapies targeting HAdV-4 is particularly urgent [
25]. This study reports the development and functional validation of a panel of nanobodies targeting the hexon protein of human adenovirus type 4 (HAdV-4). The engineered candidate nanobody, NVA17, fused with a human Fc fragment, demonstrated notable therapeutic efficacy in vitro and in vivo. Our work not only identifies a potent neutralizing antibody against HAdV-4 but also comprehensively validates the downstream immune responses activated by this antibody in vivo, as well as the intracellular immune mechanisms involved—particularly underscoring the role of TRIM21 in mediating viral neutralization.
Currently, there are no reports on nanobodies targeting HAdV-4. Here, we report the isolation of high-affinity nanobodies against HAdV-4 hexon protein via sequential immunization of an alpaca. The lead candidate, NVA17, potently neutralized both Ad4-Luc recombinant virus and authentic HAdV-4 in vitro. NVA17 (IC50 = 4.749 ng/mL) showed enhanced potency over our previously developed fully human antibody 2CF4 (IC50 = 6.545 ng/mL), indicating higher affinity and therapeutic potential. Thus, NVA17 represents a promising candidate for developing new therapeutics against HAdV-4.
Phylogenetic analysis revealed a significant correlation between VHH sequences and their functional activities, with neutralizing, binding-only, and non-binding nanobodies forming distinct clusters. This genotype-phenotype association suggests that the functional divergence of nanobodies may originate from their specific germline origins and differences in complementarity-determining region (CDR) architectures. Previous studies have reported that nanobodies can target cryptic and conserved epitopes on the viral surface through their CDRs [
26,
27]. Our findings further support this view through epitope competition assays and structure-guided mutational analysis, indicating a diverse antibody response against the hexon protein characterized by multiple non-overlapping epitopes, with key binding residues distinct from those of the previously reported humanized monoclonal antibody 2CF4. Notably, NVA17, LUM44, and 2CF4 did not compete with each other for binding, suggesting that they recognize distinct epitopes. The unique CDR sequences of these nanobodies, particularly the structural features of HCDR3—the core antigen-binding region—provide a molecular basis for their functional differences. At the molecular level, structure prediction combined with site-directed mutagenesis identified critical residues for antigen binding. Mutations of TRP110 in NVA17 and TYR100/LEU103 in LUM44 led to a significant reduction in binding affinity, underscoring the essential role of the HCDR3 loop in antigen recognition. Particularly noteworthy, the W110A mutant of NVA17 exhibited an approximately 1000-fold increase in half-maximal effective concentration (EC
50), confirming TRP110 as a linchpin residue responsible for its high affinity and potent neutralization activity. These findings align with recent studies on the targeting properties of nanobodies. A 2024 study by Ying Tianlei and Wu Yanling’s team demonstrated that the nanobody n425 binds to a highly conserved cryptic epitope at the G protein dimer interface. Rather than competing with the host receptor, it employs an allosteric inhibition mechanism that disrupts the tetrameric structure of the G protein, thereby blocking it is signaling to the downstream F protein [
13]. That study revealed the unique epitope-targeting characteristics of nanobodies compared to conventional human-derived monoclonal antibodies. Our results corroborate these findings, as the epitope recognized by NVA17 is also distinct from known human antibodies, further supporting the unique targeting strategy of nanobodies. Moreover, NVA17 and LUM44 exhibited high specificity for HAdV-4, with no cross-reactivity to HAdV-5 or HAdV-7, indicating that they target epitopes unique to the HAdV-4 hexon protein. This characteristic provides a foundation for developing serotype-specific diagnostic and therapeutic strategies, thereby minimizing off-target effects [
15]. In summary, nanobodies—through their unique CDR structures, particularly the HCDR3 region—can recognize cryptic and conserved epitopes on viral proteins, demonstrating considerable potential in countering highly mutated viral strains and providing a theoretical basis for developing next-generation antiviral strategies.
In a lethal HAdV-4 infection model using STAT1
+/− mice, NVA17 exhibited significant protective effects: a dose as low as 2 μg provided protection, while a 50 μg dose conferred complete resistance to the lethal challenge. Similarly, in a study on herpes simplex virus (HSV-2) conducted by Professor Zhu Shu’s team, a biparatopic nanobody they developed significantly suppressed viral replication in a lethal vaginal infection model at a dose of only 20 μg [
28]. These findings collectively demonstrate the considerable potential of nanobodies in antiviral therapy across different models, highlighting the broad prospects of nanobodies as a novel antiviral strategy. Although nanobodies generally exhibit a shorter half-life in vivo compared to humanized monoclonal antibodies due to their relatively low molecular weight, often requiring frequent administration or higher doses to maintain effective blood concentrations [
29], NVA17 still demonstrated notable protective activity at low doses in this study, suggesting a promising druggability profile. To further enhance its developability, improving the pharmacokinetic properties of NVA17 is of great importance. Previous studies have reported that conjugating nanobodies with serum albumin can effectively prolong their in vivo retention and improve pharmacokinetic characteristics [
30], a strategy that is also applicable to the further engineering of NVA17. Prior to advancing its preclinical development, we will systematically evaluate the pharmacokinetic behavior of NVA17 to provide a basis for optimizing its half-life and designing rational dosing regimens.
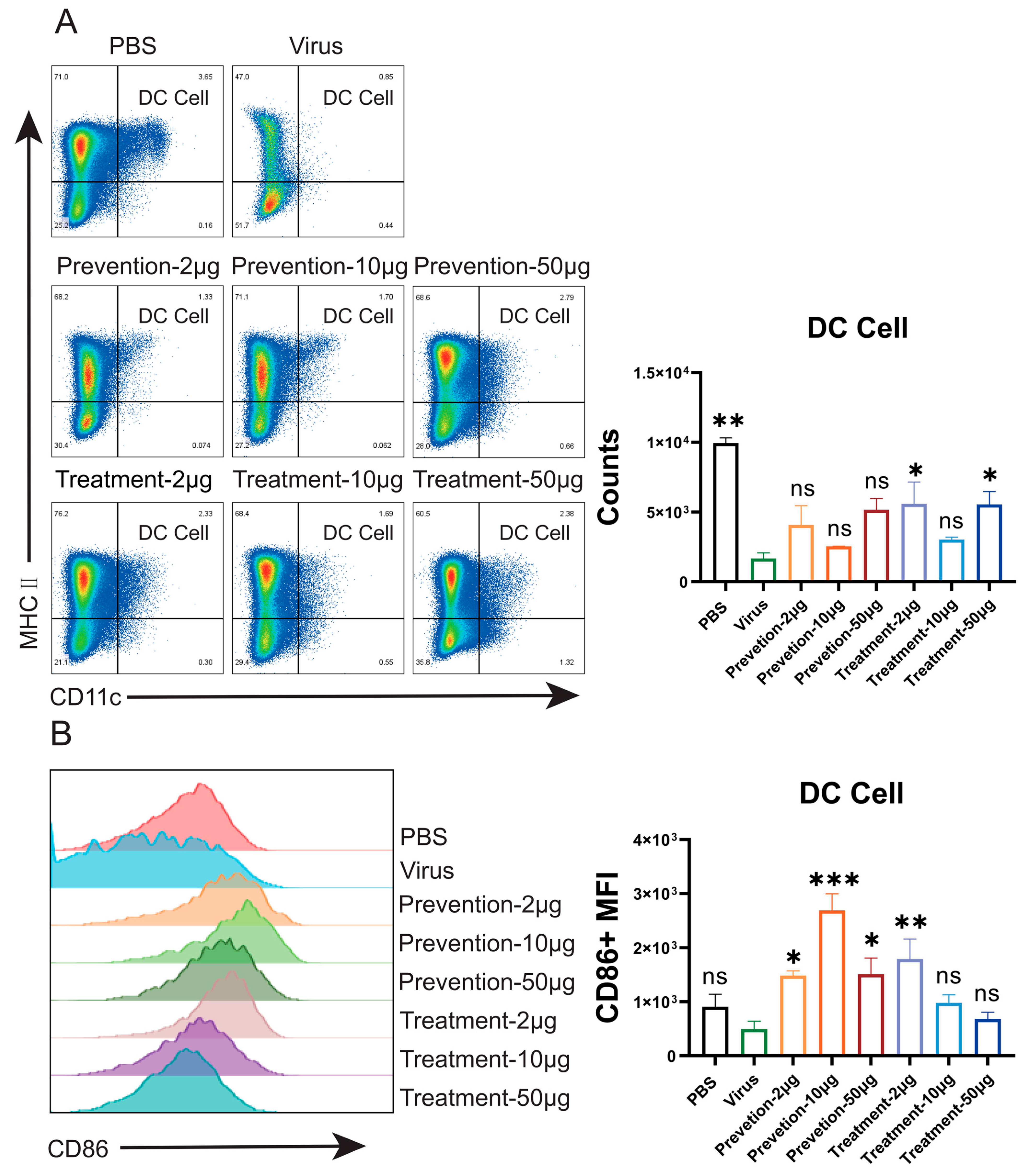
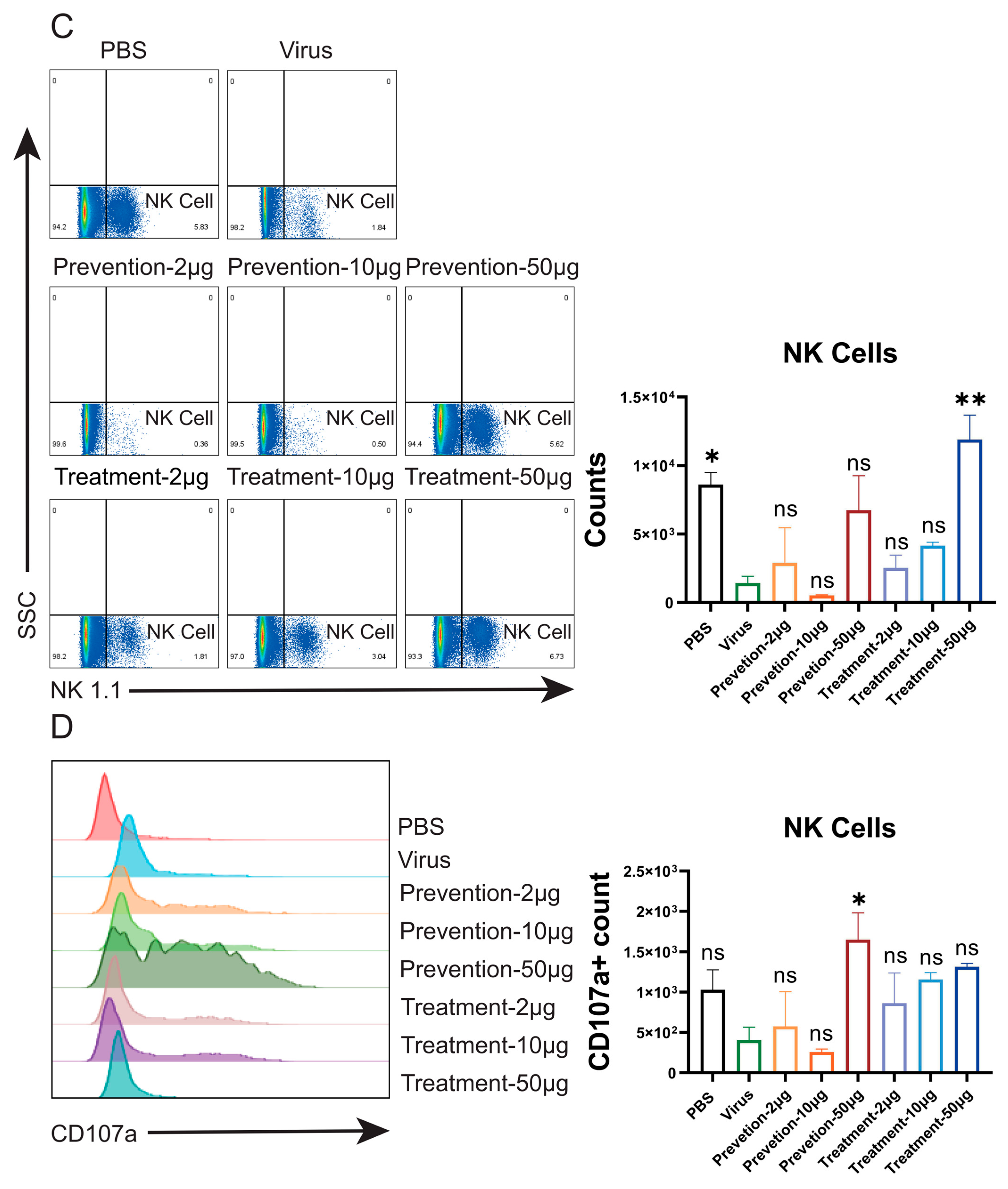
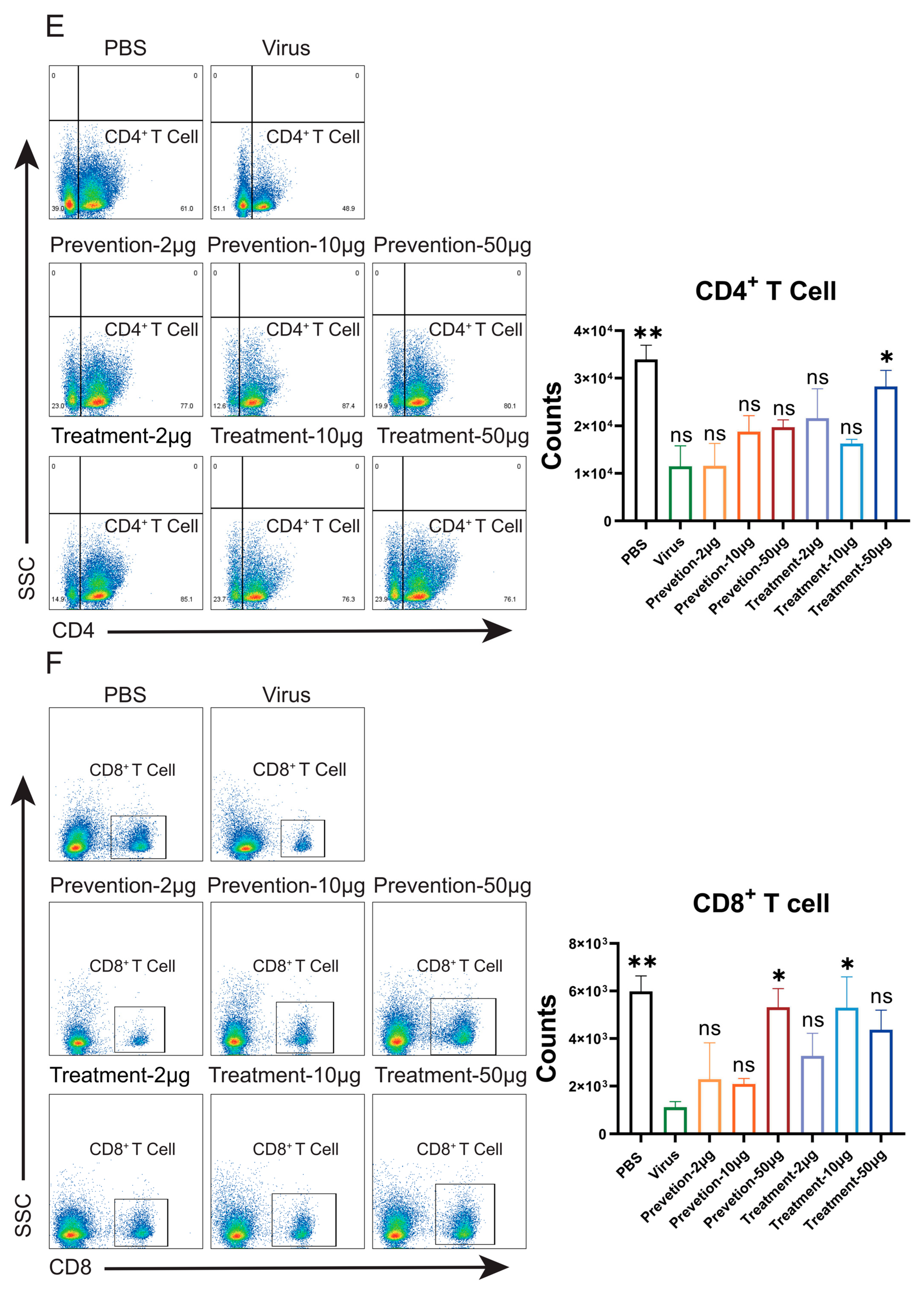
Flow cytometric analysis in mouse models demonstrated that NVA17 reversed virus-induced lymphopenia and enhanced the activation and function of key immune cells. STAT1 is a central transcription factor in the interferon signaling pathway, critical for initiating innate and adaptive immunity. The extracellular neutralizing activity of antibodies can be mechanistically compromised by STAT1 deficiency, primarily due to impaired Fc-mediated effector functions. This impairment manifests as diminished ADCC and ADCP activities resulting from downregulated Fc receptor expression, coupled with functional deficits in NK cells due to maturation defects. Concurrent attenuation of IFN-γ signaling further weakens the overall immune response. Consequently, antibody efficacy evaluated in STAT1-deficient mice may substantially underestimate its true protective potential. Notably, NVA17 achieved complete protection against lethal infection in this immunocompromised STAT1+/− model, unequivocally demonstrating its robust efficacy. It is crucial to emphasize that the lethal HAdV-4 infection model in STAT1+/− mice better recapitulates the clinical scenario of severe adenovirus infection in immunodeficient patients, who are most susceptible to adverse outcomes. Thus, this model not only enhances the clinical relevance of our findings but also provides a more stringent and credible platform for evaluating candidate therapeutics.
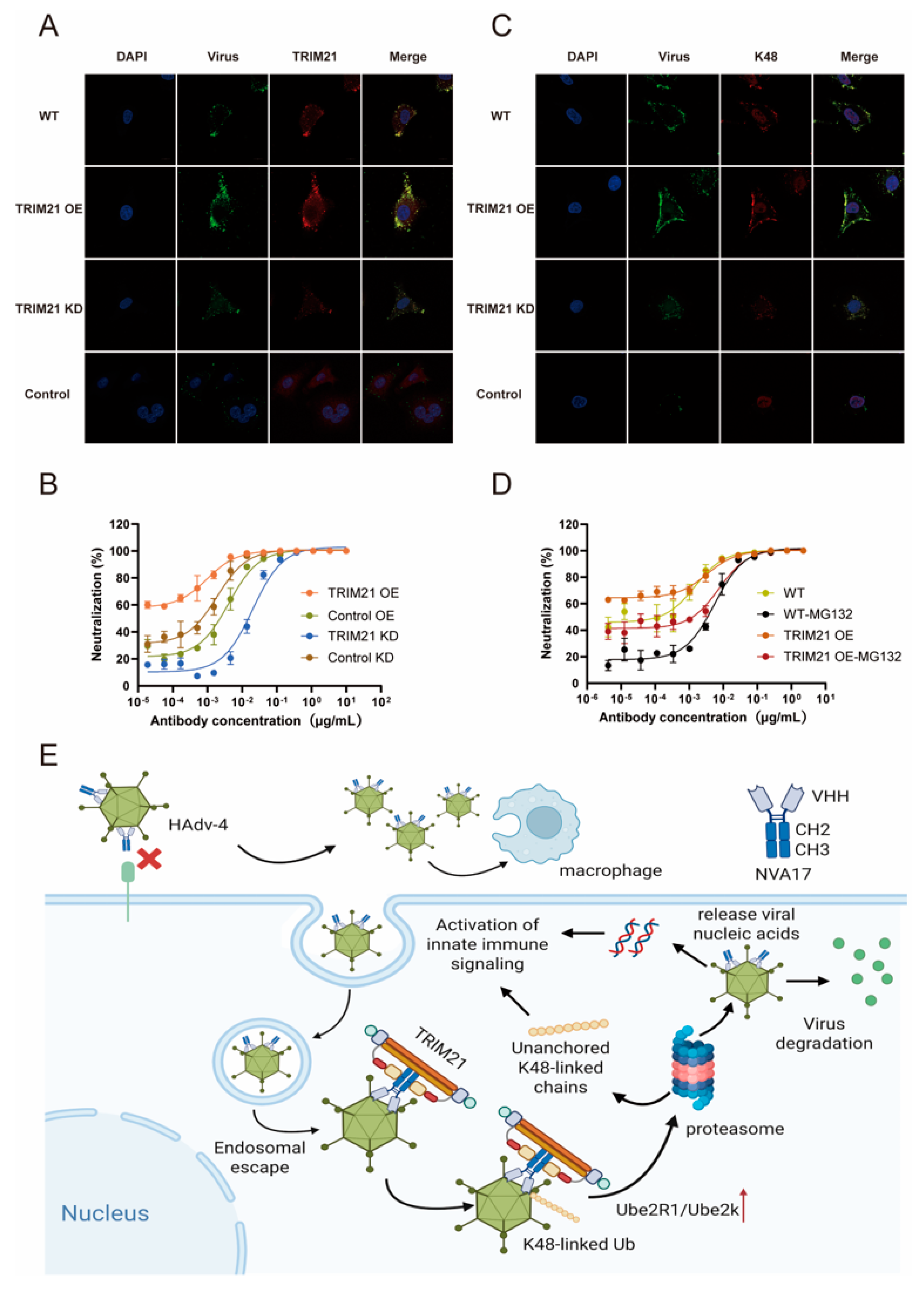
Studies have demonstrated that neutralization of non-enveloped viruses involves not only extracellular antibody-mediated blockade of viral entry but also intracellular viral clearance via the TRIM21-dependent Antibody-Dependent Intracellular Neutralization (ADIN) pathway [
31,
32]. Given that HAdV-4 is a non-enveloped virus, we hypothesized that its specific nanobody, NVA17, may also possess ADIN activity [
31]. Experimental results confirm that the antiviral activity of NVA17 is mediated not only by steric hindrance inhibiting viral entry but also significantly relies on the TRIM21-ADIN pathway. Specifically, after binding to HAdV-4 extracellularly, NVA17 is co-internalized with the virus into the cell. Within the cytoplasm, the virus-antibody complex is recognized by the E3 ubiquitin ligase TRIM21, undergoes K48-linked polyubiquitination, and is subsequently degraded by the proteasome, leading to effective viral clearance prior to replication. This indicates that NVA17 can enter cells as a virus-antibody complex and achieve intracellular viral clearance via the TRIM21-dependent proteasomal pathway, offering a novel strategy for targeting the post-entry stage of HAdV-4 infection. Notably, following the recognition of the virus-antibody complex, TRIM21 may not only activate the K48 ubiquitin-proteasome pathway but also initiate alternative ubiquitination modifications such as K63-linked ubiquitin chains, thereby triggering inflammatory signaling pathways involving NF-κB and AP-1 [
33,
34,
35]. Furthermore, although STAT1 does not directly intersect with TRIM21 in ADIN functionality, STAT1—as a key transcription factor in the interferon signaling pathway—can regulate TRIM21 expression under immune-activated conditions [
36,
37]. In STAT1
+/− mice, the intrinsic ability of TRIM21 to initiate K48/K63 ubiquitination and activate NF-κB/AP-1 signaling remains largely intact; however, due to the absence of STAT1 signaling, the overall potency of the resulting immune protection and inflammatory response may be attenuated compared to wild-type mice. Therefore, a comprehensive elucidation of the complete intracellular ADIN mechanism elicited by NVA17 requires further validation of the activation and functional contributions of these associated inflammatory signaling pathways.