The Safety and Immunogenicity of a 13-Valent Pneumococcal Polysaccharide Conjugate Vaccine (CRM197/TT) in 12–23-Month Children: A Double-Blind, Randomized, Phase III Trial in China
Abstract
1. Introduction
2. Methods
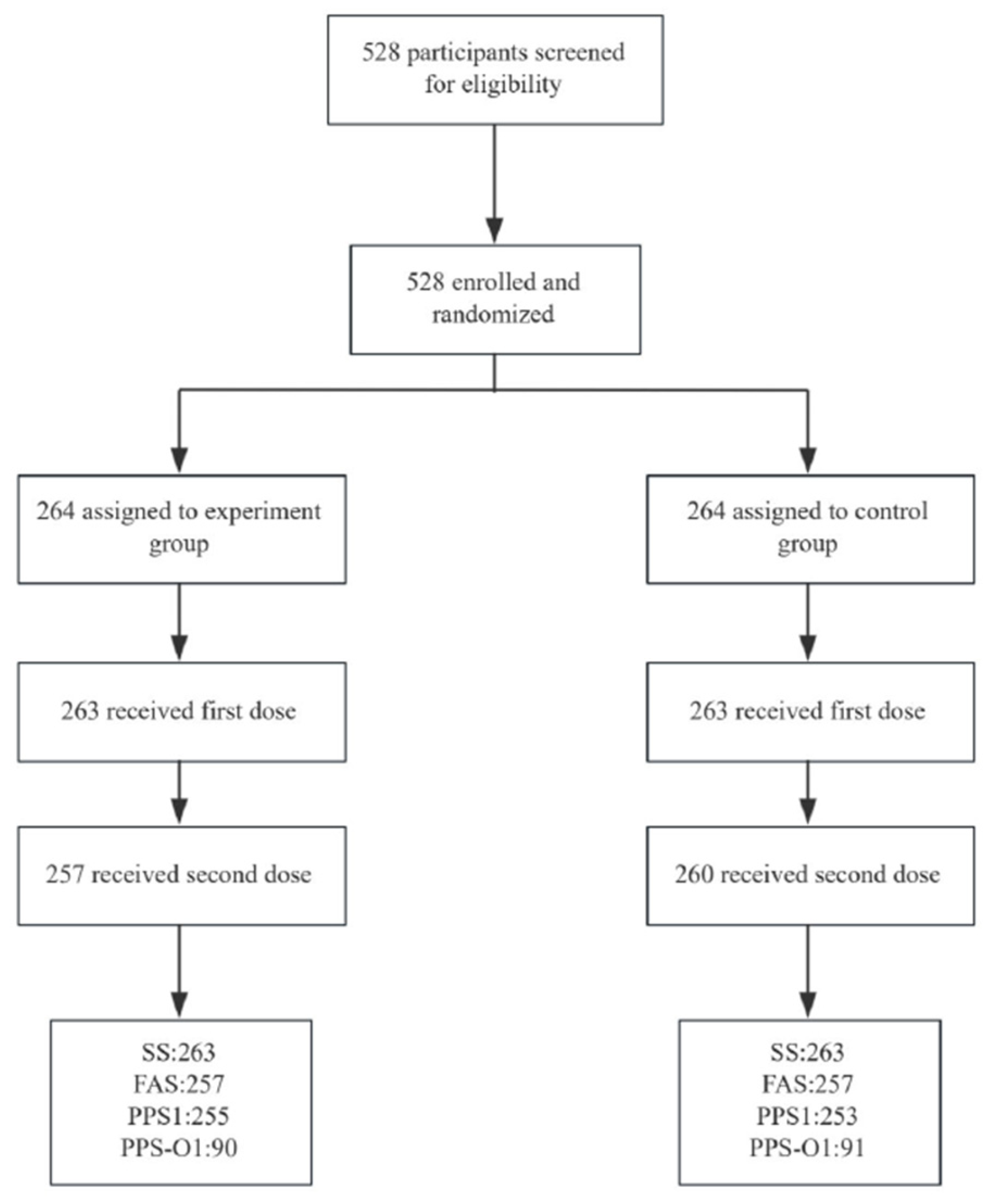
2.1. Study Plan and Participants
2.2. Vaccines and Masking
2.3. Immunogenicity Assessment
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Safety
3.2. Immunogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCV13i | 13-valent pneumococcal polysaccharide conjugate vaccine (CRM197/TT) |
| GMC | Geometric mean concentration |
| OPA | Opsonophagocytic activity |
| GMT | Geometric mean titer |
| IPD | Invasive pneumococcal disease |
| WHO | World Health Organization |
| CIES | Carrier-induced epitope suppression |
| SS | Safety set |
| BMI | Body mass index |
| FAS | Full analysis set |
| PPS1 | Per Protocol Set |
References
- Narciso, A.R.; Dookie, R.; Nannapaneni, P.; Normark, S.; Henriques-Normark, B. Streptococcus pneumoniae epidemiology, pathogenesis and control. Nat. Rev. Microbiol. 2025, 23, 256–271. [Google Scholar] [CrossRef]
- Xie, M.Z.; Dong, M.; Du, J.; Zhang, S.-S.; Huang, F.; Lu, Q.-B. Epidemiological features of Streptococcus pneumoniae in patients with acute respiratory tract infection in Beijing, China during 2009–2020. J. Infect. Public Health 2023, 16, 719–726. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Li, L.; Ma, J.; Yu, Z.; Li, M.; Zhang, W.; Sun, H. Epidemiological characteristics and antibiotic resistance mechanisms of Streptococcus pneumoniae: An updated review. Microbiol. Res. 2023, 266, 127221. [Google Scholar] [CrossRef]
- Cherazard, R.; Epstein, M.; Doan, T.L.; Salim, T.; Bharti, S.; Smith, M.A. Antimicrobial Resistant Streptococcus pneumoniae: Prevalence, Mechanisms, and Clinical Implications. Am. J. Ther. 2017, 24, e361–e369. [Google Scholar] [CrossRef]
- Hsu, H.E.; Shutt, K.A.; Moore, M.R.; Beall, B.W.; Bennett, N.M.; Craig, A.S.; Farley, M.M.; Jorgensen, J.H.; Lexau, C.A.; Petit, S.; et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 2020, 383, 1688–1690. [Google Scholar] [CrossRef]
- Kadioglu, A.; Weiser, J.N.; Paton, J.C.; Andrew, P.W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2021, 19, 250–262. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef] [PubMed]
- Klugman, K.P.; Madhi, S.A.; Huebner, R.E.; Kohberger, R.; Mbelle, N.; Pierce, N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 2003, 349, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Versluys, K.A.; Eurich, D.T.; Marrie, T.J.; Forgie, S.; Tyrrell, G.J. Invasive pneumococcal disease and long-term outcomes in children: A 20-year population cohort study. Lancet Reg. Health Am. 2022, 14, 100341. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. Recent advances in the epidemiology and prevention of Streptococcus pneumoniae infections. F1000Research 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Wantuch, P.L.; Avci, F.Y. Current status and future directions of invasive pneumococcal diseases and prophylactic approaches to control them. Hum. Vaccin. Immunother. 2018, 14, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Hu, Y.; Pan, H.; Wu, J.; Zhu, D.; Young, M.M.; Luo, L.; Yi, Z.; Giardina, P.C.; Gruber, W.C.; et al. A randomized, open-label, phase 3 study evaluating safety and immunogenicity of 13-valent pneumococcal conjugate vaccine in Chinese infants and children under 6 years of age. Hum. Vaccin. Immunother. 2023, 19, 2235926. [Google Scholar] [CrossRef]
- Marimon, J.M.; Ardanuy, C. Epidemiology of pneumococcal diseases in Spain after the introduction of pneumococcal conjugate vaccines. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2021, 39, 142–150. [Google Scholar] [CrossRef]
- WHO Position Paper: Pneumococcal Conjugate Vaccines in Infants and Children Under 5 Years of Age. Available online: https://www.who.int/publications/i/item/10665-310968 (accessed on 24 October 2025).
- National Health Commission of the People’s Republic of China. Available online: https://www.nhc.gov.cn/wjw/c100175/202103/951e284920bd4a908ba10875694f0f0e.shtml (accessed on 24 October 2025).
- Gopalakrishnan, S.; Jayapal, P.; John, J. Pneumococcal surface proteins as targets for next-generation vaccines: Addressing the challenges of serotype variation. Diagn. Microbiol. Infect. Dis. 2025, 113, 116870. [Google Scholar] [CrossRef]
- Apodaca, K.; Grant, L.R.; Perdrizet, J.; Daigle, D.; Mircus, G.; Gessner, B.D. Real-World Impact of Pneumococcal Conjugate Vaccines on Vaccine Serotypes and Potential Cross-Reacting Non-Vaccine Serotypes. Vaccines 2025, 13, 651. [Google Scholar] [CrossRef]
- Dagan, R.; Poolman, J.; Siegrist, C.A. Glycoconjugate vaccines and immune interference: A review. Vaccine 2010, 28, 5513–5523. [Google Scholar] [CrossRef]
- Ahman, H.; Käyhty, H.; Vuorela, A.; Leroy, O.; Eskola, J. Dose dependency of antibody response in infants and children to pneumococcal polysaccharides conjugated to tetanus toxoid. Vaccine 1999, 17, 2726–2732. [Google Scholar] [CrossRef]
- Xie, Z.; Li, J.; Wang, X.; Huang, L.; Gou, J.; Zhang, W.; Huang, H.; You, W.; Wang, F.; Li, X.; et al. The Safety and Immunogenicity of a 13-Valent Pneumococcal Polysaccharide Conjugate Vaccine (CRM197/TT) in Infants: A Double-Blind, Randomized, Phase III Trial. Vaccines 2024, 12, 1417. [Google Scholar] [CrossRef]
- WHO position paper: Pneumococcal conjugate vaccines in infants and children aged <5 years – September 2025. Available online: https://www.who.int/publications/i/item/who-wer10039-411-437 (accessed on 13 November 2025).
- Hsiao, A.; Lewis, N.; Hansen, J.; Timbol, J.; Suaya, J.A.; Alexander-Parrish, R.; Grant, L.R.; Gessner, B.D.; Klein, N.P. Effectiveness of 13-valent pneumococcal conjugate vaccine against vaccine-type invasive pneumococcal disease in older adults. Vaccine 2025, 44, 126543. [Google Scholar] [CrossRef]
- Lekhuleni, C.; Ndlangisa, K.; Gladstone, R.A.; Chochua, S.; Metcalf, B.J.; Li, Y.; Kleynhans, J.; de Gouveia, L.; Hazelhurst, S.; Ferreira, A.D.S.; et al. Impact of pneumococcal conjugate vaccines on invasive pneumococcal disease-causing lineages among South African children. Nat. Commun. 2024, 15, 8401. [Google Scholar] [CrossRef]
- Lucinde, R.K.; Ong’ayo, G.; Houlihan, C.; Bottomley, C.; Goldblatt, D.; Scott, J.; Gallagher, K. Pneumococcal conjugate vaccine dose-ranging studies in humans: A systematic review. Vaccine 2021, 39, 5095–5105. [Google Scholar] [CrossRef]
- WHO. Meningococcal Vaccines: WHO Position Paper, November 2011. Weekly Epidemiological Record. Wkly. Epidemiol. Rec. 2011, 86, 521–540. Available online: https://iris.who.int/handle/10665/241847 (accessed on 24 October 2025).
- Banniettis, N.; Horn, M.; Sadarangani, M.; Patel, M.S.M.; Greenberg, D.; Oberdorfer, P.; Klein, N.P.; Rupp, R.; Dagan, R.; Richmond, P.; et al. V114-031 (PNEU-LINK) study group. Safety and Tolerability of V114 Pneumococcal Vaccine in Infants: A Phase 3 Study. Pediatrics 2023, 152, e2022060428. [Google Scholar] [CrossRef] [PubMed]
- Borys, D.; Rupp, R.; Smulders, R.; Chichili, G.R.; Kovanda, L.L.; Santos, V.; Malinoski, F.; Siber, G.; Malley, R.; Sebastian, S. Safety, tolerability and immunogenicity of a novel 24-valent pneumococcal vaccine in toddlers: A phase 1 randomized controlled trial. Vaccine 2024, 42, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
| Experimental Group (n = 263) | Control Group (n = 263) | p | |
|---|---|---|---|
| Age (month) | 18.11 | 18.05 | 0.833 |
| Gender n (%) | 0.542 | ||
| Male | 135 (51.33%) | 128 (48.67%) | |
| Female | 128 (48.67%) | 135 (51.33%) | |
| Body mass index (kg/m2) | 11.67 | 11.58 | 0.477 |
| Axillary temperature before first dose (°C) | 36.46 | 36.47 | 0.656 |
| Experimental Group (n = 520) | Control Group (n = 523) | p | |
|---|---|---|---|
| Overall adverse events | 193 (37.12%) | 171 (32.70%) | 0.134 |
| Overall adverse reactions | 129 (24.81%) | 113 (21.61%) | 0.221 |
| Systemic adverse reactions | 83 (15.96%) | 86 (16.44%) | 0.833 |
| Local adverse reactions | 52 (10.00%) | 32 (6.12%) | 0.021 |
| Erythema | 41 (7.88%) | 21 (4.02%) | 0.008 |
| Swelling | 16 (3.08%) | 12 (2.29%) | 0.434 |
| Pain | 2 (0.38%) | 6 (1.15%) | 0.291 |
| Fever | 56 (10.77%) | 72 (13.77%) | 0.140 |
| Grade 3 | 0 | 1 (0.19%) | >0.999 |
| Irritability | 2 (0.38%) | 0 | 0.248 |
| Cough | 15 (2.88%) | 12 (2.29%) | 0.548 |
| Diarrhea | 12 (2.31%) | 8 (1.53%) | 0.360 |
| Vomiting | 4 (0.77%) | 3 (0.57%) | 0.994 |
| Hypersensitivity reaction | 1 (0.19%) | 0 | 0.499 |
| Pre-Vaccination | Post-Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serotype | Group | Seropositive Rate% (95%CI) | p | GMC (95%CI) | p | Seropositive Rate % (95%CI) | p | GMC (95%CI) | p |
| 1 | Experimental group | 65.49 (59.30, 71.31) | 0.323 | 0.27 (0.22, 0.32) | 0.326 | 100.00 (98.56, 100.00) | - | 3.17 (2.97, 3.38) | <0.001 |
| Control group | 61.26 (54.96, 67.30) | 0.23 (0.19, 0.29) | 100.00 (98.55, 100.00) | 3.89 (3.63, 4.18) | |||||
| 3 | Experimental group | 5.49 (3.03, 9.04) | 0.451 | 0.03 (0.03, 0.04) | 0.618 | 96.47 (93.41, 98.37) | 0.802 | 1.12 (1.04, 1.20) | <0.001 |
| Control group | 7.11 (4.27, 11.01) | 0.03 (0.02, 0.04) | 96.05 (92.85, 98.09) | 0.88 (0.81, 0.94) | |||||
| 4 | Experimental group | 5.49 (3.03, 9.04) | 0.563 | 0.02 (0.02, 0.02) | 0.653 | 100.00 (98.56, 100.00) | - | 3.47 (3.24, 3.72) | 0.363 |
| Control group | 6.72 (3.96, 10.54) | 0.02 (0.02, 0.03) | 100.00 (98.55, 100.00) | 3.31 (3.08, 3.57) | |||||
| 5 | Experimental group | 59.22 (52.91, 65.30) | 0.352 | 0.24 (0.20, 0.29) | 0.716 | 96.47 (93.41, 98.37) | 0.007 | 0.90 (0.84, 0.96) | <0.001 |
| Control group | 63.24 (56.97, 69.19) | 0.25 (0.21, 0.31) | 100.00 (98.55, 100.00) | 1.94 (1.81, 2.08) | |||||
| 6A | Experimental group | 31.76 (26.10, 37.86) | 0.802 | 0.13 (0.10, 0.16) | 0.753 | 99.22 (97.20, 99.90) | 0.499 | 1.91 (1.75, 2.09) | 0.018 |
| Control group | 32.81 (27.06, 38.97) | 0.13 (0.11, 0.16) | 100.00 (98.55, 100.00) | 2.24 (2.03, 2.47) | |||||
| 6B | Experimental group | 80.00 (74.56, 84.73) | 0.222 | 0.44 (0.37, 0.51) | 0.380 | 98.82 (96.60, 99.76) | 0.250 | 2.72 (2.47, 2.99) | 0.320 |
| Control group | 75.49 (69.72, 80.66) | 0.39 (0.33, 0.47) | 100.00 (98.55, 100.00) | 2.91 (2.64, 3.21) | |||||
| 7F | Experimental group | 30.59 (24.99, 36.64) | 0.361 | 0.07 (0.05, 0.09) | 0.886 | 100.00 (98.56, 100.00) | - | 8.10 (7.52, 8.72) | <0.001 |
| Control group | 34.39 (28.55, 40.59) | 0.07 (0.06, 0.08) | 100.00 (98.55, 100.00) | 6.07 (5.65, 6.51) | |||||
| 9V | Experimental group | 13.33 (9.41, 18.13) | 0.616 | 0.03 (0.02, 0.03) | 0.896 | 100.00 (98.56, 100.00) | - | 2.68 (2.47, 2.92) | 0.235 |
| Control group | 11.86 (8.15, 16.49) | 0.03 (0.02, 0.03) | 100.00 (98.55, 100.00) | 2.89 (2.64, 3.16) | |||||
| 14 | Experimental group | 98.04 (95.48, 99.36) | 0.391 | 1.72 (1.55, 1.90) | 0.686 | 100.00 (98.56, 100.00) | - | 10.37 (9.68, 11.11) | <0.001 |
| Control group | 96.84 (93.86, 98.63) | 1.78 (1.56, 2.02) | 100.00 (98.55, 100.00) | 14.24 (13.30, 15.25) | |||||
| 18C | Experimental group | 7.06 (4.24, 10.93) | 0.025 | 0.02 (0.02, 0.02) | 0.184 | 100.00 (98.56, 100.00) | - | 2.88 (2.67, 3.11) | 0.156 |
| Control group | 13.04 (9.15, 17.83) | 0.02 (0.02, 0.03) | 100.00 (98.55, 100.00) | 3.13 (2.87, 3.41) | |||||
| 19A | Experimental group | 91.76 (87.69, 94.83) | 0.620 | 0.83 (0.71, 0.97) | 0.577 | 100.00 (98.56, 100.00) | - | 7.24 (6.73, 7.78) | 0.420 |
| Control group | 90.51 (86.21, 93.83) | 0.89 (0.75, 1.05) | 100.00 (98.55, 100.00) | 6.94 (6.43, 7.48) | |||||
| 19F | Experimental group | 92.55 (88.61, 95.45) | 0.748 | 0.85 (0.74, 0.98) | 0.768 | 100.00 (98.56, 100.00) | - | 9.98 (9.25, 10.76) | <0.001 |
| Control group | 93.28 (89.46, 96.04) | 0.88 (0.75, 1.02) | 100.00 (98.55, 100.00) | 5.23 (4.85, 5.63) | |||||
| 23F | Experimental group | 41.18 (35.07, 47.49) | 0.033 | 0.18 (0.14, 0.22) | 0.581 | 99.61 (97.83, 99.99) | >0.999 | 2.65 (2.46, 2.85) | 0.076 |
| Control group | 50.59 (44.26, 56.91) | 0.19 (0.15, 0.24) | 99.60 (97.82, 99.99) | 2.38 (2.18, 2.61) | |||||
| Experimental Group (n = 255) | Control Group (n = 253) | |||||
|---|---|---|---|---|---|---|
| Serotype | n | Proportion % (95%CI) | n | Proportion % (95%CI) | p | |
| 1 | Pre-vaccination | 24 | 9.41 (6.12, 13.68) | 24 | 9.49 (6.17, 13.79) | 0.977 |
| Post-vaccination | 248 | 97.25 (94.43, 98.89) | 251 | 99.21 (97.17, 99.90) | 0.182 | |
| 3 | Pre-vaccination | 3 | 1.18 (0.24, 3.40) | 3 | 1.19 (0.25, 3.43) | >0.999 |
| Post-vaccination | 146 | 57.25 (50.93, 63.41) | 96 | 37.94 (31.94, 44.23) | <0.001 | |
| 4 | Pre-vaccination | 2 | 0.78 (0.10, 2.80) | 4 | 1.58 (0.43, 4.00) | 0.674 |
| Post-vaccination | 252 | 98.82 (96.60, 99.76) | 248 | 98.02 (95.45, 99.36) | 0.713 | |
| 5 | Pre-vaccination | 10 | 3.92 (1.90, 7.09) | 13 | 5.14 (2.76, 8.63) | 0.510 |
| Post-vaccination | 102 | 40.00 (33.94, 46.30) | 221 | 87.35 (82.62, 91.19) | <0.001 | |
| 6A | Pre-vaccination | 2 | 0.78 (0.10, 2.80) | 4 | 1.58 (0.43, 4.00) | 0.674 |
| Post-vaccination | 214 | 83.92 (78.83, 88.21) | 213 | 84.19 (79.10, 88.46) | 0.934 | |
| 6B | Pre-vaccination | 46 | 18.04 (13.52, 23.32) | 58 | 22.92 (17.89, 28.60) | 0.172 |
| Post-vaccination | 232 | 90.98 (86.77, 94.20) | 230 | 90.91 (86.67, 94.15) | 0.978 | |
| 7F | Pre-vaccination | 15 | 5.88 (3.33, 9.52) | 12 | 4.74 (2.47, 8.14) | 0.567 |
| Post-vaccination | 255 | 100.00 (98.56, 100.00) | 253 | 100.00 (98.55, 100.00) | - | |
| 9V | Pre-vaccination | 2 | 0.78 (0.10, 2.80) | 6 | 2.37 (0.88, 5.09) | 0.280 |
| Post-vaccination | 237 | 92.94 (89.07, 95.76) | 235 | 92.89 (88.99, 95.73) | 0.980 | |
| 14 | Pre-vaccination | 221 | 86.67 (81.87, 90.59) | 219 | 86.56 (81.73, 90.51) | 0.972 |
| Post-vaccination | 255 | 100.00 (98.56, 100.00) | 253 | 100.00 (98.55, 100.00) | - | |
| 18C | Pre-vaccination | 6 | 2.35 (0.87, 5.05) | 8 | 3.16 (1.37, 6.14) | 0.578 |
| Post-vaccination | 245 | 96.08 (92.91, 98.10) | 241 | 95.26 (91.86, 97.53) | 0.649 | |
| 19A | Pre-vaccination | 128 | 50.20 (43.89, 56.50) | 151 | 59.68 (53.36, 65.78) | 0.032 |
| Post-vaccination | 254 | 99.61 (97.83, 99.99) | 253 | 100.00 (98.55, 100.00) | >0.999 | |
| 19F | Pre-vaccination | 119 | 46.67 (40.42, 52.99) | 136 | 53.75 (47.40, 60.02) | 0.110 |
| Post-vaccination | 255 | 100.00 (98.56, 100.00) | 253 | 100.00 (98.55, 100.00) | - | |
| 23F | Pre-vaccination | 9 | 3.53 (1.63, 6.59) | 9 | 3.56 (1.64, 6.65) | 0.986 |
| Post-vaccination | 243 | 95.29 (91.92, 97.55) | 221 | 87.35 (82.62, 91.19) | 0.001 | |
| Serotype | Group | GMT (95%CI) | p | Percentage % (95%CI) | p |
|---|---|---|---|---|---|
| 1 | Experimental group | 31.82 (25.11, 40.33) | 0.075 | 91.11 (83.23, 96.08) | 0.224 |
| Control group | 43.10 (33.91, 54.77) | 95.60 (89.13, 98.79) | |||
| 3 | Experimental group | 185.42 (161.24, 213.24) | 0.483 | 100.00 (95.98, 100.00) | - |
| Control group | 172.48 (148.57, 200.24) | 100.00 (96.03, 100.00) | |||
| 4 | Experimental group | 2631.87 (2165.10, 3199.27) | 0.880 | 100.00 (95.98, 100.00) | - |
| Control group | 2688.68 (2198.68, 3287.87) | 100.00 (96.03, 100.00) | |||
| 5 | Experimental group | 13.43 (9.88, 18.26) | <0.001 | 74.44 (64.16, 83.06) | <0.001 |
| Control group | 92.95 (66.90, 129.15) | 94.51 (87.64, 98.19) | |||
| 6A | Experimental group | 5980.41 (4536.39, 7884.11) | 0.401 | 98.89 (93.96, 99.97) | 0.621 |
| Control group | 4913.92 (3385.37, 7132.63) | 96.70 (90.67, 99.31) | |||
| 6B | Experimental group | 4135.98 (3014.32, 5675.01) | 0.238 | 97.78 (92.20, 99.73) | 0.688 |
| Control group | 3046.04 (2032.74, 4564.45) | 95.60 (89.13, 98.79) | |||
| 7F | Experimental group | 39,583.25 (33,086.75, 47,355.31) | <0.001 | 100.00 (95.98, 100.00) | - |
| Control group | 17,248.59 (14,213.01, 20,932.50) | 100.00 (96.03, 100.00) | |||
| 9V | Experimental group | 3425.08 (2729.11, 4298.52) | 0.594 | 100.00 (95.98, 100.00) | - |
| Control group | 3723.45 (3010.18, 4605.73) | 100.00 (96.03, 100.00) | |||
| 14 | Experimental group | 3447.71 (2807.82, 4233.41) | 0.193 | 100.00 (95.98, 100.00) | - |
| Control group | 4138.67 (3433.06, 4989.30) | 100.00 (96.03, 100.00) | |||
| 18C | Experimental group | 1691.44 (1295.27, 2208.78) | 0.638 | 98.89 (93.96, 99.97) | >0.999 |
| Control group | 1849.70 (1417.27, 2414.07) | 98.90 (94.03, 99.97) | |||
| 19A | Experimental group | 3686.14 (3065.41, 4432.56) | 0.137 | 100.00 (95.98, 100.00) | - |
| Control group | 2995.44 (2438.89, 3678.98) | 100.00 (96.03, 100.00) | |||
| 19F | Experimental group | 1516.83 (1103.50, 2084.97) | 0.028 | 96.67 (90.57, 99.31) | 0.605 |
| Control group | 982.95 (787.96, 1226.20) | 98.90 (94.03, 99.97) | |||
| 23F | Experimental group | 8125.45 (6055.05, 10,903.79) | 0.290 | 98.89 (93.96, 99.97) | >0.999 |
| Control group | 6390.47 (4551.71, 8972.02) | 97.80 (92.29, 99.73) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Wang, F.; Huang, L.; Huang, H.; Feng, G.; Wang, X.; Tan, J.; Ma, X.; You, W.; Li, X.; et al. The Safety and Immunogenicity of a 13-Valent Pneumococcal Polysaccharide Conjugate Vaccine (CRM197/TT) in 12–23-Month Children: A Double-Blind, Randomized, Phase III Trial in China. Vaccines 2025, 13, 1190. https://doi.org/10.3390/vaccines13121190
Xie Z, Wang F, Huang L, Huang H, Feng G, Wang X, Tan J, Ma X, You W, Li X, et al. The Safety and Immunogenicity of a 13-Valent Pneumococcal Polysaccharide Conjugate Vaccine (CRM197/TT) in 12–23-Month Children: A Double-Blind, Randomized, Phase III Trial in China. Vaccines. 2025; 13(12):1190. https://doi.org/10.3390/vaccines13121190
Chicago/Turabian StyleXie, Zhiqiang, Feiyu Wang, Lili Huang, Haitao Huang, Guangwei Feng, Xue Wang, Jiebing Tan, Xiaomin Ma, Wangyang You, Xiaolong Li, and et al. 2025. "The Safety and Immunogenicity of a 13-Valent Pneumococcal Polysaccharide Conjugate Vaccine (CRM197/TT) in 12–23-Month Children: A Double-Blind, Randomized, Phase III Trial in China" Vaccines 13, no. 12: 1190. https://doi.org/10.3390/vaccines13121190
APA StyleXie, Z., Wang, F., Huang, L., Huang, H., Feng, G., Wang, X., Tan, J., Ma, X., You, W., Li, X., Gou, J., & Wang, Y. (2025). The Safety and Immunogenicity of a 13-Valent Pneumococcal Polysaccharide Conjugate Vaccine (CRM197/TT) in 12–23-Month Children: A Double-Blind, Randomized, Phase III Trial in China. Vaccines, 13(12), 1190. https://doi.org/10.3390/vaccines13121190






