Evaluation of a Cell-Adapted Live Attenuated African Swine Fever Virus Thai-Strain Vaccine Candidate: Highlighting Enhanced Virulence Risk in Co-Infected Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Animals and Disease Screening
2.3. Virus Inoculation and Challenge Study
2.4. Quantitative PCR (qPCR) Analysis
2.5. Enzyme-Linked Immunospot (ELISpot) Assay
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Histopathology
2.8. Statistical Analysis
3. Results
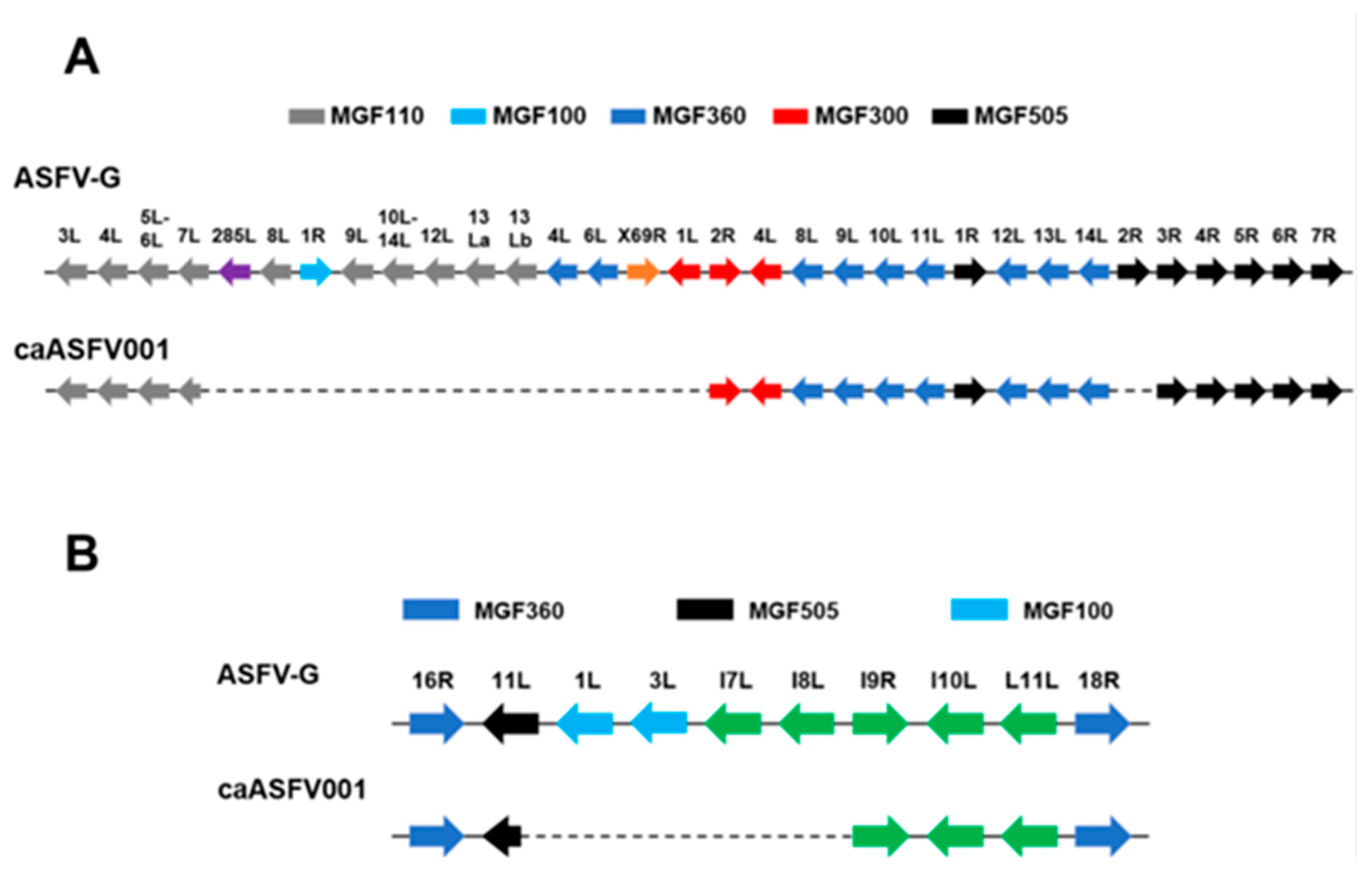
3.1. Genomic Analysis of caASFV001-MA52
3.2. Immunization with caASFV001-MA52
3.2.1. Clinical Findings Post-Vaccination
3.2.2. caASFV001-MA52 Detection and Pathogenic Viral and Bacterial Reactivation Post-Vaccination
3.3. ASFV Challenge and Clinical Outcomes
3.4. Immune Responses of Vaccinated Pigs
3.5. Disease Development and Lesion After Virulent ASFV Infection
4. Discussion
5. Limitations of the Study
6. Conclusions and Future Perspectives
7. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASFV | African swine fever virus |
| caASFV | Cell-adapted African swine fever virus |
| CHD | Challenge, high dose |
| CLD | Challenge, low dose |
| CSFV | Classical swine fever virus |
| Ct | Threshold cycles |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPV | Days post-vaccination |
| DPC | Days post-challenge |
| ELISA | Enzyme-linked immunosorbent assay |
| ELISpot | Enzyme-linked immunosorbent spot |
| HAD50 | Median hemadsorption dose |
| H&E | Hematoxylin and eosin |
| IFN-γ | Interferon gamma |
| LAV | Live attenuated vaccine |
| LVR | Left variable region |
| Mhp | Mycoplasma hyopneumoniae |
| Mhr | Mycoplasma hyorhinitis |
| MOI | Multiplicity of infection |
| NC | Negative control |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PCV | Porcine circovirus |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| RPMI-1640 | Roswell Park Memorial Institute (RPMI) 1640 medium |
| RVR | Right variable region |
| qPCR | Quantitative polymerase chain reaction |
| SPF | Specific-pathogen-free (SPF) |
| S. suis | Streptococcus suis |
| TCID50 | Median tissue culture infectious dose |
References
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV Report Consortium ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- Blome, S.; Franzke, K.; Beer, M. African Swine Fever–A Review of Current Knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Sayruamyat, S. Thai Swine Industry During African Swine Fever Outbreak in Asia. FFTC Agric. Policy Platf. FFTC-AP 2022. Available online: https://ap.fftc.org.tw/article/3011 (accessed on 31 May 2025).
- WOAH. African Swine Fever (ASF)–Situation Report 64; WOAH World Organisation for Animal Health: Paris, France, 2025. [Google Scholar]
- Gallardo, C.; Soler, A.; Nieto, R.; Sánchez, M.A.; Martins, C.; Pelayo, V.; Carrascosa, A.; Revilla, Y.; Simón, A.; Briones, V.; et al. Experimental Transmission of African Swine Fever (ASF) Low Virulent Isolate NH/P68 by Surviving Pigs. Transbound. Emerg. Dis. 2015, 62, 612–622. [Google Scholar] [CrossRef]
- Ceruti, A.; Kobialka, R.M.; Abd El Wahed, A.; Truyen, U. African Swine Fever: A One Health Perspective and Global Challenges. Animals 2025, 15, 928. [Google Scholar] [CrossRef]
- Chandana, M.S.; Nair, S.S.; Chaturvedi, V.K.; Abhishek; Pal, S.; Charan, M.S.S.; Balaji, S.; Saini, S.; Vasavi, K.; Deepa, P. Recent Progress and Major Gaps in the Vaccine Development for African Swine Fever. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2024, 55, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Rock, D.L. Challenges for African Swine Fever Vaccine Development-“… Perhaps the End of the Beginning”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Huang, S.; Qiu, H.-J. Multifaceted Immune Responses to African Swine Fever Virus: Implications for Vaccine Development. Vet. Microbiol. 2020, 249, 108832. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yu, H.; Miao, F.; Ke, J.; Hu, R. Attenuated African Swine Fever Viruses and the Live Vaccine Candidates: A Comprehensive Review. Microbiol. Spectr. 2024, 12, e0319923. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94, e02017–e02019. [Google Scholar] [CrossRef]
- Deutschmann, P.; Carrau, T.; Sehl-Ewert, J.; Forth, J.H.; Viaplana, E.; Mancera, J.C.; Urniza, A.; Beer, M.; Blome, S. Taking a Promising Vaccine Candidate Further: Efficacy of ASFV-G-ΔMGF after Intramuscular Vaccination of Domestic Pigs and Oral Vaccination of Wild Boar. Pathogens 2022, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African Swine Fever Virus Vaccine Candidate ASFV-G-ΔI177L Efficiently Protects European and Native Pig Breeds against Circulating Vietnamese Field Strain. Transbound. Emerg. Dis. 2022, 69, e497–e504. [Google Scholar] [CrossRef]
- Tran, X.H.; Phuong, L.T.T.; Huy, N.Q.; Thuy, D.T.; Nguyen, V.D.; Quang, P.H.; Ngôn, Q.V.; Rai, A.; Gay, C.G.; Gladue, D.P.; et al. Evaluation of the Safety Profile of the ASFV Vaccine Candidate ASFV-G-ΔI177L. Viruses 2022, 14, 896. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Bui, N.T.T.; Nguyen, L.T.; Ngo, T.N.T.; Van Nguyen, C.; Nguyen, L.M.; Nouhin, J.; Karlsson, E.; Padungtod, P.; Pamornchainavakul, N.; et al. An African Swine Fever Vaccine-like Variant with Multiple Gene Deletions Caused Reproductive Failure in a Vietnamese Breeding Herd. Sci. Rep. 2025, 15, 14919. [Google Scholar] [CrossRef]
- van den Born, E.; Olasz, F.; Mészáros, I.; Göltl, E.; Oláh, B.; Joshi, J.; van Kilsdonk, E.; Segers, R.; Zádori, Z. African Swine Fever Virus Vaccine Strain Asfv-G-∆I177l Reverts to Virulence and Negatively Affects Reproductive Performance. npj Vaccines 2025, 10, 46. [Google Scholar] [CrossRef]
- Rock, D.L. Thoughts on African Swine Fever Vaccines. Viruses 2021, 13, 943. [Google Scholar] [CrossRef]
- Meloni, D.; Franzoni, G.; Oggiano, A. Cell Lines for the Development of African Swine Fever Virus Vaccine Candidates: An Update. Vaccines 2022, 10, 707. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Ge, S.; Zuo, Y.; Lu, H.; Lv, Y.; Han, N.; Cai, Y.; Wu, X.; Wang, Z. Attenuated African Swine Fever Virus through Serial Passaging of Viruses in Cell Culture: A Brief Review on the Knowledge Gathered during 60 Years of Research. Virus Genes 2022, 59, 13–24. [Google Scholar] [CrossRef]
- Kim, M.H.; Subasinghe, A.; Kim, Y.; Kwon, H.-I.; Cho, Y.; Chathuranga, K.; Cha, J.-W.; Moon, J.-Y.; Hong, J.-H.; Kim, J.; et al. Development and Characterization of High-Efficiency Cell-Adapted Live Attenuated Vaccine Candidate against African Swine Fever. Emerg. Microbes Infect. 2024, 13, 2432372. [Google Scholar] [CrossRef] [PubMed]
- Suh, T.-Y.; Park, J.-H.; Hwang, S.Y.; Park, C.-R.; Kim, J.-E.; Park, J.Y.; Kim, Y.-J.; Kang, H.-E.; Kim, D.-Y.; Choi, J.-G. Safety and Efficacy of a Vero-Adapted Live Attenuated Vaccine Candidate for African Swine Fever. Vaccine 2025, 56, 127172. [Google Scholar] [CrossRef] [PubMed]
- Thaweerattanasinp, T.; Kaewborisuth, C.; Viriyakitkosol, R.; Saenboonrueng, J.; Wanitchang, A.; Tanwattana, N.; Sonthirod, C.; Sangsrakru, D.; Pootakham, W.; Tangphatsornruang, S.; et al. Adaptation of African Swine Fever Virus to MA-104 Cells: Implications of Unique Genetic Variations. Vet. Microbiol. 2024, 291, 110016. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- WOAH. African Swine Fever (Infection with African Swine Fever Virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2024; Chapter 3.9.1.; WOAH World Organisation for Animal Health: Paris, France, 2024. [Google Scholar]
- Galindo-Cardiel, I.; Ballester, M.; Solanes, D.; Nofrarías, M.; López-Soria, S.; Argilaguet, J.M.; Lacasta, A.; Accensi, F.; Rodríguez, F.; Segalés, J. Standardization of Pathological Investigations in the Framework of Experimental ASFV Infections. Virus Res. 2013, 173, 180–190. [Google Scholar] [CrossRef]
- Afonso, C.L.; Piccone, M.E.; Zaffuto, K.M.; Neilan, J.; Kutish, G.F.; Lu, Z.; Balinsky, C.A.; Gibb, T.R.; Bean, T.J.; Zsak, L.; et al. African Swine Fever Virus Multigene Family 360 and 530 Genes Affect Host Interferon Response. J. Virol. 2004, 78, 1858–1864. [Google Scholar] [CrossRef]
- Reis, A.L.; Abrams, C.C.; Goatley, L.C.; Netherton, C.; Chapman, D.G.; Sanchez-Cordon, P.; Dixon, L.K. Deletion of African Swine Fever Virus Interferon Inhibitors from the Genome of a Virulent Isolate Reduces Virulence in Domestic Pigs and Induces a Protective Response. Vaccine 2016, 34, 4698–4705. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, Y.; Quan, W.; Zheng, J.; Luo, Y.; Wang, H.; Chen, X.; Huang, Z.; Chen, X.; Xu, R.; et al. The African Swine Fever Virus with MGF360 and MGF505 Deleted Reduces the Apoptosis of Porcine Alveolar Macrophages by Inhibiting the NF-κB Signaling Pathway and Interleukin-1β. Vaccines 2021, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Holinka, L.G.; Sanford, B.; Krug, P.W.; Carlson, J.; Pacheco, J.M.; Reese, B.; Risatti, G.R.; Gladue, D.P.; Borca, M.V. African Swine Fever Virus Georgia Isolate Harboring Deletions of 9GL and MGF360/505 Genes Is Highly Attenuated in Swine but Does Not Confer Protection against Parental Virus Challenge. Virus Res. 2016, 221, 8–14. [Google Scholar] [CrossRef]
- Huang, H.; Dang, W.; Shi, Z.; Ding, M.; Xu, F.; Li, T.; Feng, T.; Zheng, H.; Xiao, S. Identification of African Swine Fever Virus MGF505-2R as a Potent Inhibitor of Innate Immunity in Vitro. Virol. Sin. 2022, 38, 84–95. [Google Scholar] [CrossRef]
- Sunwoo, S.-Y.; García-Belmonte, R.; Walczak, M.; Vigara-Astillero, G.; Kim, D.-M.; Szymankiewicz, K.; Kochanowski, M.; Liu, L.; Tark, D.; Podgórska, K.; et al. Deletion of MGF505-2R Gene Activates the cGAS-STING Pathway Leading to Attenuation and Protection against Virulent African Swine Fever Virus. Vaccines 2024, 12, 407. [Google Scholar] [CrossRef]
- Afe, A.E.; Shen, Z.-J.; Guo, X.; Zhou, R.; Li, K. African Swine Fever Virus Interaction with Host Innate Immune Factors. Viruses 2023, 15, 1220. [Google Scholar] [CrossRef]
- Orosco, F.L. Host Immune Responses against African Swine Fever Virus: Insights and Challenges for Vaccine Development. Open Vet. J. 2023, 13, 1517–1535. [Google Scholar] [CrossRef]
- Wu, L.; Yang, B.; Yuan, X.; Hong, J.; Peng, M.; Chen, J.-L.; Song, Z. Regulation and Evasion of Host Immune Response by African Swine Fever Virus. Front. Microbiol. 2021, 12, 698001. [Google Scholar] [CrossRef]
- Wang, W.; Yin, L.; Zhang, Z.; Liu, F.; Zhang, X.; Wang, Z.; Zhao, R.; Cao, M.; Zhang, Y.; Ding, L.; et al. Cell-Adapted African Swine Fever Virus Pig/HLJ/18 Is Highly Attenuated but Fails to Induce Immune Protection against a Challenge with Its Parental Virus. J. Integr. Agric. 2025. [Google Scholar] [CrossRef]
- Choi, S.A.; Kim, Y.; Lee, S.J.; Moon, S.C.; Ahn, K.S.; Zheng, X.; Kim, D.S.; Lee, S.Y.; Shin, S.P.; Tark, D.; et al. African Swine Fever Vaccine Candidate ASFV-G-ΔI177L/ΔLVR Protects Against Homologous Virulent Challenge and Exhibits Long-Term Maintenance of Antibodies. Animals 2025, 15, 473. [Google Scholar] [CrossRef]
- Truong, Q.L.; Wang, L.; Nguyen, T.A.; Nguyen, H.T.; Tran, S.D.; Vu, A.T.; Le, A.D.; Nguyen, V.G.; Hoang, P.T.; Nguyen, Y.T.; et al. A Cell-Adapted Live-Attenuated Vaccine Candidate Protects Pigs against the Homologous Strain VNUA-ASFV-05L1, a Representative Strain of the Contemporary Pandemic African Swine Fever Virus. Viruses 2023, 15, 2089. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Xu, Z.; Gao, P.; Qu, Y.; Ge, X.; Zhang, Y.; Han, J.; Guo, X.; Zhou, L.; Yang, H. African Swine Fever Virus Infection Enhances CD14-Dependent Phagocytosis of Porcine Alveolar Macrophages to Promote Bacterial Uptake and Apoptotic Body-Mediated Viral Transmission. J. Virol. 2025, 99, e0069025. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Luo, R.; Zhang, J.; Lu, Z.; Li, L.-F.; Zheng, Y.-H.; Pan, L.; Lan, J.; Zhai, H.; Huang, S.; et al. The MGF300-2R Protein of African Swine Fever Virus Is Associated with Viral Pathogenicity by Promoting the Autophagic Degradation of IKKα and IKKβ through the Recruitment of TOLLIP. PLoS Pathog. 2023, 19, e1011580. [Google Scholar] [CrossRef]
- Wang, T.; Luo, R.; Zhang, J.; Lan, J.; Lu, Z.; Zhai, H.; Li, L.-F.; Sun, Y.; Qiu, H.-J. The African Swine Fever Virus MGF300-4L Protein Is Associated with Viral Pathogenicity by Promoting the Autophagic Degradation of IKKβ and Increasing the Stability of IκBα. Emerg. Microbes Infect. 2024, 13, 2333381. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Luo, R.; Lan, J.; Chen, S.; Qiu, H.-J.; Wang, T.; Sun, Y. The MGF300-2R Protein of African Swine Fever Virus Promotes IKKβ Ubiquitination by Recruiting the E3 Ubiquitin Ligase TRIM21. Viruses 2024, 16, 949. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, S.; Xin, T.; Wang, X.; Yu, H.; Chen, S.; Jiang, Y.; Gao, X.; Jiang, Y.; Guo, X.; et al. African Swine Fever Virus MGF360-14L Negatively Regulates Type I Interferon Signaling by Targeting IRF3. Front. Cell. Infect. Microbiol. 2022, 11, 818969. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhao, P.; Cui, S.; Tao, C.; Huang, Y.; Zhu, H.; Jia, H. DNAJA3 Interacts with ASFV MGF360-14L Protein and Reduces MGF360-14L Antagonistic Role on Beta Interferon Production. Int. J. Biol. Macromol. 2025, 310, 143159. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, B.; Shen, C.; Zhang, T.; Hao, Y.; Zhang, D.; Liu, H.; Shi, X.; Li, G.; Yang, J.; et al. MGF360-9L Is a Major Virulence Factor Associated with the African Swine Fever Virus by Antagonizing the JAK/STAT Signaling Pathway. mBio 2022, 13, e0233021. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, J.; Wu, J.; Yi, J.; Wu, P.; Qi, X.; Ren, J.; Peng, G.; Duan, X.; Ru, Y.; et al. African Swine Fever Virus MGF-360-10L Is a Novel and Crucial Virulence Factor That Mediates Ubiquitination and Degradation of JAK1 by Recruiting the E3 Ubiquitin Ligase HERC5. mBio 2023, 14, e00606–e00623. [Google Scholar] [CrossRef]
- Zheng, L.; Yan, Z.; Qi, X.; Ren, J.; Ma, Z.; Liu, H.; Zhang, Z.; Li, D.; Pei, J.; Xiao, S.; et al. The Deletion of the MGF360-10L/505-7R Genes of African Swine Fever Virus Results in High Attenuation but No Protection Against Homologous Challenge in Pigs. Viruses 2025, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Assavacheep, P.; Thanawongnuwech, R. Porcine Respiratory Disease Complex: Dynamics of Polymicrobial Infections and Management Strategies after the Introduction of the African Swine Fever. Front. Vet. Sci. 2022, 9, 1048861. [Google Scholar] [CrossRef] [PubMed]
- Fehér, E.; Jakab, F.; Bányai, K. Mechanisms of Circovirus Immunosuppression and Pathogenesis with a Focus on Porcine Circovirus 2: A Review. Vet. Q. 2023, 43, 1–18. [Google Scholar] [CrossRef]
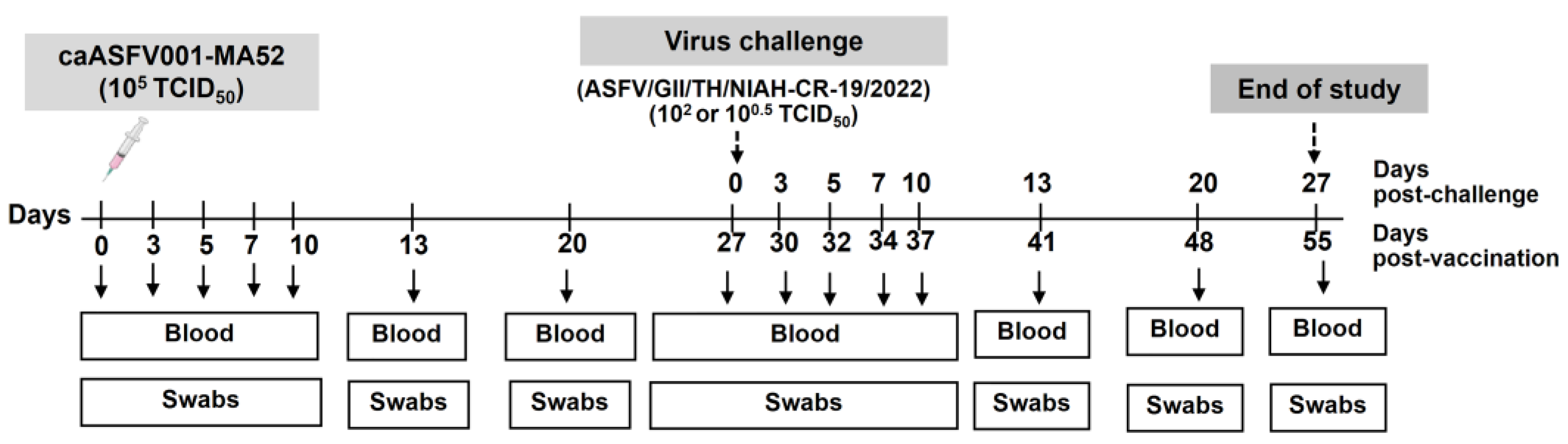


| Group | Inoculum | caASFV001-MA52 (TCID50) | No. of Pigs Pre-Vaccination | No. of Pigs Post-Vaccination | Challenge Virus (TCID50) | Abbreviation |
|---|---|---|---|---|---|---|
| A | PBS | - | 3 | 3 | - | NC |
| B | PBS | - | 3 | 3 | 102 | PC[B]-CHD |
| C | PBS | - | 3 | 3 | 100.5 | PC[C]-CLD |
| D | caASFV001-MA52 | 105 | 7 | 5 * | 102 | D[105]-CHD |
| E | caASFV001-MA52 | 105 | 7 | 7 | 100.5 | E[105]-CLD |
| Group | Label | Clinical Signs (Score) | Days Post-Vaccination | Remark |
|---|---|---|---|---|
| PC[C] | 66C | Diarrhea (1) | 6–7 | |
| D[105] | 5D | Diarrhea (1) | 6–7 | S. suis and PCV2 detected in feces |
| 7D | Fever (1) | 4, 9, 11 | Smaller size | |
| Anorexia (1) | 7–14 | |||
| 15D | Fever (1) | 17–18 | Smaller size | |
| Anorexia (1) | 7–14 | |||
| 16D | Anorexia (1) | 7–14 | Smaller size/ Non-survivor | |
| 49D | Diarrhea (1) | 6–7 | Non-survivor S. suis and caASFV001-MA52 were detected in joint fluid | |
| Joint swelling (1) | 7–10 | |||
| Joint swelling (2) | 11–12 | |||
| Anorexia (1) | 11–12 | |||
| E[105] | 45E | Diarrhea (1) | 6–8 | PCV2 detected in feces |
| 68E | Diarrhea (1) | 6–8 |
| Pathogen | 0 dpv (N = 14) | 7 dpv (N = 14) | 13 dpv (N = 14) | 20 dpv (N = 14) | 27 dpv (N = 12) |
|---|---|---|---|---|---|
| PCV2 | 0 | 35.7 (5/14) | 0 | 0 | 0 |
| PCV3 | 7 (1/14) | 21.4 (3/14) | 0 | 0 | 0 |
| PRRSV (EU) | 0 | 14.2 (2/14) | 14.2 (2/14) | 0 | 0 |
| PRRSV (US) | 0 | 14.2 (2/14) | 0 | 14.2 (2/14) | 16.6 (2/12) |
| PRRSV (HP) | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewborisuth, C.; Thaweerattanasinp, T.; Wanasen, N.; Chorpunkul, A.; Hansoongnern, P.; Tanwattana, N.; Srisutthisamphan, K.; Saenboonrueng, J.; Wanitchang, A.; Wattanaphansak, S.; et al. Evaluation of a Cell-Adapted Live Attenuated African Swine Fever Virus Thai-Strain Vaccine Candidate: Highlighting Enhanced Virulence Risk in Co-Infected Pigs. Vaccines 2025, 13, 1189. https://doi.org/10.3390/vaccines13121189
Kaewborisuth C, Thaweerattanasinp T, Wanasen N, Chorpunkul A, Hansoongnern P, Tanwattana N, Srisutthisamphan K, Saenboonrueng J, Wanitchang A, Wattanaphansak S, et al. Evaluation of a Cell-Adapted Live Attenuated African Swine Fever Virus Thai-Strain Vaccine Candidate: Highlighting Enhanced Virulence Risk in Co-Infected Pigs. Vaccines. 2025; 13(12):1189. https://doi.org/10.3390/vaccines13121189
Chicago/Turabian StyleKaewborisuth, Challika, Theeradej Thaweerattanasinp, Nanchaya Wanasen, Apidsada Chorpunkul, Payuda Hansoongnern, Nathiphat Tanwattana, Kanjana Srisutthisamphan, Janya Saenboonrueng, Asawin Wanitchang, Suphot Wattanaphansak, and et al. 2025. "Evaluation of a Cell-Adapted Live Attenuated African Swine Fever Virus Thai-Strain Vaccine Candidate: Highlighting Enhanced Virulence Risk in Co-Infected Pigs" Vaccines 13, no. 12: 1189. https://doi.org/10.3390/vaccines13121189
APA StyleKaewborisuth, C., Thaweerattanasinp, T., Wanasen, N., Chorpunkul, A., Hansoongnern, P., Tanwattana, N., Srisutthisamphan, K., Saenboonrueng, J., Wanitchang, A., Wattanaphansak, S., Tantilertcharoen, R., Suksawat, N., Kramyu, J., Liwnaree, B., Muangsanit, P., Chaikhum, K., Songkasupa, T., Chanthaworn, T., & Jongkaewwattana, A. (2025). Evaluation of a Cell-Adapted Live Attenuated African Swine Fever Virus Thai-Strain Vaccine Candidate: Highlighting Enhanced Virulence Risk in Co-Infected Pigs. Vaccines, 13(12), 1189. https://doi.org/10.3390/vaccines13121189







