Abstract
Flavivirus infections, including dengue, Zika, West Nile, and Japanese encephalitis, remain a major global health concern. Although several vaccines are licensed, the durability and qualitative features of vaccine-induced antibodies differ substantially across platforms, leading to incomplete cross-protection and the risk of antibody-dependent enhancement. Long-term durability is exemplified by YF-17D, which induces protective antibodies that have been detectable for decades, whereas the JE SA14-14-2 vaccine has achieved program-level reductions in disease in endemic regions. In contrast, CYD-TDV shows serostatus-dependent outcomes, and the investigational TAK-003 vaccine has demonstrated antibody persistence for at least four years. Recent studies have clarified how preserving quaternary envelope epitopes, minimizing prM-associated non-neutralizing specificity, and sustaining germinal center activity determine antibody affinity, breadth, and persistence. Advances in adjuvant formulations and delivery platforms have shown that engaging defined innate pathways and prolonging antigen availability enhance affinity maturation and long-lived plasma cell formation. Booster scheduling and baseline serostatus further shape the antibody quality, highlighting the importance of immune imprinting and cross-reactivity in vaccine design. Together, these findings outline the design principles for next-generation flavivirus vaccines, including stabilization of neutralization-sensitive epitopes, use of adjuvants that sustain germinal center responses, optimization of antigen persistence, and tailoring of dosing strategies to immune history to elicit durable and broadly protective humoral immunity.
1. Introduction
Flaviviruses are small enveloped positive-sense single-stranded RNA viruses belonging to the family Flaviviridae, which includes dengue, Zika, West Nile, yellow fever (YF), and Japanese encephalitis (JE) [1,2]. They continue to cause large outbreaks across tropical and temperate regions, such as human mobility, climate trends, and vector adaptation, thereby expanding transmission zones worldwide [3]. The virion encodes three structural and seven nonstructural proteins through a single open reading frame that coordinates entry, genome replication, assembly, and immune evasion, creating multiple points at which host immunity and vaccines can act [4,5,6]. The envelope glycoprotein mediates receptor binding and low pH-triggered membrane fusion and is the dominant target of neutralizing antibodies elicited by infection or vaccination [7]. The global disease burden remains high, with dengue alone causing hundreds of millions of infections annually, and with an expanding geographic footprint documented in recent mapping studies [8,9].
Vaccination demonstrates the potential for humoral protection in this genus. The YF vaccine elicits durable immunity, and JE vaccination programs have significantly reduced its incidence in regions with sustained coverage [10,11]. Population-level assessments further confirmed these reductions following the national program implementation [12,13]. Despite these successes, vaccines for dengue, Zika, and West Nile remain limited or only partially protective because antigenic diversity and immune cross-reactivity complicate the definition of reliable correlates of protection across exposure and age group [14,15,16].
Humoral immunity is the primary cause of protection against flavivirus infection and vaccination. Neutralizing antibodies targeting envelope (E) proteins block attachment and fusion to prevent systemic infections [17,18]. Antibody concentration alone does not predict vaccine performance, since qualitative features including affinity, avidity, epitope specificity, isotype distribution, and persistence of long-lived plasma cells and memory B-cells determine the durability and effectiveness of protection in real-world settings [19,20,21,22].
Cross-reactive antibodies are a hallmark of flavivirus immunity and may protect high titers. At intermediate titers, they can facilitate infection through antibody-dependent enhancement, as demonstrated in a long-term pediatric surveillance study that identified a narrow range of pre-existing titers associated with a maximal risk of severe dengue [23,24]. Heterologous interactions between flaviviruses are also relevant. Prior dengue infection generates antibodies that bind Zika virus (ZIKV) with avidity and modulate subsequent immune and clinical outcomes during sequential epidemics in endemic regions [25].
Although key aspects of T cell immunity are referenced where directly relevant to germinal center activity and affinity maturation, a detailed analysis of cellular correlates is outside the scope of this review. The primary focus is on E protein-directed humoral immunity, including the structural, adjuvant, and platform-level determinants that shape antibody specificity, avidity, breadth, and longevity. Structural virology has identified conserved epitopes on the envelope dimer interface that are recognized by broadly neutralizing human antibodies. These epitopes provide molecular templates for immunogen design aimed at increasing breadth while reducing non-neutralizing specificity [26,27]. Adjuvant research has shown that pattern recognition receptor agonists, saponin-based formulations, and optimized emulsions promote higher quality and more durable antibody responses by enhancing germinal center activity and T follicular helper (Tfh) cell support, surpassing the effects of alum [28,29]. Platform advances have contributed to this trend. mRNA vaccines and nanoparticle-based delivery systems prolong antigen availability and improve antigen display, thereby strengthening the magnitude, quality, and persistence of antibody responses in preclinical and clinical studies [30,31]. Taken together, the current evidence indicates that improving antibody quality and durability is essential for achieving broad and long-lasting protection against flaviviruses. These insights support integrated vaccine strategies that combine rational adjuvant selection, epitope-focused antigen engineering, and advanced delivery platforms to elicit high-affinity, cross-protective, and persistent humoral immunity, while maintaining safety across varied exposure histories and populations. Collectively, these observations converge on a set of design principles that guide durable and broadly protective flavivirus vaccine immunity.
- Preserving quaternary E-dimer epitopes to promote potent and broad neutralization while minimizing off-target responses;
- Reducing prM-associated or fusion loop–biased non-neutralizing specificities that contribute to ADE risk;
- Sustaining germinal center activity and Tfh support to drive affinity maturation and long-lived plasma cell development;
- Optimizing antigen persistence and delivery platform properties to reinforce durable and high-avidity antibody responses;
- Incorporating baseline serostatus and immune imprinting patterns into vaccine design can ensure safety and consistent immunogenicity across populations.
2. Overview of Humoral Immunity in Flavivirus Vaccination
Humoral immunity is the principal correlate of protection against flavivirus infections and is a major determinant of vaccine performance. Following vaccination, B-cell activation, germinal center maturation, and progressive antibody affinity improvement collectively define the quality and durability of neutralizing responses. Comparative data from licensed vaccines, including those against YF, JE, and dengue, revealed distinct profiles of antibody magnitude, persistence, and functional characteristics (Table 1). Understanding these processes provides a mechanistic foundation for interpreting vaccine-induced immunity across different platforms.
2.1. B-Cell Activation and Germinal Center Response
Effective flavivirus vaccines initiate a coordinated sequence of immune events, beginning with B-cell activation. Naïve B cells that recognize conformational epitopes on the viral E protein are activated through B-cell receptor engagement and Tfh cell assistance [22,32]. Within the germinal centers of draining lymph nodes, activated B-cells undergo proliferation, somatic hypermutation, and class-switch recombination processes that refine antibody specificity and effector potential [22,33].
Adjuvant and live-attenuated vaccine platforms strongly influence germinal center kinetics. The live-attenuated YF-17D vaccine induces extended germinal center activity and generates an antibody repertoire that has persisted for decades [34,35]. Similarly, the live-attenuated SA-14-14-2 JE vaccine elicits robust B-cell activation and durable memory responses, whereas inactivated formulations require periodic boosting to maintain sero-protection [13,36]. Adjuvants such as alum primarily increase antibody magnitude, whereas saponin-based or pattern recognition receptor agonist formulations promote more sustained germinal center activity and support higher antibody quality [28,29]. Collectively, these observations highlight that germinal center dynamics shape not only the antibody titer but also the breadth and persistence of protection.
2.2. Antibody Affinity Maturation and Long-Lived Plasma Cell Formation
Affinity maturation occurs through iterative selection of B-cell clones with improved immunoglobulin receptors, driven by activation-induced cytidine deaminase (AID)-mediated somatic hypermutation [37,38]. This process produces high-affinity antibodies that efficiently recognize quaternary epitopes on the envelope protein, improving neutralization potency and cross-serotype breadth [27,39].
Following the germinal center phase, a subset of B-cells differentiate into long-lived plasma cells that migrate to the bone marrow and continuously secrete antibodies for extended periods [40,41]. The maintenance of these plasma cells depends on stromal cell-derived survival factors, such as interleukin (IL)-6, B-cell-activating factor (BAFF), and APRIL, which sustain antibody production even after antigen clearance [42].
Among licensed flavivirus vaccines, YF-17D generates one of the most durable antibody responses, with neutralizing activity persisting for more than 30 years after a single dose [34,43]. In contrast, immunity following inactivated JE vaccines wanes within several years and requires booster doses to maintain protection [13,44]. These differences illustrate how antigen persistence and vaccine platform characteristics influence the development and longevity of plasma cells and memory B-cell compartments [30,32].
2.3. Neutralizing and Non-Neutralizing Antibody Functions
Neutralizing antibodies protect the virus primarily by blocking viral attachment, membrane fusion, and post-entry processes [45,46]. Structural analyses of broadly neutralizing antibodies (bnAbs) often recognize quaternary epitopes bridging adjacent E protein dimers, such as the envelope dimer epitope (EDE), which mediates cross-neutralization among dengue serotypes and partial activity against ZIKV [26,27].
Non-neutralizing antibodies contribute to antiviral defense by engaging Fc-gamma (Fcγ) receptors on immune cells and promoting antibody-dependent cellular cytotoxicity and phagocytosis [47,48]. At suboptimal antibody concentrations, Fc-mediated uptake can facilitate viral replication in monocytes and macrophages, resulting in antibody-dependent enhancement (ADE) [23,49].
Therefore, balancing neutralizing potency with Fc-mediated effector function is essential for flavivirus vaccine safety. Comparative serological analyses of licensed vaccines showed that YF and JE vaccines induce strong neutralizing antibodies with a likelihood of ADE, whereas dengue vaccines generate broader but more heterogeneous responses that require careful evaluation of the cross-reactive antibody quality [13,43,50]. These distinctions are summarized in Table 1, which compares the magnitude, breadth, and durability of antibody responses among licensed flavivirus vaccines and highlights how the vaccine platform, antigen conformation, and adjuvant formulation shape protective humoral immunity.

Table 1.
Comparative antibody responses to licensed flavivirus vaccines.
Table 1.
Comparative antibody responses to licensed flavivirus vaccines.
| Vaccine | Platform | Neutralizing Magnitude | Durability Tier | Breadth/Cross-Reactivity | ADE/Safe | Refs. |
|---|---|---|---|---|---|---|
| YF-17D | Live-attenuated | High titers within 2–4 weeks; polyfunctional IgG | >20 years in clinical follow up | Cross-reactive within YF genotypes; minimal heterologous activity | No ADE signal reported | [34,43] |
| JE-SA-14-14-2 | Live-attenuated | Robust titers after single dose; strong memory recall | >20 years in clinical follow up | Limited cross-neutralization to other JEV genotypes | Well-tolerated; no ADE | [13,44] |
| JE (IXIARO/inactivated) | Purified inactivated | Moderate titers; boosted by 2-dose regimen | wanes over 2–3 years | Genotype-specific; minimal heterologous response | no ADE | [44] |
| Dengue (CYD-TDV) | Chimeric yellow-fever vector | Strong neutralization; dependent on serostatus | wanes over 2–3 years | Broad but heterogeneous; partial neutralization of heterologous serotypes | Increased ADE risk in sero-negatives | [23,24,50] |
| Dengue (TAK-003) | Live-attenuated tetravalent | Balanced neutralization across serotypes | ≥4 years in clinical trials so far | Cross-neutralization with modest enhancement potential | No ADE signal to date | [51] |
| Zika (mRNA/DNA candidates) | Nucleic acid (investigational) | High titers in animals and early human trials | anticipated from platform mechanism | Strong cross-reactivity with DENV; epitope overlap | ADE potential in vitro; not confirmed in vivo | [30,52] |
Durability descriptors follow the standardized terminology used throughout the manuscript. Evidence tiers are harmonized using the categories Phase 3 clinical data, preclinical murine or NHP data, observational serology, and mechanistic inference. These terms match the terminology used throughout this manuscript. IgG, immunoglobulin G; ADE, antibody-dependent enhancement.
3. Influence of Adjuvants on Antibody Quality and Durability
Adjuvants profoundly shape the magnitude, quality, and persistence of vaccine-induced antibody responses by modulating innate sensing, antigen presentation, and germinal center formation dynamics. Their mechanisms determine whether the resulting antibodies exhibit sustained affinity maturation, long-term plasma cell support, and cross-serotype breadth [53,54]. Differences in adjuvant formulations accounted for most of the variation in antibody durability and breadth observed across vaccine platforms. Classical adjuvants, such as alum or oil-in-water emulsions, have enhanced early protective titers for decades, but these formulations show limited ability to sustain germinal center activity compared to systems that engage pattern-recognition receptors or the STING pathway. These adjuvant-driven effects align with the third design principle, which emphasizes the need to sustain germinal center activity and Tfh support to generate high-avidity antibodies and durable, long-lived plasma cell responses. To maintain a consistent and parallel structure across adjuvant classes, the subsections that follow are organized along four dimensions: enhancement of germinal center and Tfh activity, influence on IgG subclass or antibody avidity, expected durability of the antibody response, and representative flavivirus vaccine applications.
3.1. Classical Adjuvants and Their Immunomodulatory Effects
Alum, the oldest and most widely used adjuvant, improves antigen retention at the injection site and recruits monocytes and dendritic cells via local inflammasome activation [55]. This activation triggers IL-1β and IL-18 release, leading to efficient priming of naïve B-cells but relatively modest Tfh cell differentiation, which constrains affinity maturation and accelerates antibody waning [53,56]. Inactivated Japanese encephalitis and tick-borne encephalitis vaccines adjuvanted with alum induce strong early neutralizing antibody responses but show a gradual decline in antibody titers over time, necessitating periodic booster immunizations to maintain protective antibody levels [57].
Oil-in-water emulsions such as MF59 and AS03 act through distinct mechanisms. They stimulate local chemokine secretion (IL-6, and monocyte chemoattractant protein 1) and recruit antigen-presenting cells, leading to enhanced Tfh activation and affinity maturation beyond that achieved with alum [58,59,60]. In preclinical studies, these emulsions also promoted a more balanced Th1/Th2 profile and broader distribution of IgG subclasses, resulting in antibodies with improved functional quality [59,60,61]. Despite these advantages, emulsion adjuvants primarily increase the magnitude and functional breadth of early responses and still provide more limited support for prolonged germinal center reactions than replicating platforms do.
3.2. Novel Adjuvant Formulations Targeting B-Cell and T Helper Responses
Modern adjuvant discovery has focused on stimulating defined innate immune receptors that shape the magnitude and quality of B-cell selection within germinal centers. Toll-like receptor (TLR)-based adjuvants, such as CpG oligodeoxynucleotides (CPG ODN; TLR9) and monophosphoryl lipid A (TLR4), activate plasmacytoid dendritic cells and promote type I interferon and IL-12 production, favoring Th1 polarization and robust Tfh differentiation [62,63]. This cytokine milieu drives class switching toward IgG2a and IgG3 subclasses and enhances somatic hypermutation, resulting in higher affinity and improved Fc-mediated antibody function [62].
Saponin-based adjuvants, such as QS-21 and Matrix-M, act through inflammasome activation and antigen cross-presentation to expand Tfh and germinal center B-cell populations [64,65]. Evidence from preclinical studies in flaviviruses and other viral models suggests that these adjuvants can enhance antibody affinity and sustain higher titers over time, suggesting their utility in improving durability even when antigen doses are reduced. Emerging systems, such as cyclic dinucleotide STING agonists, activate follicular dendritic cells and promote extended antigen retention, which reinforces germinal center selection [66].
Lipid nanoparticle formulations used in mRNA vaccines provide both antigen delivery and intrinsic adjuvant activity through endosomal RNA sensing. This dual action leads to prolonged germinal center activity and efficient long-lived plasma cell development, as demonstrated in preclinical and early clinical studies of Zika and dengue mRNA vaccine candidates [67,68]. Collectively, these novel adjuvants demonstrate that targeted innate signaling can modulate antibody affinity, breadth, and persistence more effectively than conventional formulations.
3.3. Comparative Evaluation of Adjuvants Used in Flavivirus Vaccines
Head-to-head comparisons among flavivirus vaccine platforms illustrated clear differences in antibody durability and functional quality depending on adjuvant class. Alum-adjuvanted inactivated vaccines consistently produce high seroconversion rates but exhibit limited durability, with titers declining over several years [57,69]. In contrast, live attenuated vaccines, such as YF-17D or SA 14-14-2, generate long-lived immunity without external adjuvants because viral replication provides multifaceted innate stimulation that drives sustained Tfh activity and long-lived memory [43,70].
Preclinical data from flaviviruses and related viral vaccine models suggest that subunit- and DNA-based platforms may achieve improved antibody quality and durability when formulated with CpG, MPLA, or saponin adjuvants compared to alum alone [61,71]. Such formulations not only enhance germinal center longevity but also expand cross-neutralizing antibody repertoires that target conserved epitopes among flavivirus sero-complexes [72,73]. These effects are particularly relevant for dengue and Zika vaccines, where balancing the neutralization breadth and avoiding ADE remain key challenges.
A summary of these comparative outcomes is presented in Table 2, which outlines the major adjuvant classes, mechanisms of action, and their relative influence on antibody magnitude, affinity, and persistence in the host. Together, these results support the conclusion that rational adjuvant selection guided by systems immunology insights is central to designing next-generation flavivirus vaccines capable of eliciting high-quality, durable, and safe humoral immunity [29,74].

Table 2.
Comparative effects of classical and novel adjuvants on antibody responses to flavivirus vaccines.
4. Effects of Antigen Design and Delivery Platforms
The structure and mode of flavivirus antigen delivery critically determine how the immune system perceives, processes, and retains antigenic information. Antigen conformation dictates epitope accessibility and the type of B-cell clones that dominate the response, whereas delivery platforms influence antigen persistence and the magnitude of germinal center activity. Therefore, preserving native quaternary structures and ensuring sustained antigen availability are central requirements for generating high-affinity and durable antibody responses (Figure 1). Modern vaccine designs incorporate mRNA, viral vectors, or nanoparticles to optimize these features and elicit persistent and broadly protective humoral immunity (Table 3). These platform-dependent differences reflect the first and fourth design principles, highlighting the importance of preserving quaternary E-dimer epitopes and optimizing antigen persistence to reinforce a durable and broadly neutralizing humoral immunity.
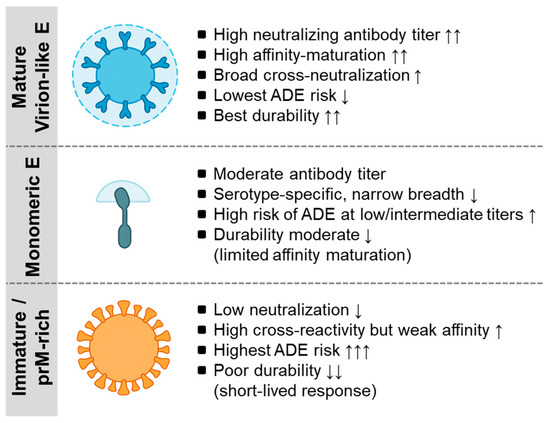
Figure 1.
Antigen configurations of the flavivirus envelope and their immunological consequences. This schematic illustrates the three major forms of E protein antigen presentation and their associated humoral profiles. Mature virion-like E (e.g., YF-17D-like or SA14-14-2-like mature particles) expose organized quaternary epitopes that drive high neutralization potency, extensive affinity maturation, minimal ADE potential, and durable long-lived plasma cell responses. Monomeric E proteins (e.g., soluble recombinant E or EDIII subunits) have limited epitope organization and elicit moderate neutralization with narrower serotype-specific breadth and a higher ADE risk at low or intermediate antibody concentrations. Immature or prM-rich antigens (e.g., incompletely matured inactivated virions) display prM-shielded surfaces that generate weak neutralization, high cross-reactivity with low affinity, the highest ADE propensity, and short-lived antibody responses. Together, these three configurations illustrate how antigen conformation governs the quality, breadth, safety profile, and durability of antibodies across flavivirus vaccine platforms. For a detailed mechanistic discussion, see Section 4 and Section 5. ADE, antibody-dependent enhancement, EDE, envelope dimer epitope. The arrows will be defined as follows: ↑ increase, ↑↑ marked increase, ↑↑↑ strong increase, ↓ decrease, and ↓↓ marked decrease.

Table 3.
Antigen and delivery platform strategies enhance antibody durability.
4.1. Structural Features of Flavivirus Envelope Proteins Influencing Antibody Quality
Flavivirus virions are composed of 180 copies of envelope glycoproteins arranged as antiparallel dimers on the viral surface [122]. This protein mediates receptor attachment and low pH-triggered membrane fusion, and its conformation governs the exposure of neutralizing epitopes. Antibodies that recognize quaternary epitopes spanning adjacent E protein dimers, such as EDE, demonstrate cross-neutralizing potential across dengue virus (DENV) serotypes and ZIKV [98,99].
In contrast, antibodies targeting the fusion loop or domain III often display strong serotype-specific neutralization but show limited breadth and carry increased ADE potential at sub-neutralizing concentrations [123,124]. These findings underscore the importance of preserving native quaternary E dimer organization to elicit antibodies with optimal quality and cross-protective potential. Structural vaccinology studies have shown that quaternary epitope exposure can be stabilized through engineered virus-like particles (VLPs) or pre-fusion-stabilized E proteins, which improve the neutralization breadth and reduce the targeting of non-protective regions [103,104]. Furthermore, the presence of prM protein in immature or partially mature particles can divert the immune response toward poorly neutralizing epitopes, emphasizing the need for antigen designs that minimize prM content and retain mature virion-like conformations [125,126].
4.2. Antigen Presentation and Delivery Mechanisms in Different Vaccine Platforms
Distinct vaccine platforms present flavivirus antigens in different structural and cellular contexts, influencing B-cell engagement and antibody persistence. Live-attenuated vaccines, such as YF-17D and SA 14-14-2 JEV, replicate transiently in vivo and provide sustained antigen exposure, a feature that supports prolonged germinal center activity and long-lived plasma cell formation [106,127]. Inactivated vaccines, which are safer for certain populations, present fixed antigens that may alter the E protein conformation and limit the induction of broadly neutralizing antibodies [128,129].
Recombinant subunit vaccines expressing soluble E or domain III proteins require potent adjuvants or multimeric presentation systems to elicit antibody responses owing to the limited capacity of monomeric antigens to crosslink B-cell receptors [130,131]. VLPs that mimic the native virion surface antibody repertoires are similar to those of natural infections and produce higher affinity responses than soluble proteins [132].
Nucleic acid-based platforms, such as mRNA vaccines, sustain antigen expression, promote prolonged germinal center activity, and promote efficient affinity maturation [96,133]. Similarly, viral vector vaccines using adenovirus or measles backbones express the E protein within host cells, supporting both humoral and T-cell immunity and providing balanced and durable protection [127]. Taken together, the balance between antigen stability, persistence, and structural fidelity determines whether the immune response favors high-avidity cross-neutralizing antibodies or short-lived strain-specific profiles.
4.3. Emerging Technologies for Improving Antigen Stability and Immune Persistence
Recent advances have aimed to enhance the stability and immunogenicity of flavivirus antigens through rational design and optimized delivery. Cryo-electron microscopy and computational modeling have identified key structural determinants of E protein flexibility, enabling targeted mutations that lock the protein in a pre-fusion conformation resistant to rearrangement [134,135,136]. This approach yielded modified E-dimer constructs that retained neutralizing epitopes, and the reduced exposure of cross-reactive regions exhibited limited neutralizing capacity.
Nanoparticle-based platforms further enhance antigen presentation by multimerizing E proteins or epitopes on scaffolds that replicate the virion geometry [137,138]. These structures efficiently engage B-cell receptors and promote strong Tfh responses, resulting in durable and high-avidity antibody production. Encapsulation of flavivirus antigens in lipid nanoparticles or biodegradable polymers can also improve thermostability and sustain antigen release, leading to extended germinal center activity over time [112,139].
Self-amplifying RNA- and replicon-based systems maintain antigen expression at lower doses and enhance both the magnitude and durability of antibody responses in dengue and Zika models [140,141]. Collectively, these emerging technologies support vaccine strategies that pair structurally stabilized antigens with delivery platforms capable of sustaining germinal center maturation to achieve broad, potent, and long-lasting humoral immunity across the flavivirus genus.
5. Cross-Reactivity and Immune Imprinting Among Flaviviruses
Cross-reactive antibody responses are hallmarks of flavivirus immunity, reflecting the high sequence and structural homology of the E protein across species [142]. These antibodies can provide cross-protection through recognition of conserved quaternary epitopes. Under sub-neutralizing conditions, they may also facilitate infection via Fcγ receptor-mediated uptake [143]. The balance between protection and enhancement depends on epitope specificity, the affinity maturation state, antibody concentration, and exposure history. These interactions underscore the fifth design principle, which incorporates baseline serostatus and immune imprinting patterns into the interpretation of vaccine-induced antibody breadth and safety.
5.1. Cross-Reactive Antibody Responses and Their Protective or Enhancing Roles
Flavivirus infections elicit a complex polyclonal antibody repertoire, some portion of which is cross-reactive with other species of the genus Flavivirus. Broadly neutralizing antibodies that target conserved quaternary epitopes, such as EDE, neutralize multiple DENV serotypes and exhibit partial cross-neutralizing activity against ZIKV [144,145]. These antibodies recognize conformational surfaces formed by adjacent E protein dimers, providing broad and robust Fc functionality [146,147].
In contrast, cross-reactive regions that exhibit limited neutralizing capacity, particularly fusion loop-directed or prM-associated epitopes, can promote the infection of Fcγ receptor-expressing cells, a process known as ADE [148,149]. Longitudinal studies in Nicaragua demonstrated a bell-shaped relationship between pre-existing antibody titers and severe dengue risk, with maximal enhancement occurring at intermediate titers, a pattern subsequently validated in multiple cohorts [24].
Dengue–Zika cross-reactivity is another aspect of this phenomenon. Antibodies elicited by prior dengue infection bind Zika E protein with high avidity but display variable neutralization potency and can enhance Zika infection of myeloid cells in vitro [148,150]. Nevertheless, high-affinity broadly neutralizing antibodies targeting conserved quaternary interfaces, including EDE or fusion loop-spanning regions, appear to be capable of conferring partial protection against these viruses.
5.2. Immune Imprinting and Its Impact on Vaccine Effectiveness
Immune imprinting, also referred to as the original antigenic sin, describes how the first flavivirus exposure biases subsequent antibody responses toward initially recognized epitopes [151]. This effect influences both infection outcomes and vaccine immunogenicity. In dengue vaccination, the history of primary infection determines the hierarchy of memory B-cell recall and the relative magnitude of type-specific versus cross-reactive antibodies. Clinical trials of the tetravalent chimeric YF dengue vaccine (CYD TDV, Dengvaxia) revealed that baseline seronegative recipients were at an elevated risk of severe disease following natural infection, whereas seropositive recipients demonstrated durable protective immunity [152].
Longitudinal sero-epidemiological studies during sequential epidemics in Central America and Southeast Asia have shown that Zika infection can modulate dengue outcomes, indicating imprinting across viral species [153]. This reciprocal relationship suggests that both natural and vaccine-induced priming can reprogram germinal center selection and shape antibody repertoire evolution during later exposures [154]. These findings the emphasize that pre-vaccination serostatus and local exposure patterns are key determinants of vaccine efficacy and safety.
5.3. Balancing Broad Protection and Safety in Vaccine-Induced Antibody Responses
Current vaccine strategies aim to favor epitopes associated with potent neutralization, while minimizing responses to regions prone to ADE. Structural vaccinology approaches that stabilize the E dimer conformation or mask the fusion loop help focus antibody responses on protective surfaces [155]. Parallel immunological studies have shown that adjuvants that promote Tfh cell activity and germinal center persistence, such as TLR- or saponin-based formulations, enhance affinity maturation and reduce the window of sub-neutralizing titers that permit ADE [156].
Incorporating baseline serological screening into dengue vaccination programs is essential to avoid enhancing the risk among seronegative individuals, and similar stratified approaches are being considered for future multivalent vaccines [157]. The next generation of flavivirus vaccines will likely integrate antigen design, adjuvant selection, and population serology to achieve broad protection with minimal risks. Among the various mitigation strategies discussed in this section, only baseline serostatus screening is currently implemented in real-world dengue vaccination programs, whereas antigen engineering, imprint-aware boosting schedules, and structure-guided epitope focusing remain investigational and context dependent. A summary of the representative cross-reactive and immune imprinting effects that influence flavivirus vaccine responses is presented in Table 4.

Table 4.
Cross-reactivity and immune imprinting effects of the flavivirus vaccination.
6. Strategies for Improving Antibody Maturation and Long-Term Maintenance
Achieving broad and durable antibody responses to flavivirus vaccines requires a coordinated strategy that optimizes the kinetics of B-cell activation, affinity maturation, and the maintenance of plasma cell and memory B-cell compartments. Recent human and animal studies have identified key determinants of antibody persistence, including booster timing, host factors such as age and immune history, and adjuvant or platform characteristics that sustain germinal center reactions [20,166,167,168]. Integrating these factors into vaccine design is essential to generate long-lasting immunity. The strategies detailed in this section expand on the third design principle, emphasizing approaches that sustain germinal center reactions and support long-lived plasma cell development to maintain durable antibody protection against pathogens.
6.1. Optimizing Booster Intervals and Heterologous Prime-Boost Regimens
The booster vaccination is a major driver of affinity, maturation, and protection. Inactivated JE vaccines show neutralizing antibody waning within 2–3 years, and periodic boosters restore protection by re-engaging memory B-cells and initiating new germinal center responses [107]. YF-17D demonstrates that a single dose can induce immunity that extends the presence of antigens associated with live attenuated replication [43,169].
Heterologous prime-boost regimes using distinct platforms can further enhance the antibody breadth and durability. Combining DNA or mRNA priming with live-attenuated or viral vector boosting increases germinal center output and promotes the selection of high-affinity B-cell clones [170,171]. In dengue and Zika models, sequential exposure to antigenically related but non-identical E proteins increased cross-neutralizing titers without broadening the enhancing antibody subsets, indicating that controlled heterologous priming can shape safe and effective immune imprinting [149,172]. These observations support the rational scheduling of booster intervals and cross-platform combinations to sustain antibody quality, while minimizing the risk of enhancement.
6.2. Host and Age-Related Factors Influencing Antibody Persistence
Host physiology and immune history strongly influence the humoral outcomes. Aging is associated with reduced germinal center size and diminished Tfh cell activity, leading to lower antibody affinity and shorter antibody half-life following vaccination [173,174]. Similar trends have been observed in infants, whose immature follicular responses and limited somatic hypermutation restrict antibody durability [175].
Pre-existing flaviviral immunity also affects viral persistence. Individuals with prior dengue exposure exhibit faster recall and higher avidity after JE or Zika vaccination than flavivirus-naïve subjects, which is consistent with antigenic imprinting that accelerates memory B-cell reactivation [162,176]. Nutritional and metabolic conditions further modulate antibody maintenance, as chronic inflammation and altered cytokine balance can reduce the availability of survival niches for long-lived plasma cells in the bone marrow [177]. Therefore, tailoring vaccine formulations and booster schedules to specific age groups and baseline serostatuses is essential to ensure durable population-level protection.
6.3. Approaches to Sustain Germinal Center Activity and Memory B-Cell Formation
Sustained germinal center activity supports the continuous selection of high-affinity clones and development of long-lived plasma cells. Vaccine formulations that prolong antigen availability or activate innate pathways that support Tfh cell functions can enhance this process. Adjuvants, such as CpG ODN and MPLA, induce IL-21 and interferon gamma (IFN-γ) production, maintain follicular dendritic cell networks, and support persistent germinal centers [178,179]. Nanoparticle and mRNA platforms extend antigen expression, resulting in durable plasma cell output and strong memory B-cell recall [180,181].
In murine flavivirus models, follicular dendritic cell activation and BAFF/APRIL signaling are critical for the survival of long-lived plasma cells, and approaches that preserve these cytokines enhance antibody half-life [182,183]. Experimental strategies, including cyclic dinucleotide STING agonists or saponin-based adjuvants, sustain germinal center activity for extended periods, yielding antibodies with greater affinity and improved Fc functional profiles [184]. Together, these findings indicate that combining platforms that provide prolonged antigen presence with adjuvants that maintain Tfh cell support and stromal signaling is central to sustaining long-term humoral immunity during flavivirus vaccination.
7. Future Perspectives and Vaccine Design Considerations
Recent advances in immunology, adjuvant chemistry, and structural vaccinology have transformed flavivirus vaccine development into a data-driven, precision-focused field. The next generation of vaccines aims to combine rational antigen design with optimized adjuvants and delivery systems to elicit antibodies with high affinity, functional diversity, and long-term persistence [129,185,186,187]. Insights gained from the dengue, JE, YF, and Zika vaccines provide a foundation for future strategies to achieve both breadth and safety in diverse populations. These developments collectively highlight the importance of integrating antigen stability, innate activation, and population-specific considerations into future vaccine designs. These forward-looking strategies integrate multiple design principles introduced in the Introduction, particularly those concerning epitope-focused antigen engineering, optimized antigen persistence, and incorporation of serostatus-driven immune imprinting.
7.1. Integration of Adjuvant and Antigen Design for Improved Humoral Immunity
One promising direction in flavivirus vaccinology is the coordinated optimization of the antigen structure and innate immune stimulation. Modern adjuvants not only boost antibody magnitude but also modulate germinal center dynamics and B-cell selection, shaping the qualitative features of humoral immunity [53,188].
Structure-guided antigen engineering has identified conserved quaternary epitopes on the envelope dimer interface that can be stabilized by targeted mutations or scaffold presentation to maintain neutralization-sensitive conformations [98,189].
Combining stabilized immunogens with potent adjuvants, such as saponins or TLR-based formulations, can prolong germinal center activity and favor the development of high-affinity cross-neutralizing antibodies with reduced likelihood of enhancement [164,190]. These integrated approaches support the coordinated activation of B-cells, Tfh cells, and long-lived plasma cell formation, thereby improving the durability and safety of the humoral responses.
7.2. Lessons from Flavivirus Vaccine Platforms for Universal Vaccine Development
Decades of experience with live attenuated, inactivated, and recombinant flavivirus vaccines have generated insights that have extended beyond a single viral genus. The durable immunity elicited by YF-17D indicates that sustained antigen expression combined with robust innate activation can provide near-lifelong protection [43,70]. In contrast, the variable outcomes observed with the tetravalent dengue vaccine (CYD-TDV) emphasize the complexity of immune imprinting and the importance of assessing baseline serostatus when evaluating vaccine performance [172,191].
These lessons inform the design principles of next-generation platforms that employ self-amplifying RNA, viral vectors, or nanoparticle-based delivery to achieve controlled antigen persistence and balanced innate and adaptive activation [141]. Cross-platform and heterologous prime-boost regimens can be tailored for distinct epidemiological settings, ensuring that responses remain durable and broad across flavivirus species [154]. The integration of these approaches provides a foundation for developing pan-flavivirus vaccines and may inform universal vaccine approaches for related viral families, including alphaviruses and bunyaviruses [192].
7.3. Outlook for Next-Generation Flavivirus Vaccines
Future vaccine development will increasingly rely on predictive analytics and high-dimensional immune profiling to define correlates of durable protection. Systems vaccinology approaches integrating transcriptomics, B-cell receptor repertoire sequencing, and machine learning can identify molecular signatures associated with long-lived plasma cell formation and high-quality antibody responses [193,194]. These insights will enable the rational selection of adjuvants and structural immunogens that maximize protective breadth while maintaining safety across diverse host immune backgrounds.
Population-tailored vaccination strategies have become increasingly important. Adjusting booster intervals, selecting appropriate adjuvants, and adapting platforms according to age, sex, and baseline serostatus can enhance both the durability and equity of vaccine impact [127].
Advances in artificial intelligence for epitope prediction and computational immunogen design have accelerated the development of optimized flavivirus antigens capable of eliciting broad neutralization with minimal risk of ADE [195]. Collectively, these developments illustrate the transition from empirical vaccine discovery to precision vaccinology guided by structure, systems biology, and population-level data. A concise summary of emerging strategies and anticipated directions for flavivirus vaccine development is presented in Table 5, which synthesizes how these approaches align with the design principles introduced at the end of the Introduction.

Table 5.
Future strategies for improving flavivirus vaccine-induced antibody response.
Table 5.
Future strategies for improving flavivirus vaccine-induced antibody response.
| Strategic Area | Emerging Concept or Approach | Expected Immunologic Impact | Development Stage or Outlook | Refs. |
|---|---|---|---|---|
| Structure-guided epitope optimization | Stabilization of E-dimer or mosaic epitopes that preserve cross-neutralizing structures | Focuses antibody response on protective epitopes and lower enhancement risk | Preclinical studies and Phase 1 early clinical testing | [98,158,196] |
| Adjuvant and antigen co-engineering | Incorporation of PRR agonists or saponin-based adjuvants into mRNA or nanoparticle platforms | Sustains germinal center activity and enhances affinity maturation | Advanced preclinical studies and Phase 1–2 clinical evaluation | [164,190,197] |
| Self-amplifying RNA and replicon platforms | Extended antigen expression with low-dose formulations | Increases magnitude and duration of antibody response | Phase 1 clinical trials | [198,199,200] |
| Pan-flavivirus vaccine design using conserved scaffolds | Use of shared E dimer and fusion loop epitope scaffolds to support broad cross protection | Enables cross serotype and cross species immunity across dengue, Zika, and JEV | Computational design with mechanistic inference | [26,101,158] |
| Systems vaccinology and immune profiling | Integration of omics signatures and antibody repertoire sequencing to define correlates of protection | Predicts antibody quality and durability for vaccine optimization | Research-stage integration with exploratory clinical programs | [201,202,203] |
| Population-tailored vaccination strategies | Adjustment of prime boost intervals and formulations based on serostatus and age | Maximizes protective efficacy while reducing enhancement risk | Program-level evaluation in endemic populations (observational evidence) | [152,157,204] |
| Combination vector or heterologous prime-boost approaches | Sequential mRNA, viral vector, or subunit vaccination | Promotes balanced B and T cell immunity with improved durability | Preclinical and translational-stage development | [205,206,207] |
| Artificial intelligence assisted immunogen design | Machine learning based prediction of stable neutralizing epitopes and escape variant targets | Accelerates design of rationally optimized immunogens | Computational modeling with mechanistic inference | [208,209,210] |
The evidence tiers in this table are standardized using preclinical murine or NHP studies, phase 1–2 early clinical trials, phase 3 clinical data, and computational or mechanistic inference, consistent with the terminology used throughout the manuscript. PRR, pattern recognition receptor.
8. Conclusions
Flavivirus vaccine development has transitioned from empirical formulations to a mechanistic understanding of how antigen structure, innate immune activation, and host history shape the quality and durability of antibody responses. Decades of work with live attenuated and inactivated vaccines have demonstrated that long-term sero-protection is achievable when antigen integrity and immune stimulation are optimally balanced. The YF-17D and JE SA 14-14-2 vaccines remain key examples of how replication-driven innate signaling and strong germinal center induction generate durable, high-avidity antibodies [70,211]. In contrast, dengue vaccination highlights the dual behavior of cross-reactive antibodies, which may mediate either broad protection or antibody-dependent enhancement depending on antibody concentration, epitope specificity, and affinity maturation state [212,213].
Modern immunology and structural biology have expanded the correlates of protection beyond neutralizing titers. Antibody affinity, subclass distribution, and Fc effector functions are now recognized as critical determinants of effective immunity. Broadly neutralizing antibodies that target conserved quaternary epitopes on the envelope dimer provide structural templates for rational immunogen design [214]. Antigen engineering approaches that stabilize the pre-fusion E conformation or display key epitopes on virus-like or nanoparticle scaffolds can directly respond to protective surfaces while limiting the exposure to enhancing epitopes. Parallel progress in adjuvant research has demonstrated that engaging pattern-recognition receptor pathways, including TLR and STING signaling, can sustain germinal center activity and promote long-lived plasma cell formation, supporting improved antibody affinity and persistence [215].
However, despite these advances, several important challenges remain. Immune imprinting, baseline serostatus, and variation in vector exposure continue to influence vaccine effectiveness, particularly in dengue- and ZIKV-endemic regions. Validated correlates of durable protection and long-term field data are needed to interpret laboratory-defined immune signatures and inform evidence-based vaccination schedules. Furthermore, thermostable, low-cost, and easily deployable vaccine formulations are essential for ensuring equitable access in regions where flavivirus transmission remains a major public health concern. Addressing these priorities will require a closer integration of immunology, epidemiology, and implementation sciences.
Future vaccine development is expected to increasingly rely on computational modeling, systems vaccinology, and artificial intelligence to predict immunogenicity and guide structural antigen optimization [216]. Combining structure-guided epitope mapping with coordinated adjuvants and platform engineering offers a path toward precision vaccines that can achieve high-quality, long-lasting, and cross-protective antibody responses. Population-tailored strategies that consider age, immune background, and prior flavivirus exposure may further enhance its safety and effectiveness.
Collectively, these developments indicate a shift toward precision vaccinology, which integrates molecular, computational, and population-level insights to achieve broad, durable, and safe humoral immunity. Next-generation flavivirus vaccines are expected to provide sustained protection against dengue, Zika, JE, and other emerging viruses, and contribute to the broader goal of designing universal vaccines for complex RNA viruses. Ensuring global accessibility and equitable deployment ultimately define the success of scientific advances.
Author Contributions
J.-Y.P.: Conceptualization, writing—original draft preparation. H.-M.L.: Conceptualization, writing—original draft preparation, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gould, E.A.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.M. Managing Japanese Encephalitis Virus as a Veterinary Infectious Disease Through Animal Surveillance and One Health Control Strategies. Life 2025, 15, 1260. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, H.M.; Jun, S.H.; Kamitani, W.; Kim, O.; Shin, H.J. Insights into the Pathogenesis and Development of Recombinant Japanese Encephalitis Virus Genotype 3 as a Vaccine. Vaccines 2024, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kamitani, W.; Shin, H.J.; Lee, H.M. Japanese Encephalitis Virus Genotype 5 Infectious Clone and Reporter System for Antiviral Evaluation. J. Med. Virol. 2025, 97, e70608. [Google Scholar] [CrossRef]
- Crill, W.D.; Chang, G.J. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 2004, 78, 13975–13986. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Sohn, Y.M. Japanese encephalitis immunization in South Korea: Past, present, and future. Emerg. Infect. Dis. 2000, 6, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774E. [Google Scholar] [CrossRef]
- Hegde, N.R.; Gore, M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccin. Immunother. 2017, 13, 1320–1337. [Google Scholar] [CrossRef]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef]
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581. [Google Scholar] [CrossRef]
- Dowd, K.A.; Pierson, T.C. Antibody-mediated neutralization of flaviviruses: A reductionist view. Virology 2011, 411, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Montoya, M.; Gresh, L.; Balmaseda, A.; Harris, E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc. Natl. Acad. Sci. USA 2016, 113, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B cells. Nat. Rev. Immunol. 2015, 15, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.A.; Stiasny, K.; Vaney, M.C.; Dellarole, M.; Heinz, F.X. The bright and the dark side of human antibody responses to flaviviruses: Lessons for vaccine design. EMBO Rep. 2018, 19, 206–224. [Google Scholar] [CrossRef]
- Viant, C.; Weymar, G.H.J.; Escolano, A.; Chen, S.; Hartweger, H.; Cipolla, M.; Gazumyan, A.; Nussenzweig, M.C. Antibody Affinity Shapes the Choice between Memory and Germinal Center B Cell Fates. Cell 2020, 183, 1298–1311.e11. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: Knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.C.; Kikuti, C.M.; Navarro Sanchez, M.E.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, K.M.; Crotty, S. Germinal center enhancement by extended antigen availability. Curr. Opin. Immunol. 2017, 47, 64–69. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Shlomchik, M.J.; Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 2012, 247, 52–63. [Google Scholar] [CrossRef]
- Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; Del Rio, C.; et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009, 183, 7919–7930. [Google Scholar] [CrossRef]
- Hou, J.; Wang, S.; Jia, M.; Li, D.; Liu, Y.; Li, Z.; Zhu, H.; Xu, H.; Sun, M.; Lu, L.; et al. A Systems Vaccinology Approach Reveals Temporal Transcriptomic Changes of Immune Responses to the Yellow Fever 17D Vaccine. J. Immunol. 2017, 199, 1476–1489. [Google Scholar] [CrossRef]
- Ghosh, D.; Basu, A. Japanese encephalitis-a pathological and clinical perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef]
- Methot, S.P.; Di Noia, J.M. Molecular Mechanisms of Somatic Hypermutation and Class Switch Recombination. Adv. Immunol. 2017, 133, 37–87. [Google Scholar] [CrossRef]
- Tas, J.M.; Mesin, L.; Pasqual, G.; Targ, S.; Jacobsen, J.T.; Mano, Y.M.; Chen, C.S.; Weill, J.C.; Reynaud, C.A.; Browne, E.P.; et al. Visualizing antibody affinity maturation in germinal centers. Science 2016, 351, 1048–1054. [Google Scholar] [CrossRef]
- De Alwis, R.; Smith, S.A.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E., Jr.; et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. USA 2012, 109, 7439–7444. [Google Scholar] [CrossRef] [PubMed]
- Halliley, J.L.; Tipton, C.M.; Liesveld, J.; Rosenberg, A.F.; Darce, J.; Gregoretti, I.V.; Popova, L.; Kaminiski, D.; Fucile, C.F.; Albizua, I.; et al. Long-Lived Plasma Cells Are Contained within the CD19(-)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 2015, 43, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef]
- Slamanig, S.A.; Nolte, M.A. The Bone Marrow as Sanctuary for Plasma Cells and Memory T-Cells: Implications for Adaptive Immunity and Vaccinology. Cells 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Wieten, R.W.; Jonker, E.F.; van Leeuwen, E.M.; Remmerswaal, E.B.; Ten Berge, I.J.; de Visser, A.W.; van Genderen, P.J.; Goorhuis, A.; Visser, L.G.; Grobusch, M.P.; et al. A Single 17D Yellow Fever Vaccination Provides Lifelong Immunity; Characterization of Yellow-Fever-Specific Neutralizing Antibody and T-Cell Responses After Vaccination. PLoS ONE 2016, 11, e0149871. [Google Scholar] [CrossRef]
- Hills, S.L.; Walter, E.B.; Atmar, R.L.; Fischer, M.; Group, A.J.E.V.W. Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2019, 68, 1–33. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert. Rev. Mol. Med. 2008, 10, e12. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Vogt, M.R.; Dowd, K.A.; Engle, M.; Tesh, R.B.; Johnson, S.; Pierson, T.C.; Diamond, M.S. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J. Virol. 2011, 85, 11567–11580. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003, 60, 421–467. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016, 14, 45–54. [Google Scholar] [CrossRef]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Grigoryan, L.; Feng, Y.; Bellusci, L.; Lai, L.; Wali, B.; Ellis, M.; Yuan, M.; Arunachalam, P.S.; Hu, M.; Kowli, S.; et al. AS03 adjuvant enhances the magnitude, persistence, and clonal breadth of memory B cell responses to a plant-based COVID-19 vaccine in humans. Sci. Immunol. 2024, 9, eadi8039. [Google Scholar] [CrossRef]
- Reinke, S.; Thakur, A.; Gartlan, C.; Bezbradica, J.S.; Milicic, A. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.C.; Lin, C.Y.; Chen, C.; Cheng, H.Y.; Hsieh, E.F.; Liu, L.T.; Chiu, C.H.; Huang, L.M. Long-Term Immunogenicity Study of an Aluminum Phosphate-Adjuvanted Inactivated Enterovirus A71 Vaccine in Children: An Extension to a Phase 2 Study. Vaccines 2024, 12, 985. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lee, P.I. Safety of Japanese encephalitis vaccines. Hum. Vaccin. Immunother. 2021, 17, 4259–4264. [Google Scholar] [CrossRef]
- Calabro, S.; Tritto, E.; Pezzotti, A.; Taccone, M.; Muzzi, A.; Bertholet, S.; De Gregorio, E.; O’Hagan, D.T.; Baudner, B.; Seubert, A. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 2013, 31, 3363–3369. [Google Scholar] [CrossRef]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Rossi Paccani, S.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- Ko, E.J.; Kang, S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef]
- Kovarik, J.; Bozzotti, P.; Tougne, C.; Davis, H.L.; Lambert, P.H.; Krieg, A.M.; Siegrist, C.A. Adjuvant effects of CpG oligodeoxynucleotides on responses against T-independent type 2 antigens. Immunology 2001, 102, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014, 32, 6377–6389. [Google Scholar] [CrossRef]
- Ragupathi, G.; Gardner, J.R.; Livingston, P.O.; Gin, D.Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert. Rev. Vaccines 2011, 10, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, N.; Zhang, X.; Wang, M.; Liu, Y.; Shi, Y. Potentials of saponins-based adjuvants for nasal vaccines. Front. Immunol. 2023, 14, 1153042. [Google Scholar] [CrossRef]
- Yoon, M.; Choi, Y.; Wi, T.; Choi, Y.S.; Choi, J. The role of cGAMP via the STING pathway in modulating germinal center responses and CD4 T cell differentiation. Front. Immunol. 2024, 15, 1340001. [Google Scholar] [CrossRef] [PubMed]
- Laczko, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castano, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H., 3rd; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses Against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, R.; Eriksson, H. Aluminium adjuvants in vaccines—A way to modulate the immune response. Semin. Cell Dev. Biol. 2021, 115, 3–9. [Google Scholar] [CrossRef]
- Sanchez-Felipe, L.; Alpizar, Y.A.; Ma, J.; Coelmont, L.; Dallmeier, K. YF17D-based vaccines—Standing on the shoulders of a giant. Eur. J. Immunol. 2024, 54, e2250133. [Google Scholar] [CrossRef]
- Wang, P. Natural and Synthetic Saponins as Vaccine Adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Shan, C.; Xie, X.; Shi, P.Y. Zika Virus Vaccine: Progress and Challenges. Cell Host Microbe 2018, 24, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; P, S.A.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Willingham, S.B.; Ting, J.P.; Re, F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008, 181, 17–21. [Google Scholar] [CrossRef]
- Franchi, L.; Nunez, G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008, 38, 2085–2089. [Google Scholar] [CrossRef]
- Unger, B.; Barrett, P.N. Tick-borne encephalitis vaccines. Intern. Med. J. 2013, 43, 838–839. [Google Scholar] [CrossRef]
- Firbas, C.; Jilma, B. Product review on the JE vaccine IXIARO. Hum. Vaccin. Immunother. 2015, 11, 411–420. [Google Scholar] [CrossRef]
- Dupuis, M.; Denis-Mize, K.; LaBarbara, A.; Peters, W.; Charo, I.F.; McDonald, D.M.; Ott, G. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur. J. Immunol. 2001, 31, 2910–2918. [Google Scholar] [CrossRef]
- Li, A.P.Y.; Cohen, C.A.; Leung, N.H.L.; Fang, V.J.; Gangappa, S.; Sambhara, S.; Levine, M.Z.; Iuliano, A.D.; Perera, R.; Ip, D.K.M.; et al. Immunogenicity of standard, high-dose, MF59-adjuvanted, and recombinant-HA seasonal influenza vaccination in older adults. NPJ Vaccines 2021, 6, 25. [Google Scholar] [CrossRef]
- Lin, L.; Lyke, K.E.; Koren, M.; Jarman, R.G.; Eckels, K.H.; Lepine, E.; McArthur, M.A.; Currier, J.R.; Friberg, H.; Moris, P.; et al. Safety and Immunogenicity of an AS03(B)-Adjuvanted Inactivated Tetravalent Dengue Virus Vaccine Administered on Varying Schedules to Healthy U.S. Adults: A Phase 1/2 Randomized Study. Am. J. Trop. Med. Hyg. 2020, 103, 132–141. [Google Scholar] [CrossRef]
- Diaz, C.; Koren, M.; Lin, L.; Martinez, L.J.; Eckels, K.H.; Campos, M.; Jarman, R.G.; De La Barrera, R.; Lepine, E.; Febo, I.; et al. Safety and Immunogenicity of Different Formulations of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico: Final Results after 3 Years of Follow-Up from a Randomized, Placebo-Controlled Phase I Study. Am. J. Trop. Med. Hyg. 2020, 102, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef]
- Katsenelson, N.; Kanswal, S.; Puig, M.; Mostowski, H.; Verthelyi, D.; Akkoyunlu, M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur. J. Immunol. 2007, 37, 1785–1795. [Google Scholar] [CrossRef]
- Demento, S.L.; Bonafe, N.; Cui, W.; Kaech, S.M.; Caplan, M.J.; Fikrig, E.; Ledizet, M.; Fahmy, T.M. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J. Immunol. 2010, 185, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Thotathil, N.; Zhao, Z.; Mitragotri, S. Vaccine adjuvants for infectious disease in the clinic. Bioeng. Transl. Med. 2024, 9, e10663. [Google Scholar] [CrossRef]
- Toussi, D.N.; Massari, P. Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands. Vaccines 2014, 2, 323–353. [Google Scholar] [CrossRef]
- Robert Putnak, J.; Coller, B.A.; Voss, G.; Vaughn, D.W.; Clements, D.; Peters, I.; Bignami, G.; Houng, H.S.; Chen, R.C.; Barvir, D.A.; et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine 2005, 23, 4442–4452. [Google Scholar] [CrossRef] [PubMed]
- Visciano, M.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J. Transl. Med. 2012, 10, 4. [Google Scholar] [CrossRef]
- Al-Osaimi, H.M.; Kanan, M.; Marghlani, L.; Al-Rowaili, B.; Albalawi, R.; Saad, A.; Alasmari, S.; Althobaiti, K.; Alhulaili, Z.; Alanzi, A.; et al. A systematic review on malaria and dengue vaccines for the effective management of these mosquito borne diseases: Improving public health. Hum. Vaccin. Immunother. 2024, 20, 2337985. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Investig. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.A.; Beatty, P.R.; Reiner, G.L.; Sivick, K.E.; Hix Glickman, L.; Dubensky, T.W., Jr.; Harris, E. Cyclic Dinucleotide-Adjuvanted Dengue Virus Nonstructural Protein 1 Induces Protective Antibody and T Cell Responses. J. Immunol. 2019, 202, 1153–1162. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, W.; Nie, J.; Zhang, L. Progress Update on STING Agonists as Vaccine Adjuvants. Vaccines 2025, 13, 371. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Wollner, C.J.; Richner, J.M. mRNA Vaccines against Flaviviruses. Vaccines 2021, 9, 148. [Google Scholar] [CrossRef]
- Satchidanandam, V. Japanese Encephalitis Vaccines. Curr. Treat. Options Infect. Dis. 2020, 12, 375–386. [Google Scholar] [CrossRef]
- Kudlacek, S.T.; Metz, S.; Thiono, D.; Payne, A.M.; Phan, T.T.N.; Tian, S.; Forsberg, L.J.; Maguire, J.; Seim, I.; Zhang, S.; et al. Designed, highly expressing, thermostable dengue virus 2 envelope protein dimers elicit quaternary epitope antibodies. Sci. Adv. 2021, 7, eabg4084. [Google Scholar] [CrossRef]
- Sharma, A.; Zhang, X.; Dejnirattisai, W.; Dai, X.; Gong, D.; Wongwiwat, W.; Duquerroy, S.; Rouvinski, A.; Vaney, M.C.; Guardado-Calvo, P.; et al. The epitope arrangement on flavivirus particles contributes to Mab C10’s extraordinary neutralization breadth across Zika and dengue viruses. Cell 2021, 184, 6052–6066.e18. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Widman, D.G.; Yount, B.L.; Wahala, W.M.; Durbin, A.; Whitehead, S.; Sariol, C.A.; Crowe, J.E., Jr.; de Silva, A.M.; Baric, R.S. A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 2015, 6, e01461-15. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Hvasta, M.G.; Kudlacek, S.T.; Thiono, D.J.; Tripathy, A.; Nicely, N.I.; de Silva, A.M.; Kuhlman, B. A conserved set of mutations for stabilizing soluble envelope protein dimers from dengue and Zika viruses to advance the development of subunit vaccines. J. Biol. Chem. 2022, 298, 102079. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Sanchez-San Martin, C.; Zheng, A.; Kielian, M. In vitro reconstitution reveals key intermediate states of trimer formation by the dengue virus membrane fusion protein. J. Virol. 2010, 84, 5730–5740. [Google Scholar] [CrossRef]
- Shen, W.F.; Galula, J.U.; Liu, J.H.; Liao, M.Y.; Huang, C.H.; Wang, Y.C.; Wu, H.C.; Liang, J.J.; Lin, Y.L.; Whitney, M.T.; et al. Epitope resurfacing on dengue virus-like particle vaccine preparation to induce broad neutralizing antibody. eLife 2018, 7, e38970. [Google Scholar] [CrossRef]
- Metz, S.W.; Gallichotte, E.N.; Brackbill, A.; Premkumar, L.; Miley, M.J.; Baric, R.; de Silva, A.M. In Vitro Assembly and Stabilization of Dengue and Zika Virus Envelope Protein Homo-Dimers. Sci. Rep. 2017, 7, 4524. [Google Scholar] [CrossRef]
- Whitehead, S.S.; Durbin, A.P.; Pierce, K.K.; Elwood, D.; McElvany, B.D.; Fraser, E.A.; Carmolli, M.P.; Tibery, C.M.; Hynes, N.A.; Jo, M.; et al. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl. Trop. Dis. 2017, 11, e0005584. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.A.; Nivarthi, U.K.; Graham, N.R.; Eisenhauer, P.; Delacruz, M.J.; Pierce, K.K.; Whitehead, S.S.; Boyson, J.E.; Botten, J.W.; Kirkpatrick, B.D.; et al. Stimulation of B Cell Immunity in Flavivirus-Naive Individuals by the Tetravalent Live Attenuated Dengue Vaccine TV003. Cell Rep. Med. 2020, 1, 100155. [Google Scholar] [CrossRef] [PubMed]
- Kadlecek, V.; Borja-Tabora, C.F.; Eder-Lingelbach, S.; Gatchalian, S.; Kiermayr, S.; Sablan, B., Jr.; Kundi, M.; Taucher, C.; Dubischar, K.L. Antibody Persistence up to 3 Years After Primary Immunization with Inactivated Japanese Encephalitis Vaccine IXIARO in Philippine Children and Effect of a Booster Dose. Pediatr. Infect. Dis. J. 2018, 37, e233–e240. [Google Scholar] [CrossRef]
- Paulke-Korinek, M.; Kollaritsch, H.; Kundi, M.; Zwazl, I.; Seidl-Friedrich, C.; Jelinek, T. Persistence of antibodies six years after booster vaccination with inactivated vaccine against Japanese encephalitis. Vaccine 2015, 33, 3600–3604. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Recombinant Protein-Based Dengue Vaccines. Front. Immunol. 2018, 9, 1919. [Google Scholar] [CrossRef]
- Georgiev, G.I.; Malonis, R.J.; Wirchnianski, A.S.; Wessel, A.W.; Jung, H.S.; Cahill, S.M.; Nyakatura, E.K.; Vergnolle, O.; Dowd, K.A.; Cowburn, D.; et al. Resurfaced ZIKV EDIII nanoparticle immunogens elicit neutralizing and protective responses in vivo. Cell Chem. Biol. 2022, 29, 811–823.e7. [Google Scholar] [CrossRef]
- Van Hoeven, N.; Wiley, S.; Gage, E.; Fiore-Gartland, A.; Granger, B.; Gray, S.; Fox, C.; Clements, D.E.; Parks, D.E.; Winram, S.; et al. A combination of TLR-4 agonist and saponin adjuvants increases antibody diversity and protective efficacy of a recombinant West Nile Virus antigen. NPJ Vaccines 2018, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified mRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 702–712. [Google Scholar] [CrossRef]
- Saunders, K.O.; Pardi, N.; Parks, R.; Santra, S.; Mu, Z.; Sutherland, L.; Scearce, R.; Barr, M.; Eaton, A.; Hernandez, G.; et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines 2021, 6, 50. [Google Scholar] [CrossRef]
- Raviprakash, K.; Wang, D.; Ewing, D.; Holman, D.H.; Block, K.; Woraratanadharm, J.; Chen, L.; Hayes, C.; Dong, J.Y.; Porter, K. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J. Virol. 2008, 82, 6927–6934. [Google Scholar] [CrossRef]
- Bullard, B.L.; Corder, B.N.; Gordon, D.N.; Pierson, T.C.; Weaver, E.A. Characterization of a Species E Adenovirus Vector as a Zika virus vaccine. Sci. Rep. 2020, 10, 3613. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Wu, K.; Kim, G.N.; Saeedian, N.; Seon, S.H.; Park, G.; Jung, D.I.; Jeong, H.W.; Kim, N.H.; Seo, S.H.; et al. Induction of protective immune responses against a lethal Zika virus challenge post-vaccination with a dual serotype of recombinant vesicular stomatitis virus carrying the genetically modified Zika virus E protein gene. J. Gen. Virol. 2021, 102, 001588. [Google Scholar] [CrossRef] [PubMed]
- Taslem Mourosi, J.; Awe, A.; Jain, S.; Batra, H. Nucleic Acid Vaccine Platform for DENGUE and ZIKA Flaviviruses. Vaccines 2022, 10, 834. [Google Scholar] [CrossRef]
- Comes, J.D.G.; Pijlman, G.P.; Hick, T.A.H. Rise of the RNA machines—Self-amplification in mRNA vaccine design. Trends Biotechnol. 2023, 41, 1417–1429. [Google Scholar] [CrossRef]
- Casmil, I.C.; Jin, J.; Won, E.J.; Huang, C.; Liao, S.; Cha-Molstad, H.; Blakney, A.K. The advent of clinical self-amplifying RNA vaccines. Mol. Ther. 2025, 33, 2565–2582. [Google Scholar] [CrossRef]
- Metz, S.W.; Thomas, A.; Brackbill, A.; Xianwen, Y.; Stone, M.; Horvath, K.; Miley, M.J.; Luft, C.; DeSimone, J.M.; Tian, S.; et al. Nanoparticle delivery of a tetravalent E protein subunit vaccine induces balanced, type-specific neutralizing antibodies to each dengue virus serotype. PLoS Negl. Trop. Dis. 2018, 12, e0006793. [Google Scholar] [CrossRef]
- Silva, E.F.; Orsi, M.; Andrade, A.L.; Domingues, R.Z.; Silva, B.M.; de Araujo, H.R.; Pimenta, P.F.; Diamond, M.S.; Rocha, E.S.; Kroon, E.G.; et al. A tetravalent dengue nanoparticle stimulates antibody production in mice. J. Nanobiotechnol. 2012, 10, 13. [Google Scholar] [CrossRef]
- Sevvana, M.; Kuhn, R.J. Mapping the diverse structural landscape of the flavivirus antibody repertoire. Curr. Opin. Virol. 2020, 45, 51–64. [Google Scholar] [CrossRef]
- Gunawardana, S.A.; Shaw, R.H. Cross-reactive dengue virus-derived monoclonal antibodies to Zika virus envelope protein: Panacea or Pandora’s box? BMC Infect. Dis. 2018, 18, 641. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.A.; van der Schaar, H.M.; da Silva Voorham, J.M.; van der Ende-Metselaar, H.; Lei, H.Y.; Wilschut, J.; Smit, J.M. Immature dengue virus: A veiled pathogen? PLoS Pathog. 2010, 6, e1000718. [Google Scholar] [CrossRef]
- Wirawan, M.; Fibriansah, G.; Marzinek, J.K.; Lim, X.X.; Ng, T.S.; Sim, A.Y.L.; Zhang, Q.; Kostyuchenko, V.A.; Shi, J.; Smith, S.A.; et al. Mechanism of Enhanced Immature Dengue Virus Attachment to Endosomal Membrane Induced by prM Antibody. Structure 2019, 27, 253–267.e8. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, M.; Cao, J.; Shen, J.; Zhou, X.; Wang, D.; Cao, J. Flavivirus: From Structure to Therapeutics Development. Life 2021, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Larena, M.; Prow, N.A.; Hall, R.A.; Petrovsky, N.; Lobigs, M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody. J. Virol. 2013, 87, 4395–4402. [Google Scholar] [CrossRef]
- Araujo, S.C.; Pereira, L.R.; Alves, R.P.S.; Andreata-Santos, R.; Kanno, A.I.; Ferreira, L.C.S.; Goncalves, V.M. Anti-Flavivirus Vaccines: Review of the Present Situation and Perspectives of Subunit Vaccines Produced in Escherichia coli. Vaccines 2020, 8, 492. [Google Scholar] [CrossRef]
- Martin, J.; Hermida, L. Dengue vaccine: An update on recombinant subunit strategies. Acta Virol. 2016, 60, 3–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fahimi, H.; Mohammadipour, M.; Haddad Kashani, H.; Parvini, F.; Sadeghizadeh, M. Dengue viruses and promising envelope protein domain III-based vaccines. Appl. Microbiol. Biotechnol. 2018, 102, 2977–2996. [Google Scholar] [CrossRef]
- Wong, S.H.; Jassey, A.; Wang, J.Y.; Wang, W.C.; Liu, C.H.; Lin, L.T. Virus-Like Particle Systems for Vaccine Development against Viruses in the Flaviviridae Family. Vaccines 2019, 7, 123. [Google Scholar] [CrossRef]
- Unali, G.; Douam, F. Orthoflavivirus Vaccine Platforms: Current Strategies and Challenges. Vaccines 2025, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ebel, H.; Benecke, T.; Vollmer, B. Stabilisation of Viral Membrane Fusion Proteins in Pre-fusion Conformation by Structure-Based Design for Structure Determination and Vaccine Development. Viruses 2022, 14, 1816. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, F.; Lin, S.; Yang, F.; Cheng, Y.; Cao, Y.; Chen, Z.; Lu, G. Crystal structure of Usutu virus envelope protein in the pre-fusion state. Virol. J. 2018, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.H.; Klein, D.E.; Schmidt, A.G.; Pena, J.M.; Harrison, S.C. Sequential conformational rearrangements in flavivirus membrane fusion. eLife 2014, 3, e04389. [Google Scholar] [CrossRef]
- He, J.; Ding, X.; Zhao, J.; Zeng, J.; Zhou, Y.; Xiao, W.; Hua, D.; Liu, M.; Guo, H.; Zhang, Y.; et al. A novel pan-epitope based nanovaccine self-assembled with CpG enhances immune responses against flavivirus. J. Nanobiotechnol. 2024, 22, 738. [Google Scholar] [CrossRef]
- Ripoll, D.R.; Khavrutskii, I.; Wallqvist, A.; Chaudhury, S. Modeling the Role of Epitope Arrangement on Antibody Binding Stoichiometry in Flaviviruses. Biophys. J. 2016, 111, 1641–1654. [Google Scholar] [CrossRef]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef]
- Silva-Pilipich, N.; Beloki, U.; Salaberry, L.; Smerdou, C. Self-Amplifying RNA: A Second Revolution of mRNA Vaccines against COVID-19. Vaccines 2024, 12, 318. [Google Scholar] [CrossRef]
- Battisti, P.; Ykema, M.R.; Kasal, D.N.; Jennewein, M.F.; Beaver, S.; Weight, A.E.; Hanson, D.; Singh, J.; Bakken, J.; Cross, N.; et al. A bivalent self-amplifying RNA vaccine against yellow fever and Zika viruses. Front. Immunol. 2025, 16, 1569454. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St John, A.L. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef]
- Maciejewski, S.; Pierson, T.C. Cross-Reactive Flavivirus Antibody: Friend and Foe? Cell Host Microbe 2018, 24, 622–624. [Google Scholar] [CrossRef]
- Swanstrom, J.A.; Plante, J.A.; Plante, K.S.; Young, E.F.; McGowan, E.; Gallichotte, E.N.; Widman, D.G.; Heise, M.T.; de Silva, A.M.; Baric, R.S. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. mBio 2016, 7, e01123-16. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Stuart, J.B.; Levoir, L.M.; Belmont, L.; Goo, L. Defining the impact of flavivirus envelope protein glycosylation site mutations on sensitivity to broadly neutralizing antibodies. mBio 2024, 15, e03048-23. [Google Scholar] [CrossRef] [PubMed]
- Fibriansah, G.; Ibarra, K.D.; Ng, T.S.; Smith, S.A.; Tan, J.L.; Lim, X.N.; Ooi, J.S.; Kostyuchenko, V.A.; Wang, J.; de Silva, A.M.; et al. DENGUE VIRUS. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 2015, 349, 88–91. [Google Scholar] [CrossRef]
- Fernandez, E.; Dejnirattisai, W.; Cao, B.; Scheaffer, S.M.; Supasa, P.; Wongwiwat, W.; Esakky, P.; Drury, A.; Mongkolsapaya, J.; Moley, K.H.; et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat. Immunol. 2017, 18, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Sun, J.; Du, S.; Zheng, Z.; Cheng, G.; Jin, X. Defeat Dengue and Zika Viruses with a One-Two Punch of Vaccine and Vector Blockade. Front. Microbiol. 2020, 11, 362. [Google Scholar] [CrossRef]
- Lin, H.H.; Yang, S.P.; Tsai, M.J.; Lin, G.C.; Wu, H.C.; Wu, S.C. Dengue and Zika Virus Domain III-Flagellin Fusion and Glycan-Masking E Antigen for Prime-Boost Immunization. Theranostics 2019, 9, 4811–4826. [Google Scholar] [CrossRef]
- Midgley, C.M.; Bajwa-Joseph, M.; Vasanawathana, S.; Limpitikul, W.; Wills, B.; Flanagan, A.; Waiyaiya, E.; Tran, H.B.; Cowper, A.E.; Chotiyarnwong, P.; et al. An in-depth analysis of original antigenic sin in dengue virus infection. J. Virol. 2011, 85, 410–421. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.E.; Kalimuddin, S. Insights into dengue immunity from vaccine trials. Sci. Transl. Med. 2023, 15, eadh3067. [Google Scholar] [CrossRef] [PubMed]
- Dussupt, V.; Modjarrad, K.; Krebs, S.J. Landscape of Monoclonal Antibodies Targeting Zika and Dengue: Therapeutic Solutions and Critical Insights for Vaccine Development. Front. Immunol. 2020, 11, 621043. [Google Scholar] [CrossRef]
- St John, A.L.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar] [CrossRef]
- World Health Organization. Dengue vaccine: WHO position paper, September 2018—Recommendations. Vaccine 2019, 37, 4848–4849. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.C.; Medits, I.; Sharma, A.; Simon-Loriere, E.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Haouz, A.; et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536, 48–53. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Katzelnick, L.C.; Burger-Calderon, R.; Gallini, J.; Moore, R.H.; Kuan, G.; Balmaseda, A.; Pinsky, B.A.; Harris, E. Antibody-Dependent Enhancement of Severe Disease Is Mediated by Serum Viral Load in Pediatric Dengue Virus Infections. J. Infect. Dis. 2020, 221, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-Garcia, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Laydon, D.J.; Dorigatti, I.; Hinsley, W.R.; Nedjati-Gilani, G.; Coudeville, L.; Ferguson, N.M. Efficacy profile of the CYD-TDV dengue vaccine revealed by Bayesian survival analysis of individual-level phase III data. eLife 2021, 10, e65131. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Zambrana, J.V.; Elizondo, D.; Collado, D.; Garcia, N.; Arguello, S.; Mercado, J.C.; Miranda, T.; Ampie, O.; Mercado, B.L.; et al. Dengue and Zika virus infections in children elicit cross-reactive protective and enhancing antibodies that persist long term. Sci. Transl. Med. 2021, 13, eabg9478. [Google Scholar] [CrossRef]
- Slon-Campos, J.L.; Dejnirattisai, W.; Jagger, B.W.; Lopez-Camacho, C.; Wongwiwat, W.; Durnell, L.A.; Winkler, E.S.; Chen, R.E.; Reyes-Sandoval, A.; Rey, F.A.; et al. A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection. Nat. Immunol. 2019, 20, 1291–1298. [Google Scholar] [CrossRef]
- Silva, M.; Kato, Y.; Melo, M.B.; Phung, I.; Freeman, B.L.; Li, Z.; Roh, K.; Van Wijnbergen, J.W.; Watkins, H.; Enemuo, C.A.; et al. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. Sci. Immunol. 2021, 6, eabf1152. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Zaman, K.; Yunus, M.; Aziz, A.B.; Feser, J.; Mooney, J.; Tang, Y.; Ellison, D.W.; Thaisomboonsuk, B.; Zhang, L.; Neuzil, K.M.; et al. Antibody persistence and immune memory response following primary vaccination and boosting with live attenuated SA 14-14-2 Japanese encephalitis vaccine (CD-JEV) in Bangladesh: A phase 4 open-label clinical trial. Vaccine X 2022, 10, 100143. [Google Scholar] [CrossRef]
- Wec, A.Z.; Haslwanter, D.; Abdiche, Y.N.; Shehata, L.; Pedreno-Lopez, N.; Moyer, C.L.; Bornholdt, Z.A.; Lilov, A.; Nett, J.H.; Jangra, R.K.; et al. Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine. Proc. Natl. Acad. Sci. USA 2020, 117, 6675–6685. [Google Scholar] [CrossRef] [PubMed]
- Bouckenooghe, A.; Bailleux, F.; Feroldi, E. Modeling the long-term persistence of neutralizing antibody in children and toddlers after vaccination with live attenuated Japanese encephalitis chimeric virus vaccine. Hum. Vaccin. Immunother. 2019, 15, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kling, K.; Domingo, C.; Bogdan, C.; Duffy, S.; Harder, T.; Howick, J.; Kleijnen, J.; McDermott, K.; Wichmann, O.; Wilder-Smith, A.; et al. Duration of Protection After Vaccination Against Yellow Fever: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 2266–2274. [Google Scholar] [CrossRef]
- Keelapang, P.; Ketloy, C.; Puttikhunt, C.; Sriburi, R.; Prompetchara, E.; Sae-Lim, M.; Siridechadilok, B.; Duangchinda, T.; Noisakran, S.; Charoensri, N.; et al. Heterologous prime-boost immunization induces protection against dengue virus infection in cynomolgus macaques. J. Virol. 2023, 97, e0096323. [Google Scholar] [CrossRef]
- George, J.A.; Eo, S.K. Distinct Humoral and Cellular Immunity Induced by Alternating Prime-boost Vaccination Using Plasmid DNA and Live Viral Vector Vaccines Expressing the E Protein of Dengue Virus Type 2. Immune Netw. 2011, 11, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.M.; Galula, J.U.; Wu, S.R.; Liu, J.H.; Chen, Y.H.; Wang, W.H.; Wang, S.F.; Song, C.S.; Chen, F.C.; Abarientos, A.B.; et al. Antibodies from dengue patients with prior exposure to Japanese encephalitis virus are broadly neutralizing against Zika virus. Commun. Biol. 2024, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.S.; Marcial-Juarez, E.; Linterman, M.A. The cellular factors that impair the germinal center in advanced age. J. Immunol. 2025, 214, 862–871. [Google Scholar] [CrossRef]
- Lee, J.L.; Linterman, M.A. Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol. Lett. 2022, 241, 1–14. [Google Scholar] [CrossRef]
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.J.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of pre-existing dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef]
- Palacios-Pedrero, M.A.; Osterhaus, A.; Becker, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Aging and Options to Halt Declining Immunity to Virus Infections. Front. Immunol. 2021, 12, 681449. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert. Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into vaccines for elderly individuals: From the impacts of immunosenescence to delivery strategies. NPJ Vaccines 2024, 9, 77. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Lotspeich-Cole, L.; Parvathaneni, S.; Sakai, J.; Liu, L.; Takeda, K.; Lee, R.C.; Akkoyunlu, M. Sustained antigen delivery improves germinal center reaction and increases antibody responses in neonatal mice. NPJ Vaccines 2024, 9, 92. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Liu, Y.; Li, Q.; Xue, Y.; Liu, L.; Liang, R.; Xiong, Y.; Zhao, J.; Chen, J.; et al. BAFF promotes follicular helper T cell development and germinal center formation through BR3 signal. JCI Insight 2024, 9, e183400. [Google Scholar] [CrossRef]
- Zhang, Y.; Tech, L.; George, L.A.; Acs, A.; Durrett, R.E.; Hess, H.; Walker, L.S.K.; Tarlinton, D.M.; Fletcher, A.L.; Hauser, A.E.; et al. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J. Exp. Med. 2018, 215, 1227–1243. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Taylor, D.W.; Compans, R.W. Combination of STING Pathway Agonist with Saponin Is an Effective Adjuvant in Immunosenescent Mice. Front. Immunol. 2019, 10, 3006. [Google Scholar] [CrossRef]
- Anasir, M.I.; Poh, C.L. Structural Vaccinology for Viral Vaccine Design. Front. Microbiol. 2019, 10, 738. [Google Scholar] [CrossRef]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing immunization: A comprehensive review of mRNA vaccine technology and applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Flores, H.E.; Pinzon Burgos, E.F.; Camacho Ortega, S.; Heredia, A.; Chua, J.V. From Antibodies to Immunity: Assessing Correlates of Flavivirus Protection and Cross-Reactivity. Vaccines 2025, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; McEntee, C.P. Vaccine adjuvants: Tailoring innate recognition to send the right message. Immunity 2024, 57, 772–789. [Google Scholar] [CrossRef]
- Yang, C.; Gong, R.; de Val, N. Development of Neutralizing Antibodies against Zika Virus Based on Its Envelope Protein Structure. Virol. Sin. 2019, 34, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.S.; Baillet, J.; Filsinger Interrante, M.V.; Adamska, J.Z.; Zhou, X.; Saouaf, O.M.; Yan, J.; Klich, J.H.; Jons, C.K.; Meany, E.L.; et al. Saponin nanoparticle adjuvants incorporating Toll-like receptor agonists drive distinct immune signatures and potent vaccine responses. Sci. Adv. 2024, 10, eadn7187. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Q.; Yang, X.; Wei, Y.; Chen, J.T.; Wang, X.J.; Peng, H.J. A Review on Dengue Vaccine Development. Vaccines 2020, 8, 63. [Google Scholar] [CrossRef]
- Cui, J.Z.; Xiong, X.H.; Wang, Q.Y.; Dong, H.L.; Liu, G.; Chen, H.P. Insect-Specific Flaviviruses Have Potential Applications as a Scaffold for Pathogenic Flavivirus Vaccines. Vaccines 2025, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, J.A.; Duffy, D. Systems vaccinology studies—Achievements and future potential. Microbes Infect. 2024, 26, 105318. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Recent updates on correlates of vaccine-induced protection. Front. Immunol. 2022, 13, 1081107. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.B.; Yang, Q.W.; Huang, H.W.; Chiu, K.C.; Su, S.L.; Liao, C.L.; Yen, L.C. A novel immunogen comprising a bc loop and mutant fusion loop epitopes generates potent neutralization and protective abilities against flaviviruses without risk of disease enhancement. Vaccine 2025, 57, 127219. [Google Scholar] [CrossRef]
- Thomas, A.; Thiono, D.J.; Kudlacek, S.T.; Forsberg, J.; Premkumar, L.; Tian, S.; Kuhlman, B.; de Silva, A.M.; Metz, S.W. Dimerization of Dengue Virus E Subunits Impacts Antibody Function and Domain Focus. J. Virol. 2020, 94, e00745-20. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Rasheed, M.A.U.; Havenar-Daughton, C.; Pham, M.; Legere, T.; Sher, Z.J.; Kovalenkov, Y.; Gumber, S.; Huang, J.Y.; Gottardo, R.; et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci. Immunol. 2020, 5, eabb1025. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef]
- Luisi, K.; Morabito, K.M.; Burgomaster, K.E.; Sharma, M.; Kong, W.P.; Foreman, B.M.; Patel, S.; Fisher, B.; Aleshnick, M.A.; Laliberte, J.; et al. Development of a potent Zika virus vaccine using self-amplifying messenger RNA. Sci. Adv. 2020, 6, eaba5068. [Google Scholar] [CrossRef]
- Essink, B.; Chu, L.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.D.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Gerritsen, B.; Tomalin, L.E.; Fourati, S.; Mule, M.P.; Chawla, D.G.; Rychkov, D.; Henrich, E.; Miller, H.E.R.; Diray-Arce, J.; et al. Transcriptional atlas of the human immune response to 13 vaccines reveals a common predictor of vaccine-induced antibody responses. Nat. Immunol. 2022, 23, 1788–1798. [Google Scholar] [CrossRef]
- Gonzalez-Dias, P.; Lee, E.K.; Sorgi, S.; de Lima, D.S.; Urbanski, A.H.; Silveira, E.L.; Nakaya, H.I. Methods for predicting vaccine immunogenicity and reactogenicity. Hum. Vaccin. Immunother. 2020, 16, 269–276. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4.5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef]
- Valdes, I.; Izquierdo, A.; Cobas, K.; Thao, P.; Anh Duc, H.; Duc Loc, H.; Dung, L.T.; Lazo, L.; Suzarte, E.; Perez, Y.; et al. A heterologous prime-boost strategy for immunization against Dengue virus combining the Tetra DIIIC subunit vaccine candidate with the TV005 live-attenuated tetravalent vaccine. J. Gen. Virol. 2019, 100, 975–984. [Google Scholar] [CrossRef]
- Sun, P.; Jani, V.; Johnson, A.; Cheng, Y.; Nagabhushana, N.; Williams, M.; Morrison, B.J.; Defang, G. T cell and memory B cell responses in tetravalent DNA, tetravalent inactivated and tetravalent live-attenuated prime-boost dengue vaccines in rhesus macaques. Vaccine 2021, 39, 7510–7520. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, G.; Tandavanitj, R.; Preciado-Llanes, L.; Sanchez-Velazquez, R.; Prado Rocha, R.; Kim, Y.C.; Reyes-Sandoval, A.; Patel, A.H. Heterologous prime-boost Zika virus vaccination induces comprehensive humoral and cellular immunity in mouse models. Front. Immunol. 2025, 16, 1578427. [Google Scholar] [CrossRef]
- Natsrita, P.; Charoenkwan, P.; Shoombuatong, W.; Mahalapbutr, P.; Faksri, K.; Chareonsudjai, S.; Rungrotmongkol, T.; Pipattanaboon, C. Machine-learning-assisted high-throughput identification of potent and stable neutralizing antibodies against all four dengue virus serotypes. Sci. Rep. 2024, 14, 17165. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Fassi, E.M.A.; Romanato, A.; D’Annessa, I.; Odinolfi, M.T.; Brambilla, D.; Damin, F.; Chiari, M.; Gori, A.; Colombo, G.; et al. Computational Analysis of Dengue Virus Envelope Protein (E) Reveals an Epitope with Flavivirus Immunodiagnostic Potential in Peptide Microarrays. Int. J. Mol. Sci. 2019, 20, 1921. [Google Scholar] [CrossRef]
- Deepthi, V.; Sasikumar, A.; Mohanakumar, K.P.; Rajamma, U. Computationally designed multi-epitope vaccine construct targeting the SARS-CoV-2 spike protein elicits robust immune responses in silico. Sci. Rep. 2025, 15, 9562. [Google Scholar] [CrossRef]
- Schnyder, J.L.; de Jong, H.K.; Bache, B.E.; Schaumburg, F.; Grobusch, M.P. Long-term immunity following yellow fever vaccination: A systematic review and meta-analysis. Lancet Glob. Health 2024, 12, e445–e456. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue virus neutralizing antibody: A review of targets, cross-reactivity, and antibody-dependent enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef]
- Chan, K.R.; Ismail, A.A.; Thergarajan, G.; Raju, C.S.; Yam, H.C.; Rishya, M.; Sekaran, S.D. Serological cross-reactivity among common flaviviruses. Front. Cell Infect. Microbiol. 2022, 12, 975398. [Google Scholar] [CrossRef]
- Parra-Gonzalez, M.; Najera-Maldonado, L.; Peralta-Cuevas, E.; Gutierrez-Onofre, A.J.; Garcia-Atutxa, I.; Villanueva-Flores, F. Overcoming dengue vaccine challenges through next-generation virus-like particle immunization strategies. Front. Cell Infect. Microbiol. 2025, 15, 1614805. [Google Scholar] [CrossRef] [PubMed]
- Santos-Peral, A.; Zaucha, M.; Nikolova, E.; Yaman, E.; Puzek, B.; Winheim, E.; Goresch, S.; Scheck, M.K.; Lehmann, L.; Dahlstroem, F.; et al. Basal T cell activation predicts yellow fever vaccine response independently of cytomegalovirus infection and sex-related immune variations. Cell Rep. Med. 2025, 6, 101946. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).