A Multi-Epitope Recombinant Vaccine Candidate Against Bovine Alphaherpesvirus 1 and 5 Elicits Robust Immune Responses in Mice and Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Silico Prediction of Immunodominant Epitopes and Construction of Synthetic Genes
2.3. In Silico Evaluation of the Immunogenic Coverage of the RecBoAHV via T- and B-Cell Epitope Prediction
2.4. Cloning of the Synthetic Gene RecBoAHV for Expression in a Prokaryotic Vector
2.5. Construction of the Recombinant MVA-RecBoAHV Vector
2.6. Immunization of New Zealand Rabbits
2.6.1. Immunization with RecBoAHV
2.6.2. Immunization with MVA-RecBoAHV in Prime-Boost-Boost Homologous Protocol, and Evaluation of Previous Anti-Poxvirus Immunity Interference
2.7. Immunization of Mice with MVA-RecBoAHV in Prime-Boost-Boost Protocols
2.8. Indirect ELISA
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Design of the Recombinant Multi-Epitope Protein and Expression
3.2. Expression and Characterization of the Recombinant Multi-Epitope Protein
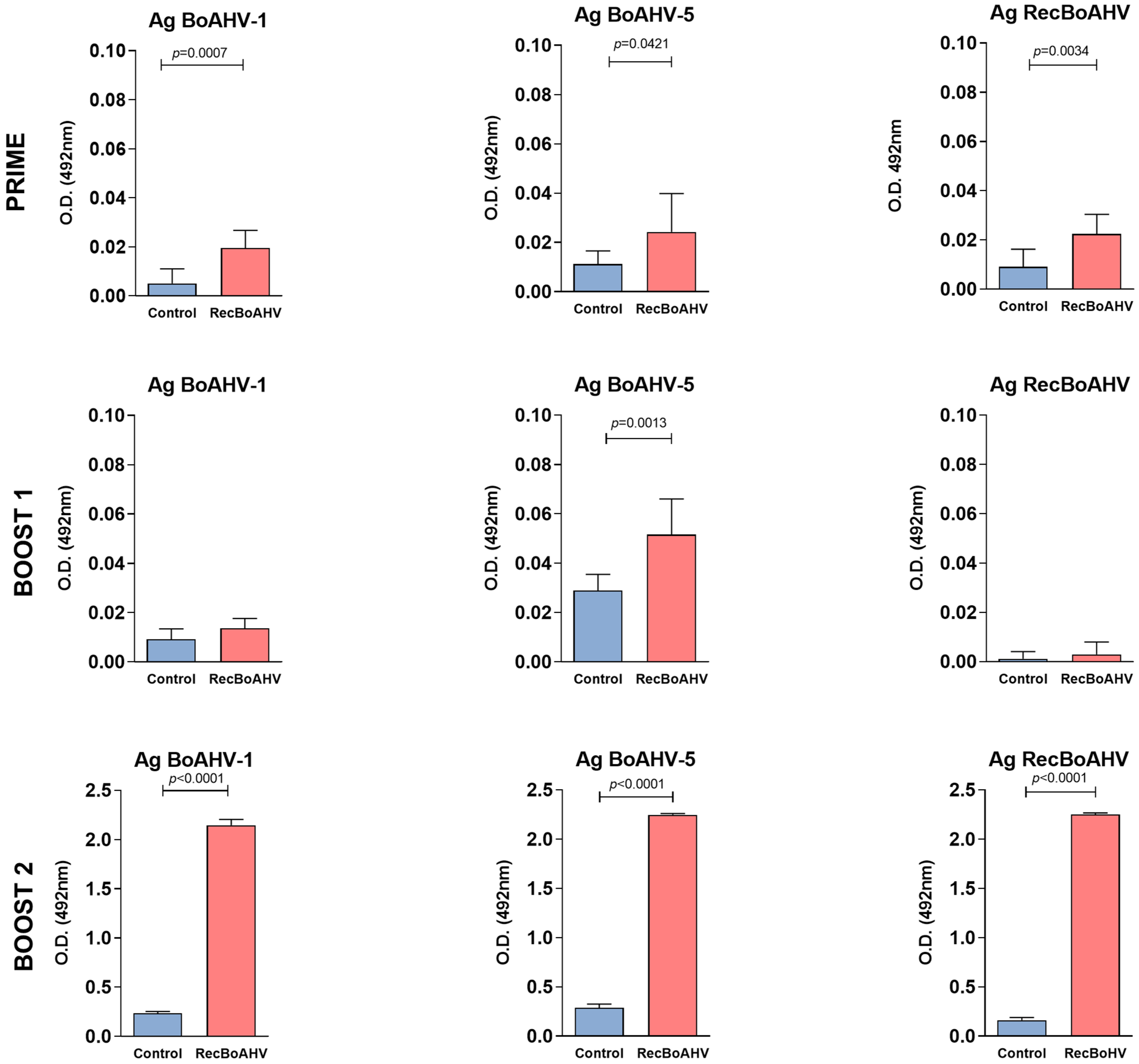
3.3. Immunogenicity of the RecBoAHV in a Rabbit Model
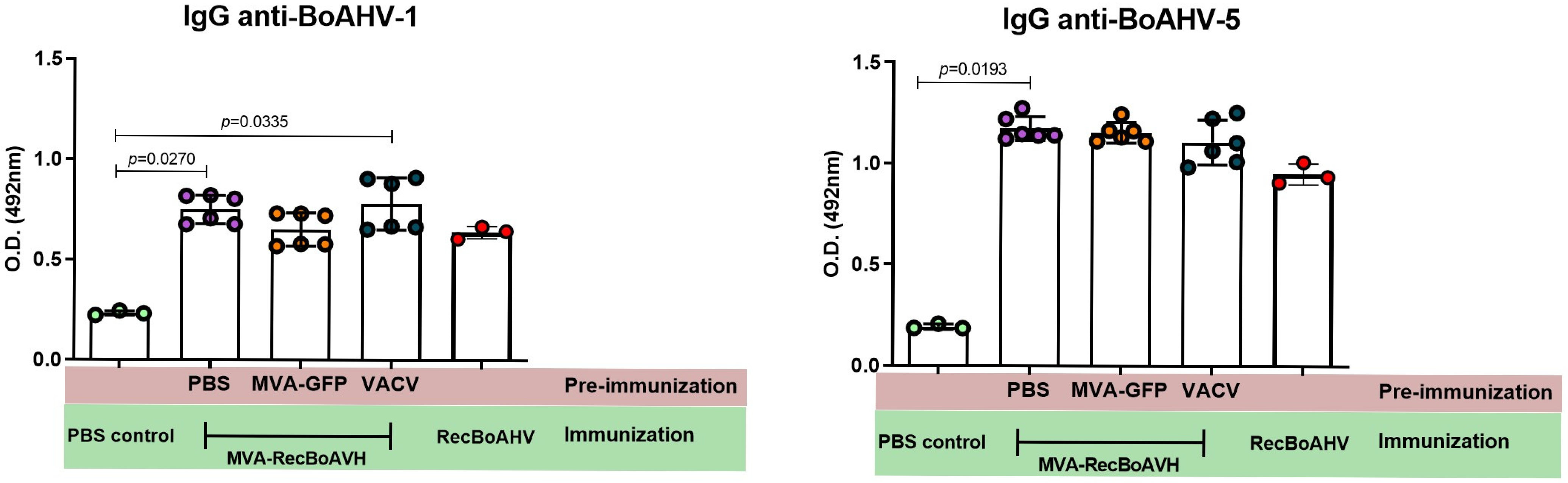
3.4. Immunogenicity of the MVA-RecBoAHV in a Rabbit Model
3.5. Immunogenicity of the RecBoAHV in a Mouse Model
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rimayanti, R.; Khairullah, A.; Lestari, T.; Moses, I.; Utama, S.; Damayanti, R.; Mulyati, S.; Raharjo, H.; Kusala, M.; Raissa, R.; et al. Infectious Bovine Rhinotracheitis (IBR): Unveiling the Hidden Threat to Livestock Productivity and Global Trade. Open Vet. J. 2024, 14, 2525. [Google Scholar] [CrossRef]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef]
- Dotto-Maurel, A.; Arzul, I.; Morga, B.; Chevignon, G. Herpesviruses: Overview of Systematics, Genomic Complexity and Life Cycle. Virol. J. 2025, 22, 155. [Google Scholar] [CrossRef]
- Campos, F.S.; Franco, A.C.; Hübner, S.O.; Oliveira, M.T.; Silva, A.D.; Esteves, P.A.; Roehe, P.M.; Rijsewijk, F.A.M. High Prevalence of Co-Infections with Bovine herpesvirus 1 and 5 Found in Cattle in Southern Brazil. Vet. Microbiol. 2009, 139, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 Infection and Infectious Bovine Rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.S.; Lemos, R.A.A.; Rech, R.R.; Barros, C.S.L.; Rissi, D.R. Bovine Herpesviral Meningoencephalitis: Large Case Study and Literature Review. J. Vet. Diagn. Investig. 2025, 37, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Herpesvirus Latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Walz, P.H.; Passler, T.; Palomares, R.; Newcomer, B.W.; Riddell, K.P.; Gard, J.; Zhang, Y.; Galik, P. Efficacy of Four Commercially Available Multivalent Modified-Live Virus Vaccines against Clinical Disease, Viremia, and Viral Shedding in Early-Weaned Beef Calves Exposed Simultaneously to Cattle Persistently Infected with Bovine Viral Diarrhea Virus and Cattle Acutely Infected with Bovine herpesvirus 1. Am. J. Vet. Res. 2016, 77, 88–97. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Zhuang, L.; Ye, Z.; Li, L.; Yang, L.; Gong, W. Next-Generation TB Vaccines: Progress, Challenges, and Prospects. Vaccines 2023, 11, 1304. [Google Scholar] [CrossRef]
- Daian e Silva, D.S.d.O.; da Fonseca, F.G. The Rise of Vectored Vaccines: A Legacy of the COVID-19 Global Crisis. Vaccines 2021, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.A.F.; Versiani, A.F.; Barbosa-Stancioli, E.F.; dos Reis, J.K.P.; dos Reis, J.G.A.C.; da Fonseca, F.G. Developing a Feline Immunodeficiency Virus Subtype B Vaccine Prototype Using a Recombinant MVA Vector. Vaccines 2022, 10, 1717. [Google Scholar] [CrossRef]
- Daian e Silva, D.S.O.; Cox, L.J.; Rocha, A.S.; Lopes-Ribeiro, Á.; Souza, J.P.C.; Franco, G.M.; Prado, J.L.C.; Pereira-Santos, T.A.; Martins, M.L.; Coelho-dos-Reis, J.G.A.; et al. Preclinical Assessment of an Anti-HTLV-1 Heterologous DNA/MVA Vaccine Protocol Expressing a Multiepitope HBZ Protein. Virol. J. 2023, 20, 304. [Google Scholar] [CrossRef] [PubMed]
- Daian e Silva, D.S.O.; Pinho, T.M.G.; Rocha, R.P.; Oliveira, S.B.; Franco, G.M.; Barbosa-Stancioli, E.F.; Da Fonseca, F.G. Preclinical Evaluation of a Recombinant MVA Expressing the Hemagglutinin-Neuraminidase Envelope Protein of Parainfluenza Virus 5 (Mammalian orthorubulavirus 5). Vet. Vaccine 2023, 2, 100027. [Google Scholar] [CrossRef]
- Nave, L.; Margalit, I.; Tau, N.; Cohen, I.; Yelin, D.; Lienert, F.; Yahav, D. Immunogenicity and Safety of Modified Vaccinia Ankara (MVA) Vaccine—A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines 2023, 11, 1410. [Google Scholar] [CrossRef]
- Franceschi, V.; Parker, S.; Jacca, S.; Crump, R.W.; Doronin, K.; Hembrador, E.; Pompilio, D.; Tebaldi, G.; Estep, R.D.; Wong, S.W.; et al. BoHV-4-based vector single heterologous antigen delivery protects STAT1(−/−) mice from monkeypoxvirus lethal challenge. PLoS Negl. Trop. Dis. 2015, 9, e0003850. [Google Scholar] [CrossRef]
- Del Medico Zajac, M.P.; Zanetti, F.A.; Esusy, M.S.; Federico, C.R.; Zabal, O.; Valera, A.R.; Calamante, G. Induction of both local immune response in mice and protection in a rabbit model by intranasal immunization with modified vaccinia Ankara virus expressing a secreted form of bovine herpesvirus 1 glycoprotein D. Viral Immunol. 2017, 30, 70–76. [Google Scholar] [CrossRef]
- Silva, D.G.D.; Carvalho, I.L.Q.; Toscano, E.C.B.; Santos, B.A.S.S.; Oliveira, B.S.; Campos, M.A.; Fonseca, F.G.; Camargos, Q.M.; Sousa, G.F.; Caliari, M.V.; et al. Brain-derived neurotrophic factor is downregulated after bovine alpha-herpesvirus 5 infection in both wild-type and TLR3/7/9 deficient mice. J. Vet. Med. Sci. 2021, 83, 180–186. [Google Scholar] [CrossRef]
- Dong, N.; Nichols, H.; Sun, Q.; Chen, X.; Zheng, J.; Guan, Z.; Zhang, H.; Davison, A.; Wezel, Y.; Li, Z.; et al. Bovine herpesvirus-4 based vaccine provides protective immunity against Streptococcus suis disease in a rabbit model. Vaccines 2023, 11, 1004. [Google Scholar] [CrossRef]
- Wen, X.; Tong, X.; Wang, M.; Wang, J.; Ni, H.; Ran, X. Protective immunity following vaccination with a recombinant multiple-epitope protein of bovine herpesvirus type 1 in a rabbit model. Appl. Microbiol. Biotechnol. 2020, 104, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [Google Scholar] [CrossRef]
- Schuler, M.M.; Nastke, M.-D.; Stevanović, S. SYFPEITHI. In Immunoinformatics; Springer: Totowa, NJ, USA, 2007; pp. 75–93. [Google Scholar]
- Cserző, M.; Eisenhaber, B.; Eisenhaber, F.; Magyar, C.; Simon, I. The First Quarter Century of the Dense Alignment Surface Transmembrane Prediction Method. Int. J. Mol. Sci. 2023, 24, 14016, Erratum in Int. J. Mol. Sci. 2024, 25, 3422. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER Server: New Development for Protein Structure and Function Predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved Predictions of MHC Antigen Presentation by Concurrent Motif Deconvolution and Integration of MS MHC Eluted Ligand Data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.N.; Høie, M.H.; Deleuran, S.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred 3.0: Improved B-cell Epitope Prediction Using Protein Language Models. Protein Sci. 2022, 31, e4497. [Google Scholar] [CrossRef] [PubMed]
- Heydari Zarnagh, H.; Hassanpour, K.; Rasaee, M.J. Constructing Chimeric Antigen for Precise Screening of HTLV-I Infection. Iran. J. Allergy Asthma Immunol. 2015, 14, 427–436. [Google Scholar]
- Van Drunen Littel-Van Den Hurk, S.; Garzon, S.; Van Den Hurk, J.V.; Babiuk, L.A.; Tijssen, P. The Role of the Major Tegument Protein VP8 of Bovine herpesvirus-1 in Infection and Immunity. Virology 1995, 206, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Boni, M.; Diluca, D.; Salvatori, D.; Vita, A.; Cassai, E. A Combined Bovine herpesvirus 1 GB-GD DNA Vaccine Induces Immune Response in Mice. Comp. Immunol. Microbiol. Infect. Dis. 2005, 28, 155–166. [Google Scholar] [CrossRef]
- Kumar, S.; Nan, L.; Kalodimou, G.; Jany, S.; Freudenstein, A.; Brandmüller, C.; Müller, K.; Girl, P.; Ehmann, R.; Guggemos, W.; et al. Implementation of an immunoassay based on the MVA-T7pol-expression system for rapid identification of immunogenic SARS-CoV-2 antigens: A proof-of-concept study. Int. J. Mol. Sci. 2024, 25, 10898. [Google Scholar] [CrossRef]
- Pérez, P.; Lázaro-Frías, A.; Zamora, C.; Sánchez-Cordón, P.J.; Astorgano, D.; Luczkowiak, J.; Delgado, R.; Casasnovas, J.M.; Esteban, M.; García-Arriaza, J. A single dose of an MVA vaccine expressing a prefusion-stabilized SARS-CoV-2 spike protein neutralizes variants of concern and protects mice from a lethal SARS-CoV-2 infection. Front. Immunol. 2022, 12, 824728. [Google Scholar] [CrossRef]
- Tscherne, A.; Kalodimou, G.; Kupke, A.; Rohde, C.; Freudenstein, A.; Jany, S.; Kumar, S.; Sutter, G.; Krähling, V.; Becker, S.; et al. Rapid development of modified vaccinia virus Ankara (MVA)-based vaccine candidates against Marburg virus suitable for clinical use in humans. Vaccines 2024, 12, 1316. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Palhares, R.M.; Andrade, G.I.; Piscitelli Mansur, A.A.; Barbosa-Stancioli, E.F. Improvement of viral recombinant protein-based immunoassays using nanostructured hybrids as solid support. J. Mater. Sci. Mater. Med. 2009, 20, 513–519. [Google Scholar] [CrossRef]
- Jurak, I.; Griffiths, A.; Coen, D.M. Mammalian alphaherpesvirus MiRNAs. Biochim. Biophys. Acta Gene Regul. Mech. 2011, 1809, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Ostler, J.B.; Jones, C. The Bovine herpesvirus 1 Latency-Reactivation Cycle, a Chronic Problem in the Cattle Industry. Viruses 2023, 15, 552. [Google Scholar] [CrossRef]
- Vidor, E. The Nature and Consequences of Intra- and Inter-Vaccine Interference. J. Comp. Pathol. 2007, 137, S62–S66. [Google Scholar] [CrossRef]
- Yahaya, A.A.; Sanusi, S.; Malo, F.U. Computer-Assisted Multi-Epitopes T-Cell Subunit COVID-19 Vaccine Design. Biomed. Biotechnol. Res. J. 2021, 5, 27–34. [Google Scholar] [CrossRef]
- Khairkhah, N.; Bolhassani, A.; Agi, E.; Namvar, A.; Nikyar, A. Immunological Investigation of a Multiepitope Peptide Vaccine Candidate Based on Main Proteins of SARS-CoV-2 Pathogen. PLoS ONE 2022, 17, e0268251. [Google Scholar] [CrossRef]
- Hojo-Souza, N.S.; de Castro, J.T.; Rivelli, G.G.; Azevedo, P.O.; Oliveira, E.R.; Faustino, L.P.; Salazar, N.; Bagno, F.F.; Carvalho, A.F.; Rattis, B.; et al. SpiN-Tec: A T Cell-Based Recombinant Vaccine That Is Safe, Immunogenic, and Shows High Efficacy in Experimental Models Challenged with SARS-CoV-2 Variants of Concern. Vaccine 2024, 42, 126394. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Bi, R.; Liu, X.; Han, Z.; Li, M.; Liao, X.; Xie, T.; Bai, S.; Xie, Q.; et al. A Broad-Spectrum Multiepitope Vaccine against Seasonal Influenza A and B Viruses in Mice. eBioMedicine 2024, 106, 105269. [Google Scholar] [CrossRef]
- Fatoba, A.J.; Adeleke, V.T.; Maharaj, L.; Okpeku, M.; Adeniyi, A.A.; Adeleke, M.A. Design of a Multiepitope Vaccine against Chicken Anemia Virus Disease. Viruses 2022, 14, 1456. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Peptide-Based Synthetic Vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef]
- Behl, J.D.; Verma, N.K.; Tyagi, N.; Mishra, P.; Behl, R.; Joshi, B.K. The Major Histocompatibility Complex in Bovines: A Review. ISRN Vet. Sci. 2012, 2012, 872710. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, S.; Earley, B.; Johnston, D.; McCabe, M.S.; Kim, J.W.; Taylor, J.F.; Duffy, C.; Lemon, K.; McMenamy, M.; Cosby, S.L.; et al. Whole Blood Transcriptome Analysis in Dairy Calves Experimentally Challenged with Bovine herpesvirus 1 (BoHV-1) and Comparison to a Bovine Respiratory Syncytial Virus (BRSV) Challenge. Front. Genet. 2023, 14, 1092877. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.C.; Maccari, G.; Heimeier, D.; Hammond, J.A. Highly-contiguous Bovine Genomes Underpin Accurate Functional Analyses and Updated Nomenclature of MHC Class I. HLA 2022, 99, 167–182. [Google Scholar] [CrossRef]
- Romera, S.A.; Perez, R.; Marandino, A.; LuciaTau, R.; Campos, F.; Roehe, P.M.; Thiry, E.; Maidana, S.S. Whole-genome analysis of natural interspecific recombinant viruses between bovine alphaherpesviruses 1 and 5. Virus Res. 2022, 302, 198472. [Google Scholar] [CrossRef]
- Traesel, C.K.; Bernardes, L.M.; Spilki, F.R.; Weiblen, R.; Flores, E.F. Sequence analysis of the 5′ third of glycoprotein C gene of South American bovine herpesviruses 1 and 5. Braz. J. Med. Biol. Res. 2015, 48, 470–478. [Google Scholar] [CrossRef]
- Guo, W.; Xie, J.; Liu, J.; Chen, H.; Jung, Y.S. The full-genome characterization and phylogenetic analysis of Bovine herpesvirus type 1.2 isolated in China. Front. Microbiol. 2022, 13, 1033008. [Google Scholar] [CrossRef]
- Draper, S.J.; Heeney, J.L. Viruses as Vaccine Vectors for Infectious Diseases and Cancer. Nat. Rev. Microbiol. 2010, 8, 62–73. [Google Scholar] [CrossRef]
- Volz, A.; Sutter, G. Modified Vaccinia Virus Ankara. In Vaccinia Virus and Poxvirology: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 187–243. [Google Scholar]
- Franco-Luiz, A.P.M.; Oliveira, D.B.; Pereira, A.F.; Gasparini, M.C.S.; Bonjardim, C.A.; Ferreira, P.C.P.; de Souza Trindade, G.; Puentes, R.; Furtado, A.; Abrahão, J.S.; et al. Detection of Vaccinia Virus in Dairy Cattle Serum Samples from 2009, Uruguay. Emerg. Infect. Dis. 2016, 22, 2174–2177. [Google Scholar] [CrossRef]
- Costa, G.B.; Augusto, L.T.S.; Leite, J.A.; Ferreira, P.C.P.; Bonjardim, C.A.; Abrahão, J.S.; Kroon, E.G.; Moreno, E.C.; de Souza Trindade, G. Seroprevalence of Orthopoxvirus in Rural Brazil: Insights into Anti-OPV Immunity Status and Its Implications for Emergent Zoonotic OPV. Virol. J. 2016, 13, 121. [Google Scholar] [CrossRef]
- Abrahão, J.S.; de Souza Trindade, G.; Pereira-Oliveira, G.; de Oliveira Figueiredo, P.; Costa, G.; Moreira Franco-Luiz, A.P.; Lopes Assis, F.; Bretas de Oliveira, D.; Mattos Paim, L.R.; de Araújo Oliveira, C.E.; et al. Detection of Vaccinia Virus during an Outbreak of Exanthemous Oral Lesions in Brazilian Equids. Equine Vet. J. 2017, 49, 221–224. [Google Scholar] [CrossRef]
- Au, W.Y.; Cheung, P.P.-H. Effectiveness of Heterologous and Homologous Covid-19 Vaccine Regimens: Living Systematic Review with Network Meta-Analysis. BMJ 2022, 377, e069989. [Google Scholar] [CrossRef]
- Quinan, B.R.; Flesch, I.E.A.; Pinho, T.M.G.; Coelho, F.M.; Tscharke, D.C.; da Fonseca, F.G. An Intact Signal Peptide on Dengue Virus E Protein Enhances Immunogenicity for CD8+ T Cells and Antibody When Expressed from Modified Vaccinia Ankara. Vaccine 2014, 32, 2972–2979. [Google Scholar] [CrossRef]
- Gherardi, M.M.; Esteban, M. Recombinant Poxviruses as Mucosal Vaccine Vectors. J. Gen. Virol. 2005, 86, 2925–2936. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, I.M.; Moss, B.; Strober, W.; Berzofsky, J.A. Mucosal Vaccination Overcomes the Barrier to Recombinant Vaccinia Immunization Caused by Preexisting Poxvirus Immunity. Proc. Natl. Acad. Sci. USA 1999, 96, 4512–4517. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.F.; Del Médico Zajac, M.P.; Zanetti, F.A.; Valera, A.R.; Zabal, O.; Calamante, G. Recombinant MVA Expressing Secreted Glycoprotein D of BoHV-1 Induces Systemic and Mucosal Immunity in Animal Models. Viral Immunol. 2011, 24, 331–339. [Google Scholar] [CrossRef] [PubMed]



| Group | Prime | Boost 1 | Boost 2 |
|---|---|---|---|
| 1 | PBS | PBS | PBS |
| 2 | MVA-GFP 1.0 × 107 PFU | MVA-GFP 1.0 × 107 PFU | MVA-GFP 1.0 × 107 PFU |
| 3 | MVA-RecBoAHV 1.0 × 107 PFU | MVA-RecBoAHV 1.0 × 107 PFU | MVA-RecBoAHV 1.0 × 107 PFU |
| 4 | RecBoAHV 1.0 µg | MVA-RecBoAHV 1.0 × 107 PFU | MVA-RecBoAHV 1.0 × 107 PFU |
| 5 | RecBoAHV 1.0 µg | RecBoAHV 1.0 µg | RecBoAHV 1.0 µg |
| 6 | BoAHV-1 + BoAHV-5 (inactivated antigens) 1 × 106 TCID50 | BoAHV-1 + BoAHV-5 (inactivated antigens) 1 × 106 TCID50 | BoAHV-1 + BoAHV-5 (inactivated antigens) 1 × 106 TCID50 |
| Protein | Virus | Peptide | Amino Acid Sequence | Position (Protein ID) |
|---|---|---|---|---|
| gB (B and T cells) | BoAHV-1 | Pep1 | HREHTSYSPERFQQIEGYYKRDMATGRRLKEPVSRNFL | 319–356 (CAA06106.1) |
| BoAHV-5 | 326–363 (AAR86134.1) | |||
| gD (B cells) | BoAHV-1 | Pep2 | EAVRRHARAYNATVI | 92–106 (CAA06145.1) |
| BoAHV-5 | IADPQVGRTLWGAVRRNARTYNATVIWYKIESGCA | 82–116 (AAR86173.1) | ||
| gD (T cells) | BoAHV-1 | Pep3 | IMAAPARLVEGQ | 161–172 (CAA06145.1) |
| BoAHV-5 | FAYPTDDELGLVMAAPARLAEGQYRRALYIDG | 151–182 (AAR86173.1) | ||
| Tegument phosphoprotein (T cells) | BoAHV-1 | Pep4 | DEDTSEDENVYDYIDGDSSD | 62–81 (CAA06087.1) |
| BoAHV-5 | 62–81 (AAR86115.1) | |||
| RecBoAHV | HREHTSYSPERFQQIEGYYKRDMATGRRLKEPVSRNFLGSGSGEAVRRHARAYNATVIGSGSGIMAAPARLVEGQGSGSGDEDTSEDENVYDYIDGDSSD | |||
| Animal Model | Immunizing Agent | ELISA IgG | Prime | Boost 1 | Boost 2 | ELISA IgM | Prime | Boost 1 | Boost 2 |
|---|---|---|---|---|---|---|---|---|---|
| Rabbit | RecBoAHV | Anti-BoAHV-1 | 1.14 | 0.77 | 7.89 | - | - | - | - |
| Anti-BoAHV-5 | 1.11 | 1.22 | 6.22 | - | - | - | - | ||
| MVA HO | Anti-BoAHV-1 | - | - | 2.94 | - | - | - | - | |
| Anti-BoAHV-5 | - | - | 5.39 | - | - | - | - | ||
| Mice | RecBoAHV | Anti-BoAHV-1 | 1.08 | 1.45 | 1.60 | Anti-BoAHV-1 | 1.28 | 1.38 | 1.72 |
| Anti-BoAHV-5 | 1.35 | 1.38 | 1.75 | Anti-BoAHV-5 | 1.45 | 1.65 | 1.71 | ||
| MVA-RecBoAHV HO | Anti-BoAHV-1 | 1.11 | 1.27 | 1.39 | Anti-BoAHV-1 | 1.19 | 1.32 | 1.57 | |
| Anti-BoAHV-5 | 1.22 | 1.40 | 1.85 | Anti-BoAHV-5 | 1.18 | 1.19 | 1.43 | ||
| MVA-RecBoAHV HE | Anti-BoAHV-1 | 1.43 | 1.68 | 2.74 | Anti-BoAHV-1 | 1.81 | 1.93 | 2.05 | |
| Anti-BoAHV-5 | 1.93 | 2.49 | 3.90 | Anti-BoAHV-5 | 4.18 | 4.80 | 2.98 | ||
| Inactivated BoAHV1/5 | Anti-BoAHV-1 | 1.27 | 1.48 | 1.83 | Anti-BoAHV-1 | 1.29 | 1.63 | 1.90 | |
| Anti-BoAHV-5 | 1.25 | 1.41 | 2.00 | Anti-BoAHV-5 | 2.16 | 1.86 | 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, A.A.S.; Pereira, S.H.; Laguardia-Nascimento, M.; Ferrari, A.B.; Cox, L.J.; Rocha, R.P.; Leocádio, V.A.T.; Ribeiro, Á.L.; Lourenço, K.L.; Da Fonseca, F.G.; et al. A Multi-Epitope Recombinant Vaccine Candidate Against Bovine Alphaherpesvirus 1 and 5 Elicits Robust Immune Responses in Mice and Rabbits. Vaccines 2025, 13, 1115. https://doi.org/10.3390/vaccines13111115
Barbosa AAS, Pereira SH, Laguardia-Nascimento M, Ferrari AB, Cox LJ, Rocha RP, Leocádio VAT, Ribeiro ÁL, Lourenço KL, Da Fonseca FG, et al. A Multi-Epitope Recombinant Vaccine Candidate Against Bovine Alphaherpesvirus 1 and 5 Elicits Robust Immune Responses in Mice and Rabbits. Vaccines. 2025; 13(11):1115. https://doi.org/10.3390/vaccines13111115
Chicago/Turabian StyleBarbosa, Aline Aparecida Silva, Samille Henriques Pereira, Mateus Laguardia-Nascimento, Amanda Borges Ferrari, Laura Jorge Cox, Raissa Prado Rocha, Victor Augusto Teixeira Leocádio, Ágata Lopes Ribeiro, Karine Lima Lourenço, Flávio Guimarães Da Fonseca, and et al. 2025. "A Multi-Epitope Recombinant Vaccine Candidate Against Bovine Alphaherpesvirus 1 and 5 Elicits Robust Immune Responses in Mice and Rabbits" Vaccines 13, no. 11: 1115. https://doi.org/10.3390/vaccines13111115
APA StyleBarbosa, A. A. S., Pereira, S. H., Laguardia-Nascimento, M., Ferrari, A. B., Cox, L. J., Rocha, R. P., Leocádio, V. A. T., Ribeiro, Á. L., Lourenço, K. L., Da Fonseca, F. G., & Barbosa-Stancioli, E. F. (2025). A Multi-Epitope Recombinant Vaccine Candidate Against Bovine Alphaherpesvirus 1 and 5 Elicits Robust Immune Responses in Mice and Rabbits. Vaccines, 13(11), 1115. https://doi.org/10.3390/vaccines13111115








