The DNA Vaccines for the Gn and Gc Heterologous Polymer of Severe Fever with Thrombocytopenia Syndrome Virus Induce Potent Immunogenicity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Pseudoviruses, and Proteins
2.2. Main Reagents and Instruments
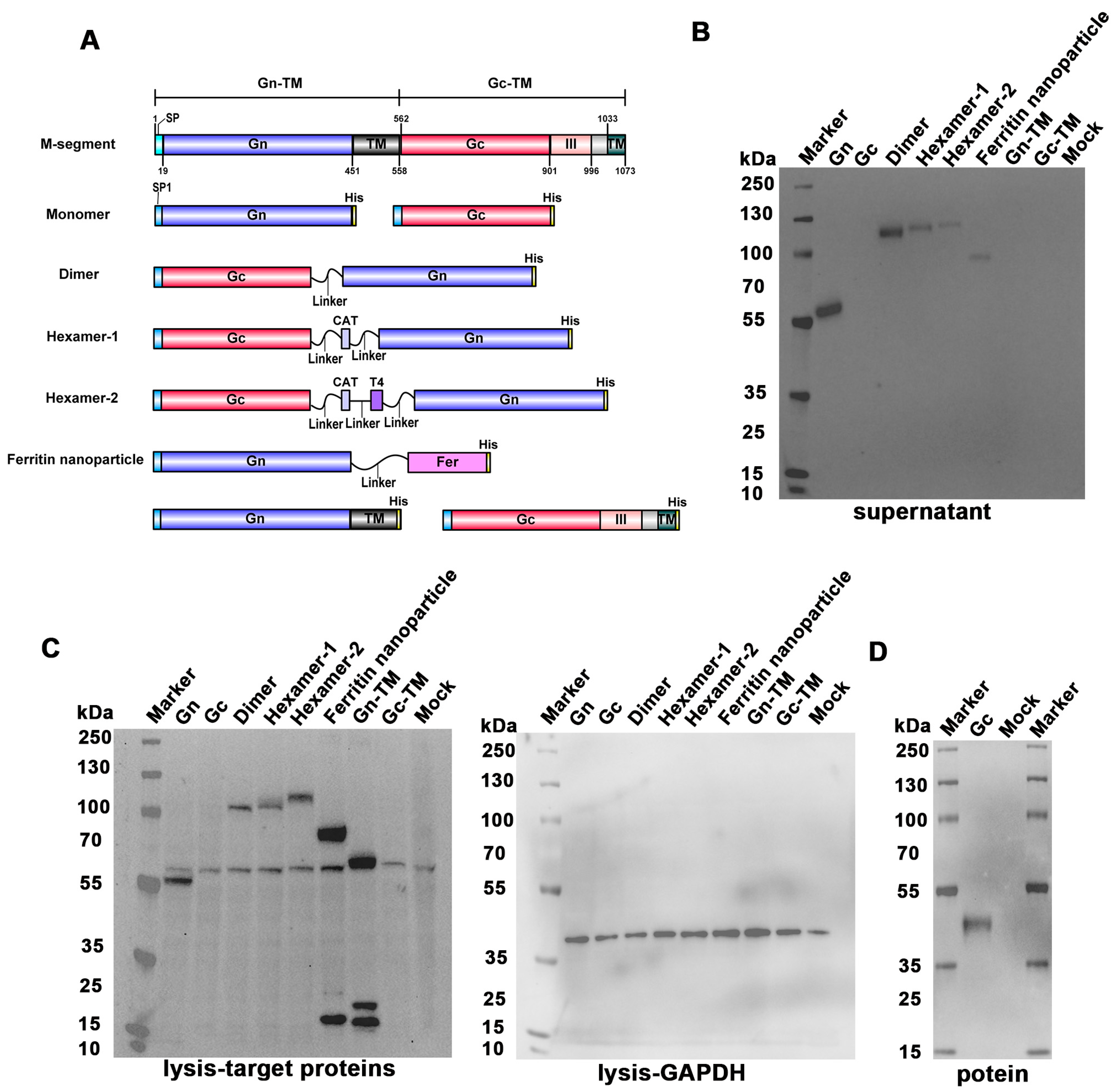
2.3. DNA Vaccines Design and Construction
2.4. Western Blot
2.5. The Feed and Vaccination of Experimental Animals
2.6. Neutralizing Antibody Detection by Pseudovirus System
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. IFN-γ and IL-4 Enzyme-Linked Immunosorbent Assay (ELISpot)
2.9. Antibody-Dependent Cellular Cytotoxicity (ADCC) Response
2.10. Statistical Analysis
3. Results
3.1. Design and Construct the SFTSV DNA Vaccines
3.2. Validation of the DNA Vaccines In Vitro
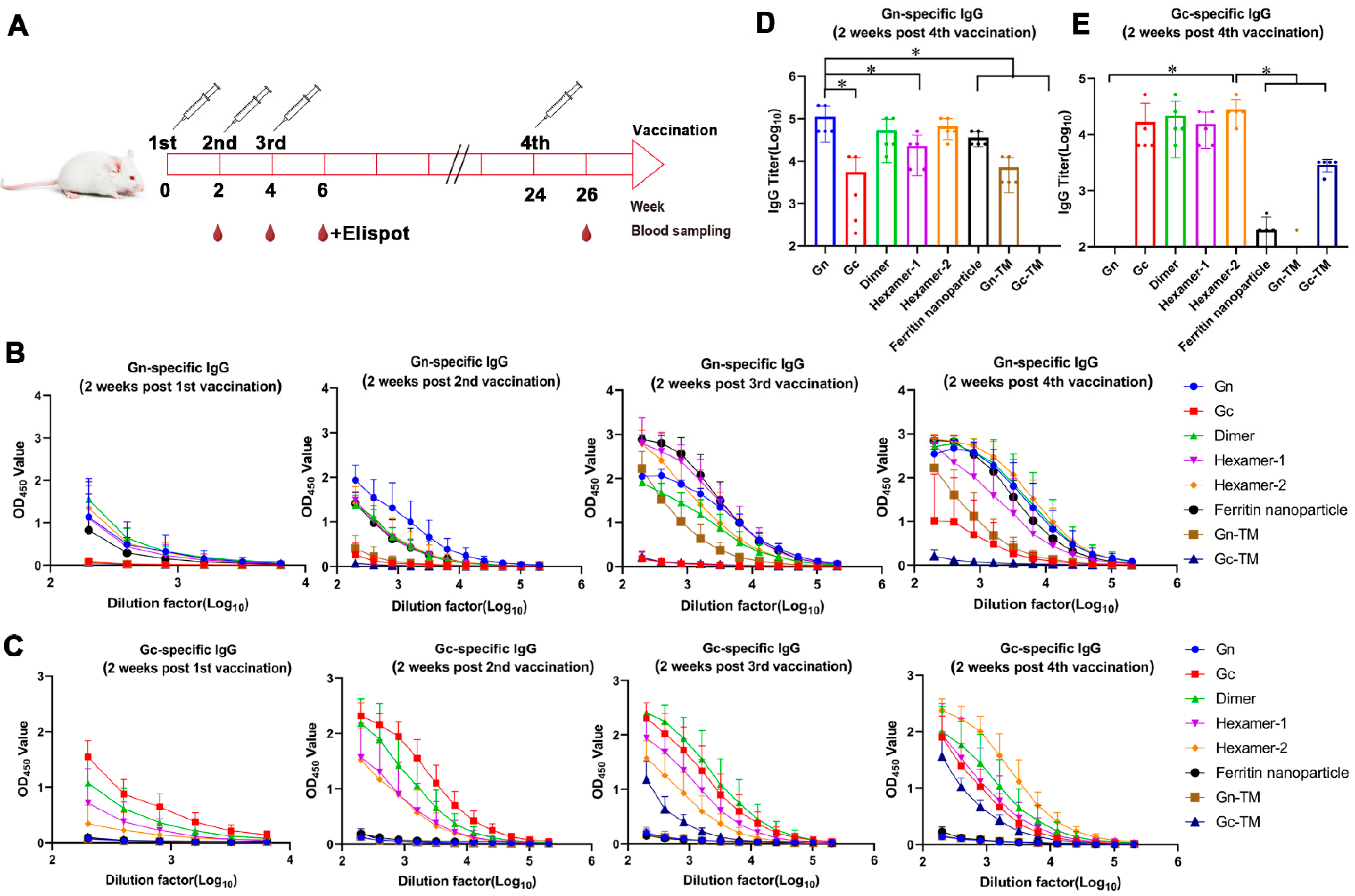
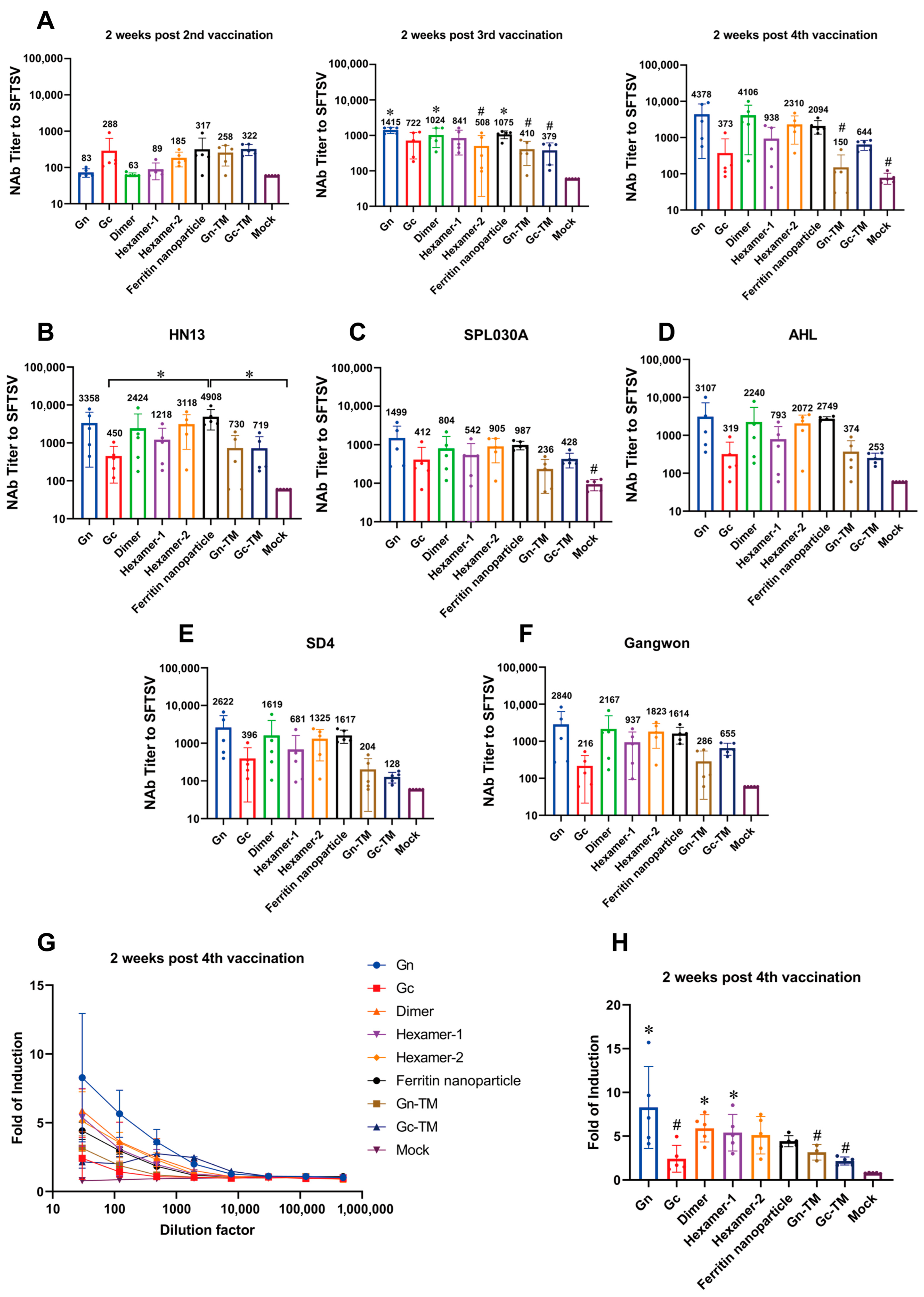
3.3. The Humoral Response of the SFTSV DNA Vaccines In Vivo
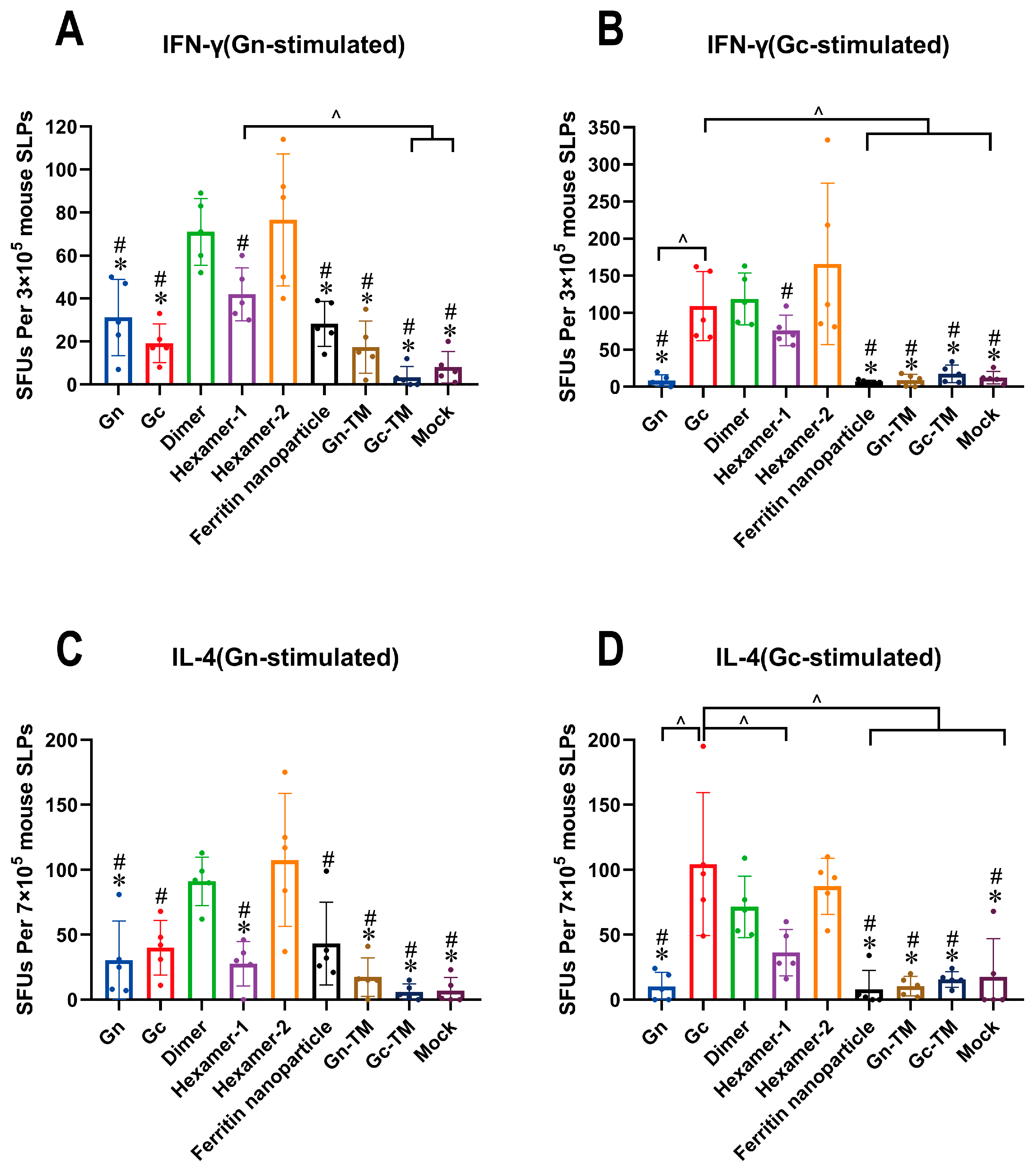
3.4. The Cellular Response of the SFTSV DNA Vaccines In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, B.; Huang, S.Y.; Wei, F.; Zhu, X.Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhao, L.; Li, B.; Yu, H.; Wen, H.; Yu, X.J. An emerging hemorrhagic fever in China caused by a novel bunyavirus SFTSV. Sci. China Life Sci. 2013, 56, 697–700. [Google Scholar] [CrossRef]
- Yuan, F.; Zhu, L.; Tian, D.; Xia, M.; Zheng, M.H.; Zhang, Q.; Zhang, T.; Zhang, X.; Zheng, A. The first discovery of severe fever with thrombocytopenia virus in the center of metropolitan Beijing, China. Virol. Sin. 2024, 39, 875–881. [Google Scholar] [CrossRef] [PubMed]
- You, E.; Wang, L.; Zhang, L.; Wu, J.; Zhao, K.; Huang, F. Epidemiological characteristics of severe fever with thrombocytopenia syndrome in Hefei of Anhui province: A population-based surveillance study from 2011 to 2018. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 929–939. [Google Scholar] [CrossRef]
- Guo, C.T.; Lu, Q.B.; Ding, S.J.; Hu, C.Y.; Hu, J.G.; Wo, Y.; Fan, Y.D.; Wang, X.J.; Qin, S.L.; Cui, N.; et al. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome (SFTS) in China: An integrated data analysis. Epidemiol. Infect. 2016, 144, 1345–1354. [Google Scholar] [CrossRef]
- Woo, D.; Michelow, I.C.; Choi, Y.; Lee, H.; Park, S. Transmission of severe fever with thrombocytopenia syndrome (SFTS) to humans: A systematic review of individual participant data and meta-analysis. J. Infect. Public Health 2025, 18, 102685. [Google Scholar] [CrossRef]
- Xu, A.L.; Xue, H.; Li, Y.; Wang, X.; Zheng, J.X.; Shi, F.Y.; Cui, Q.X.; Lu, Y.; Cun, D.J.; Li, L.H. Comprehensive meta-analysis of severe fever with thrombocytopenia syndrome virus infections in humans, vertebrate hosts and questing ticks. Parasites Vectors 2024, 17, 265. [Google Scholar] [CrossRef]
- Robles, N.; Han, H.J.; Park, S.J.; Choi, Y.K. Epidemiology of severe fever and thrombocytopenia syndrome virus infection and the need for therapeutics for the prevention. Clin. Exp. Vaccine Res. 2018, 7, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, D.; Zhang, Y.; Zhu, M.; Chen, N.; Yushan, Z. Transmission and mortality risk assessment of severe fever with thrombocytopenia syndrome in China: Results from 11-years’ study. Infect. Dis. Poverty 2022, 11, 30–40. [Google Scholar] [CrossRef]
- Casel, M.A.; Park, S.J.; Choi, Y.K. Severe fever with thrombocytopenia syndrome virus: Emerging novel phlebovirus and their control strategy. Exp. Mol. Med. 2021, 53, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Adkins, S.; Alkhovsky, S.V.; Avsic-Zupanc, T.; Ayllon, M.A.; Bahl, J.; Balkema-Buschmann, A.; Ballinger, M.J.; Bandte, M.; Beer, M.; et al. 2022 taxonomic update of phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2022, 167, 2857–2906. [Google Scholar] [CrossRef]
- Lei, X.Y.; Liu, M.M.; Yu, X.J. Severe fever with thrombocytopenia syndrome and its pathogen SFTSV. Microbes Infect. 2015, 17, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zheng, A. Entry of severe fever with thrombocytopenia syndrome virus. Virol. Sin. 2017, 32, 44–50. [Google Scholar] [CrossRef]
- Halldorsson, S.; Behrens, A.J.; Harlos, K.; Huiskonen, J.T.; Elliott, R.M.; Crispin, M.; Brennan, B.; Bowden, T.A. Structure of a phleboviral envelope glycoprotein reveals a consolidated model of membrane fusion. Proc. Natl. Acad. Sci. USA 2016, 113, 7154–7159. [Google Scholar] [CrossRef]
- Du, S.; Peng, R.; Xu, W.; Qu, X.; Wang, Y.; Wang, J.; Li, L.; Tian, M.; Guan, Y.; Wang, J.; et al. Cryo-EM structure of severe fever with thrombocytopenia syndrome virus. Nat. Commun. 2023, 14, 6333. [Google Scholar] [CrossRef]
- Mishra, A.K.; Hellert, J.; Freitas, N.; Guardado-Calvo, P.; Haouz, A.; Fels, J.M.; Maurer, D.P.; Abelson, D.M.; Bornholdt, Z.A.; Walker, L.M.; et al. Structural basis of synergistic neutralization of Crimean-Congo hemorrhagic fever virus by human antibodies. Science 2022, 375, 104–109. [Google Scholar] [CrossRef]
- Sun, Z.; Cheng, J.; Bai, Y.; Cao, L.; Xie, D.; Deng, F.; Zhang, X.; Rao, Z.; Lou, Z. Architecture of severe fever with thrombocytopenia syndrome virus. Protein Cell 2023, 14, 914–918. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, H.; Zhang, M.; Yang, Y.; Xiao, H.; He, Q.; Li, H.; Wang, Y.; Li, Z. Gc glycoprotein trimer vaccine elicits robust neutralizing antibodies against severe fever with thrombocytopenia syndrome virus in mice. Int. Immunopharmacol. 2025, 165, 115470. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, F. A comprehensive comparison of DNA and RNA vaccines. Adv. Drug Deliv. Rev. 2024, 210, 115340. [Google Scholar] [CrossRef] [PubMed]
- Guthe, S.; Kapinos, L.; Moglich, A.; Meier, S.; Grzesiek, S.; Kiefhaber, T. Very fast folding and association of a trimerization domain from bacteriophage T4 fibritin. J. Mol. Biol. 2004, 337, 905–915. [Google Scholar] [CrossRef]
- Bukreyev, A.; Camargo, E.; Collins, P.L. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J. Virol. 1996, 70, 6634–6641. [Google Scholar] [CrossRef]
- Kim, D.; Lai, C.J.; Cha, I.; Jung, J.U. Current progress of severe fever with thrombocytopenia syndrome virus (SFTSV) vaccine development. Viruses 2024, 16, 128. [Google Scholar] [CrossRef]
- Yu, K.M.; Park, S.J.; Yu, M.A.; Kim, Y.I.; Choi, Y.; Jung, J.U.; Brennan, B.; Choi, Y.K. Cross-genotype protection of live-attenuated vaccine candidate for severe fever with thrombocytopenia syndrome virus in a ferret model. Proc. Natl. Acad. Sci. USA 2019, 116, 26900–26908. [Google Scholar] [CrossRef]
- Hicks, P.; Manzoni, T.B.; Westover, J.B.; Petch, R.J.; Roper, B.; Gowen, B.B.; Bates, P. Safety, immunogenicity, and efficacy of a recombinant vesicular stomatitis virus vectored vaccine against severe fever with thrombocytopenia syndrome virus and heartland bandavirus. Vaccines 2024, 12, 1403. [Google Scholar] [CrossRef]
- Dong, F.; Li, D.; Wen, D.; Li, S.; Zhao, C.; Qi, Y.; Jangra, R.K.; Wu, C.; Xia, D.; Zhang, X.; et al. Single dose of a rVSV-based vaccine elicits complete protection against severe fever with thrombocytopenia syndrome virus. npj Vaccines 2019, 4, 5. [Google Scholar] [CrossRef]
- Manzoni, T.B.; Westover, J.B.; Lundgreen, K.A.; Hicks, P.D.; Petch, R.J.; Ort, J.T.; Weissman, D.; Fan, S.; Hensley, S.E.; Pardi, N.; et al. Homologous and heterologous vaccination regimens with mRNA and rVSV platforms induce potent immune responses against SFTSV glycoprotein. Viruses 2025, 17, 1095. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Huang, D.D.; Bai, J.Y.; Zhuang, L.; Lu, Q.B.; Zhang, X.A.; Liu, W.; Wang, J.Y.; Cao, W.C. Immunization with recombinant SFTSV/NSS protein does not promote virus clearance in SFTSV-infected c57bl/6j mice. Viral Immunol. 2015, 28, 113–122. [Google Scholar] [CrossRef]
- Kim, D.; Kim, E.; Kim, S.; Chung, Y.; Lai, C.; Cha, I.; Cho, S.; Choi, Y.; Dai, X.; Kim, S.; et al. Self-assembling gn head ferritin nanoparticle vaccine provides full protection from lethal challenge of Dabie bandavirus in aged ferrets. MBio 2023, 14, e0186823. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Hu, J. The integrated consideration of vaccine platforms, adjuvants, and delivery routes for successful vaccine development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef]
- Kwak, J.E.; Kim, Y.I.; Park, S.J.; Yu, M.A.; Kwon, H.I.; Eo, S.; Kim, T.S.; Seok, J.; Choi, W.S.; Jeong, J.H.; et al. Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nat. Commun. 2019, 10, 3836. [Google Scholar] [CrossRef]
- Kang, J.G.; Jeon, K.; Choi, H.; Kim, Y.; Kim, H.I.; Ro, H.J.; Seo, Y.B.; Shin, J.; Chung, J.; Jeon, Y.K.; et al. Vaccination with single plasmid DNA encoding IL-12 and antigens of severe fever with thrombocytopenia syndrome virus elicits complete protection in IFNAR knockout mice. PLoS Negl. Trop. Dis. 2020, 14, e0007813. [Google Scholar] [CrossRef]
- Wu, J.; Liang, J.; Li, S.; Lu, J.; Zhou, J.; Gao, M.; Zhang, Y.; Chen, J. DNA nanovaccines derived from ferritin-modified glycogens for targeted delivery to immature dendritic cells and for promotion of Th1 cell differentiation. Acta Biomater. 2025, 196, 436–452. [Google Scholar] [CrossRef]
- Qiao, Y.; Jin, S.; Nie, J.; Chang, Y.; Wang, B.; Guan, S.; Li, Q.; Shi, Y.; Kong, W.; Shan, Y. Hemagglutinin-based DNA vaccines containing trimeric self-assembling nanoparticles confer protection against influenza. J. Leukoc. Biol. 2022, 112, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, S.; Qiao, Y.; Fu, Y.; Nie, J.; Jiang, S.; Yao, X.; Pan, Y.; Zhao, L.; Wu, C.; et al. Self-assembling ferritin nanoparticles coupled with linear sequences from canine distemper virus haemagglutinin protein elicit robust immune responses. J. Nanobiotechnol. 2022, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lai, C.J.; Cha, I.; Kang, S.; Yang, W.S.; Choi, Y.; Jung, J.U. SFTSV Gn-Head mRNA vaccine confers efficient protection against lethal viral challenge. J. Med. Virol. 2023, 95, e29203. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ma, S.; Xu, Y.; Zhou, Z.; Zhang, P.; Peng, W.; Yang, H.; Tan, C.; Chen, J.; Pan, P. Development of a novel multi-epitope mRNA vaccine candidate to combat SFTSV pandemic. PLoS Negl. Trop. Dis. 2025, 19, e0012815. [Google Scholar] [CrossRef]
- Neeli, P.; Chai, D.; Roy, D.; Prajapati, S.; Bonam, S.R. DNA vaccines in the post-mRNA era: Engineering, applications, and emerging innovations. Int. J. Mol. Sci. 2025, 26, 8716. [Google Scholar] [CrossRef]
- Papadatou, I.; Michos, A. Advances in biotechnology and the development of novel human vaccines. Vaccines 2025, 13, 989. [Google Scholar] [CrossRef]
- Pagliari, S.; Dema, B.; Sanchez-Martinez, A.; Montalvo, Z.G.; Rollier, C.S. DNA vaccines: History, molecular mechanisms and future perspectives. J. Mol. Biol. 2023, 435, 168297. [Google Scholar] [CrossRef]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Ledwith, B.J.; Manam, S.; Troilo, P.J.; Barnum, A.B.; Pauley, C.J.; Griffiths, T.N.; Harper, L.B.; Beare, C.M.; Bagdon, W.J.; Nichols, W.W. Plasmid DNA vaccines: Investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology 2000, 43, 258–272. [Google Scholar] [CrossRef]
- Silveira, M.M.; Moreira, G.; Mendonca, M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021, 267, 118919. [Google Scholar] [CrossRef]
- Mori, T.; Kanda, Y.; Takenaka, K.; Okamoto, S.; Kato, J.; Kanda, J.; Yoshimoto, G.; Gondo, H.; Doi, S.; Inaba, M.; et al. Safety of asp0113, a cytomegalovirus DNA vaccine, in recipients undergoing allogeneic hematopoietic cell transplantation: An open-label phase 2 trial. Int. J. Hematol. 2017, 105, 206–212. [Google Scholar] [CrossRef]
- Silva, C.L.; Bonato, V.L.; Dos, S.R.; Zarate-Blades, C.R.; Sartori, A. Recent advances in DNA vaccines for autoimmune diseases. Expert. Rev. Vaccines 2009, 8, 239–252. [Google Scholar] [CrossRef]
- Yun, S.M.; Park, S.J.; Park, S.W.; Choi, W.; Jeong, H.W.; Choi, Y.K.; Lee, W.J. Molecular genomic characterization of tick- and human-derived severe fever with thrombocytopenia syndrome virus isolates from South Korea. PLoS Negl. Trop. Dis. 2017, 11, e0005893. [Google Scholar] [CrossRef]
- Fu, Y.; Li, S.; Zhang, Z.; Man, S.; Li, X.; Zhang, W.; Zhang, C.; Cheng, X. Phylogeographic analysis of severe fever with thrombocytopenia syndrome virus from Zhoushan islands, China: Implication for transmission across the ocean. Sci. Rep. 2016, 6, 19563. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.; Ismail, H.; Li, B.; Jin, T. Molecular and structural basis of DNA sensors in antiviral innate immunity. Front. Immunol. 2020, 11, 613039. [Google Scholar] [CrossRef] [PubMed]
- Suschak, J.J.; Wang, S.; Fitzgerald, K.A.; Lu, S. A CGAS-independent STING/IRF7 pathway mediates the immunogenicity of DNA vaccines. J. Immunol. 2016, 196, 310–316. [Google Scholar] [CrossRef]
- Suschak, J.J.; Wang, S.; Fitzgerald, K.A.; Lu, S. Identification of aim2 as a sensor for DNA vaccines. J. Immunol. 2015, 194, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Moseman, J.E.; Shim, D.; Jeon, D.; Rastogi, I.; Schneider, K.M.; McNeel, D.G. Messenger RNA and plasmid DNA vaccines for the treatment of cancer. Vaccines 2025, 13, 976. [Google Scholar] [CrossRef]
- Saggini, R.; Pellegrino, R. MAPK is implicated in sepsis, immunity, and MAPK is implicated in sepsis, immunity, and inflammation. Int. J. Infect. 2024, 3, 100–104. [Google Scholar]
- Frank, I.; Li, S.S.; Grunenberg, N.; Overton, E.T.; Robinson, S.T.; Zheng, H.; Seaton, K.E.; Heptinstall, J.R.; Allen, M.A.; Mayer, K.H.; et al. Safety and immunogenicity of a polyvalent DNA-protein HIV vaccine with matched env immunogens delivered as a prime-boost regimen or coadministered in HIV-uninfected adults in the USA (HVTN 124): A phase 1, placebo-controlled, double-blind randomised controlled trial. Lancet Hiv. 2024, 11, e285–e299. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Liu, X.; Tong, J.; Li, H.; Zhang, H.; Chen, J.; Zhang, M.; Li, Z.; Li, Q. The DNA Vaccines for the Gn and Gc Heterologous Polymer of Severe Fever with Thrombocytopenia Syndrome Virus Induce Potent Immunogenicity in Mice. Vaccines 2025, 13, 1186. https://doi.org/10.3390/vaccines13121186
He Q, Liu X, Tong J, Li H, Zhang H, Chen J, Zhang M, Li Z, Li Q. The DNA Vaccines for the Gn and Gc Heterologous Polymer of Severe Fever with Thrombocytopenia Syndrome Virus Induce Potent Immunogenicity in Mice. Vaccines. 2025; 13(12):1186. https://doi.org/10.3390/vaccines13121186
Chicago/Turabian StyleHe, Qiuju, Xiaojuan Liu, Jincheng Tong, Huan Li, Heng Zhang, Jiamin Chen, Mengyi Zhang, Zhihua Li, and Qianqian Li. 2025. "The DNA Vaccines for the Gn and Gc Heterologous Polymer of Severe Fever with Thrombocytopenia Syndrome Virus Induce Potent Immunogenicity in Mice" Vaccines 13, no. 12: 1186. https://doi.org/10.3390/vaccines13121186
APA StyleHe, Q., Liu, X., Tong, J., Li, H., Zhang, H., Chen, J., Zhang, M., Li, Z., & Li, Q. (2025). The DNA Vaccines for the Gn and Gc Heterologous Polymer of Severe Fever with Thrombocytopenia Syndrome Virus Induce Potent Immunogenicity in Mice. Vaccines, 13(12), 1186. https://doi.org/10.3390/vaccines13121186




