Clinical Trial Safety Surveillance in Africa: Experts’ Perspectives on Current Practices and Opportunities
Abstract
1. Background
2. Approach
3. Results
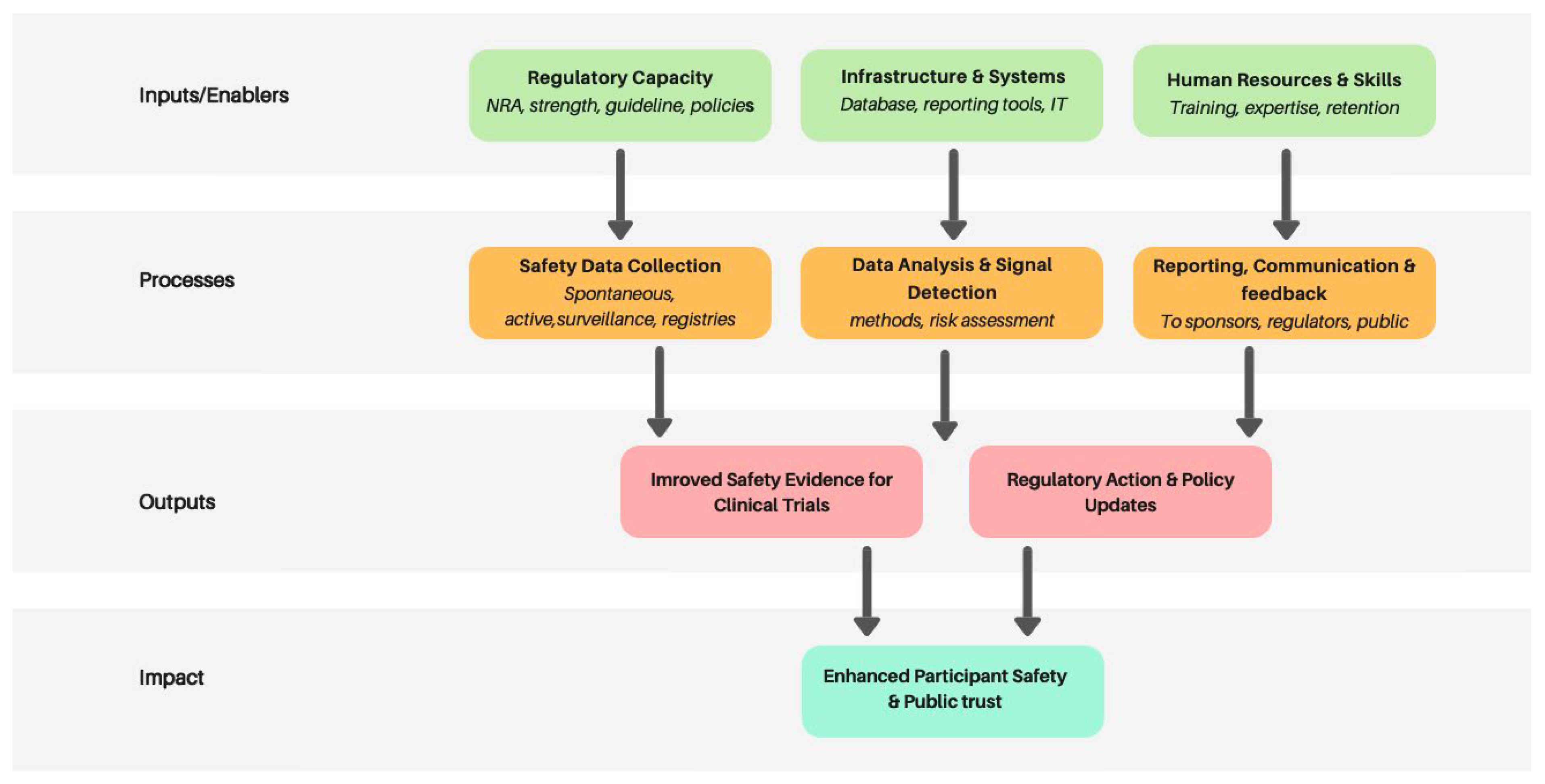
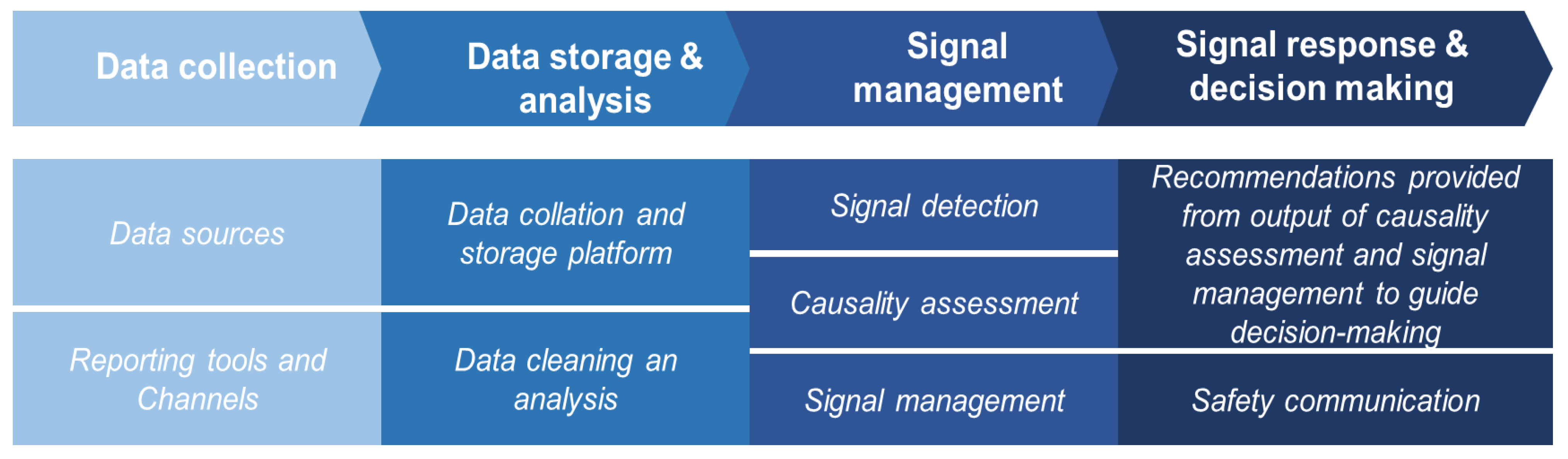
3.1. Safety Surveillance System for Clinical Trial in Africa
3.1.1. Findings from the Electronic Survey
3.1.2. Findings from the Virtual Meetings
3.2. Challenges Identified with Clinical Trial Safety Monitoring System in the African Region
4. Discussion and Recommendations
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMRH | African Medicines Regulatory Harmonization |
| AVAREF | African Vaccine Regulatory Forum |
| CIOMS | Council for International Organizations of Medical Sciences |
| CRO | Clinical Research Organization |
| CT | Clinical Trial |
| GCP | Good Clinical Practice |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| IRB | Institutional Review Board |
| LMIC | Low–middle-income Country |
| NEC | National Ethics Committee |
| NRA | National Regulatory Authority |
Appendix A
| Themes | Questions Asked |
|---|---|
| CT safety data reporting and custodianship |
|
| CT safety data collation, storage, and analysis |
|
| Causality assessment, signal generation, and management |
|
References
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guidelines for Good Clinical Practice (GCP) E6(R3); ICH: Geneva, Switzerland, 2025. Available online: https://database.ich.org/sites/default/files/ICH_E6%28R3%29_Step4_FinalGuideline_2025_0106.pdf (accessed on 19 April 2025).
- World Health Organization (WHO). Guidance for Best Practices for Clinical Trials; WHO: Geneva, Switzerland, 2024.
- Council for International Organizations of Medical Sciences (CIOMS). Management of Safety Information from Clinical Trials: Report of CIOMS Working Group VI; CIOMS: Geneva, Switzerland, 2005.
- World Health Organization (WHO). Clinical Trials Geneva: World Health Organization; WHO: Geneva, Switzerland, 2022. Available online: https://www.who.int/health-topics/clinical-trials#tab=tab_1 (accessed on 10 December 2024).
- African Vaccine Regulators Forum (AVAREF). Annual Reports on Clinical Trial Oversight and Harmonization in Africa; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Samara, C.; Garcia, A.; Henry, C.; Vallotton, L.; Cariolato, L.; Desmeules, J.; Pinçon, A. Safety Surveillance during drug development: Comparative evaluation of existing regulations. Adv. Ther. 2023, 40, 2147–2185. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, P.L.; DeStefano, F. The complementary roles of Phase 3 trials and post-licensure surveillance in the evaluation of new vaccines. Vaccine 2015, 33, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Reporting Safety Information on Clinical Trials: Reporting Requirements Under the Clinical Trial Regulation. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/clinical-trials-human-medicines/reporting-safety-information-clinical-trials (accessed on 20 November 2024).
- Rajman, I.; Knapp, L.; Morgan, T.; Masimirembwa, C. African Genetic Diversity: Implications for Cytochrome P450-mediated Drug Metabolism and Drug Development. eBioMedicine 2017, 17, 67–74. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline: General Considerations for Clinical Studies E8(R1) 6 October 2021; ICH: Geneva, Switzerland, 2021. Available online: https://www.ema.europa.eu/en/ich-e8-general-considerations-clinical-studies-scientific-guideline (accessed on 1 March 2025).
- Bottazzi, M.E.; Hotez, P.J. “Running the Gauntlet”: Formidable challenges in advancing neglected tropical diseases vaccines from development through licensure, and a “Call to Action”. Hum. Vaccin. Immunother. 2019, 15, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Africa. Neglected Tropical Diseases and Vaccine Development in Africa; WHO AFRO: Brazzaville, Congo, 2019.
- African Medicines Agency (AMA). Operational Framework and Integration with AVAREF and AMRH; African Union: Addis Ababa, Ethiopia, 2022.
- Biswas, P.; Justice, N.; Biswas, H. Clinical Trials Safety Data. In Principles and Practice of Pharmacovigilance and Drug Safety; Springer International Publishing: Cham, Switzerland, 2024; pp. 163–189. [Google Scholar]
- WHO AVAREF Secretariat. Guidelines and Templates for Good Clinical Practice (GCP) in Africa; WHO AFRO: Brazzaville, Congo, 2022. Available online: https://www.afro.who.int/health-topics/immunization/avaref/guidelines (accessed on 17 November 2024).
- Abiri, O.T.; Bah, A.J.; Lahai, M.; Lisk, D.R.; Komeh, J.P.; Johnson, J.; Johnson, W.C.; Mansaray, S.S.; Kanu, J.S.; Russell, J.B.; et al. Regulating clinical trials in a resource-limited setting during the Ebola public health emergency in Sierra Leone. Trials 2022, 23, 466. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejekam, C.S.; Nyarko, K.A.; Abiri, O.T.; Beno, Y.N.; Adechina Adehan, R.M. Clinical Trial Safety Surveillance in Africa: Experts’ Perspectives on Current Practices and Opportunities. Vaccines 2025, 13, 1139. https://doi.org/10.3390/vaccines13111139
Ejekam CS, Nyarko KA, Abiri OT, Beno YN, Adechina Adehan RM. Clinical Trial Safety Surveillance in Africa: Experts’ Perspectives on Current Practices and Opportunities. Vaccines. 2025; 13(11):1139. https://doi.org/10.3390/vaccines13111139
Chicago/Turabian StyleEjekam, Chioma S., Kwasi A. Nyarko, Onome T. Abiri, Yakubu N. Beno, and Rhanda M. Adechina Adehan. 2025. "Clinical Trial Safety Surveillance in Africa: Experts’ Perspectives on Current Practices and Opportunities" Vaccines 13, no. 11: 1139. https://doi.org/10.3390/vaccines13111139
APA StyleEjekam, C. S., Nyarko, K. A., Abiri, O. T., Beno, Y. N., & Adechina Adehan, R. M. (2025). Clinical Trial Safety Surveillance in Africa: Experts’ Perspectives on Current Practices and Opportunities. Vaccines, 13(11), 1139. https://doi.org/10.3390/vaccines13111139




