Self-Assembled Peptides: A New Generation of Vaccine Adjuvant Platform
Abstract
1. Introduction
2. Vaccine Adjuvants: From Empirical Approaches to Rational Design
2.1. The Core Mechanism of Action of Adjuvants
2.2. Classification and Development History of Traditional Vaccine Adjuvants
2.3. Current Limitations of Existing Adjuvants and the Need for Developing Novel Adjuvants
3. The Basic Theory of SAPs
3.1. The Driving Force of Peptide Self-Assembly
3.2. Environmental Factors Affecting Peptide Self-Assembly
3.2.1. Temperature
3.2.2. pH
3.2.3. Ionic Concentration
3.3. The Unique Advantages of SAPs as Adjuvants
3.4. The Theoretical Basis of SAPs for Vaccine Research and Design
4. Application of SAPs in Vaccine Adjuvants
4.1. Adjuvant Properties of SAPs: Expanding and Enhancing Immune Responses Beyond Traditional Adjuvants
4.1.1. Synergistic Activation of Balanced Th1/Th2 Immune Responses
4.1.2. Efficient Activation of CTL Responses
4.1.3. Efficient Induction of Mucosal Immune Responses
4.1.4. Precise Immune Polarization and Epitope Synergy
4.2. The Role of SAPs’ Physical and Chemical Properties in Immune Regulation
4.2.1. Size-Dependent Immune Activation
4.2.2. Surface Charge as a Key Determinant of Intracellular Transport and Delivery Efficiency
4.2.3. Molecular Conformation and the Diversity of Self-Assembly Driving Forces
4.3. SAPs: A Transition Toward Intelligent and Personalized Therapeutics
4.3.1. Multifunctional Scaffolds for Co-Delivery and Immune Activation
4.3.2. Integrated Antigen-Adjuvant Systems for Coordinated Delivery
4.3.3. Toward Clinically Relevant Smart Responsive and Personalized Platforms
5. Challenges and Future Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xing, J.Y.; Zhao, X.X.; Li, X.T.; Fang, R.; Sun, M.R.; Zhang, Y.; Song, N.N. The recent advances in vaccine adjuvants. Front. Immunol. 2025, 16, 1557415. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Liang, X.L.; Zhou, J.Y.; Wang, M.M.; Wang, J.; Song, H.H.; Xu, Y.G.; Li, Y. Progress and prospect of polysaccharides as adjuvants in vaccine development. Virulence 2024, 15, 2435373. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Yuan, L.L.; Gao, X.D.; Xia, Y.F. Optimising the oil phases of aluminium hydrogel-stabilised emulsions for stable, safe and efficient vaccine adjuvant. Front. Chem. Sci. Eng. 2022, 16, 973–984. [Google Scholar] [CrossRef]
- Bajoria, S.; Kaur, K.; Kumru, O.S.; Van Slyke, G.; Doering, J.; Novak, H.; Aponte, S.A.R.; Dalvie, N.C.; Naranjo, C.A.; Johnston, R.S.; et al. Antigen-adjuvant interactions, stability, and immunogenicity profiles of a SARS-CoV-2 receptor-binding domain (RBD) antigen formulated with aluminum salt and CpG adjuvants. Hum. Vaccines Immunother. 2022, 18, 2079346. [Google Scholar] [CrossRef]
- Moyer, T.J.; Kato, Y.; Abraham, W.; Chang, J.Y.H.; Kulp, D.W.; Watson, N.; Turner, H.L.; Menis, S.; Abbott, R.K.; Bhiman, J.N.; et al. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020, 26, 430–440, Erratum in Nat. Med. 2020, 26, 804. https://doi.org/10.1038/s41591-020-0861-0. [Google Scholar] [CrossRef]
- Nouri, A.; Laraba-Djebari, F. Enhancement of long-lasting immunoprotective effect against envenomation using safe antigens: Comparative role of MF59 and Alum adjuvants. Vaccine 2015, 33, 5756–5763. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.M.; Safhi, M.M.; Kannadasan, M.; Sukumaran, N. Vaccine adjuvants—Current status and prospects on controlled release adjuvancity. Saudi Pharm. J. 2011, 19, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, D.; Lu, S.H.; Zheng, B. Toxoplasmosis vaccines: What we have and where to go? npj Vaccines 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Seena, S.; Gagliardi, T.; Palma, P.J. Advances in the design of amino acid and peptide synthesized gold nanoparticles for their applications. Adv. Colloid Interface Sci. 2023, 318, 102951. [Google Scholar] [CrossRef]
- Li, L.L.; Qiao, Z.Y.; Wang, L.; Wang, H. Programmable Construction of Peptide-Based Materials in Living Subjects: From Modular Design and Morphological Control to Theranostics. Adv. Mater. 2019, 31, e1804971. [Google Scholar] [CrossRef]
- Zhang, S.G. Discovery and design of self-assembling peptides. Interface Focus 2017, 7, 20170028. [Google Scholar] [CrossRef] [PubMed]
- Gelain, F.; Luo, Z.L.; Zhang, S.G. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2021, 121, 5093. [Google Scholar] [CrossRef]
- Yokoi, H.; Kinoshita, T.; Zhang, S.G. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mater. 2006, 18, 1365. [Google Scholar] [CrossRef]
- Rudra, J.S.; Tian, Y.F.; Jung, J.P.; Collier, J.H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. USA 2010, 107, 622–627. [Google Scholar] [CrossRef]
- Rudra, J.S.; Sun, T.; Bird, K.C.; Daniels, M.D.; Gasiorowski, J.Z.; Chong, A.S.; Collier, J.H. Modulating Adaptive Immune Responses to Peptide Self-Assemblies. ACS Nano 2012, 6, 1557–1564. [Google Scholar] [CrossRef]
- Pompano, R.R.; Chen, J.J.; Verbus, E.A.; Han, H.F.; Fridman, A.; McNeely, T.; Collier, J.H.; Chong, A.S. Titrating T-Cell Epitopes within Self-Assembled Vaccines Optimizes CD4+ Helper T Cell and Antibody Outputs. Adv. Healthc. Mater. 2014, 3, 1898–1908. [Google Scholar] [CrossRef]
- Wen, Y.; Waltman, A.; Han, H.F.; Collier, J.H. Switching the Immunogenicity of Peptide Assemblies Using Surface Properties. ACS Nano 2016, 10, 9274–9286. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef]
- Rad-Malekshahi, M.; Lempsink, L.; Amidi, M.; Hennink, W.E.; Mastrobattista, E. Biomedical Applications of Self-Assembling Peptides. Bioconjugate Chem. 2016, 27, 3–18. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Lv, G.T.; An, H.W.; Zhang, N.Y.; Wang, H. In Situ Self-Assembly of Bispecific Peptide for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2022, 61, e202113649. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Chen, X.Y.; Zhao, Y.; Yang, Y.M.; Wang, W.J.; Wu, C.; Yang, B.Z.; Zhang, Z.T.; Zhang, L.S.; Liu, Y.; et al. pH-Switchable Antimicrobial Nanofiber Networks of Hydrogel Eradicate Biofilm and Rescue Stalled Healing in Chronic Wounds. Acs Nano 2019, 13, 11686–11697. [Google Scholar] [CrossRef]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef]
- Bookstaver, M.L.; Tsai, S.J.; Bromberg, J.S.; Jewell, C.M. Improving Vaccine and Immunotherapy Design Using Biomaterials. Trends Immunol. 2018, 39, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Hartgerink, J.D.; Young, S. Self-assembling peptides as immunomodulatory biomaterials. Front. Bioeng. Biotechnol. 2023, 11, 1139782. [Google Scholar] [CrossRef]
- Wen, Y.; Collier, J.H. Supramolecular peptide vaccines: Tuning adaptive immunity. Curr. Opin. Immunol. 2015, 35, 73–79. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Kelly, S.H.; Sanchez-Perez, L.; Sampson, J.H.; Collier, J.H. Comparative study of α-helical and β-sheet self-assembled peptide nanofiber vaccine platforms: Influence of integrated T-cell epitopes. Biomater. Sci. 2020, 8, 3522–3535. [Google Scholar] [CrossRef]
- Roe, E.F.; Freire Haddad, H.; Lazar, K.M.; Liu, P.Y.; Collier, J.H. Tuning Helical Peptide Nanofibers as a Sublingual Vaccine Platform for a Variety of Peptide Epitopes. Adv. Healthc. Mater. 2024, 14, e2402055. [Google Scholar] [CrossRef]
- Sis, M.J.; Webber, M.J. Drug Delivery with Designed Peptide Assemblies. Trends Pharmacol. Sci. 2019, 40, 747–762. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.L.; Shrimali, P.C.; Clapacs, Z.E.; Files, M.A.; Rudra, J.S. Peptide-based supramolecular vaccine systems. Acta Biomater. 2021, 133, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wu, J.; Qing, S.; Zhang, X.; Zhang, L.L.; Yue, H.; Zeng, M.; Wang, B.; Yuan, Z.; Qiu, Y.F.; et al. Biosynthesis of Self-Assembled Proteinaceous Nanoparticles for Vaccination. Adv. Mater. 2020, 32, e2002940. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.M.; Cai, Y.L.; Jiang, Y.J.; He, X.M.; Wei, Y.Q.; Yu, Y.F.; Tian, X.H. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct Tar. 2023, 8, 283. [Google Scholar] [CrossRef]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Choi, J.; Oh, Y.; Cho, Y.; Lee, S. Vaccine adjuvants: Smart components to boost the immune system. Arch. Pharm. Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Plotkin, S.L. The development of vaccines: How the past led to the future. Nat. Rev. Microbiol. 2011, 9, 889–893. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Sun, L.X.; Nie, M.F.; Li, J.C.; Huang, X.F.; Heng, S.J.; Zhang, W.L.; Xia, T.; Guo, Z.L.; Zhao, Q.J.; et al. Modulation of Skin Inflammatory Responses by Aluminum Adjuvant. Pharmaceutics 2023, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Martiñón, S.; Cisneros, A.; Villicaña, S.; Hernández-Miramontes, R.; Mixcoha, E.; Calderón-Vargas, P. Chemical and Immunological Characteristics of Aluminum-Based, Oil-Water Emulsion, and Bacterial-Origin Adjuvants. J. Immunol. Res. 2019, 2019, 3974127. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Omer, S.B. Why and How Vaccines Work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Cui, Y.; Ho, M.G.; Hu, Y.J.; Shi, Y. Vaccine adjuvants: Current status, research and development, licensing, and future opportunities. J. Mater. Chem. B 2024, 12, 4118–4137. [Google Scholar] [CrossRef]
- Chen, W.H.; Kozlovsky, B.F.; Effros, R.B.; Grubeck-Loebenstein, B.; Edelman, R.; Sztein, M.B. Vaccination in the elderly: An immunological perspective. Trends Immunol. 2009, 30, 351–359. [Google Scholar] [CrossRef]
- de la Rica, R.; Matsui, H. Applications of peptide and protein-based materials in bionanotechnology. Chem. Soc. Rev. 2010, 39, 3499–3509. [Google Scholar] [CrossRef]
- Luo, D.; Yan, C.; Wang, T. Interparticle Forces Underlying Nanoparticle Self-Assemblies. Small 2015, 11, 5984–6008. [Google Scholar] [CrossRef]
- Bishop, K.J.M.; Wilmer, C.E.; Soh, S.; Grzybowski, B.A. Nanoscale Forces and Their Uses in Self-Assembly. Small 2009, 5, 1600–1630. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Xing, R.R.; Yan, X.H. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
- Boucenna, I.; Guedeau-Boudeville, M.A.; Lapp, A.; Colinart, P.; Proag, A.; Royon, L.; Mourchid, A. Temperature directed-assembly of coated-laponite nanoparticles in pluronic micellar solutions. Soft Matter 2013, 9, 170–176. [Google Scholar] [CrossRef]
- Mahmoud, Z.N.; Grundy, D.J.; Channon, K.J.; Woolfson, D.N. The non-covalent decoration of self-assembling protein fibers. Biomaterials 2010, 31, 7468–7474. [Google Scholar] [CrossRef]
- Rad-Malekshahi, M.; Visscher, K.M.; Rodrigues, J.P.G.L.M.; de Vries, R.; Hennink, W.E.; Baldus, M.; Bonvin, A.M.J.J.; Mastrobattista, E.; Weingarth, M. The Supramolecular Organization of a Peptide-Based Nanocarrier at High Molecular Detail. J. Am. Chem. Soc. 2015, 137, 7775–7784. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Silva, T.L.L.; Carrejo, N.C.; Marmolejo, C.A.O.; Li, I.C.; Hartgerink, J.D. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 2018, 161, 154–163. [Google Scholar] [CrossRef]
- Lu, L.; Armstrong, E.A.; Yager, J.Y.; Unsworth, L.D. Sustained Release of Dexamethasone from Sulfobutyl Ether β-cyclodextrin Modified Self-Assembling Peptide Nanoscaffolds in a Perinatal Rat Model of Hypoxia-Ischemia. Adv. Healthc. Mater. 2019, 8, e1900083. [Google Scholar] [CrossRef] [PubMed]
- Lampel, A. Biology-Inspired Supramolecular Peptide Systems. Chem 2020, 6, 1222–1236. [Google Scholar] [CrossRef]
- Zhou, H.R.; Zhu, Y.H.; Yang, B.B.; Huo, Y.H.; Yin, Y.Y.; Jiang, X.M.; Ji, W. Stimuli-responsive peptide hydrogels for biomedical applications. J. Mater. Chem. B 2024, 12, 1748–1774. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Yang, C.; Jian, Y.H.; Deng, H.B.; Du, Y.M.; Shi, X.W. Ion-responsive chitosan hydrogel actuator inspired by carrotwood seed pod. Carbohydr. Polym. 2022, 276, 118759. [Google Scholar] [CrossRef]
- Xie, F.J.; Li, R.X.; Shu, W.K.; Zhao, L.; Wan, J.J. Self-assembly of Peptide dendrimers and their bio-applications in theranostics. Mater. Today Bio 2022, 14, 100239. [Google Scholar] [CrossRef]
- Rapis, E. Self-organization and supramolecular chemistry of protein films from the nano- to the macroscale. Tech. Phys. 2004, 49, 494–498. [Google Scholar] [CrossRef]
- Rajagopal, K.; Schneider, J.P. Self-assembling peptides and proteins for nanotechnological applications. Curr. Opin. Struct. Biol. 2004, 14, 480–486. [Google Scholar] [CrossRef]
- Knowles, T.P.; Fitzpatrick, A.W.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef]
- Ozbas, B.; Kretsinger, J.; Rajagopal, K.; Schneider, J.P.; Pochan, D.J. Salt-triggered peptide folding and consequent self-assembly into hydrogels with tunable modulus. Macromolecules 2004, 37, 7331–7337. [Google Scholar] [CrossRef]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-Diphenylalanine self assembles to a hydrogel via a novel architecture based on π-π interlocked β-sheets. Adv. Mater. 2008, 20, 37. [Google Scholar] [CrossRef]
- Ranganathan, D.; Haridas, V.; Gilardi, R.; Karle, I.L. Self-assembling aromatic-bridged serine-based cyclodepsipeptides (serinophanes): A demonstration of tubular structures formed through aromatic π-π interactions. J. Am. Chem. Soc. 1998, 120, 10793–10800. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, R.; Zhang, P.C.; Fern, J.; Cheetham, A.G.; Patel, K.; Schulman, R.; Kan, C.Y.; Cui, H.G. Electrostatic-Driven Lamination and Untwisting of β-Sheet Assemblies. ACS Nano 2016, 10, 880–888. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhang, S.G. Molecular designer self-assembling peptides. Chem. Soc. Rev. 2006, 35, 1105–1110. [Google Scholar] [CrossRef]
- Wu, H.Q.; Ruan, L.P. The influence of temperature on the stability and self-assembly performance of designed peptides. Chem. Res. Appl. 2016, 28, 42–46. [Google Scholar]
- Rani, A.; Kavianini, I.; De Leon-Rodriguez, L.M.; McGillivray, D.J.; Williams, D.E.; Brimble, M.A. Nanoribbon self-assembly and hydrogel formation from an NOctanoyl octapeptide derived from the antiparallel β-Interface of a protein homotetramer. Acta Biomater. 2020, 114, 233–243. [Google Scholar] [CrossRef]
- Weitzhandler, I.; Dzuricky, M.; Hoffmann, I.; Quiroz, F.G.; Gradzielski, M.; Chilkoti, A. Micellar Self-Assembly of Recombinant Resilin-/Elastin-Like Block Copolypeptides. Biomacromolecules 2017, 18, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, S.; Suyama, K.; Tanaka, N.; Andoh, T.; Nagata, A.; Tomohara, K.; Taniguchi, S.; Maeda, I.; Nose, T. Development of truncated elastin-like peptide analogues with improved temperature-response and self-assembling properties. Sci. Rep. 2022, 12, 19414. [Google Scholar] [CrossRef]
- Goldberger, J.E.; Berns, E.J.; Bitton, R.; Newcomb, C.J.; Stupp, S.I. Electrostatic Control of Bioactivity. Angew. Chem. Int. Ed. 2011, 50, 6292–6295. [Google Scholar] [CrossRef]
- Taghizadeh Pirposhteh, R.; Mohajel, N.; Arashkia, A.; Azadmanesh, K.; Masoumi, M. Central position of histidine in the sequence of designed alternating polarity peptides enhances pH-responsive assembly with DNA. BMC Biotechnol. 2025, 25, 54. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, H.S. A pH-Responsive Protein Assembly through Clustering of a Charge-Tunable Single Amino Acid Repeat. ACS Appl. Mater. Interfaces 2024, 16, 47100–47109. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nishimura, K.; Morita, K.; Kanemitsu, S.; Nishida, Y.; Morimoto, T.; Aoi, T.; Tamura, A.; Maruyama, T. Microenvironment pH-Induced Selective Cell Death for Potential Cancer Therapy Using Nanofibrous Self-Assembly of a Peptide Amphiphile. Biomacromolecules 2021, 22, 2524–2531. [Google Scholar] [CrossRef]
- Ye, X.W.; Tian, W.; Han, L.; Li, Y.J.; Liu, S.; Lai, W.J.; Liu, Y.X.; Wang, L.; Yang, P.P.; Wang, H. High-Throughput Screening of pH-Dependent β-sheet Self-Assembling Peptide. Small 2024, 20, e2307963. [Google Scholar] [CrossRef]
- Tabandeh, S.; Leon, L. Engineering Peptide-Based Polyelectrolyte Complexes with Increased Hydrophobicity. Molecules 2019, 24, 868. [Google Scholar] [CrossRef]
- Ghosh, P.; Torner, J.; Arora, P.S.; Maayan, G. Dual Control of Peptide Conformation with Light and Metal Coordination. Chem.-Eur. J. 2021, 27, 8956–8959. [Google Scholar] [CrossRef] [PubMed]
- Abul-Haija, Y.M.; Scott, G.G.; Sahoo, J.K.; Tuttle, T.; Ulijn, R.V. Cooperative, ion-sensitive co-assembly of tripeptide hydrogels. Chem. Commun. 2017, 53, 9562–9565. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.X.; Liu, A.H.; Guan, Y.; Zheng, J.; Shen, Z.H.; Wan, X.H. Tuning the Helicity of Self-Assembled Structure of a Sugar-Based Organogelator by the Proper Choice of Cooling Rate. Langmuir 2010, 26, 3615–3622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, L.; Wang, J.; Xu, H.; Lu, J.R. Solvent Controlled Structural Transition of KI4K Self-Assemblies: From Nanotubes to Nanofibrils. Langmuir 2015, 31, 12975–12983. [Google Scholar] [CrossRef]
- Gao, J.; Zhan, J.; Yang, Z.M. Enzyme-Instructed Self-Assembly (EISA) and Hydrogelation of Peptides. Adv. Mater. 2020, 32, e1805798. [Google Scholar] [CrossRef]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. npj Vaccines 2021, 6, 70. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.Z.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wang, J.; Wang, G.S.; Su, Z.P.; Li, Y.Z.; Chen, Y.D.; Meng, J.G.; Yao, Y.; Wang, L.F.; Wilkens, S.; et al. Chimeric nanobody-decorated liposomes by self-assembly. Nat. Nanotechnol. 2024, 19, 818–824. [Google Scholar] [CrossRef]
- Jia, S.R.; Ji, S.L.; Zhao, J.; Lv, Y.H.; Wang, J.Y.; Sun, D.Q.; Ding, D. A Fluorinated Supramolecular Self-Assembled Peptide as Nanovaccine Adjuvant for Enhanced Cancer Vaccine Therapy. Small Methods 2023, 7, e2201409. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.S.; Li, W.W.; Xu, R.; Xu, C.; Li, X.Y.; Li, N.; Xu, F.D.; Yang, K.; Yuan, B. Multivalent Co-assembly of LL37-CpG nanoparticles: Enhanced immune response through activating multiple cell internalization pathways. Mater. Today Bio 2025, 33, 102011. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, Z.Y.; Ma, Z.N.; Han, L.L.; Zhou, Y.R.; Liang, T.C.; Yang, K.S.; Zhao, L.; Chen, X.Y.; Zhang, P.F. Condensate nanovaccine adjuvants augment CD8 T-Cell-dependent antitumor immunity through mtDNA leakage-triggered cGAS-STING axis activation. Signal Transduct. Target. Ther. 2025, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Hu, Y.H.; Ding, Y.H.; Zhang, X.Y.; Dong, X.; Xie, L.M.; Yang, Z.M.; Hu, Z.W. Dual-Enzyme-Instructed Peptide Self-Assembly to Boost Immunogenic Cell Death by Coordinating Intracellular Calcium Overload and Chemotherapy. ACS Nano 2025, 19, 488–503. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, X.Y.; Mao, C.Q.; Jiang, Y.H. The Distinct Properties of Polysaccharide Nanoparticles Tune Immune Responses against mRNA Antigen via Stimulator of Interferon Genes-Mediated Autophagy and Inflammasome. ACS Nano 2023, 17, 21782–21798. [Google Scholar] [CrossRef]
- Alonso, S.; Julio, D.; Caballero, J.C.; Caballero, D.; Zato, E.; Mj, P.; J, G.; Barez, A.; Vidriales, M.; Orfao, A.; et al. Infiltrating T-Cells in Hodgkin Lymphoma Lymph Nodes: A New Biological Marker Related to Disease Outcome. Haematologica 2015, 100, 761. [Google Scholar]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Z.; Chandrudu, S.; Skwarczynski, M.; Toth, I. The application of self-assembled nanostructures in peptide-based subunit vaccine development. Eur. Polym. J. 2017, 93, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Acar, H.; Srivastava, S.; Chung, E.J.; Schnorenberg, M.R.; Barrett, J.C.; LaBelle, J.L.; Tirrell, M. Self-assembling peptide-based building blocks in medical applications. Adv. Drug Deliv. Rev. 2017, 110, 65–79. [Google Scholar] [CrossRef]
- Si, Y.H.; Tian, Q.M.; Zhao, F.; Kelly, S.H.; Shores, L.S.; Camacho, D.F.; Sperling, A.I.; Andrade, M.S.; Collier, J.H.; Chong, A.S. Adjuvant-free nanofiber vaccine induces in situ lung dendritic cell activation and T17 responses. Sci. Adv. 2020, 6, eaba0995. [Google Scholar] [CrossRef]
- Serdar, N.G.; Pospisil, T.; Sisic, M.; Crnolatac, I.; Males, P.; Frkanec, R.; Frkanec, L. Self-assembled Ac-FFA-NH based hydrogels with strong immunostimulating activity for vaccine delivery. Nanoscale Adv. 2025, 7, 4660–4672. [Google Scholar] [CrossRef]
- Kaba, S.A.; Brando, C.; Guo, Q.; Mittelholzer, C.; Raman, S.; Tropel, D.; Aebi, U.; Burkhard, P.; Lanar, D.E. A Nonadjuvanted Polypeptide Nanoparticle Vaccine Confers Long-Lasting Protection against Rodent Malaria. J. Immunol. 2009, 183, 7268–7277. [Google Scholar] [CrossRef]
- Luo, Z.C.; Wu, Q.J.; Yang, C.B.; Wang, H.M.; He, T.; Wang, Y.Z.; Wang, Z.Y.; Chen, H.; Li, X.Y.; Gong, C.Y.; et al. A Powerful CD8 T-Cell Stimulating D-Tetra-Peptide Hydrogel as a Very Promising Vaccine Adjuvant. Adv. Mater 2017, 29, 1601776. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.Z.; Yang, Q.L.; Liu, Z.J.; Xiao, Z.Q.; Le, Z.C.; Yang, Z.M.; Yang, C.B. A versatile supramolecular nanoadjuvant that activates NF-κB for cancer immunotherapy. Theranostics 2019, 9, 3388–3397. [Google Scholar] [CrossRef]
- Si, Y.H.; Wen, Y.; Kelly, S.H.; Chong, A.S.; Collier, J.H. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8 T cell responses. J. Control. Release 2018, 282, 120–130. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Norberg, P.K.; Reap, E.A.; Congdon, K.L.; Fries, C.N.; Kelly, S.H.; Sampson, J.H.; Conticello, V.P.; Collier, J.H. A Supramolecular Vaccine Platform Based on α-Helical Peptide Nanofibers. ACS Biomater. Sci. Eng. 2017, 3, 3128–3132. [Google Scholar] [CrossRef]
- Zottig, X.; Al-Halifa, S.; Côté-Cyr, M.; Calzas, C.; Le Goffic, R.; Chevalier, C.; Archambault, D.; Bourgault, S. Self-assembled peptide nanorod vaccine confers protection against influenza A virus. Biomaterials 2021, 269, 120672. [Google Scholar] [CrossRef] [PubMed]
- Curvino, E.J.; Woodruff, M.E.; Roe, E.F.; Haddad, H.F.; Alvarado, P.C.; Collier, J.H. Supramolecular Peptide Self-Assemblies Facilitate Oral Immunization. ACS Biomater. Sci. Eng. 2024, 10, 3041–3056. [Google Scholar] [CrossRef]
- Song, H.J.; Su, Q.; Nie, Y.; Zhang, C.N.; Huang, P.S.; Shi, S.B.; Liu, Q.; Wang, W.W. Supramolecular assembly of a trivalent peptide hydrogel vaccine for cancer immunotherapy. Acta Biomater. 2023, 158, 535–546. [Google Scholar] [CrossRef]
- Su, Q.; Song, H.J.; Huang, P.S.; Zhang, C.N.; Yang, J.; Kong, D.L.; Wang, W.W. Supramolecular co-assembly of self-adjuvanting nanofibrious peptide hydrogel enhances cancer vaccination by activating MyD88-dependent NF-κB signaling pathway without inflammation. Bioact. Mater. 2021, 6, 3924–3934. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Rad-Malekshahi, M.; Fransen, M.F.; Krawczyk, M.; Mansourian, M.; Bourajjaj, M.; Chen, J.; Ossendorp, F.; Hennink, W.E.; Mastrobattista, E.; Amidi, M. Self-Assembling Peptide Epitopes as Novel Platform for Anticancer Vaccination. Mol. Pharm. 2017, 14, 1482–1493. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Bektas, E.I.; Muerner, M.; Sapudom, J.; Srejber, M.; Airoldi, M.; Schmidt, R.; Vernengo, A.J.; Edwards-Gayle, C.J.C.; Tipay, P.S.; et al. Effect of Tyrosine-Containing Self-Assembling β-Sheet Peptides on Macrophage Polarization and Inflammatory Response. ACS Appl. Mater. Interfaces 2025, 17, 27740–27758. [Google Scholar] [CrossRef]
- Kopec, K.; Pedziwiatr, M.; Gront, D.; Sztatelman, O.; Slawski, J.; Lazick, M.; Worch, R.; Zawada, K.; Makarova, K.; Nyk, M.; et al. Comparison of α-Helix and β-Sheet Structure Adaptation to a Quantum Dot Geometry: Toward the Identification of an Optimal Motif for a Protein Nanoparticle Cover. ACS Omega 2019, 4, 13086–13099. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W. Self-assembling amphiphilic peptides. J. Pept. Sci. 2014, 20, 453–467. [Google Scholar] [CrossRef]
- Files, M.A.; Naqvi, K.F.; Saito, T.B.; Clover, T.M.; Rudra, J.S.; Endsley, J.J. Self-adjuvanting nanovaccines boost lung-resident CD4 T cell immune responses in BCG-primed mice. npj Vaccines 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Shrimali, P.C.; Chen, S.; Das, A.; Dreher, R.; Howard, M.K.; Ryan, J.J.; Buck, J.; Kim, D.; Sprunger, M.L.; Rudra, J.S.; et al. Amyloidogenic propensity of self-assembling peptides and their adjuvant potential for use as DNA vaccines. Acta Biomater. 2023, 169, 464–476. [Google Scholar] [CrossRef]
- Mathes, T.G.; Kim, U.; Jeon, K.; Estevez, P.J.; Terasaki, M.; Ermis, M.; O’Raw, A.; Jucaud, V.; Khademhosseini, A.; Falcone, N. Lipopeptide Hydrogel Possesses Adjuvant-Like Properties for the Delivery of the GPC-3 Peptide-derived Antigen. Adv. Funct. Mater. 2025, 35, 2413870. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.X.; Song, H.J.; Qin, Y.B.; Huang, P.S.; Zhang, C.N.A.; Kong, D.L.; Wang, W.W. Engineering Dendritic-Cell-Based Vaccines and PD-1 Blockade in Self-Assembled Peptide Nanofibrous Hydrogel to Amplify Antitumor T-Cell Immunity. Nano Lett. 2018, 18, 4377–4385. [Google Scholar] [CrossRef]
- Xing, R.R.; Li, S.K.; Zhang, N.; Shen, G.Z.; Möhwald, H.; Yan, X.H. Self-Assembled Injectable Peptide Hydrogels Capable of Triggering Antitumor Immune Response. Biomacromolecules 2017, 18, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Z.J.; Wang, C.R.; Su, Q.; Song, H.J.; Zhang, C.N.; Huang, P.S.; Liang, X.J.; Dong, A.J.; Kong, D.L.; et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 2020, 230, 119649. [Google Scholar] [CrossRef]
- Zashikhina, N.N.; Volokitina, M.V.; Korzhikov-Vlakh, V.A.; Tarasenko, I.I.; Lavrentieva, A.; Scheper, T.; Rühl, E.; Orlova, R.V.; Tennikova, T.B.; Korzhikova-Vlakh, E.G. Self-assembled polypeptide nanoparticles for intracellular irinotecan delivery. Eur. J. Pharm. Sci. 2017, 109, 1–12. [Google Scholar] [CrossRef]
- He, J.X.; Ding, X.Y.; Zhao, J.; Zeng, J.; Zhou, Y.X.; Xiao, W.; Hua, D.; Liu, M.C.; Guo, H.X.; Zhang, Y.; et al. A novel pan-epitope based nanovaccine self-assembled with CpG enhances immune responses against flavivirus. J. Nanobiotechnol. 2024, 22, 738. [Google Scholar] [CrossRef]
- Trent, A.; Ulery, B.D.; Black, M.J.; Barrett, J.C.; Liang, S.; Kostenko, Y.; David, N.A.; Tirrell, M.V. Peptide Amphiphile Micelles Self-Adjuvant Group A Streptococcal Vaccination. AAPS J. 2015, 17, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, J.; Lu, S.; Igweze, J.; Xu, W.; Kuang, D.; Zealey, C.; Liu, D.H.; Gregor, A.; Bozorgzad, A.; et al. Self-assembling peptide for co-delivery of HIV-1 CD8+T cells epitope and Toll-like receptor 7/8 agonists R848 to induce maturation of monocyte derived dendritic cell and augment polyfunctional cytotoxic T lymphocyte (CTL) response. J. Control. Release 2016, 236, 22–30. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zeng, Y.H.; Xiao, Q.Q.; Wu, Y.Y.; Liu, J.L.; Wang, H.C.; Luo, Y.T.; Zhan, J.; Liao, N.; Cai, Y.B. An in-situ peptide-antibody self-assembly to block CD47 and CD24 signaling enhances macrophage-mediated phagocytosis and anti-tumor immune responses. Nat. Commun. 2024, 15, 5670. [Google Scholar] [CrossRef]
- Wang, T.T.; Wang, D.G.; Yu, H.J.; Feng, B.; Zhou, F.Y.; Zhang, H.W.; Zhou, L.; Jiao, S.; Li, Y.P. A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat. Commun. 2018, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Rudra, J.S.; Mishra, S.; Chong, A.S.; Mitchell, R.A.; Nardin, E.H.; Nussenzweig, V.; Collier, J.H. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials 2012, 33, 6476–6484. [Google Scholar] [CrossRef] [PubMed]
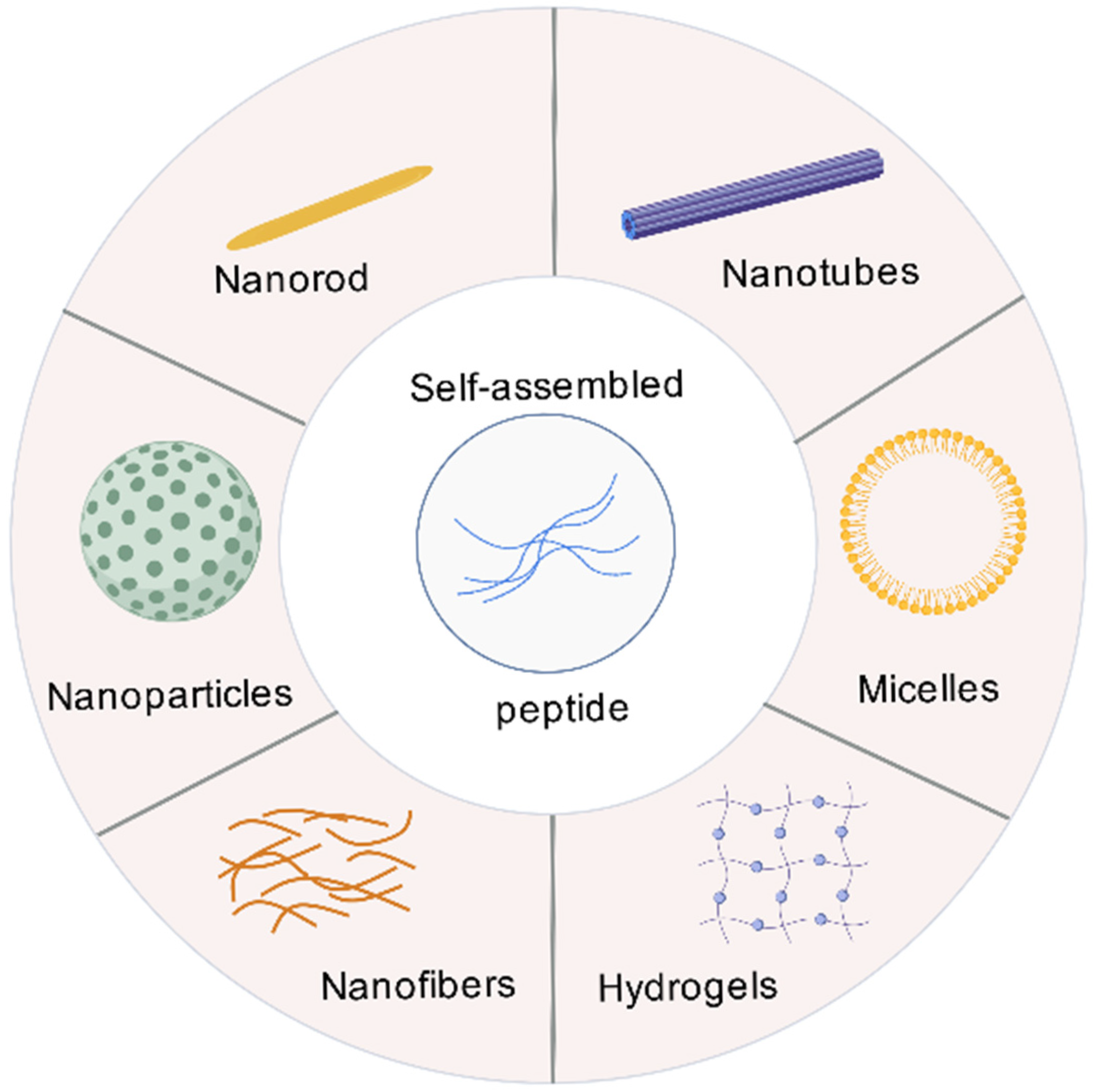
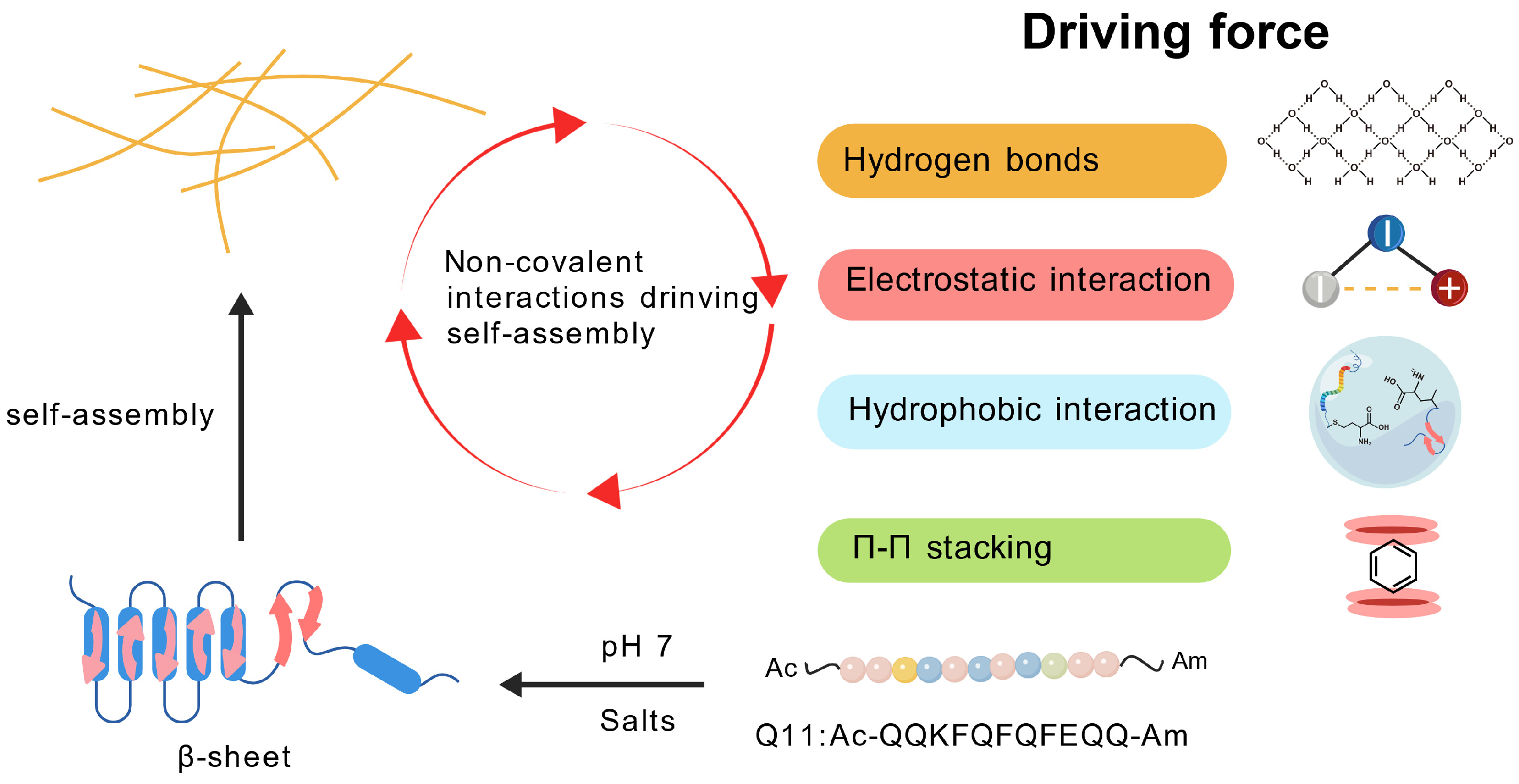
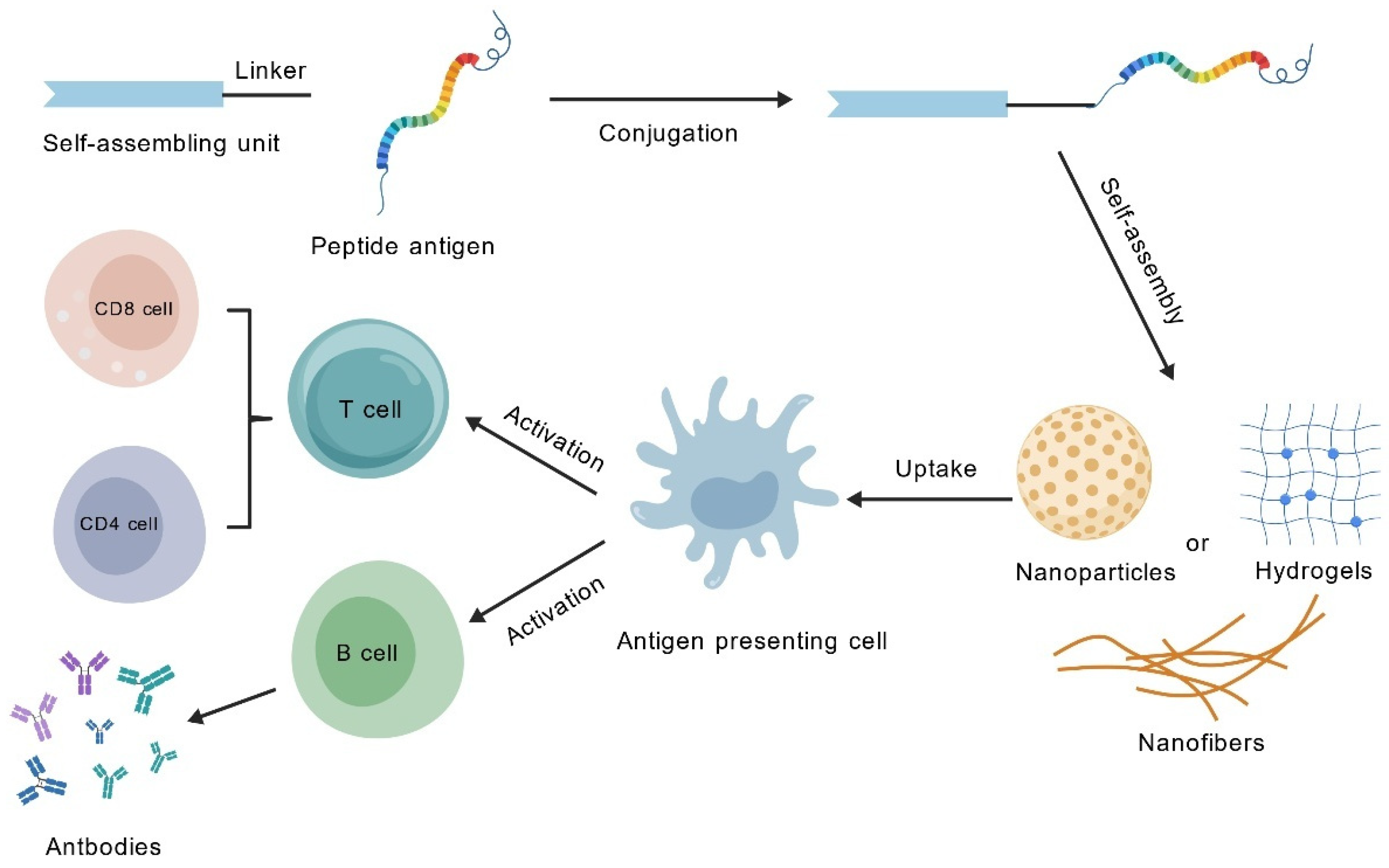
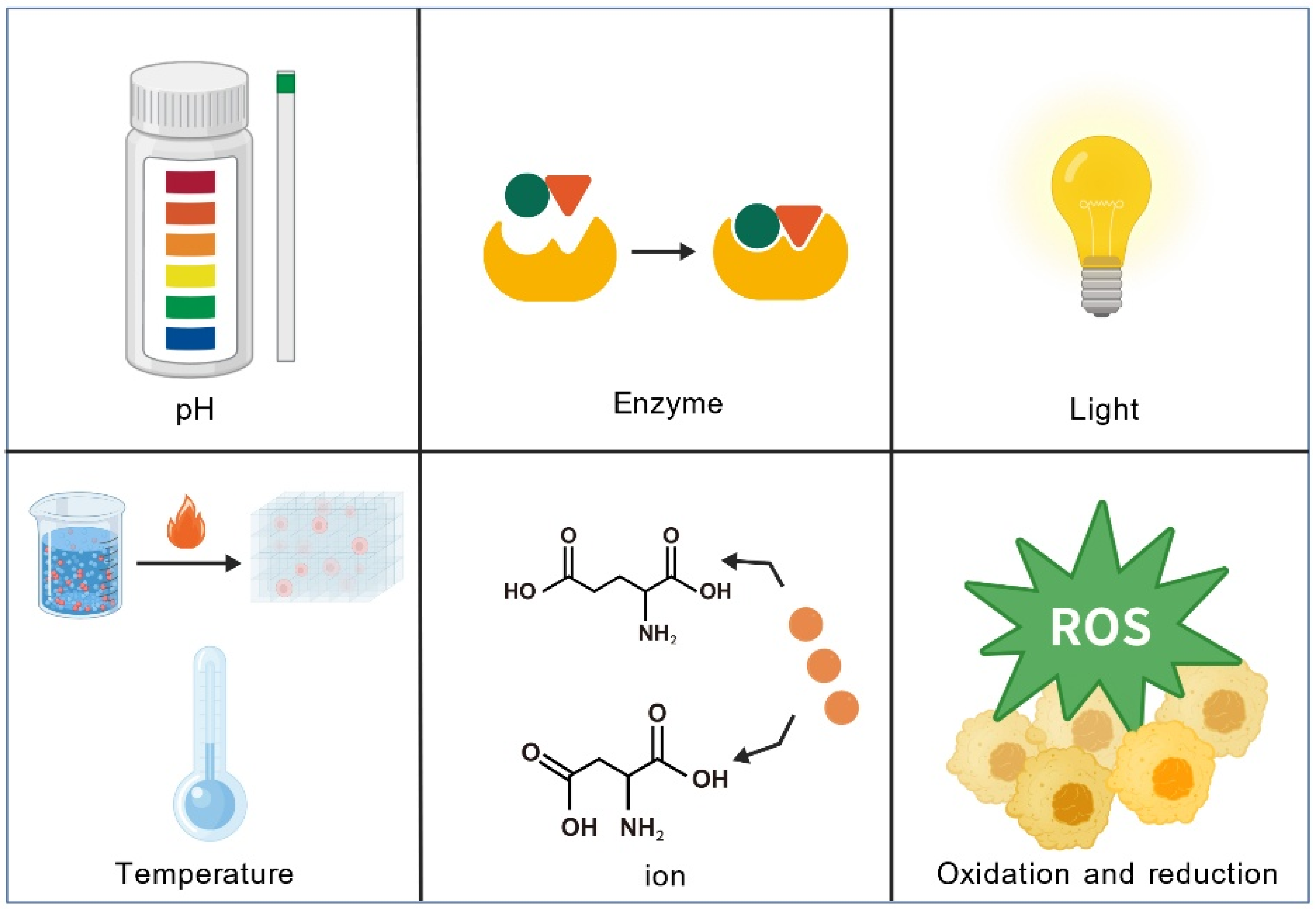
| Adjuvant Classification | Examples | Mode of Action | Approved Vaccines |
|---|---|---|---|
| Delivery system adjuvants | Aluminum adjuvants (aluminum hydroxide, aluminum phosphate) | Mainly induces Th2 type humoral immune response, but has weak cellular immune induction ability, which may cause local inflammatory response | Diphtheria, Tetanus, Pertussis (DTP) Vaccine and Hepatitis B Vaccine |
| Emulsions (MF59, AS03) | Oil in water (o/w) or water in oil (w/o) type can enhance humoral and certain cellular immunity | AS03 is used for influenza vaccines and H5N1 avian influenza vaccines | |
| Microparticle/Nanoparticle Delivery Systems (VLPs, Lipid Nanoparticles, Liposomes) | Simulating pathogens, easily ingested by APCs, with both delivery and immune stimulation functions | COVID-19 Vaccine, Shingles Vaccine | |
| Immunostimulants | TLR Agonists (MPLA, CpG ODN, Flagellin) | Specific activation of PRRs, induction of chemokine and inflammatory cytokine production, T cell immune bias | Combined with aluminum for hepatitis B vaccine and COVID-19 vaccine (CpG 1018) |
| Cytokines (IL-2, IL-12) | Regulate the function, activation, and differentiation direction of immune cells | _ | |
| Chitosan | Activation of cGAS STING and NLRP3 inflammasome pathways can enhance Th1 type immune response | _ | |
| Compound adjuvants | AS01 | Improve antibody titers and enhance Th1 type immune responses | Shingles Vaccine, Malaria Vaccine |
| AS02 | _ | ||
| AS04 | Activate TLR4, promote the maturation of APCs, and improve humoral and cellular immunity | Human papilloma (HPV) vaccine, hepatitis B vaccine | |
| Novel Biomaterial Adjuvants | Self-Assembling Peptides, Polymeric Nanoparticles | Degradable, structurally and functionally designable, capable of integrating antigen delivery and immune regulatory functions | _ |
| Peptide Sequence | Nanostructure | Features | Ref. |
|---|---|---|---|
| PSFCFKFEP | Nanofiber hydrogel | Temperature < 80 °C, nanofiber; Temperature > 80 °C, Irregular aggregates | [69] |
| C8-HEFISTAH-NH2 | Hydrogel | Stable in pH 4–8 and temperature 25–50 °C range | [70] |
| Elastin-like polypeptides (ELPs) G-(QYPSDGRG)n-(XGVPG)m-Y | Spherical micelle, Cylindrical micelles | temperature response | [71] |
| (FPGVG)n | Micron-sized spherical aggregates | temperature response | [72] |
| RFH: NH3+-RFRHRHRFR-COO− | Nanostructure | pH response | [74] |
| Histidine repeat sequence (12×His) | Spherical nanoparticles | pH response | [75] |
| C16-VVAEEE | Nanofiber hydrogel or solution | pH ≤ 6.8, nanofiber hydrogel; pH ≥ 7.0, solution | [76] |
| P1 (LVEFRHY) | Nanofiber | pH 7.5, nanofibers; pH 6.5, nanoparticles | [77] |
| FFD (Phe-Phe-Asp) GHK (Gly-His-Lys) | Hydrogel | At pH 7.4, GHK formed amorphous aggregates, while FFD assembled into nanofibers. When Cu2+ (30 mM) was added, the FFD/GHK mixture rapidly formed a three-dimensional nanofiber network, and transformed into a hydrogel | [80] |
| VKVKVKVK-VPPPT-KVKVKVKV | Hydrogel | At pH 7.4, adding salts (e.g., NaCl or KF) shielded the electrostatic repulsion of lysine (K) residues, thereby inducing protein folding | [64] |
| Peptide Name and Sequence | Self-Assembly Type | Immunization Strategy/Application | Key Results | Reference |
|---|---|---|---|---|
| Q11 (Ac-QQKFQFQFEQQ-Am) | β-sheet nanofibers | Insert hydrophilic SGSG linker between OVA and Q11 domains to form O-Q11; Subcutaneous injection | Elicited high IgG titers similar to CFA; dependent on self-assembly; no significant T cell help involved. | [19] |
| Connecting pEα peptide antigen through SGSG to form Eα52–58-Q11, Intranasal immunization | Induced lung dendritic cell activation and migration to lymph nodes; primed TH17 responses independently in lung and lymph nodes. | [97] | ||
| Oral immunization with PASylation modifications for mucosal delivery against peptide (OVA323–339) and small molecule (phosphorylcholine) epitopes. | Enabled oral immunization by resisting protease degradation and enhancing muco-penetration; induced systemic and local immune responses without inflammation; effective in DSS colitis models. | [105] | ||
| Nanofibers | The Q11 domain and acidic polymerase (PA224–233, SSLENFRAYV) are linked by SGSG to form the PAQ11 peptide; Intranasal delivery; influenza vaccine. | Elicited resident CD8+ T cells in lung, non-inflammatory, provided protection against influenza challenge. | [102] | |
| Coil29 (QARILEADAEILR-AYARILEAHAEILRAQ) | α-helical nanofibers | Sublingual immunization with epitopes (e.g., OVA, 2C7, FP) | Raised robust immune responses; PASylation required for hydrophobic epitopes to reduce mucin complexation and enhance epithelial penetration. | [33] |
| Subcutaneous immunization in mice with epitope-bearing nanofibers (e.g., PEPvIII, PADRE, SIINFEKL) for cancer and model antigens. | Induced robust antibody, CD4+ T-cell, and CD8+ T-cell responses without supplemental adjuvants; antibody titers higher than CFA-adjuvanted groups; promoted epitope uptake by APCs. | [103] | ||
| Nano-B5 platform (e.g., CTB-Tri, LTB-Tri, StxB-Tri) | Nanoparticle | Based on AB5 toxins and trimer-forming peptides; used for prophylactic and therapeutic vaccines against infections and tumors | Induced strong humoral and cellular immune responses in mice and monkeys; excellent lymph node targeting and safety. | [36] |
| 4RDP (F5) (containing di-pentafluorophenylalanine (F5) and tetra-arginine (4R)) | Nanoparticle | Fluorinated supramolecular self-assembly, as adjuvant for cancer therapy (e.g., with OVA antigen) | Enhanced antigen uptake, lysosomal escape, and cross-presentation; elicited TH1-biased cellular immunity; combined with anti-PD-L1 for tumor inhibition. | [87] |
| Ac-FFA-NH2 | Hydrogel | Subcutaneous delivery with OVA antigen; vaccine adjuvant | Induced high IgG titers, robust humoral and cellular immune responses; composite with liposomes showed sustained antigen release. | [98] |
| P4c-Mal (100 amino acid monomeric linear peptide) | Nanoparticles | Subcutaneous injection without adjuvant; malaria vaccine | Long-lasting protection against Plasmodium berghei, high antibody titers, CD4+ T cell-dependent response. | [99] |
| Nap-GFFY | Hydrogel | Subcutaneous immunization; vaccine adjuvant for cancer and infectious diseases | Stimulated strong CD8+ T cell responses, enhanced antigen uptake by dendritic cells, non-inflammatory. | [100] |
| 3DSNA (Ada-GDFDFDYGDKDKDK-NH2) | Nanofibers (pH-triggered self-assembly) | Subcutaneous injection; cancer immunotherapy adjuvant | Activated NF-κB, enhanced antigen presentation, induced CD8+ T cell responses, inhibited tumor growth. | [101] |
| KKI10 (KKGSGSSNNFGAILSS) | β-sheet nanorods | Highly conserved epitopes connecting extracellular domains of matrix protein 2 (M2e); Subcutaneous and intranasal immunization. | Elicited M2e-specific IgG responses; provided complete protection against influenza A virus (H1N1); self-adjuvanting with efficient APC uptake and TLR-2 activation. | [104] |
| F peptide (FEFEFKFK) | Nanofibrous hydrogel | F peptide co-assembled with gp100209–217, Tyr369–377, MART-126–35, Subcutaneous immunization in mice for cancer immunotherapy (B16 melanoma). | Enhanced DC maturation and antigen presentation; elicited broad-spectrum CD8+ T-cell responses; inhibited tumor growth without additional adjuvants. | [106] |
| K-peptide (KWKAKAKAKWK)and E-peptide (EWEAEAEAEWE) | Nanofibrous hydrogel | OVA323–336 is covalently linked to the C-terminus of K or E peptides via GGG linkers, forming epitope conjugated peptides (ECPs); Subcutaneous immunization in mice for cancer immunotherapy (E.G7-OVA lymphoma). | Activated MyD88-dependent NF-κB pathway in DCs without inflammation; enhanced T-cell immunity and tumor inhibition; self-adjuvanting. | [107] |
| EF8 (EFEFKFEFK) | β-sheet nanofibers | In vitro treatment of THP-1 derived macrophages or PBMC-derived macrophages at 2 mM or 20 mM | Induced M2c polarization (anti-inflammatory response) | [110] |
| YEF8 (YEFEFKFEFK) | β-sheet nanofibers | In vitro treatment of THP-1 derived macrophages or PBMC-derived macrophages at 2 mM or 20 mM Intratracheal booster in BCG-primed mice | Induced M1 polarization (pro-inflammatory response) | |
| EF8Y (EFEFKFEFKY) | No strong inflammatory response, tends towards M2a-like state | |||
| YEF8Y (YEFEFKFEFKY) | No significant inflammatory response | |||
| EYF8 (EYEFKFEFK) | No significant inflammatory response, tends towards M2a-like state | |||
| KFE8-Ag85B (KFE8: FKFEFKFE conjugated to Ag85B240–254via cleavable linker) | Increased frequency of Ag85B-specific CD4+T cells, including tissue-resident memory cells; no improved protection against Mtb challenge | |||
| KFE32-GFP (KFE32: 4 repeats of KFE8 with linkers, fused to GFP) | Nanofibers (in vivo likely) | DNA vaccine, intramuscular injection in mice | Elicited anti-GFP antibodies and CD8+ T cell responses; balanced Th1/Th2 response | [114] |
| Myr-FF (Myristic acid-Phe-Phe) | Lipopeptide hydrogel | Used as an adjuvant for delivering GPC-3 peptide antigen in cancer vaccines | Acted as a TLR2 agonist, upregulates costimulatory molecules (CD80, CD83, CD86) on DCs, induced cytokine secretion (e.g., IL-6, TNF-α), and promoted leukocyte infiltration in lymph nodes without toxicity. | [115] |
| Myr-FFY (Myristic acid-Phe-Phe-Tyr) | Lipopeptide hydrogel | Same as above; adjuvant for GPC-3 peptide delivery | Showed higher TLR2 activation compared to Myr-FF, enhanced DC maturation, and sustained release of antigen with robust immune response. | |
| RADA16 (Ac-RADARADARADARADA-NH2) | Nanofibrous hydrogel | Simple physical mixture of peptide nanofiber hydrogel, anti PD-1 antibody, dendritic cells and tumor antigen is injected subcutaneously | Enhanced DC maturation and antigen presentation, recruited host DCs, promotes T-cell proliferation and cytokine secretion (e.g., IFN-γ), and suppressed tumor growth in prophylactic and therapeutic models. | [116] |
| Fmoc-FF (Fmoc-Phe-Phe) | Hydrogel | Injectable peptide hydrogels with adjustable mechanical and rheological properties were obtained by electrostatic coupling and co assembly with positively charged poly lysine (PLL) | Induced T cell activation (increasing CD4+ and CD8+T cells), inhibited tumor growth, and exhibited biocompatibility and biodegradability in vivo without the addition of antigens, immune regulatory factors, and adjuvants. | [117] |
| TBT (KYVKQNTLKLAT-GGVDRGWGNGCGLFGKG-LL-LEYIPEITLPVIAALSIAES) | Nanoparticles | Combining TBT with adjuvant CpG to form nanovaccine; Subcutaneous immunization in mice | Enhanced antigen-specific IgG, increased IFN-γ and IL-4 expression, protection against DENV and ZIKV | [120] |
| J8 (QAEDKVKQSREAKKQVEKALKQLEDKVQK) | Cylindrical micelles | J8 peptide covalently couples with dipalmitoylglutamic acid (diC16) to form J8-diC16, Subcutaneous vaccination in mice | Induced strong IgG1 antibody response comparable to conventional adjuvants | [121] |
| EAK16-II: AEAEAKAKAEAEAKAK | Nanofibers | The coupling of the EAK16-II peptide with the HIV-1-specific CTL epitope SLYNTVATL produced SL9-EAK16-II. This was then co-assembled with the TLR7/8 agonist R848 to create a tripartite formulation, In vitro DC stimulation and mouse immunization | Promoted DC maturation and specific CTL response | [122] |
| PAC-SABI (FFVLKAWSATWSNpYWRH) | Nanofiber network | In vitro and in vivo tumor models | Simultaneous blocking of CD47/CD24 signals enhanced macrophage phagocytosis | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-M.; Zhu, J.; Wang, Z.-Y.; Bai, Y.-L.; Li, H.-B.; Nashun, B.; Jiang, Y. Self-Assembled Peptides: A New Generation of Vaccine Adjuvant Platform. Vaccines 2025, 13, 1183. https://doi.org/10.3390/vaccines13121183
Zhang M-M, Zhu J, Wang Z-Y, Bai Y-L, Li H-B, Nashun B, Jiang Y. Self-Assembled Peptides: A New Generation of Vaccine Adjuvant Platform. Vaccines. 2025; 13(12):1183. https://doi.org/10.3390/vaccines13121183
Chicago/Turabian StyleZhang, Miao-Miao, Ji Zhu, Zhao-Yi Wang, Yu-Lun Bai, Hai-Bo Li, Buhe Nashun, and Yue Jiang. 2025. "Self-Assembled Peptides: A New Generation of Vaccine Adjuvant Platform" Vaccines 13, no. 12: 1183. https://doi.org/10.3390/vaccines13121183
APA StyleZhang, M.-M., Zhu, J., Wang, Z.-Y., Bai, Y.-L., Li, H.-B., Nashun, B., & Jiang, Y. (2025). Self-Assembled Peptides: A New Generation of Vaccine Adjuvant Platform. Vaccines, 13(12), 1183. https://doi.org/10.3390/vaccines13121183






