Evaluation of the Safety and Efficacy of the Respiratory Syncytial Virus FG Chimeric Vaccine KD-409 in Rodent Models for Maternal and Pediatric Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Antigens, Viruses, Cells, and Animals
2.2. Immunization
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) and Cell-ELISA
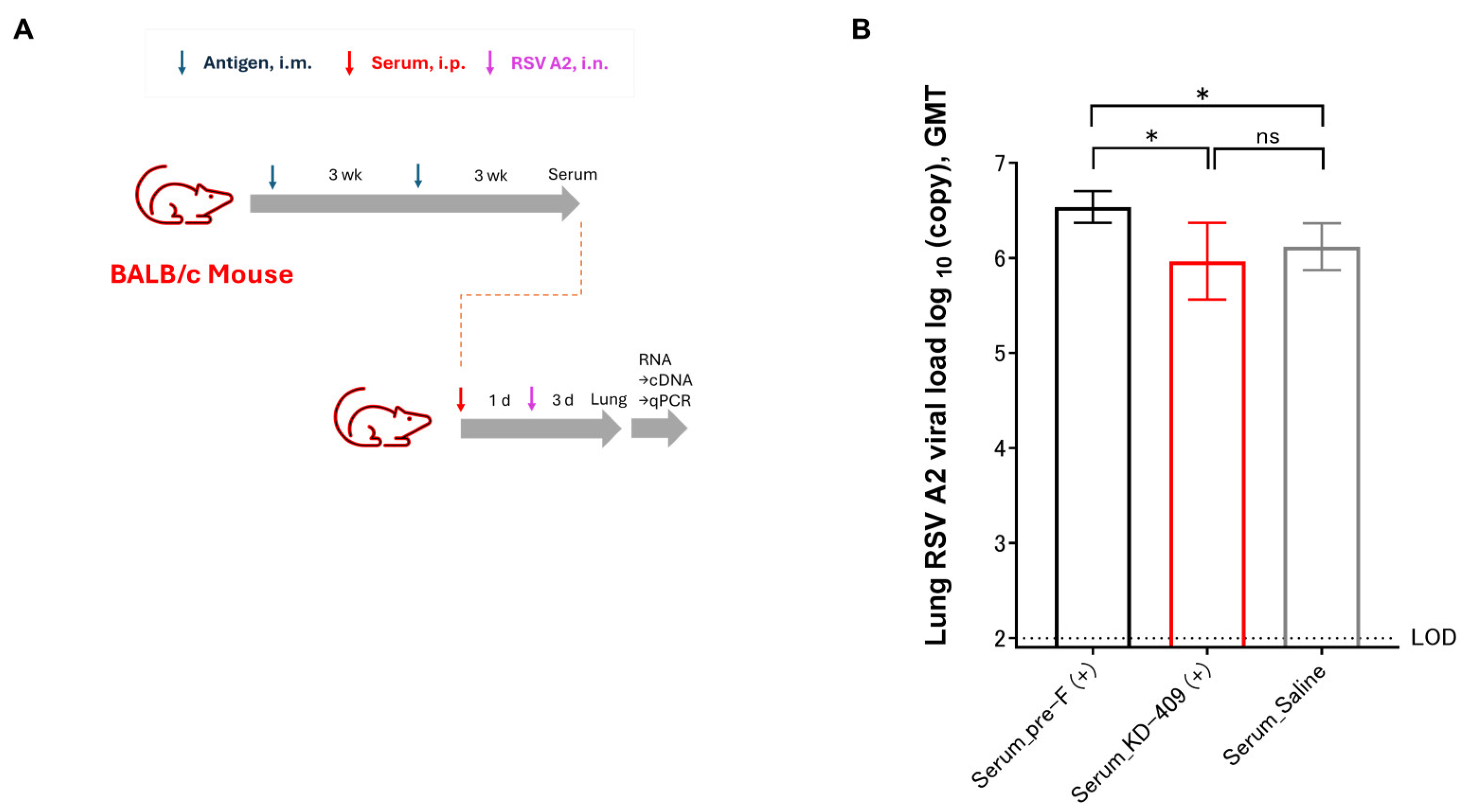
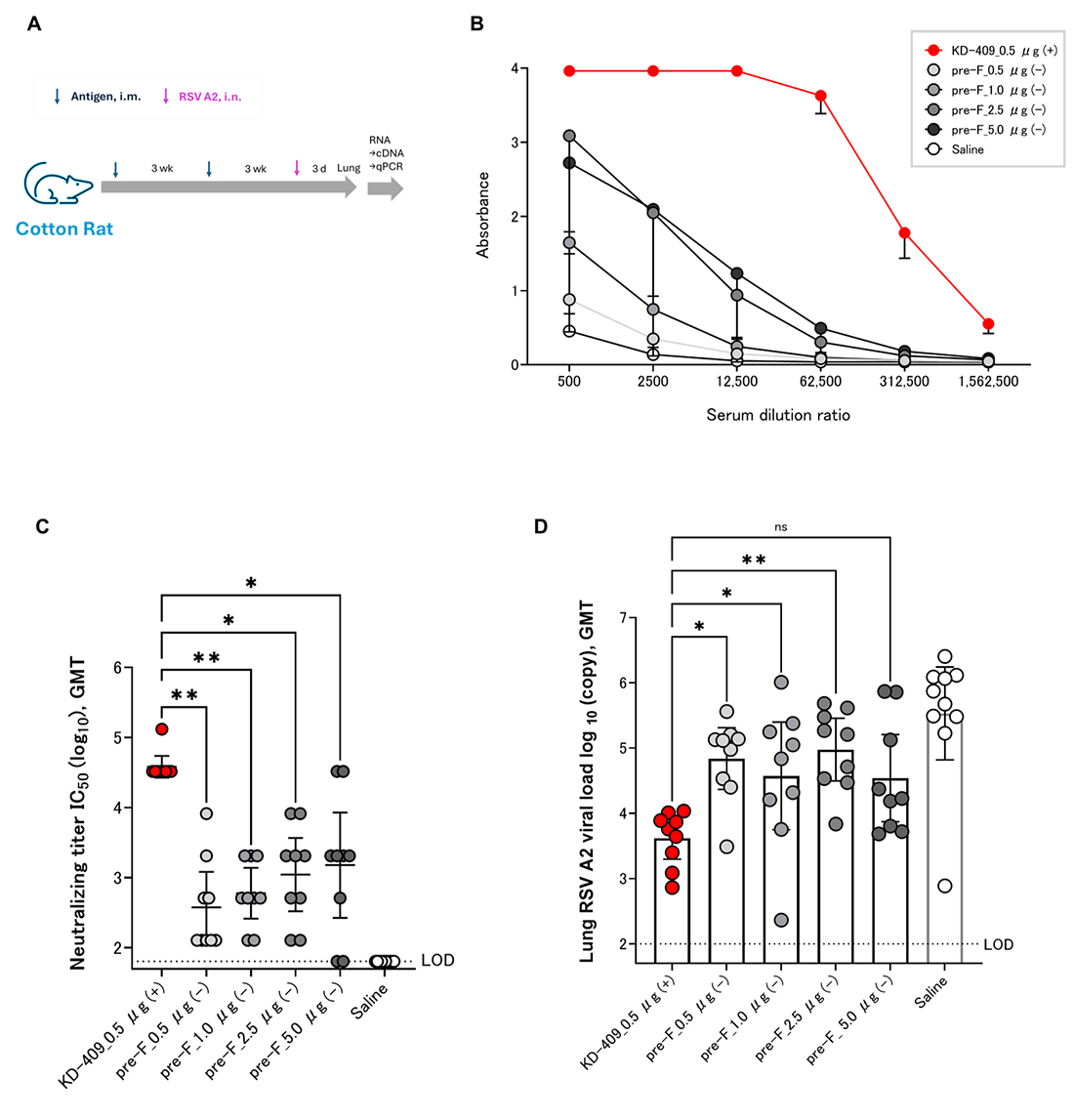
2.4. Virus Challenge and Copy Number Analysis
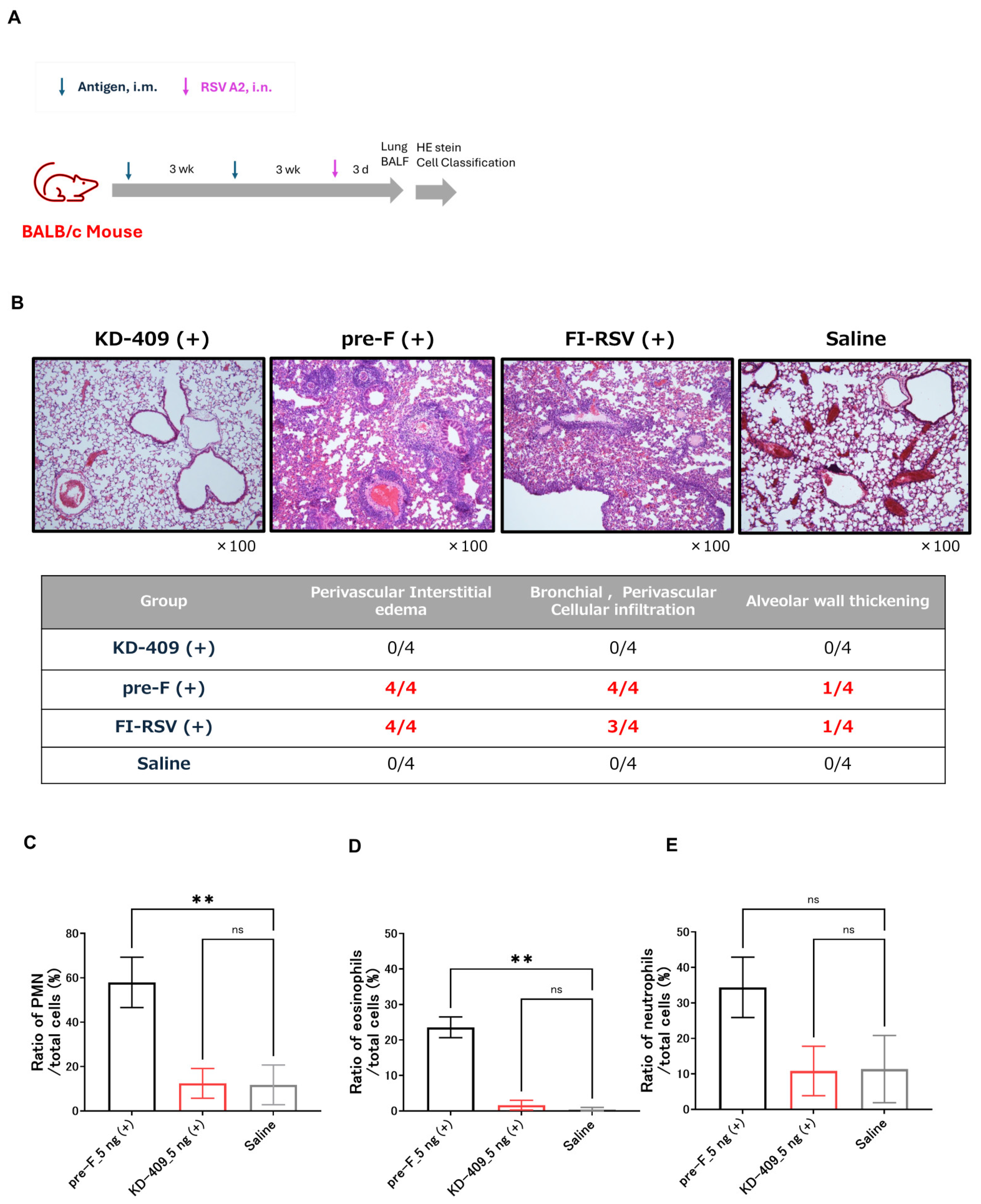
2.5. Inflammation Assessment
2.6. Statistical Analysis
3. Results
3.1. Antibody Transfer in a Guinea Pig Pregnancy Model
3.2. Evaluation of Infection Exacerbation Following Passive Immunization in Mice
3.3. Evaluation of Symptom Exacerbation Following Active Immunization in Mice
3.4. Efficacy Evaluation by Active Immunization of Cotton Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RSV | Respiratory syncytial virus |
| ALRI | Acute lower respiratory infection |
| FI-RSV | Formalin-inactivated respiratory syncytial virus |
| VED | Vaccine-enhanced disease |
| FDA | Food and Drug Administration |
| CCD | Conserved central domain |
| BBG2Na | Fusion protein of RSV G protein and streptococcal G protein albumin-binding domain |
| DTP | Diphtheria, tetanus, and pertussis |
| ADE | Antibody-dependent enhancement |
| CX3C | Specific motif sequence in G protein |
| CX3CR1 | CX3C chemokine receptor 1 |
| PMN | Polymorphonuclear leukocytes |
| BALF | Bronchoalveolar lavage fluid |
| ELISA | Enzyme-linked immunosorbent assay |
| HEp-2 | Human epithelial type 2 cells |
| PFU | Plaque-forming units |
| MGB | Minor groove binder |
| PCR | Polymerase chain reaction |
| ANOVA | Analysis of variance |
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef]
- Approval-Letter-ABRYSVO-(STN-125769-26). 2023. Available online: https://www.fda.gov/media/171523/download?attachment (accessed on 21 August 2023).
- FDA Advisory Committee Votes in Support of Approval for Pfizer’s Vaccine Candidate to Help Prevent RSV in Infants Through Maternal Immunization. 2023. Available online: https://www.pfizer.com/news/press-release/press-release-detail/fda-advisory-committee-votes-support-approval-pfizers (accessed on 18 May 2023).
- U.S. FDA Accepts Biologics License Application for Pfizer’s Respiratory Syncytial Virus Maternal Vaccine Candidate for Priority Review. 2023. Available online: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-accepts-biologics-license-application-pfizers (accessed on 21 February 2023).
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.Y.; Mitzner, W.; et al. Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Olszewska, W.; Wang, B.; Tregoning, J.S.; Helson, R.; Sattentau, Q.J.; Openshaw, P.J.M. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 2006, 12, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, E.; Senpuku, K.; Kawaguchi, Y.; Yamamoto, S.; Yasuda, K.; Kuroda, E.; Ouji-Sageshima, N.; Ito, T.; Hirai, T.; Shibata, T.; et al. Recombinant RSV G protein vaccine induces enhanced respiratory disease via IL-13 and mucin overproduction. NPJ Vaccine 2024, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, K.M.; Kosanovich, J.L.; Gidwani, S.V.; Zomback, A.; Lipp, M.A.; Perkins, T.N.; Oury, T.D.; Petrovsky, N.; Marshall, C.P.; Yondola, M.A.; et al. Prefusion RSV F immunization elicits Th2-mediated lung pathology in mice when formulated with a Th2 (but not a Th1/Th2-balanced) adjuvant despite complete viral protection. Front. Immunol. 2020, 11, 1673. [Google Scholar] [CrossRef] [PubMed]
- Caidi, H.; Miao, C.; Thornburg, N.J.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model. Antiviral Res. 2018, 154, 149–157. [Google Scholar] [CrossRef]
- Johnson, S.M.; McNally, B.A.; Ioannidis, I.; Flano, E.; Teng, M.N.; Oomens, A.G.; Walsh, E.E.; Peeples, M.E. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLOS Pathog. 2015, 11, e1005318. [Google Scholar] [CrossRef]
- Chirkova, T.; Lin, S.; Oomens, A.G.P.; Gaston, K.A.; Boyoglu-Barnum, S.; Meng, J.; Stobart, C.C.; Cotton, C.U.; Hartert, T.V.; Moore, M.L.; et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J. Gen. Virol. 2015, 96, 2543–2556. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Power, U.F.; Robert, A.; Haeuw, J.F.; Helffer, K.; Perez, A.; Asin, M.A.; Corvaia, N.; Libon, C. The respiratory syncytial virus G protein conserved domain induces a persistent and protective antibody response in rodents. PLoS ONE 2012, 7, e34331. [Google Scholar] [CrossRef]
- Hall, C.B.; Simőes, E.A.F.; Anderson, L.J. Clinical and epidemiologic features of respiratory syncytial virus. Curr. Top. Microbiol. Immunol. 2013, 372, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.C.; McLaurin, K.K.; Margulis, A.V.; Mauskopf, J.; Ambrose, C.S.; Pavilack, M.; Candrilli, S.D. Chronologic age at hospitalization for respiratory syncytial virus among preterm and term infants in the United States. Infect. Dis. Ther. 2017, 6, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Khan, R.A.; Walker, B.; Bennett, E.; Gent, N. Respiratory syncytial virus-associated hospitalisation in children aged ≤ 5 years: A scoping review of literature from 2009 to 2021. ERJ Open Res 2022, 8, 00593–2021. [Google Scholar] [CrossRef] [PubMed]
- Oguti, B.; Ali, A.; Andrews, N.; Barug, D.; Anh Dang, D.; Halperin, S.A.; Thu Hoang, H.T.; Holder, B.; Kampmann, B.; Kazi, A.M.; et al. The half-life of maternal transplacental antibodies against diphtheria, tetanus, and pertussis in infants: An individual participant data meta-analysis. Vaccine 2022, 40, 450–458. [Google Scholar] [CrossRef]
- Yamaue, R.; Torikai, M.; Terashima, M.; Mori, H. KD-409, a respiratory syncytial virus FG chimeric protein without the CX3C chemokine motif, is an efficient respiratory syncytial virus vaccine preparation for passive and active immunization in mice. Vaccines 2024, 12, 753. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrot, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Enders, A.C.; Blankenship, T.N. Comparative placental structure. Adv. Drug Deliv. Rev. 1999, 38, 3–15. [Google Scholar] [CrossRef]
- Glenn, G.M.; Fries, L.F.; Smith, G.; Kpamegan, E.; Lu, H.; Guebre-Xabier, M.; Hickman, S.P.; Flyer, D. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Vaccine 2015, 33, 6488–6492. [Google Scholar] [CrossRef] [PubMed]
- van Erp, E.A.; van Kasteren, P.B.; Guichelaar, T.; Ahout, I.M.L.; de Haan, C.A.M.; Luytjes, W.; Ferwerda, G.; Wicht, O. In vitro enhancement of respiratory syncytial virus infection by maternal antibodies does not explain disease severity in infants. J. Virol. 2017, 91, e00851-17. [Google Scholar] [CrossRef] [PubMed]
- Bebia, Z.; Reyes, O.; Jeanfreau, R.; Kantele, A.; De Leon, R.G.; Sánchez, M.G.; Banooni, P.; Gardener, G.J.; Rasero, J.L.B.; Pardilla, M.B.E.; et al. Safety and immunogenicity of an investigational respiratory syncytial virus vaccine (RSVPreF3) in mothers and their infants: A Phase 2 randomized trial. J. Infect. Dis. 2023, 228, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wildenbeest, J.G.; Billard, M.N.; Zuurbier, R.P.; Korsten, K.; Langedijk, A.C.; van de Ven, P.M.; Snape, M.D.; Drysdale, S.B.; Pollard, A.J.; Robinson, H.; et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: A prospective birth cohort study. Lancet Respir. Med. 2023, 11, 341–353. [Google Scholar] [CrossRef]
- Openshaw, P.J.M.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and harmful immunity to RSV infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Steinhoff, M.C.; Magaret, A.; Zaman, K.; Roy, E.; Langdon, G.; Formica, M.A.; Walsh, E.E.; Englund, J.A. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J. Infect. Dis. 2014, 210, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.T.; Satav, A.; Gill, C.J.; Omer, S.B.; Pieciak, R.C.; Kazi, A.M.; Simões, E.A.; Polack, F.P. Challenges of assessing community mortality due to respiratory viruses in children aged less than 5 years. Clin. Infect. Dis. 2021, 73 (Suppl. S3), S248–S254. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Paredes, A.; Allison, J.E.; Taber, L.H.; Frank, A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 1981, 98, 708–715. [Google Scholar] [CrossRef]
- Gervassi, A.L.; Horton, H. Is infant immunity actively suppressed or immature? Virology 2014, 5, VRT-S12248. [Google Scholar] [CrossRef]
- van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef]
- Schneider-Ohrum, K.; Cayatte, C.; Bennett, A.S.; Rajani, G.M.; McTamney, P.; Nacel, K.; Hostetler, L.; Cheng, L.; Ren, K.; O’Day, T.; et al. Immunization with low doses of recombinant postfusion or prefusion respiratory syncytial virus F Primes for vaccine-enhanced disease in the cotton rat model independently of the presence of a Th1-biasing (GLA-SE) or Th2-biasing (alum) adjuvant. J. Virol. 2017, 91, e02180-16. [Google Scholar] [CrossRef]
- Polack, F.P.; Alvarez-Paggi, D.; Libster, R.; Caballero, M.T.; Blair, R.V.; Hijano, D.R.; de la Iglesia Niveyro, P.X.; Menendez, D.R.; Gladwell, W.; Avendano, L.M.; et al. Fatal enhanced respiratory syncytial virus disease in toddlers. Sci. Transl. Med. 2021, 13, eabj7843. [Google Scholar] [CrossRef]
- Zhivaki, D.; Lemoine, S.; Lim, A.; Morva, A.; Vidalain, P.O.; Schandene, L.; Casartelli, N.; Rameix-Welti, M.A.; Hervé, P.L.; Dériaud, E.; et al. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity 2017, 46, 301–314. [Google Scholar] [CrossRef]
- Fedechkin, S.O.; George, N.L.; Wolff, J.T.; Kauvar, L.M.; DuBois, R.M. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci. Immunol. 2018, 3, eaar3534. [Google Scholar] [CrossRef]
- Che, Y.; Gribenko, A.V.; Song, X.; Handke, L.D.; Efferen, K.S.; Tompkins, K.; Kodali, S.; Nunez, L.; Prasad, A.K.; Phelan, L.M.; et al. Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine. Sci. Transl. Med. 2023, 15, eade6422. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.E.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA Briefing Document: Considerations for Respiratory Syncytial Virus (RSV) Vaccine Safety in Pediatric Populations, Vaccines and Related Biological Products Advisory Committee Meeting December 12, 2024. Available online: https://www.fda.gov/media/184301/download (accessed on 12 December 2024).
- Zaghouani, H.; Hoeman, C.M.; Adkins, B. Neonatal immunity: Faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009, 30, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Yamaguchi, T.; Ishida, W.; Fukata, K.; Taniguchi, T.; Liu, F.T.; Ueno, H. Genetic background determines susceptibility to experimental immune-mediated blepharoconjunctivitis: Comparison of BALB/c and C57BL/6 mice. Exp. Eye Res. 2006, 82, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Animal models of respiratory syncytial virus infection. Vaccine 2017, 35, 469–480. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaue, R.; Terashima, M.; Soejima, K.; Torikai, M. Evaluation of the Safety and Efficacy of the Respiratory Syncytial Virus FG Chimeric Vaccine KD-409 in Rodent Models for Maternal and Pediatric Vaccination. Vaccines 2025, 13, 1170. https://doi.org/10.3390/vaccines13111170
Yamaue R, Terashima M, Soejima K, Torikai M. Evaluation of the Safety and Efficacy of the Respiratory Syncytial Virus FG Chimeric Vaccine KD-409 in Rodent Models for Maternal and Pediatric Vaccination. Vaccines. 2025; 13(11):1170. https://doi.org/10.3390/vaccines13111170
Chicago/Turabian StyleYamaue, Ryo, Madoka Terashima, Kenji Soejima, and Masaharu Torikai. 2025. "Evaluation of the Safety and Efficacy of the Respiratory Syncytial Virus FG Chimeric Vaccine KD-409 in Rodent Models for Maternal and Pediatric Vaccination" Vaccines 13, no. 11: 1170. https://doi.org/10.3390/vaccines13111170
APA StyleYamaue, R., Terashima, M., Soejima, K., & Torikai, M. (2025). Evaluation of the Safety and Efficacy of the Respiratory Syncytial Virus FG Chimeric Vaccine KD-409 in Rodent Models for Maternal and Pediatric Vaccination. Vaccines, 13(11), 1170. https://doi.org/10.3390/vaccines13111170




