Factors Associated with Vaccination Status of Neonates in the Tertiary Referral Department of Neonatology and Neonatal Intensive Care in the North-Eastern Region of Poland
Abstract
1. Introduction
2. Material and Methods
2.1. Recommendations on Vaccination of Neonates in Poland
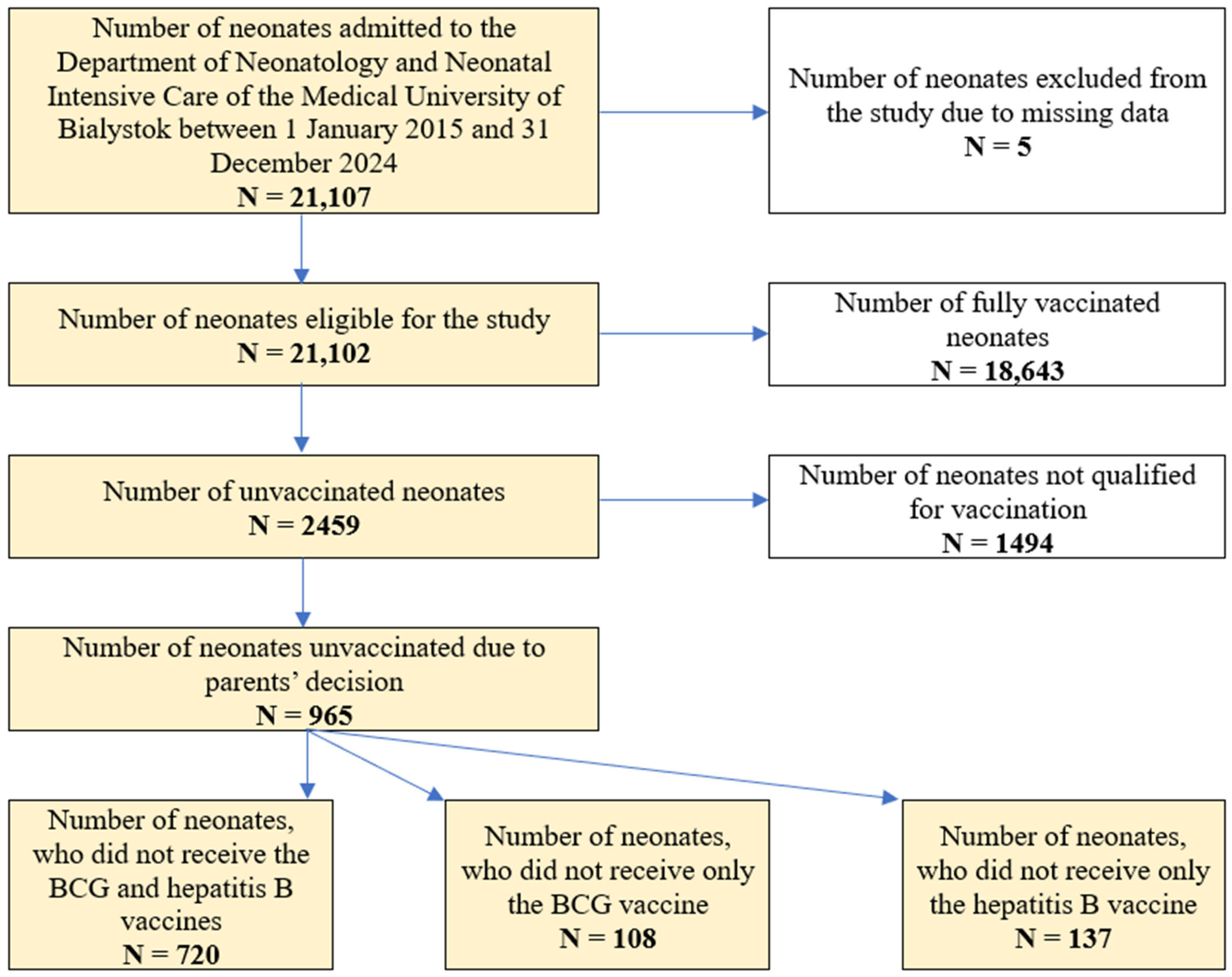
2.2. Subjects and Data Collection
2.3. Study Covariates
2.4. Assessment of the Neonatal Immunization Before, During and After the COVID-19 Pandemic
2.5. Data Management Plan
2.6. Sample Size and Missing Data Points
2.7. Data Quality Control
2.8. Operational Definitions
2.9. Statistical Analysis
2.10. Ethical Considerations
3. Results
3.1. The Characteristics of the Study Group
3.2. The Characteristics of the Neonates Unvaccinated Due to Parents’ Decision
3.3. Factors Associated with the Immunization Status—The Multivariate Logistic Regression Analysis
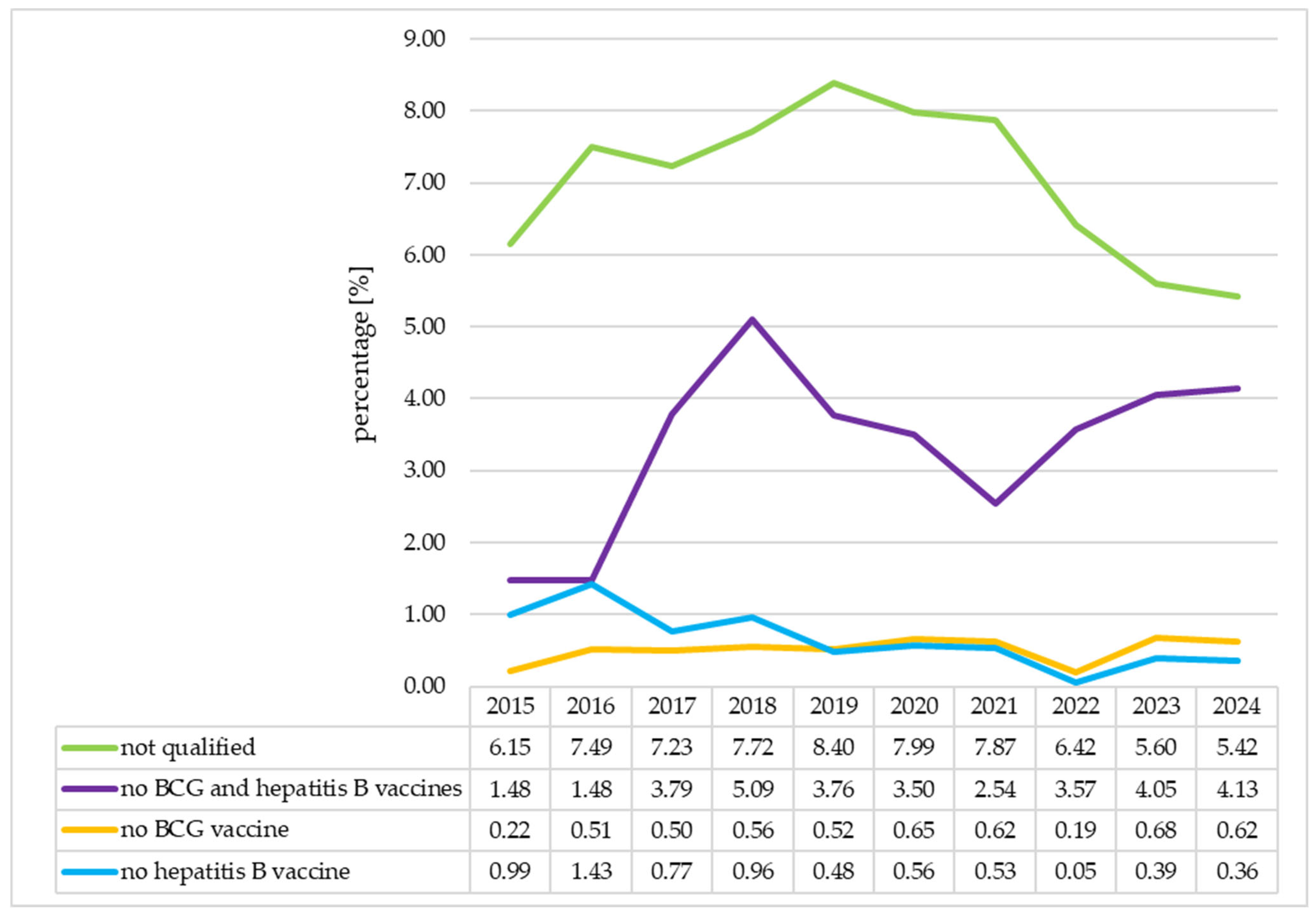
3.4. The Analysis of the Immunization Status of the Neonates in Individual Years
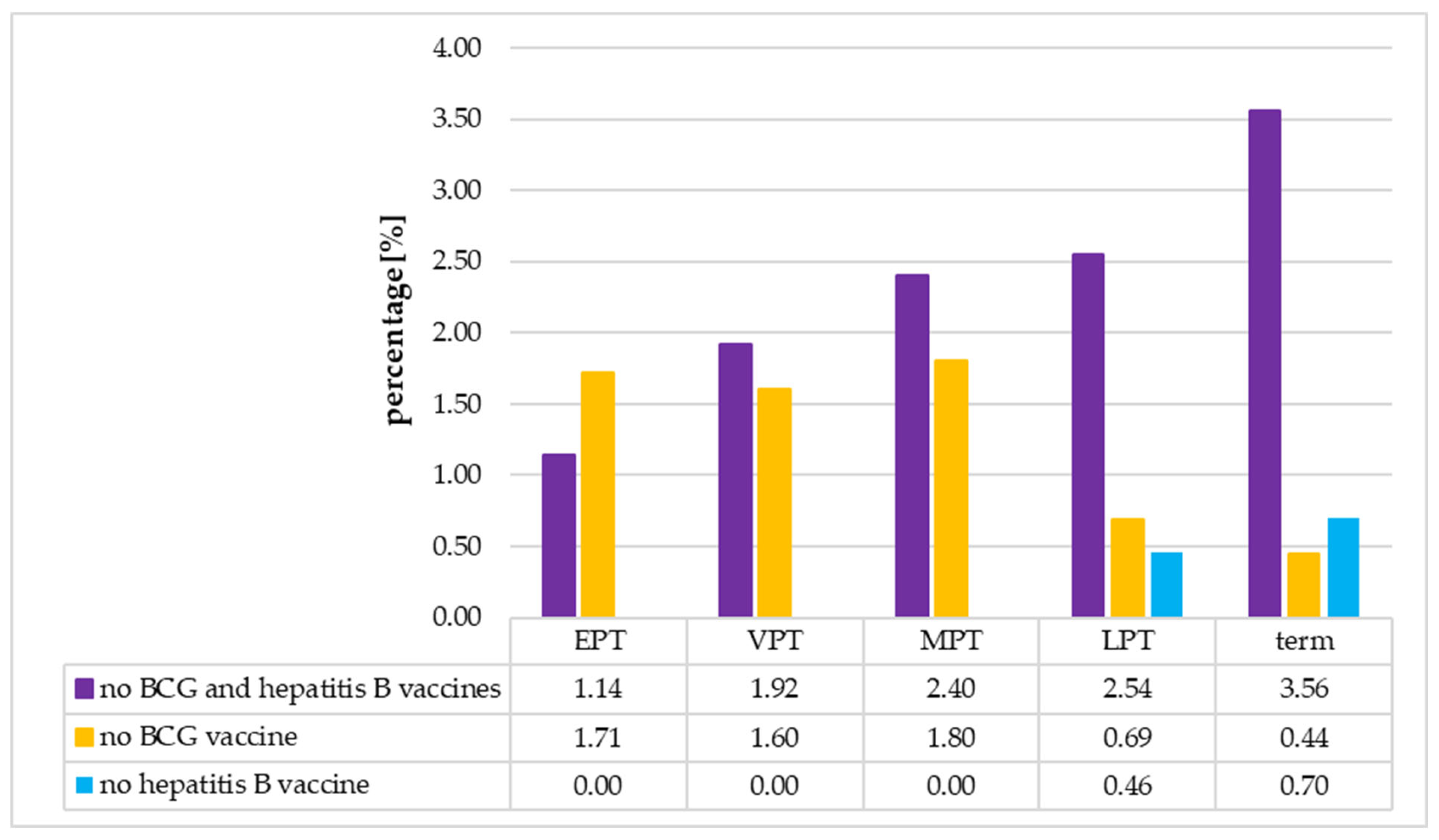
3.5. The Analysis of the Immunization Status of the Neonates According to Their Gestational Age
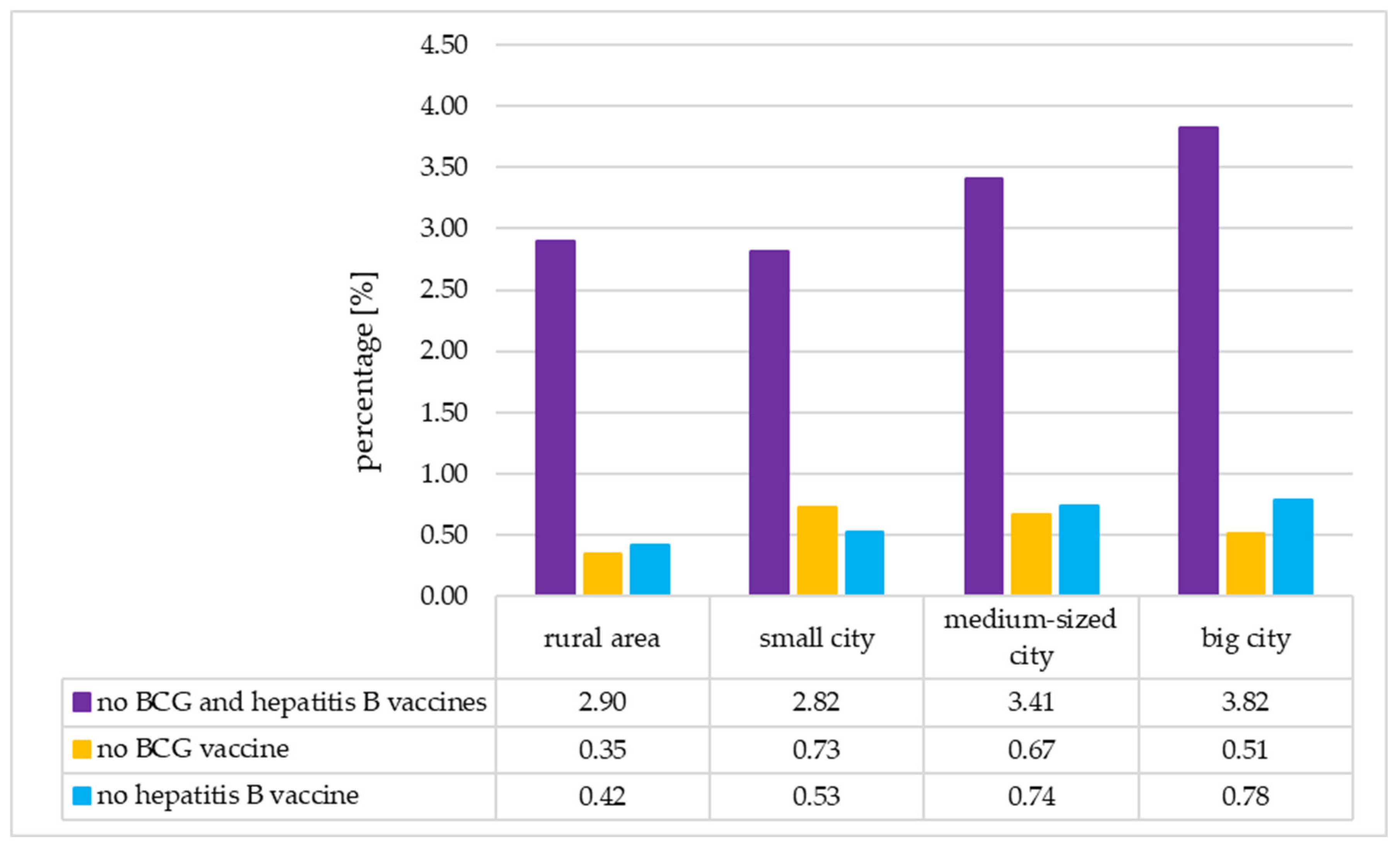
3.6. The Analysis of the Immunization Status of the Neonates According to Their Place of Residence
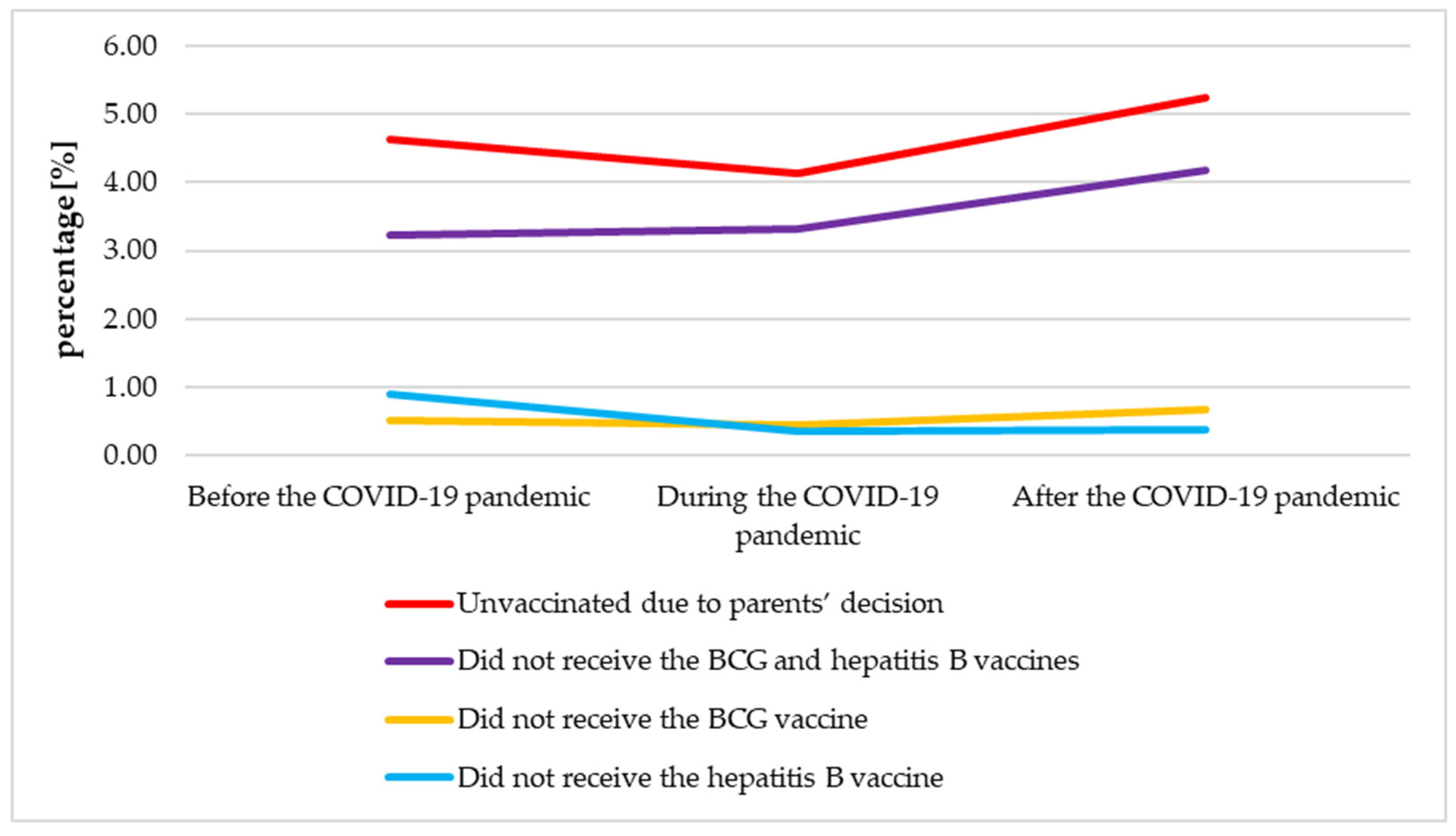
3.7. The Analysis of the Neonatal Immunization Before, During and After the COVID-19 Pandemic
3.8. Summary of Findings
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Farina, S.; Maio, A.; Gualano, M.R.; Ricciardi, W.; Villani, L. Childhood Mandatory Vaccinations: Current Situation in European Countries and Changes Occurred from 2014 to 2024. Vaccines 2024, 12, 1296. [Google Scholar] [CrossRef]
- Mourão, M.L.; Baca-Arzaga, A.; Castellanos, M.; Johnstone, R.; Miedema, D.; Ozonoff, A.; Odumade, O.A.; Angelidou, A. Trends in Neonatal Vaccination: A Ten-year Retrospective Study in a Large Delivery Center. Pediatr. Infect. Dis. J. 2025, 44, S97–S100. [Google Scholar] [CrossRef]
- Kurpas, D.; Stefanicka–Wojtas, D.; Soll–Morka, A.; Lomper, K.; Uchmanowicz, B.; Blahova, B.; Bredelytė, A.; Dumitra, G.G.; Hudáčková, V.; Javorska, K.; et al. Vaccine Hesitancy and Immunization Patterns in Central and Eastern Europe: Sociocultural, Economic, Political, and Digital Influences Across Seven Countries. Risk Manag. Healthc. Policy 2025, 18, 1911–1934. [Google Scholar] [CrossRef] [PubMed]
- Szalast, K.; Nowicki, G.J.; Pietrzak, M.; Mastalerz-Migas, A.; Biesiada, A.; Grochans, E.; Ślusarska, B. Parental Attitudes, Motivators and Barriers Toward Children’s Vaccination in Poland: A Scoping Review. Vaccines 2025, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Mrożek-Budzyn, D.; Kiełtyka, A.; Mróz, E. Opinions about vaccination among mothers who delivered newborns in two hospitals in Krakow and Myślenice. Przegląd Epidemiol. 2016, 70, 471–478. [Google Scholar]
- Szymoniak, K.; Cholewa, D.; Fryc, D.; Ćwiek, D. Parents’ opinion on the refusal of childhood vaccines. Piel. Pol. 2016, 3, 159–165. [Google Scholar]
- McKee, C.; Bohannon, K. Exploring the Reasons Behind Parental Refusal of Vaccines. J. Pediatr. Pharmacol. Ther. 2016, 21, 104–109. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine hesitancy: An overview. Hum. Vaccin. Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef]
- Damnjanović, K.; Graeber, J.; Ilić, S.; Lam, W.Y.; Lep, Ž.; Morales, S.; Pulkkinen, T.; Vingerhoets, L. Parental Decision-Making on Childhood Vaccination. Front. Psychol. 2018, 9, 735. [Google Scholar] [CrossRef]
- Harmsen, I.A.; Ruiter, R.A.; Paulussen, T.G.; Mollema, L.; Kok, G.; de Melker, H.E. Factors that influence vaccination decision-making by parents who visit an anthroposophical child welfare center: A focus group study. Adv. Prev. Med. 2012, 2012, 175694. [Google Scholar] [CrossRef]
- Szczepienia.Info. 2025. Available online: https://szczepienia.pzh.gov.pl/kalendarz-szczepien-2025/ (accessed on 23 September 2025).
- Sarker, R.; Roknuzzaman, A.S.M.; Hossain, M.J.; Bhuiyan, M.A.; Islam, M.R. The WHO declares COVID-19 is no longer a public health emergency of international concern: Benefits, challenges, and necessary precautions to come back to normal life. Int. J. Surg. 2023, 109, 2851–2852. [Google Scholar] [CrossRef] [PubMed]
- Bujang, M.A.; Sa’at, N.; Sidik, T.M.I.T.A.B.; Joo, L.C. Sample Size Guidelines for Logistic Regression from Observational Studies with Large Population: Emphasis on the Accuracy Between Statistics and Parameters Based on Real Life Clinical Data. Malays. J. Med. Sci. 2018, 25, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Statistics Poland. Vaccinations of Children and Youth. 2024. Available online: https://stat.gov.pl/en/topics/health/health/vaccinations-of-children-and-youth,5,5.html (accessed on 23 September 2025).
- Łoś-Rycharska, E.; Popielarz, M.; Wolska, J.; Sobieska-Poszwa, A.; Dziembowska, I.; Krogulska, A. The antivaccination movement and the perspectives of Polish parents. Pediatr. Pol.—Pol. J. Paediatr. 2022, 97, 183–192. [Google Scholar] [CrossRef]
- van de Berg, S.; Coyer, L.; von Both, U.; Scheuerer, T.; Kolberg, L.; Hoch, M.; Böhmer, M.M. Coverage and determinants of COVID-19 child vaccination in Munich, Germany in October 2022–January 2023: Results of the COVIP-Virenwächter study. Eur. J. Pediatr. 2024, 183, 3727–3738. [Google Scholar] [CrossRef]
- Nozaki, I.; Hachiya, M.; Kitamura, T. Factors influencing basic vaccination coverage in Myanmar: Secondary analysis of 2015 Myanmar demographic and health survey data. BMC Public Health 2019, 19, 242. [Google Scholar] [CrossRef]
- Imran, W.; Abbas, F.; Javed, S.A. What is causing high polio vaccine dropout among Pakistani children? Public Health 2018, 164, 16–25. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Shida, N.; Izumi, S.; Ogasawara, M.; Kakinuma, W.; Tsujiuchi, T.; Machida, K. Factors associated with mothers not vaccinating their children against mumps in Japan. Public Health 2016, 137, 95–105. [Google Scholar] [CrossRef]
- Gowda, C.; Dempsey, A.F. The rise (and fall?) of parental vaccine hesitancy. Hum. Vaccin. Immunother. 2013, 9, 1755–1762. [Google Scholar] [CrossRef]
- Garcia, É.M.; de Souza, E.L.; Matozinhos, F.P.; da Silva, T.M.R.; Waldman, E.A.; Sato, A.P.S. Associated factors with vaccine hesitancy in mothers of children up to two years old in a Brazilian city. PLoS Glob. Public Health 2023, 3, e0002026. [Google Scholar] [CrossRef]
- Corben, P.; Leask, J. Vaccination hesitancy in the antenatal period: A cross-sectional survey. BMC Public Health 2018, 18, 566. [Google Scholar] [CrossRef]
- Sharif-Nia, H.; She, L.; Allen, K.-A.; Marôco, J.; Kaur, H.; Arslan, G.; Gorgulu, O.; Osborne, J.W.; Rahmatpour, P.; Fomani, F.K. Parental hesitancy toward children vaccination: A multi-country psychometric and predictive study. BMC Public Health 2024, 24, 1348. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; A Alanazi, M.F.; Al-Hazmi, A.H.; Alruwaili, B.F.; Alsaidan, A.A.; Alruwaili, T.A.M.; Algaed, M.A.M.; Alsharari, A.K.; Alenazi, R.H.; Alshalan, A.M.; et al. Maternal Perception, Hesitancy, and Satisfaction Toward Childhood Immunization in Primary Health Centers, Hafr Al-Batin: A Multicenter Cross-Sectional Study from Eastern Saudi Arabia. Risk Manag. Healthc. Policy 2023, 16, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Soysal, G.; Akdur, R. Investigating Vaccine Hesitancy and Refusal Among Parents of Children Under Five: A Community-based Study. J. Curr. Pediatr. 2022, 20, 339–348. [Google Scholar] [CrossRef]
- Khaliq, A.; Elahi, A.A.; Zahid, A.; Lassi, Z.S. A Survey Exploring Reasons behind Immunization Refusal among the Parents and Caregivers of Children under Two Years Living in Urban Slums of Karachi, Pakistan. Int. J. Environ. Res. Public Health. 2022, 19, 11631. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, O.A.; Omoloja, O.; Zibiri, A.O.; Dinehin, A.V.; Adewusi, A.O.; Nwabueze, J. Determinants of vaccine hesitancy among pregnant women in South-West Nigeria: An explanatory sequential mixed method design. BMJ Open 2025, 15, e101767. [Google Scholar] [CrossRef]
- Gołębiewska, B.; Stefańczyk, J.; Jaska, E. Social media adoption by rural residents. Ann. Pol. Assoc. Agric. Agribus. Econ. 2020, 22, 95–103. [Google Scholar] [CrossRef]
- Nylen, E.; Telega, G.; Nagorska, M. Attitudes of new mothers toward childhood vaccinations in Rzeszow, Poland. Pediatr. Pol. Pol. J. Paediatr. 2023, 98, 133–139. [Google Scholar] [CrossRef]
- Ji, C.; Senthinathan, A.; Apajee, J.; Dubey, V.; Forte, M.; Kwong, J.C.; Morris, S.K.; Piche-Renaud, P.-P.; Wilson, S.E.; Tu, K. Impact of the COVID-19 pandemic on routine immunization coverage of children and teenagers in Ontario, Canada. Vaccine 2025, 49, 126811. [Google Scholar] [CrossRef]
- Parmar, K.; Siddiqui, A.; Nugent, K. Bacillus Calmette-Guerin Vaccine and Nonspecific Immunity. Am. J. Med. Sci. 2021, 361, 683–689. [Google Scholar] [CrossRef]
- Gong, W.; Mao, Y.; Li, Y.; Qi, Y. BCG Vaccination: A potential tool against COVID-19 and COVID-19-like Black Swan incidents. Int. Immunopharmacol. 2022, 108, 108870. [Google Scholar] [CrossRef]
- Gong, W.; An, H.; Wang, J.; Cheng, P.; Qi, Y. The Natural Effect of BCG Vaccination on COVID-19: The Debate Continues. Front. Immunol. 2022, 13, 953228. [Google Scholar] [CrossRef]
- Lee, S.K.; Sun, J.; Jang, S.; Connelly, S. Misinformation of COVID-19 vaccines and vaccine hesitancy. Sci. Rep. 2022, 12, 13681. [Google Scholar] [CrossRef]
- Kamianowski, A.; Kamianowski, C.; Szpica, G.; Jakubas, A.; Wasilewska, A.; Kamianowska, M. Analysis of the in-hospital mortality in the tertiary referral department of neonatology and neonatal intensive care. Sci. Rep. 2025, 15, 24849. [Google Scholar] [CrossRef]
- Tuckerman, J.; Kaufman, J.; Danchin, M. Effective Approaches to Combat Vaccine Hesitancy. Pediatr. Infect. Dis. J. 2022, 41, e243–e245. [Google Scholar] [CrossRef]
- Cagnotta, C.; Lettera, N.; Cardillo, M.; Pirozzi, D.; Catalán-Matamoros, D.; Capuano, A.; Scavone, C. Parental vaccine hesitancy: Recent evidences support the need to implement targeted communication strategies. J. Infect. Public Health 2025, 18, 102648. [Google Scholar] [CrossRef]
| Terminology | Abbreviation | Gestational Age (Weeks) |
|---|---|---|
| term | - | 37 0/7–41 6/7 |
| late preterm (LPT) | LPT | 34 0/7–36 6/7 |
| moderate preterm | MPT | 32 0/7–33 6/7 |
| very preterm | VPT | 28 0/7–31 6/7 |
| extremely preterm | EPT | ≤27 6/7 |
| Characteristic | All Neonates (N = 21,102) | Group A: Vaccinated (N = 18,643) | Unvaccinated (N = 2459) | p | |||
|---|---|---|---|---|---|---|---|
| Group B: Parents’ Decision (N = 965) | Group C: Not Qualified (N = 1494) | ||||||
| Frequency (Percentage of all Neonates, %) | p1 | p2 | p3 | ||||
| male | 10,750 (50.94) | 9358 (44.35) | 500 (2.37) | 892 (4.23) | 0.133 | 0.600 | 0.000 |
| female | 10,349 (49.04) | 9285 (44.00) | 465 (2.20) | 599 (2.84) | |||
| vaginal delivery | 11,094 (52.57) | 9811 (46.49) | 509 (2.41) | 774 (3.67) | 0.917 | 0.834 | 0.567 |
| cesarean delivery | 10,008 (47.43) | 8832 (41.85) | 456 (2.16) | 720 (3.41) | |||
| single pregnancy | 19,443 (92.14) | 17,211 (81.56) | 882 (4.18) | 1350 (6.40) | 0.502 | 0.405 | 0.014 |
| multiple pregnancy | 1659 (7.86) | 1432 (6.79) | 83 (0.39) | 144 (0.68) | |||
| place of residence: city | 15,627 (74.05) | 13,796 (65.38) | 764 (3.62) | 1067 (5.06) | 0.903 | 0.000 | 0.025 |
| place of residence: rural area | 5475 (25.95) | 4847 (22.97) | 201 (0.95) | 427 (2.02) | |||
| primigravida | 8222 (38.96) | 7356 (34.86) | 277 (1.31) | 589 (2.79) | 0.314 | 0.000 | 0.724 |
| multigravida | 12,880 (61.04) | 11,287 (53.49) | 688 (3.26) | 905 (4.29) | |||
| primiparous | 9603 (45.51) | 8573 (40.63) | 335 (1.59) | 695 (3.29) | 0.340 | 0.000 | 0.448 |
| multiparous | 11,499 (54.49) | 10,070 (47.72) | 630 (2.99) | 799 (3.79) | |||
| term birth | 18,553 (87.92) | 16,712 (79.20) | 871 (4.13) | 970 (4.60) | 0.000 | 0.029 | 0.000 |
| preterm birth | 2549 (12.08) | 1931 (9.15) | 94 (0.45) | 524 (2.48) | |||
| maternal age: <35 years | 15,814 (74.94) | 14,051 (66.59) | 672 (3.18) | 1091 (5.17) | 0.324 | 0.000 | 0.099 |
| maternal age: ≥35 years | 5288 (25.06) | 4592 (21.76) | 293 (1.39) | 403 (1.91) | |||
| Median (Q1–Q3) | p1 | p2 | p3 | ||||
| birth weight (g) | 3370 (2980 −3710) | 3400 (3000–3730) | 3360 (3000–3700) | 3120 (2200–3650) | 0.004 (0.017) | 0.963 (0.001) | 0.000 (0.208) |
| gestational age (weeks) | 39.00 (38.00–40.00) | 39 (38–40) | 39 (38–40) | 38 (35–40) | 0.001 (0.019) | 0.274 (0.021) | 0.000 (0.250) |
| 1 min Apgar score | 10.00 (9.00–10.00) | 10 (9–10) | 10 (9–10) | 9 (6–10) | 0.000 (0.026) | 0.334 (0.018) | 0.000 (0.336) |
| Characteristic | All Neonates (N = 21,102) | Unvaccinated—Parents’ Decision (N = 965) | p | ||||
|---|---|---|---|---|---|---|---|
| Subgroup B1: Did not Receive the BCG and Hepatitis B Vaccines (N = 720) | Subgroup B2: Did not Receive the BCG Vaccine (N = 108) | Subgroup B3: Did not Receive the Hepatitis B Vaccine (N = 137) | |||||
| Frequency (Percentage of all Neonates, %) | p1 | p2 | p3 | ||||
| male | 10,750 (50.94) | 371 (1.76) | 59 (0.28) | 70 (0.33) | 0.761 | 0.446 | 0.973 |
| female | 10,349 (49.04) | 349 (1.64) | 49 (0.23) | 67 (0.32) | |||
| vaginal delivery | 11,094 (52.57) | 384 (1.82) | 56 (0.27) | 69 (0.33) | 0.689 | 0.881 | 0.606 |
| cesarean delivery | 10,008 (47.43) | 336 (1.59) | 52 (0.25) | 68 (0.32) | |||
| single pregnancy | 19,443 (92.14) | 660 (3.13) | 93 (0.44) | 129 (0.61) | 0.644 | 0.020 | 0.380 |
| multiple pregnancy | 1659 (7.86) | 60 (0.28) | 15 (0.07) | 8 (0.04) | |||
| place of residence: city | 15,627 (74.05) | 561 (2.66) | 89 (0.42) | 114 (0.54) | 0.020 | 0.048 | 0.015 |
| place of residence: rural area | 5475 (25.95) | 159 (0.75) | 19 (0.09) | 23 (0.11) | |||
| primigravida | 8222 (38.96) | 198 (0.94) | 24 (0.11) | 55 (0.26) | 0.000 | 0.000 | 0.777 |
| multigravida | 12,880 (61.04) | 522 (2.47) | 84 (0.40) | 82 (0.39) | |||
| primiparous | 9603 (45.51) | 236 (1.12) | 33 (0.16) | 66 (0.31) | 0.000 | 0.002 | 0.532 |
| multiparous | 11,499 (54.49) | 484 (2.29) | 75 (0.36) | 71 (0.34) | |||
| term birth | 18,553 (87.92) | 660 (3.13) | 82 (0.39) | 129 (0.61) | 0.002 | 0.000 | 0.025 |
| preterm birth | 2549 (12.08) | 60 (0.28) | 26 (0.12) | 8 (0.04) | |||
| maternal age: <35 years | 15,814 (74.94) | 495 (2.35) | 75 (0.36) | 102 (0.48) | 0.000 | 0.189 | 0.869 |
| maternal age: ≥35 years | 5288 (25.06) | 225 (1.07) | 33 (0.16) | 35 (0.17) | |||
| Median (Q1–Q3) | p1 (r1) | p2 (r2) | p3 (r3) | ||||
| birth weight (g) | 3370 (2980 −3710) | 3400 (3050–3700) | 3175 (2610–3700) | 3300 (2950–3700) | 0.195 (0.028) | 0.007 (0.148) | 0.596 (0.026) |
| gestational age (weeks) | 39.00 (38.00–40.00) | 39 (38–40) | 39 (37–40) | 39 (38–40) | 0.205 (0.028) | 0.072 (0.100) | 0.109 (0.079) |
| 1 min Apgar score | 10.00 (9.00–10.00) | 10 (9–10) | 9 (8–10) | 10 (9–10) | 0.291 (0.023) | 0.008 (0.151) | 0.010 (0.127) |
| Variables | Categories | Univariate Logistic Regression | Multivariate Logistic Regression (Backward Stepwise Model) | ||
|---|---|---|---|---|---|
| COR (CI = 95%) | p-Value | AOR (CI = 95%) | p-Value | ||
| Immunization status: “did not receive the BCG and hepatitis B vaccines” (N = 720) | |||||
| mother’s age | ≥35 years | 1.439 (1.224–1.691) | 0.000 | 1.280 (1.084–1.511) | 0.004 |
| <35 years | 1 | 1 | |||
| place of residence | city | 1.245 (1.041–1.489) | 0.016 | 1.260 (1.053–1.507) | 0.012 |
| rural area | 1 | 1 | |||
| gravidity | multigravida | 1.712 (1.450–2.021) | 0.000 | - | - |
| primigravida | 1 | ||||
| parity | multiparous | 1.733 (1.480–2.030) | 0.000 | 1.524 (1.053–2.029) | 0.002 |
| primiparous | 1 | 1 | |||
| type of pregnancy | multiple | 1.069 (0.817–1.399) | 0.626 | - | - |
| single | 1 | ||||
| way of delivery | cesarean section | 0.968 (0.834–1.124) | 0.671 | - | - |
| vaginal | 1 | ||||
| gestational age | term | 1.529 (1.170–1.998) | 0.002 | 1.658 (1.410–1.950) | 0.000 |
| preterm | 1 | 1 | |||
| sex | male | 1.024 (0.883–1.189) | 0.751 | - | - |
| female | 1 | ||||
| Immunization status: “did not receive the BCG vaccine” (N = 108) | |||||
| mother’s age | ≥35 years | 1.413 (0.937–2.130) | 0.099 | - | - |
| <35 years | 1 | ||||
| place of residence | city | 1.645 (1.002–2.702) | 0.049 | 1.749 (1.064–2.875) | 0.028 |
| rural area | 1 | 1 | |||
| gravidity | multigravida | 2.242 (1.423–3.533) | 0.001 | 2.265 (1.437–3.569) | 0.000 |
| primigravida | 1 | 1 | |||
| parity | multiparous | 1.904 (1.263–2.871) | 0.002 | - | - |
| primiparous | 1 | ||||
| type of pregnancy | multiple | 1.901 (1.099–3.287) | 0.022 | - | - |
| single | 1 | ||||
| way of delivery | cesarean section | 1.194 (0.818–1.743) | 0.358 | - | - |
| vaginal | 1 | ||||
| gestational age | term | 0.430 (0.276–0.670) | 0.000 | 0.471 (0.291–0.763) | 0.003 |
| preterm | 1 | 1 | |||
| sex | male | 0.443 (0.794–1.696) | 0.443 | - | - |
| female | 1 | ||||
| Immunization status: “did not receive the hepatitis B vaccine” (N = 137) | |||||
| mother’s age | ≥35 years | 1.058 (0.717–1.561) | 0.775 | - | - |
| <35 years | 1 | ||||
| place of residence | city | 1.742 (1.112–2.730) | 0.015 | 1.714 (1.094–2.686) | 0.019 |
| rural area | 1 | 1 | |||
| gravidity | multigravida | 0.951 (0.675–1.340) | 0.776 | - | - |
| primigravida | 1 | ||||
| parity | multiparous | 0.898 (0.642–1.257) | 0.531 | - | - |
| primiparous | 1 | ||||
| type of pregnancy | multiple | 0.726 (0.355–1.486) | 0.381 | - | - |
| single | 1 | ||||
| way of delivery | cesarean section | 1.125 (0.804–1.574) | 0.492 | - | - |
| vaginal | 1 | ||||
| gestational age | term | 2.222 (1.087–4.544) | 0.029 | 2.170 (1.061–4.438) | 0.034 |
| preterm | 1 | 1 | |||
| sex | male | 1.006 (0.719–1.408) | 0.972 | - | - |
| Group of Neonates | Before the COVID-19 Pandemic (N = 11265) | During the COVID-19 Pandemic (N = 6591) | After the COVID-19 Pandemic (N = 3246) | p | ||
|---|---|---|---|---|---|---|
| Frequency (Percentage of all Neonates, %) | p1 | p2 | p3 | |||
| Group A: Vaccinated | 9908 (87.95) | 5827 (88.41) | 2908 (89.59) | 0.365 | 0.081 | 0.011 |
| Group B: Unvaccinated due to parents’ decision | 523 (4.64) | 272 (4.13) | 170 (5.24) | 0.107 | 0.012 | 0.162 |
| Subgroup B1: Did not receive the BCG and hepatitis B vaccines | 365 (3.24) | 219 (3.32) | 136 (4.19) | 0.765 | 0.030 | 0.009 |
| Subgroup B2: Did not receive the BCG vaccine | 57 (0.51) | 29 (0.44) | 22 (0.68) | 0.539 | 0.123 | 0.241 |
| Subgroup B3: Did not receive the hepatitis B vaccine | 101 (0.90) | 24 (0.36) | 12 (0.37) | 0.000 | 0.966 | 0.003 |
| Group C: Not qualified for vaccination | 834 (7.40) | 492 (7.46) | 168 (5.18) | 0.880 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamianowski, A.; Kamianowski, C.; Szpica, G.; Jakubas, A.; Wasilewska, A.; Kamianowska, M. Factors Associated with Vaccination Status of Neonates in the Tertiary Referral Department of Neonatology and Neonatal Intensive Care in the North-Eastern Region of Poland. Vaccines 2025, 13, 1191. https://doi.org/10.3390/vaccines13121191
Kamianowski A, Kamianowski C, Szpica G, Jakubas A, Wasilewska A, Kamianowska M. Factors Associated with Vaccination Status of Neonates in the Tertiary Referral Department of Neonatology and Neonatal Intensive Care in the North-Eastern Region of Poland. Vaccines. 2025; 13(12):1191. https://doi.org/10.3390/vaccines13121191
Chicago/Turabian StyleKamianowski, Aleksander, Cezary Kamianowski, Gabriela Szpica, Angelika Jakubas, Anna Wasilewska, and Monika Kamianowska. 2025. "Factors Associated with Vaccination Status of Neonates in the Tertiary Referral Department of Neonatology and Neonatal Intensive Care in the North-Eastern Region of Poland" Vaccines 13, no. 12: 1191. https://doi.org/10.3390/vaccines13121191
APA StyleKamianowski, A., Kamianowski, C., Szpica, G., Jakubas, A., Wasilewska, A., & Kamianowska, M. (2025). Factors Associated with Vaccination Status of Neonates in the Tertiary Referral Department of Neonatology and Neonatal Intensive Care in the North-Eastern Region of Poland. Vaccines, 13(12), 1191. https://doi.org/10.3390/vaccines13121191






