Evolution and Vaccine Strain Match of HA and NA Genes of Influenza A/H3N2 Subtype in Riyadh, Saudi Arabia, 2020–2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Consent Forms, and Ethics
2.2. Identification and Typing of IAV
2.3. Amplification of HA and NA Genes
2.4. DNA Sequencing of HA and NA Genes
2.5. Statistical Analysis
3. Results
3.1. Demographic Analysis and IAV Prevalence
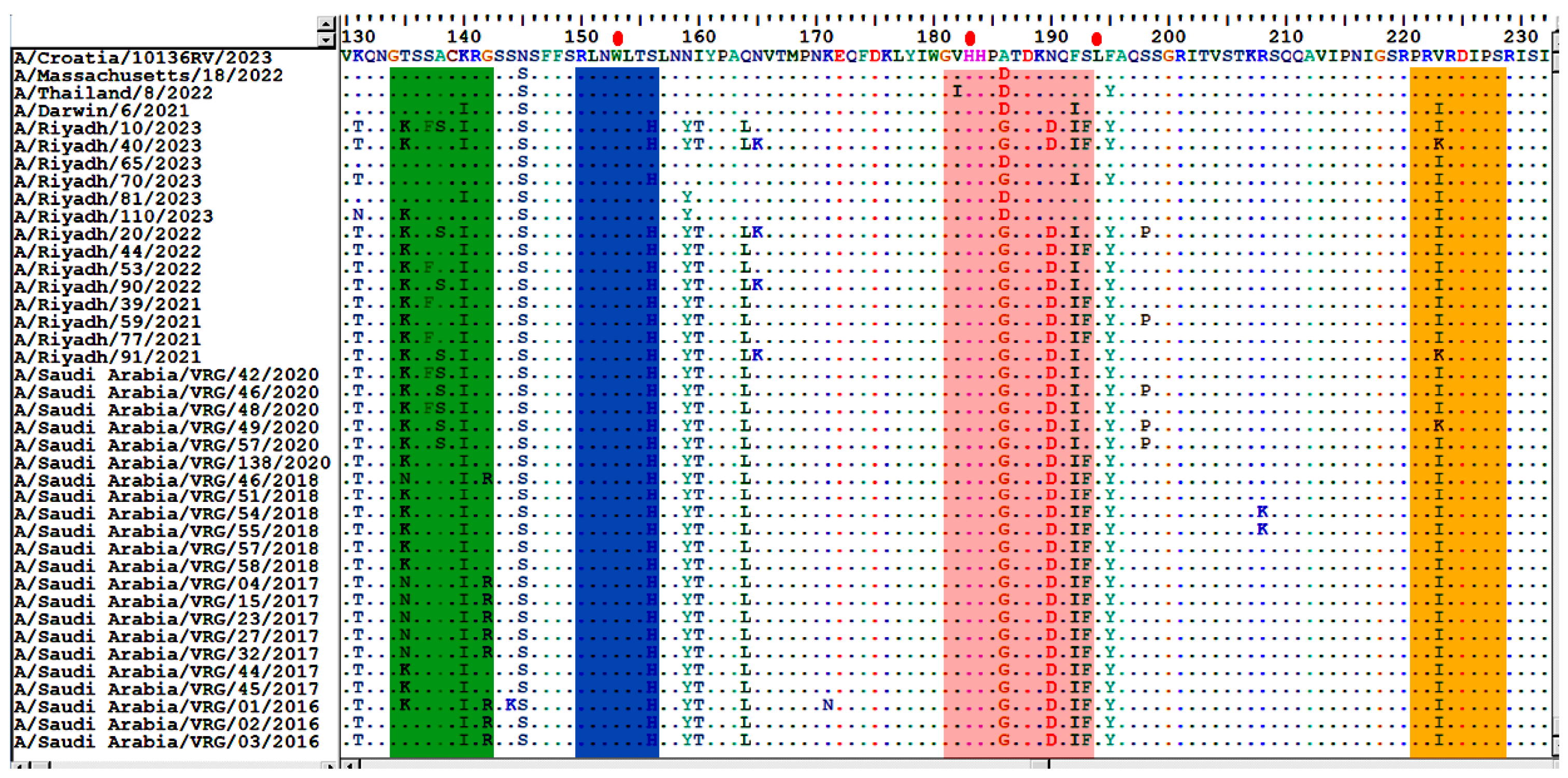
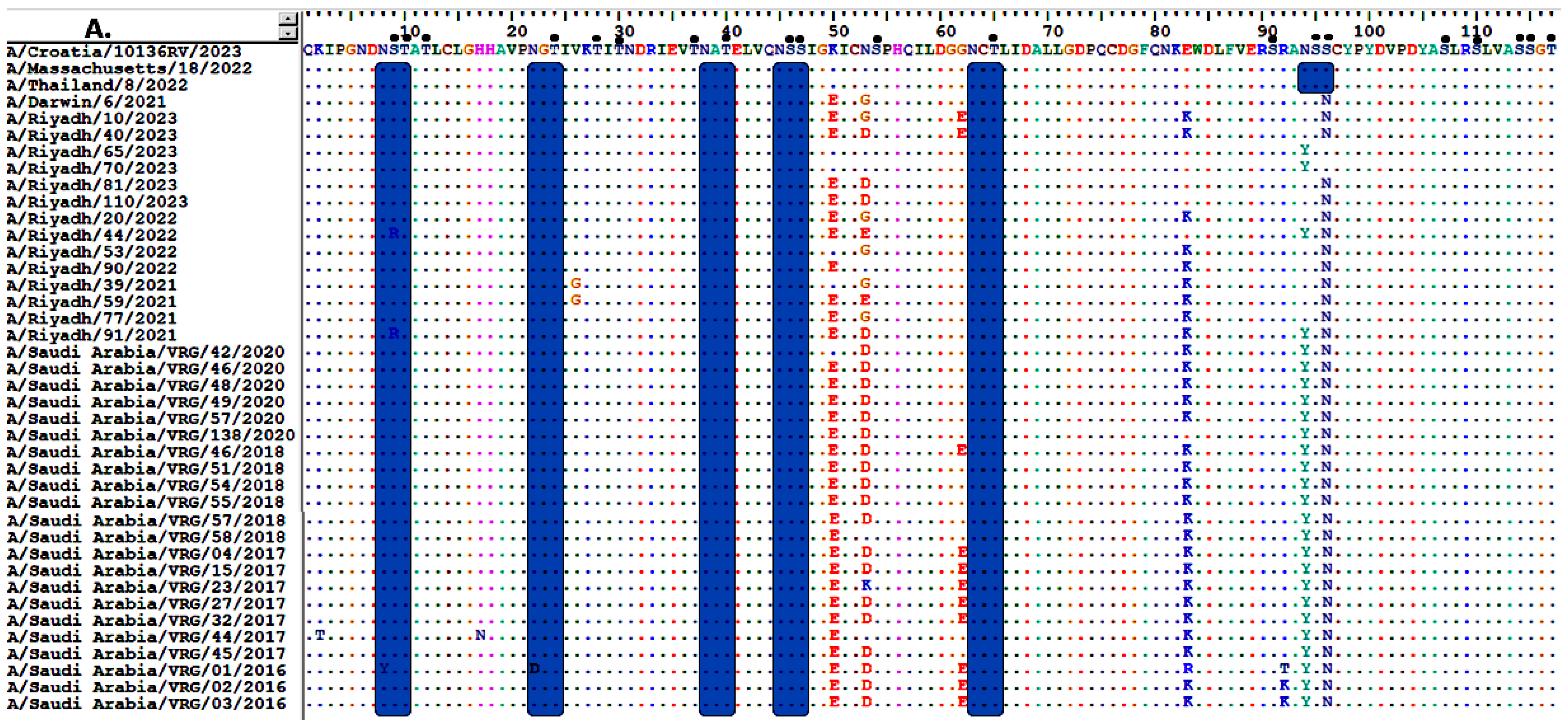
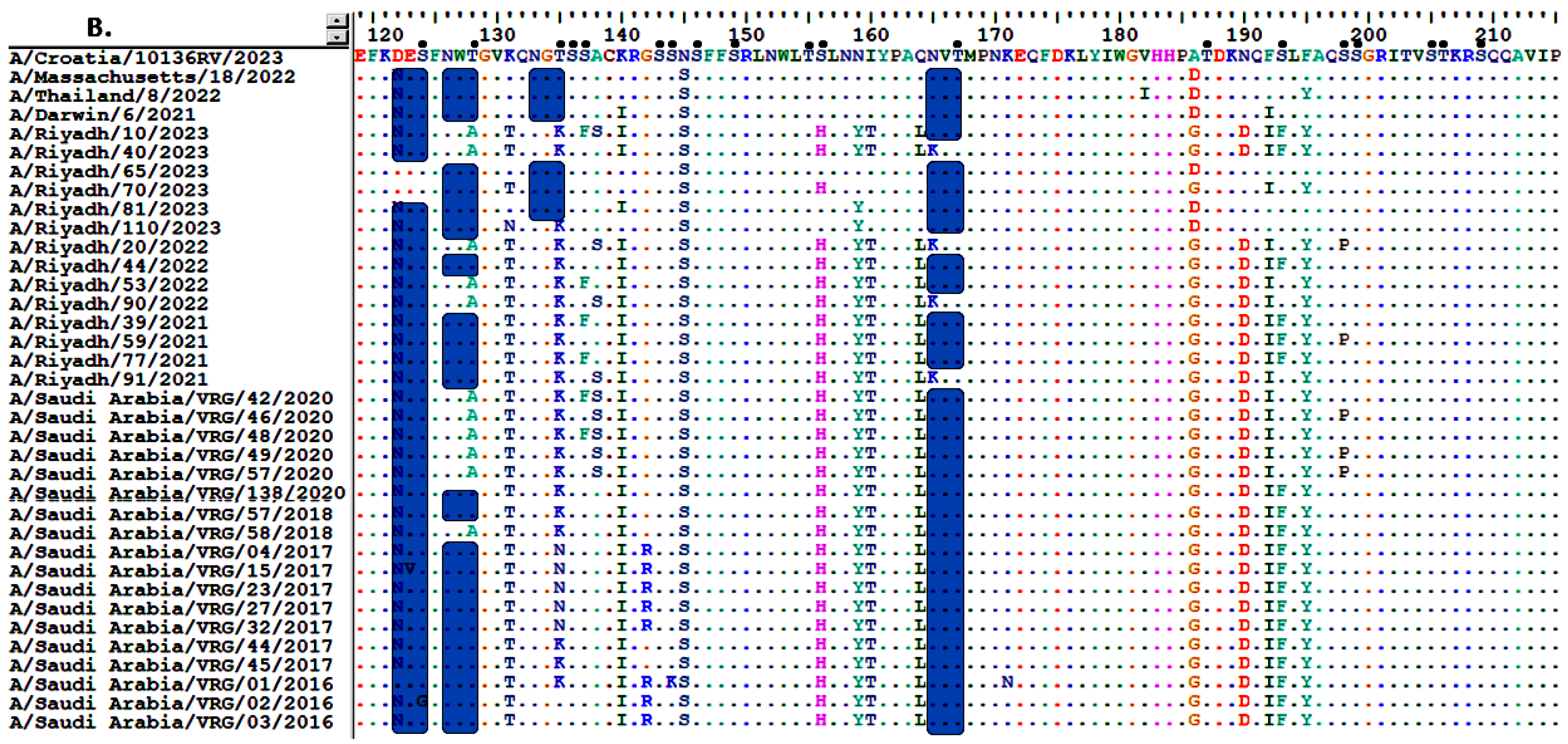
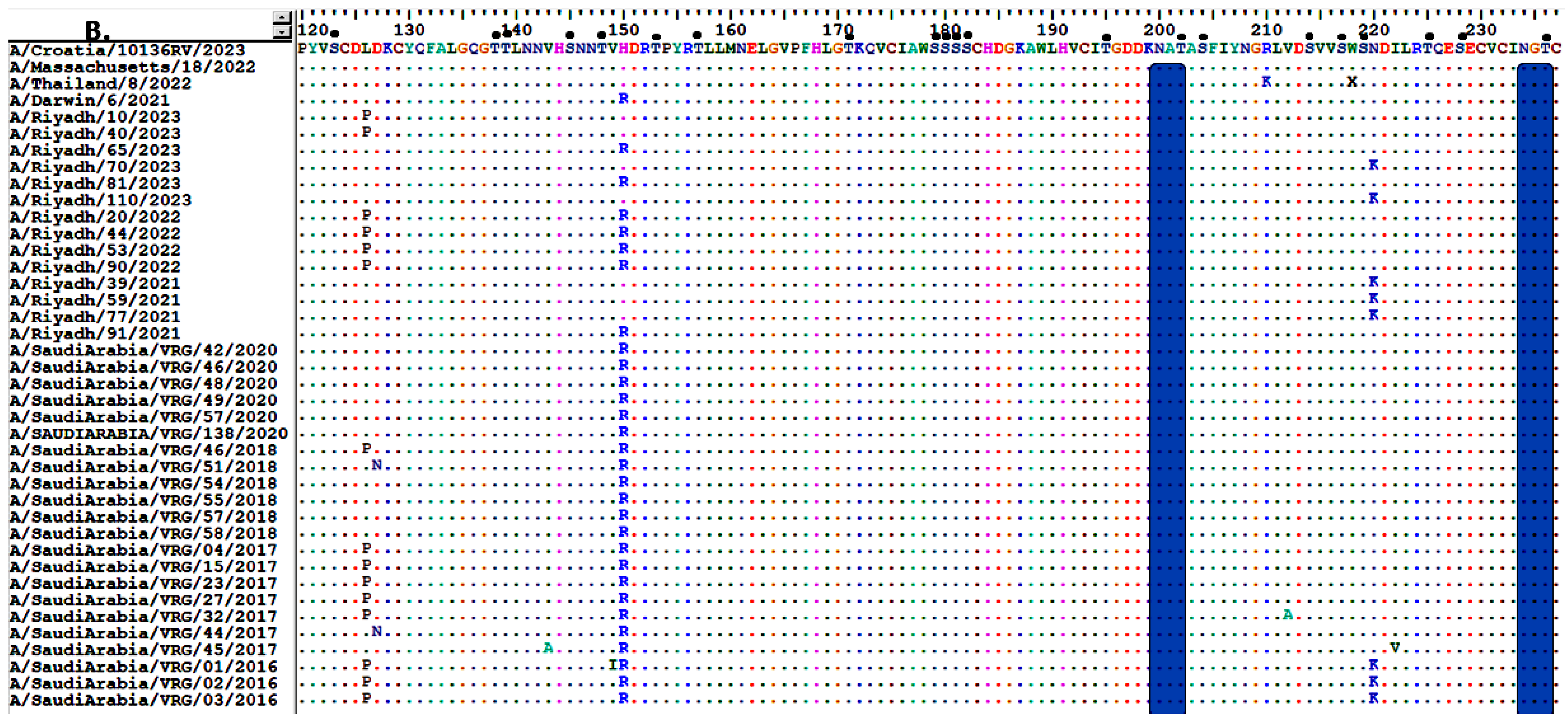
3.2. Analysis of Nucleotide and Amino Acid Sequences of the HA Gene
3.3. Sequence Homology of IAV A/H3N2 HA and NA Genes
3.4. Comparative Analysis of RBD’s Antigenicity-Related Homologous Sequences
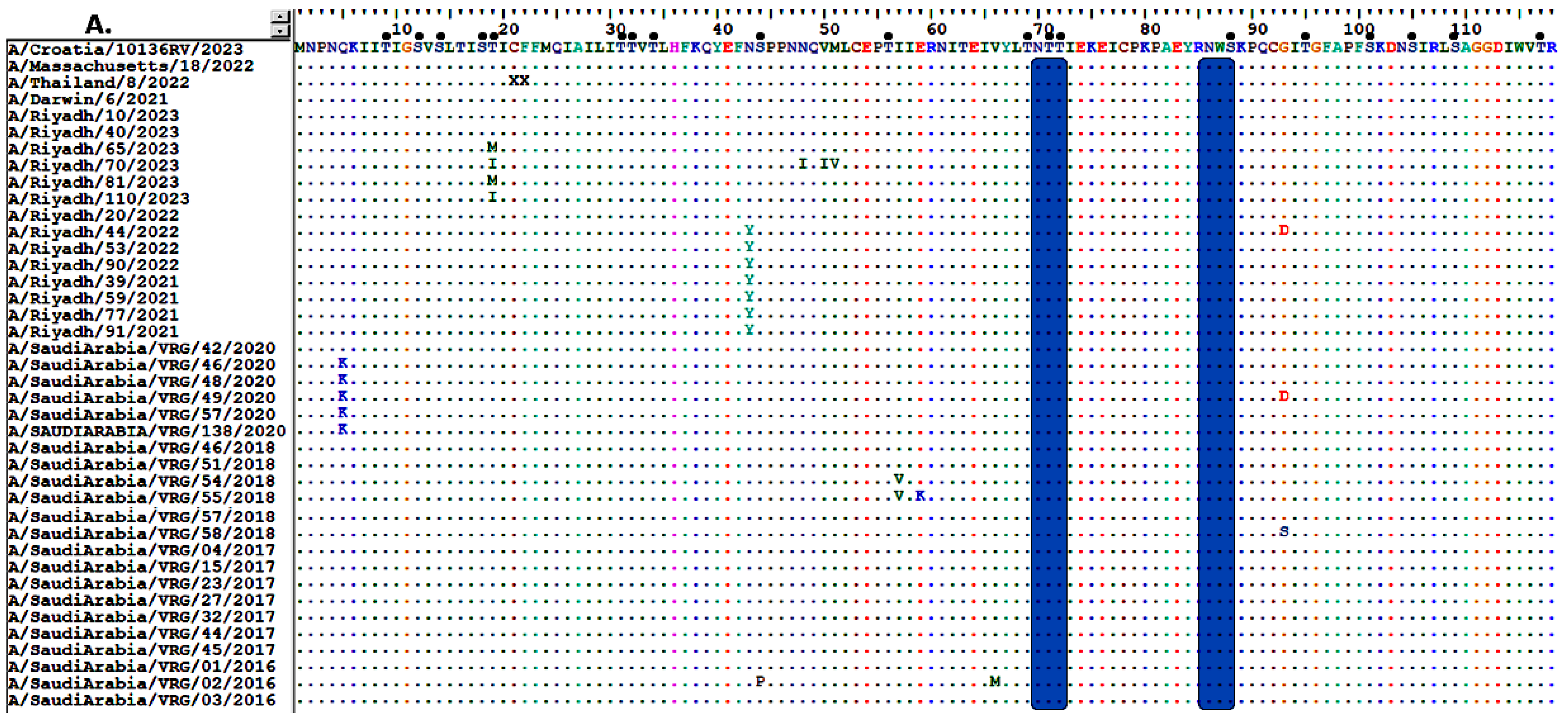
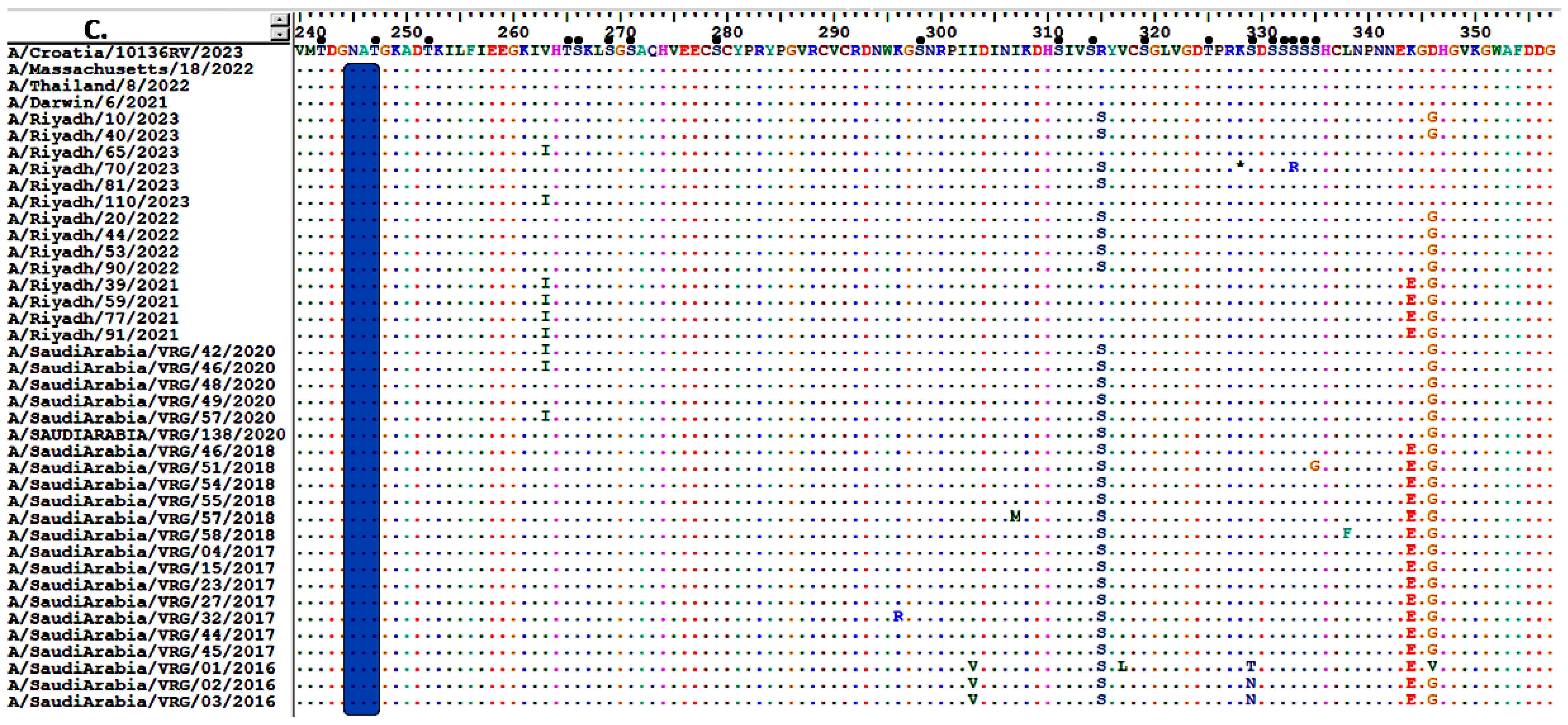
3.5. Sequence Analysis of the NA Gene
3.6. Glycosylation Profiles of HA and NA Proteins
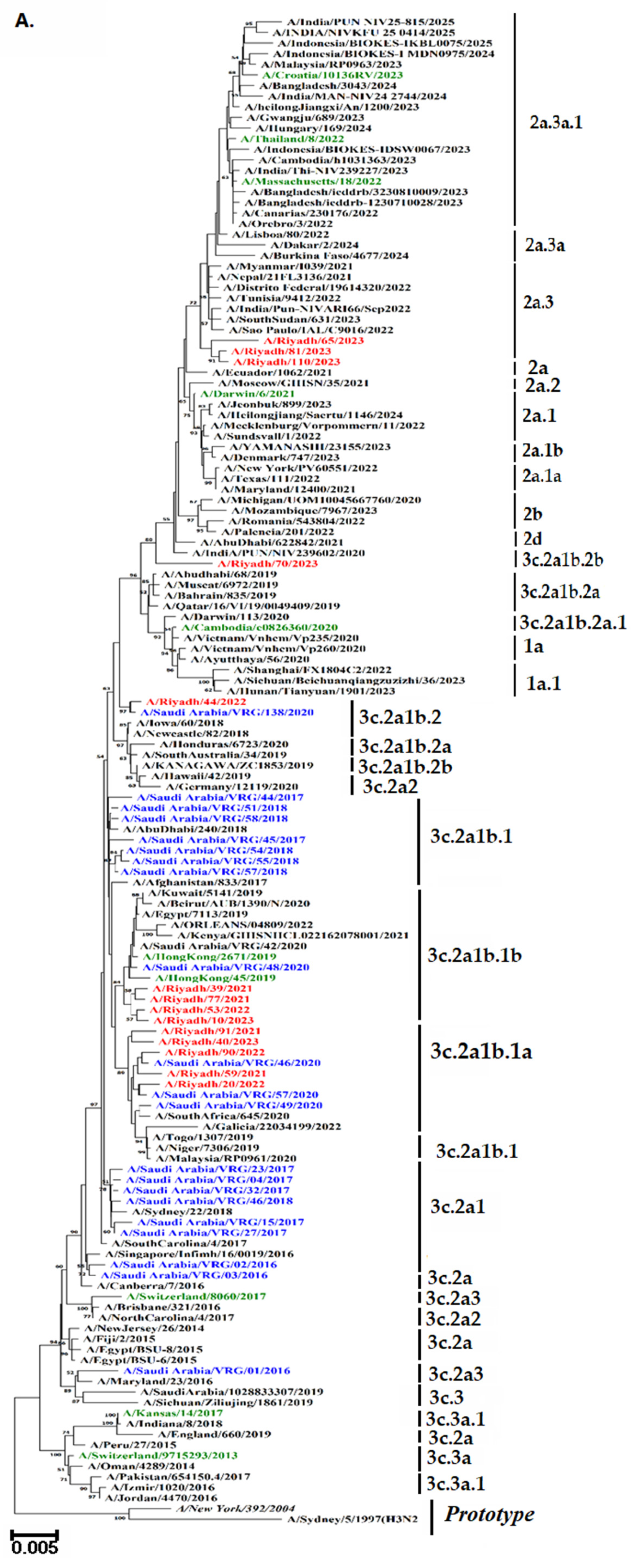
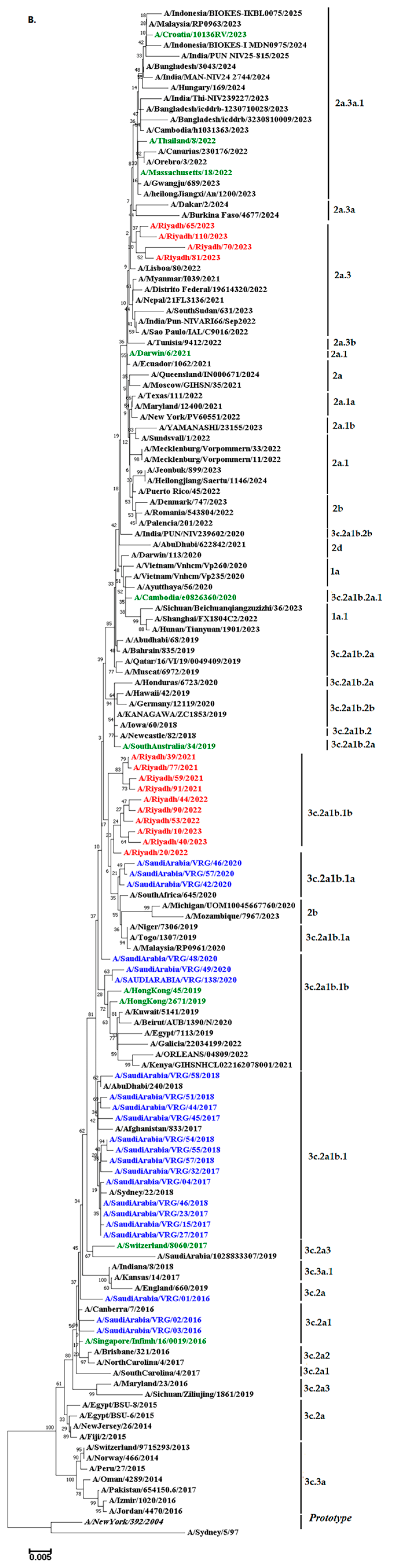
3.7. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization; World Organisation for Animal Health. Herramienta Operacional de Vigilancia e In-Tercambio de Información: Una Herramienta Operacional de la Guía Tripartita de Zoonosis; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Bimrew, S.; Abera, M. Review on classification and nomenclature of viruses. Int. J. Appl. Agric. Sci 2023, 11, 11–23. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Xue, J.; Xiang, Z.; Guo, J.; Zhan, L.; Wei, Q.; Kong, Q. Flu-CED: A comparative transcriptomics database of influenza virus-infected human and animal models. Anim. Models Exp. Med. 2024, 7, 881–892. [Google Scholar] [CrossRef]
- Kratsch, C.; Klingen, T.R.; Mümken, L.; Steinbrück, L.; McHardy, A.C. Determination of antigenicity-altering patches on the major surface protein of human influenza A/H3N2 viruses. Virus Evol. 2016, 2, vev025. [Google Scholar] [CrossRef]
- Brown, M.; Hampson, A.W.; Gerrard, J. The forgotten pandemic: Hong Kong influenza in Australia (1968–1970). Med. J. Aust. 2025, 223, 400–403. [Google Scholar] [CrossRef]
- Linster, M.; Schrauwen, E.; Van Der Vliet, S.; Burke, D.; Lexmond, P.; Bestebroer, T.; Smith, D.J.; Herfst, S.; Koel, B.; Fouchier, R. The molecular basis for antigenic drift of human A/H2N2 influenza viruses. J. Virol. 2019, 93, e01907-18. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.M. Antigenic Evolution and Correlates of Protection in Seasonal Influenza: Insights from H1N1 and H3N2 Circulation. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2025. [Google Scholar]
- Mawaddah, A. Comparison of the Effectiveness of Pneumococcal and Influenza Vaccines in Preventing Pneumonia in the Elderly. Formosa J. Multidiscip. Res. 2025, 4, 5065–5084. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Vincent Baker, A.L.; Van Reeth, K. Influenza Viruses. In Diseases of Swine; John Wiley & Sons: Hoboken, NJ, USA, 2025; pp. 645–664. [Google Scholar]
- Zhirnov, O.; Chernyshova, A. The uncleaved viral hemagglutinin HA0 increases influenza A virus resistance to thermal pasteurization. Virology 2025, 604, 110389. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, B.O.; Adeboboye, C.F. Influenza Viruses: Targetting Conserved Viral Ha-Stem, Matrix and Nucleo-Proteins to Disarm a Resilient and Recurring Pandemic. In RNA Viruses Infection; IntechOpen: London, UK, 2022. [Google Scholar]
- Parvez, S.; Pathrathota, A.; Uppar, A.L.; Yadagiri, G.; Mudavath, S.L. Influenza Virus: Global Health Impact, Strategies, Challenges, Role of Nanotechnolgy in Influenza Vaccine Development. Vaccines 2025, 13, 890. [Google Scholar] [CrossRef]
- Al-Hajjar, S. The 2024–2025 influenza season: Decoding the rise in severe illness and hospitalizations. Int. J. Pediatr. Adolesc. Med. 2025, 12, 1–3. [Google Scholar] [CrossRef]
- Althaqafi, A.; Farahat, F.; Alsaedi, A.; Alshamrani, M.; Alsaeed, M.S.; AlhajHussein, B.; El-Kafrawy, S.A.; Azhar, E.I. Molecular detection of influenza A and B viruses in four consecutive influenza seasons 2015–16 to 2018–19 in a tertiary center in Western Saudi Arabia. J. Epidemiol. Glob. Health 2021, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.E.; Alkadi, H.; Alarjani, M.; Al-Anazi, A.E.; Ibrahim, M.A.; ALOhali, T.A.; Enani, M.; Alturaiki, W.; Alosaimi, B. Moderately low effectiveness of the influenza quadrivalent vaccine: Potential mismatch between circulating strains and vaccine strains. Vaccines 2023, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Dudin, G.A.; Aziz, I.M.; Alzayed, R.M.; Ahmed, A.; Hussain, T.; Somily, A.M.; Alsaadi, M.M.; Almajhdi, F.N. Genetic diversity and evolutionary kinetics of influenza A virus H3N2 subtypes circulating in Riyadh, Saudi Arabia. Vaccines 2023, 11, 702. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2001, 2002, 310–322. [Google Scholar]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef]
- Benkouiten, S.; Al-Tawfiq, J.A.; Memish, Z.A.; Albarrak, A.; Gautret, P. Clinical respiratory infections and pneumonia during the Hajj pilgrimage: A systematic review. Travel Med. Infect. Dis. 2019, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Al-Dorzi, H.M.; Alsafwani, Z.A.; Alsalahi, E.; Aljulayfi, A.S.; Alshaer, R.; Alanazi, S.; Aldossari, M.A.; Alsahoo, D.A.; Khan, R. Patients with influenza admitted to a tertiary-care hospital in Riyadh between 2018 and 2022: Characteristics, outcomes and factors associated with ICU admission and mortality. BMC Pulm. Med. 2024, 24, 464. [Google Scholar] [CrossRef]
- Nelson, M.I.; Edelman, L.; Spiro, D.J.; Boyne, A.R.; Bera, J.; Halpin, R.; Sengamalay, N.; Ghedin, E.; Miller, M.A.; Simonsen, L.; et al. Correction: Molecular Epidemiology of A/H3N2 and A/H1N1 Influenza Virus during a Single Epidemic Season in the United States. PLoS Pathog. 2008, 4, 10-1371. [Google Scholar] [CrossRef]
- Gomaa, M.R.; Badra, R.; El Rifay, A.S.; Kandeil, A.; Kamel, M.N.; Abo Shama, N.M.; El-Shesheny, R.; Barakat, A.B.; Ali, M.A.; Kayali, G. Incidence and seroprevalence of seasonal influenza a viruses in Egypt: Results of a community-based cohort study. Influenza Other Respir. Viruses 2022, 16, 749–755. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Ayora-Talavera, G. Sialic acid receptors: Focus on their role in influenza infection. J. Recept. Ligand Channel Res. 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Koutsakos, M.; Nguyen, T.H.; Barclay, W.S.; Kedzierska, K. Knowns and unknowns of influenza B viruses. Future Microbiol. 2016, 11, 119–135. [Google Scholar] [CrossRef]
- Gilchuk, I.M.; Dong, J.; Irving, R.P.; Buchman, C.D.; Armstrong, E.; Turner, H.L.; Li, S.; Ward, A.B.; Carnahan, R.H.; Crowe, J.E., Jr. Pan-H7 influenza human antibody virus neutralization depends on avidity and steric hindrance. JCI Insight 2025, 10, e186182. [Google Scholar] [CrossRef]
- Thompson, A.J.; Wu, N.C.; Canales, A.; Kikuchi, C.; Zhu, X.; de Toro, B.F.; Worth, C.; Wang, S.; McBride, R.; Peng, W. Evolution of human H3N2 influenza virus receptor specificity has substantially expanded the receptor-binding domain site. Cell Host Microbe 2024, 32, 261–275.e264. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza viruses and vaccines: The role of vaccine effectiveness studies for evaluation of the benefits of influenza vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef]
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing seasonal influenza—The need for a universal influenza vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Wojcik, R.; Moore, C.; de Lejarazu, R.O.; de Lusignan, S.; Montomoli, E.; Rossi, A.; Pérez-Rubio, A.; Trilla, A.; Baldo, V.; et al. The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus. Vaccine 2020, 38, 6047–6056. [Google Scholar] [CrossRef]
- Ampofo, W.K.; Azziz-Baumgartner, E.; Bashir, U.; Cox, N.J.; Fasce, R.; Giovanni, M.; Grohmann, G.; Huang, S.; Katz, J.; Mironenko, A.; et al. Strengthening the influenza vaccine virus selection and development process: Report of the 3rd WHO Informal Consultation for Improving Influenza Vaccine Virus Selection held at WHO headquarters, Geneva, Switzerland, 1–3 April 2014. Vaccine 2015, 33, 4368–4382. [Google Scholar] [CrossRef]
- Chambers, B.S.; Parkhouse, K.; Ross, T.M.; Alby, K.; Hensley, S.E. Identification of Hemagglutinin Residues Responsible for H3N2 Antigenic Drift during the 2014–2015 Influenza Season. Cell Rep. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Sci. Rep. 2021, 11, 4554. [Google Scholar] [CrossRef]
- Kissling, E.; Pozo, F.; Buda, S.; Vilcu, A.-M.; Rizzo, C.; Gherasim, A.; Horváth, J.K.; Brytting, M.; Domegan, L.; Meijer, A.; et al. Effectiveness of influenza vaccine against influenza A in Europe in seasons of different A (H1N1) pdm09 and the same A (H3N2) vaccine components (2016–17 and 2017–18). Vaccine X 2019, 3, 100042. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Sabaiduc, S.; Chambers, C.; Eshaghi, A.; Gubbay, J.B.; Krajden, M.; Drews, S.J.; Martineau, C.; De Serres, G.; Dickinson, J.A.; et al. Mutations acquired during cell culture isolation may affect antigenic characterisation of influenza A (H3N2) clade 3C. 2a viruses. Eurosurveillance 2016, 21, 30112. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Jang, Y.H.; Kwon, S.B.; Lee, C.M.; Han, G.; Seong, B.L. Glycosylation of hemagglutinin and neuraminidase of influenza A virus as signature for ecological spillover and adaptation among influenza reservoirs. Viruses 2018, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Murakami, S.; Nakatsu, S.; Imai, H.; Muramoto, Y.; Shindo, K.; Sagara, H.; Kawaoka, Y. Importance of the 1+7 configuration of ribonucleoprotein complexes for influenza A virus genome packaging. Nat. Commun. 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, C.; Yamayoshi, S.; Akimoto, M.; Nakamura, K.; Miura, H.; Fujisaki, S.; Pattinson, D.J.; Shimizu, K.; Ozawa, H.; Momoki, T.; et al. Genetic and antigenic characterisation of influenza A (H3N2) viruses isolated in Yokohama during the 2016/17 and 2017/18 influenza seasons. Eurosurveillance 2019, 24, 1800467. [Google Scholar] [CrossRef]
- Chang, D.; Hackett, W.E.; Zhong, L.; Wan, X.-F.; Zaia, J. Measuring site-specific glycosylation similarity between influenza a virus variants with statistical certainty. Mol. Cell. Proteom. 2020, 19, 1533–1545. [Google Scholar] [CrossRef]
- Rambaut, A.; Pybus, O.G.; Nelson, M.I.; Viboud, C.; Taubenberger, J.K.; Holmes, E.C. The genomic and epidemiological dynamics of human influenza A virus. Nature 2008, 453, 615–619. [Google Scholar] [CrossRef]
- Nelson, M.I.; Simonsen, L.; Viboud, C.; Miller, M.A.; Taylor, J.; George, K.S.; Griesemer, S.B.; Ghedin, E.; Sengamalay, N.A.; Spiro, D.J.; et al. Stochastic processes are key determinants of short-term evolution in influenza a virus. PLoS Pathog. 2006, 2, e125. [Google Scholar]


| No. of samples N (%) | Positive for IAV N (%) | Positive for | |||
|---|---|---|---|---|---|
| A/H1N1 pdm09 N (%) | A/H3N2 N (%) | ||||
| Total | 380 | 65 (17.11) | 30 (7.89) | 35 (9.21) | |
| Season | 2020/21 | 120 (31.57) | 14 (11.67) | 6 (5.00) | 8 (6.67) |
| 2021/22 | 130 (34.21) | 17(14.17) | 6 (4.62) | 11 (8.46) | |
| 2022/23 | 130 (34.21) | 34 (26.15) | 18 (13.84) | 16 (12.30) | |
| Gender | Male | 170 (44.74) | 44 (25.88) a | 16 (9.41) a | 28 (16.47) a |
| Female | 210 (55.26) | 21 (10.00) | 14 (6.67) | 7 (3.33) | |
| Age in years | 0–4 | 95 (20.00) | 27 (28.42) b | 13 (13.68) b | 14 (14.75) b |
| 5–14 | 110 (28.95) | 19 (17.27) | 7 (6.35) | 12 (10.91) | |
| 15–64 | 120 (31.57) | 9 (7.50) | 4 (3.33) | 5 (4.16) | |
| ≥65 | 55 (14.48) | 10 (18.18) | 6 (10.91) | 4(7.27) | |
| Mutation Site | 19 | 49 | 61 | 64 | 66 | 73 | 108 | 110 | 136 | 151 | 156 | 160 | 174 | 189 | 232 | 294 | 328 | 377 | 390 | 420 | 466 | 500 | 505 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/New York/392/2004 | L | Q | S | T | G | E | K | Y | N | T | K | N | K | K | S | N | N | T | N | I | R | G | D |
| A/Riyadh/10/2023 | I | R | N | I | E | . | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/40/2023 | I | R | N | I | E | . | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/65/2023 | I | R | N | I | K | G | R | . | K | . | . | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/70/2023 | I | R | N | I | K | G | R | . | K | . | . | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/81/2023 | I | R | N | I | E | G | R | N | K | . | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/110/2023 | I | R | N | I | E | G | R | N | K | K | . | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/20/2022 | I | R | N | I | E | G | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/44/2022 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/53/2022 | I | R | N | I | K | G | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/90/2022 | I | R | N | I | E | G | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/39/2021 | I | R | N | I | K | G | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/59/2021 | I | R | N | I | E | G | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/77/2021 | I | R | N | I | E | G | R | N | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Riyadh/91/2021 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/42/2020 | I | R | N | I | K | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/46/2020 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/48/2020 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/49/2020 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/57/2020 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/138/2020 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/46/2018 | I | R | N | I | E | E | R | . | K | N | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/51/2018 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/54/2018 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/55/2018 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/57/2018 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/58/2018 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/04/2017 | I | R | N | I | E | . | R | . | K | N | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/15/2017 | I | R | N | I | E | . | R | . | K | N | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/23/2017 | I | R | N | I | E | . | R | . | K | N | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/27/2017 | I | R | N | I | E | . | R | . | K | N | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/32/2017 | I | R | N | I | E | . | R | . | K | N | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/44/2017 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/45/2017 | I | R | N | I | E | G | R | . | K | K | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/01/2016 | I | R | N | I | E | . | T | . | K | K | I | K | N | Q | N | K | S | R | D | . | K | . | N |
| A/Saudi Arabia/VRG/02/2016 | I | R | N | I | E | . | . | . | K | . | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| A/Saudi Arabia/VRG/03/2016 | I | R | N | I | E | . | . | . | K | . | I | S | N | Q | N | K | S | R | D | V | K | E | N |
| Mutation Site | 43 | 77 | 81 | 93 | 126 | 147 | 150 | 194 | 215 | 220 | 221 | 245 | 247 | 263 | 267 | 303 | 310 | 329 | 339 | 344 | 367 | 464 | 505 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/New York/392/2004 | N | M | L | D | P | D | H | V | I | K | K | S | S | I | T | V | Y | N | D | E | S | I | P |
| A/Riyadh/10/2023 | . | I | P | G | . | N | . | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | . |
| A/Riyadh/40/2023 | . | I | P | G | . | N | . | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | . |
| A/Riyadh/65/2023 | . | I | P | G | L | N | R | I | V | N | D | N | T | I | K | I | H | S | N | K | N | L | H |
| A/Riyadh/70/2023 | . | I | P | G | L | N | . | I | V | . | D | N | T | V | K | I | H | S | N | K | N | L | H |
| A/Riyadh/81/2023 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | H |
| A/Riyadh/110/2023 | . | I | P | G | L | N | . | I | V | K | D | N | T | . | K | I | H | S | N | K | N | L | H |
| A/Riyadh/20/2022 | . | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | H |
| A/Riyadh/44/2022 | Y | I | P | . | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | H |
| A/Riyadh/53/2022 | Y | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | H |
| A/Riyadh/90/2022 | Y | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | H |
| A/Riyadh/39/2021 | Y | I | P | G | L | N | . | I | V | . | D | N | T | . | K | I | H | S | N | . | N | L | . |
| A/Riyadh/59/2021 | Y | I | P | G | L | N | . | I | V | . | D | N | T | . | K | I | H | S | N | . | N | L | . |
| A/Riyadh/77/2021 | Y | I | P | G | L | N | . | I | V | . | D | N | T | . | K | I | H | S | N | . | N | L | . |
| A/Riyadh/91/2021 | Y | I | P | G | L | N | R | I | V | N | D | N | T | . | K | I | H | S | N | . | N | L | . |
| A/Saudi Arabia/VRG/42/2020 | . | I | P | G | L | N | R | I | V | N | D | N | T | . | K | I | H | S | N | K | N | L | . |
| A/Saudi Arabia/VRG/46/2020 | . | I | P | G | L | N | R | I | V | N | D | N | T | . | K | I | H | S | N | K | N | L | . |
| A/Saudi Arabia/VRG/48/2020 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | . |
| A/Saudi Arabia/VRG/49/2020 | . | I | P | . | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | . |
| A/Saudi Arabia/VRG/57/2020 | . | I | P | G | L | N | R | I | V | N | D | N | T | . | K | I | H | S | N | K | N | L | . |
| A/Saudi Arabia/VRG/138/2020 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | K | N | L | . |
| A/Saudi Arabia/VRG/46/2018 | . | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/51/2018 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/54/2018 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/55/2018 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/57/2018 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/58/2018 | . | I | P | S | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/04/2017 | . | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/15/2017 | . | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/23/2017 | . | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/27/2017 | . | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/32/2017 | . | I | P | G | . | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/44/2017 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/45/2017 | . | I | P | G | L | N | R | I | V | N | D | N | T | V | K | I | H | S | N | . | N | L | H |
| A/Saudi Arabia/VRG/01/2016 | . | I | P | G | . | N | R | I | V | . | D | N | T | V | K | . | H | T | N | . | N | L | H |
| A/Saudi Arabia/VRG/02/2016 | . | I | P | G | . | N | R | I | V | . | D | N | T | V | K | . | H | . | N | . | N | L | H |
| A/Saudi Arabia/VRG/03/2016 | . | I | P | G | . | N | R | I | V | . | D | N | T | V | K | . | H | . | N | . | N | L | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkubaisi, N.A.; Aziz, I.M.; Farrag, M.A.; Aljowaie, R.M.; Alsaleh, A.N.; Alanazi, F.N.; Almajhdi, F.N. Evolution and Vaccine Strain Match of HA and NA Genes of Influenza A/H3N2 Subtype in Riyadh, Saudi Arabia, 2020–2023. Vaccines 2025, 13, 1184. https://doi.org/10.3390/vaccines13121184
Alkubaisi NA, Aziz IM, Farrag MA, Aljowaie RM, Alsaleh AN, Alanazi FN, Almajhdi FN. Evolution and Vaccine Strain Match of HA and NA Genes of Influenza A/H3N2 Subtype in Riyadh, Saudi Arabia, 2020–2023. Vaccines. 2025; 13(12):1184. https://doi.org/10.3390/vaccines13121184
Chicago/Turabian StyleAlkubaisi, Noorah A., Ibrahim M. Aziz, Mohamed A. Farrag, Reem M. Aljowaie, Asma N. Alsaleh, Fatimah N. Alanazi, and Fahad N. Almajhdi. 2025. "Evolution and Vaccine Strain Match of HA and NA Genes of Influenza A/H3N2 Subtype in Riyadh, Saudi Arabia, 2020–2023" Vaccines 13, no. 12: 1184. https://doi.org/10.3390/vaccines13121184
APA StyleAlkubaisi, N. A., Aziz, I. M., Farrag, M. A., Aljowaie, R. M., Alsaleh, A. N., Alanazi, F. N., & Almajhdi, F. N. (2025). Evolution and Vaccine Strain Match of HA and NA Genes of Influenza A/H3N2 Subtype in Riyadh, Saudi Arabia, 2020–2023. Vaccines, 13(12), 1184. https://doi.org/10.3390/vaccines13121184







