Abstract
Cancer immunotherapy has redefined oncology’s goals, aiming for durable systemic immunity rather than mere cytoreduction. However, many solid tumors remain refractory due to immunosuppressive microenvironments and antigenic heterogeneity. Local tumor ablation techniques—including radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, irreversible electroporation (IRE), and high-intensity focused ultrasound (HIFU)—are being re-evaluated beyond their historic cytoreductive role. This comprehensive review synthesizes the paradigm of tumor ablation as an in situ vaccination strategy, a concept that leverages the tumor itself as a source of antigens and the ablation process to generate endogenous adjuvants. We detail the mechanistic underpinnings, highlighting how ablation induces immunogenic cell death (ICD), releasing damage-associated molecular patterns (DAMPs) such as calreticulin, ATP, HMGB1, and cytosolic DNA. These signals activate innate immunity via pathways like cGAS-STING, promote dendritic cell maturation, and facilitate epitope spreading. We critically examine the determinants of efficacy, including the critical impact of ablation modality on the “DAMP signature,” the necessity of complete ablation, and the pivotal role of the host’s immune contexture. Furthermore, we explore the induction of tertiary lymphoid structures (TLS) as a key anatomical site for sustained immune priming. Translational strategies are extensively discussed, focusing on optimizing procedural techniques, rationally combining ablation with immune checkpoint inhibitors (ICIs) and innate immune agonists, and developing a robust biomarker framework. By adopting the core principles of vaccinology—meticulous attention to antigen, adjuvant, route, and schedule—ablation can be engineered into a reproducible platform for systemic immunotherapy. This review concludes by addressing current limitations and outlining a roadmap for clinical translation, positioning interventional oncology as a central discipline in the future of immuno-oncology.
1. Introduction
The landscape of oncology has been fundamentally reshaped by immunotherapy, shifting the therapeutic paradigm from direct tumor cytotoxicity to empowering the host’s immune system to achieve durable, systemic disease control. Immune checkpoint inhibitors (ICIs), which target regulatory pathways such as PD-1/PD-L1 and CTLA-4, have demonstrated unprecedented long-term survival benefits across a spectrum of malignancies [1,2]. Similarly, bispecific T-cell engagers and adoptive cell therapies, such as CAR-T cells, represent a new frontier of engineered immunity [3]. Despite these advances, a significant proportion of patients with solid tumors derive little or no benefit. Primary and acquired resistance remain formidable challenges, often driven by an immunosuppressive tumor microenvironment (TME), low tumor mutational burden (TMB), poor antigen presentation, and profound antigenic heterogeneity that allows for immune escape [4,5].
Historically, local tumor ablation techniques were developed as minimally invasive tools for cytoreduction. Modalities such as radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, irreversible electroporation (IRE), and high-intensity focused ultrasound (HIFU) offered a means to destroy tumors percutaneously with minimal morbidity, particularly for patients who were poor surgical candidates [6,7]. The primary endpoint was local control, with any systemic effect considered an ancillary benefit, if considered at all [7].
Over the past decade, this perspective has undergone a radical transformation. A growing body of evidence suggests that the therapeutic effect of ablation extends far beyond the physical destruction of tissue [8,9]. It is now understood that ablating a tumor in situ can initiate a cascade of immunologic events that closely mimic a conventional vaccination. This has given rise to the powerful concept of “in situ vaccination” [10].
The in situ vaccination paradigm posits that the controlled destruction of tumor tissue in its native anatomical and immunological context provides the two fundamental components of a classical vaccine:
- Antigen Source: The tumor itself serves as a comprehensive, personalized library of tumor-associated antigens (TAAs) and neoantigens. This library reflects the full clonal heterogeneity of the malignancy, presenting a diverse antigenic repertoire to the immune system that is impossible to replicate with exogenously manufactured vaccines fully [11].
- Endogenous Adjuvant: The ablation procedure induces a specific form of cell death known as immunogenic cell death (ICD). ICD is characterized by the spatiotemporal release of endogenous danger signals, or damage-associated molecular patterns (DAMPs) [12]. These include pre-apoptotic surface exposure of calreticulin (an “eat-me” signal), extracellular release of ATP and HMGB1, and the leakage of cytosolic DNA [13,14]. These DAMPs bind to pattern recognition receptors (PRRs) on innate immune cells, activating critical pathways such as cGAS-STING and triggering robust type I interferon (IFN-I) responses, which are essential for effective dendritic cell (DC) licensing and T-cell priming [15,16,17].
From a vaccinology perspective, ablation thus serves a sophisticated function: it transforms the tumor into an in situ depot that co-delivers a personalized antigen payload with a potent, locally released adjuvant cocktail [18,19]. In clinical oncology, the most striking manifestation of this systemic immunity is the abscopal effect (from the Latin ab—“away from” and scopus—“target”)—the regression of metastatic lesions outside the localized treatment field following local therapy [19]. This conceptual reorientation aligns with the broader trend in oncology of re-interpreting local physical interventions—most notably radiotherapy—in immunologic terms. The abscopal effect, a phenomenon in which localized treatment induces regression of metastatic disease at distant sites, is the classic manifestation of systemic immune activation [20,21,22,23].
The unique appeal of ablation as an in situ vaccine lies in its universality and simplicity. Unlike peptide, DNA, or dendritic-cell vaccines that require prior antigen discovery, prediction, synthesis, and complex ex vivo manufacturing, ablation is agnostic to tumor genotype and histology [24,25]. Each ablated lesion automatically provides the patient’s complete, native antigenic repertoire in its natural conformation and post-translational state, presented within the powerful inflammatory context of tissue disruption and DAMP release [24,26]. Consequently, ablation-induced immunity has the potential to overcome the significant challenges posed by inter- and intra-tumoral heterogeneity, a major limitation of current targeted immunotherapies [27]. However, the efficacy of this approach can be modulated by tumor-intrinsic factors, such as the suppression of key innate immune sensing pathways, which may predict resistance and require additional combinatorial strategies [28,29,30]. Table 1 compares classic systemic vaccination with in situ vaccination via tumor ablation, highlighting their similarities, differences, advantages, and disadvantages.

Table 1.
Comparison Between Classic Systemic Vaccination and In Situ Vaccination by Tumor Ablation.
In this extensive review, we delve into the mechanistic foundations underlying tumor ablation as an in situ vaccination platform. We critically examine the key determinants of its efficacy, drawing explicit parallels to classical vaccinology principles of antigen, adjuvant, route, and schedule. We then propose and elaborate on advanced translational strategies to standardize and enhance this approach, focusing on combination therapies with ICIs and innate immune agonists, as well as on the development of a rigorous biomarker framework. Finally, we survey the evolving clinical trial landscape, discuss safety considerations, address current limitations, and offer a forward-looking perspective on integrating this promising modality into the next generation of cancer immunotherapy.
2. Mechanistic Basis of Ablation as Vaccination
2.1. Antigenic Breadth and the Catalyst of Epitope Spreading
A fundamental tenet of successful vaccination is the exposure of the immune system to a sufficient breadth of antigens to stimulate a diverse, durable, and robust immune response capable of overcoming escape variants. Tumor ablation achieves this organically by simultaneously releasing a vast array of canonical tumor-associated antigens (e.g., MUC1, CEA, HER2, NY-ESO-1) alongside a unique set of patient-specific neoantigens generated by the tumor’s individual mutational landscape [26,31]. This stands in stark contrast to synthetic peptide or mRNA vaccines, which are inherently restricted to a limited set of predefined epitopes, often selected based on bioinformatic predictions that may not accurately reflect actual immunogenicity or clonal representation [32,33].
This comprehensive antigenic exposure is the critical catalyst for a process known as epitope spreading (also called antigen spreading or determinant spreading) [34]. This is a phenomenon in which the immune response, initially directed against a limited number of immunodominant epitopes released by the ablation, expands over time to recognize additional, secondary antigens present on tumor cells [35]. This occurs through the efficient phagocytosis of apoptotic/necrotic tumor debris by antigen-presenting cells (APCs), which process and present a much wider array of antigens than were initially targeted [36,37]. Epitope spreading is a hallmark of a productive, broadening immune response and a key defense against tumor escape by downregulating single antigens [34,38].
In preclinical models, ablation-induced epitope spreading correlates directly with the development of a more diverse T-cell receptor (TCR) repertoire intratumorally and peripherally, and is a strong predictor of improved local and systemic tumor control [39]. Clinically, epitope spreading has been identified as a key correlate of response and durable clinical benefit in patients treated with ICIs and other immunotherapies [40]. It may account for the long-term, ongoing regression seen in a subset of patients. Thus, the value of ablation lies not only in the quantitative release of a large antigenic payload but also in its qualitative ability to drive the diversification of immune recognition, thereby significantly reducing the likelihood of clonal escape and tumor recurrence [41].
2.2. Immunogenic Cell Death: The Engine of Endogenous Adjuvanticity
The immunologic potency of ablation is not merely a function of antigen release; it is critically dependent on the quality of cell death [42]. Immunogenic cell death (ICD) is a functionally unique form of cell death that activates the adaptive immune system against dead-cell-associated antigens. It is characterized by the stereotyped, sequential emission of stress and damage signals that, together, act as a powerful endogenous adjuvant system [43,44].
The well-defined biochemical hallmarks of ICD include:
- Pre-apoptotic exposure of calreticulin (CRT): Normally residing in the endoplasmic reticulum, CRT translocates to the cell surface during early ICD. It acts as a potent “eat-me” signal, engaging CD91 receptors on phagocytes such as dendritic cells and macrophages, thereby promoting the phagocytosis of tumor cell corpses and subsequent cross-presentation of tumor antigens to CD8+ T cells [43,45].
- Extracellular release of adenosine triphosphate (ATP): Released from dying cells, ATP serves a dual function. First, it acts as a potent chemoattractant for monocytes and dendritic cells by engaging P2Y2 receptors [46,47]. Second, it binds to the P2RX7 purinergic receptor on the surface of dendritic cells, triggering the assembly and activation of the NLRP3 inflammasome [43,48]. This leads to the secretion of pro-inflammatory cytokines, such as IL-1β, which is critical for the polarization of IFN-γ-producing CD8+ T cells [49,50].
- Release of high-mobility group box 1 (HMGB1): This nuclear protein is released passively during late-stage necrosis. HMGB1 binds to Toll-like receptor 4 (TLR4) on dendritic cells, promoting their maturation, enhancing antigen processing, and stimulating the production of pro-inflammatory cytokines such as TNF-α and IL-6 [51]. The interaction between HMGB1 and TLR4 is crucial for effective cross-priming [51].
- Leakage of cytosolic and nuclear DNA: The loss of membrane integrity leads to the release of DNA into the extracellular space and the TME [52,53,54]. This DNA is sensed by the cyclic GMP-AMP synthase (cGAS), which catalyzes the production of cyclic GMP-AMP (cGAMP) [55]. cGAMP acts as a second messenger that activates the stimulator of interferon genes (STING) pathway in surrounding dendritic cells and other immune cells [56]. STING activation is a master regulator of type I interferon (IFN-I) production, arguably the most critical cytokine linking innate immune sensing to adaptive T-cell immunity [57].
Collectively, this coordinated cascade of DAMPs licenses conventional type 1 dendritic cells (cDC1s)—a specialized subset equipped for cross-presentation—to efficiently phagocytose tumor debris, process the released antigens, and present them on MHC-I molecules to prime naive CD8+ T cells in the draining lymph nodes [58]. In the language of vaccinology, the ablation procedure provides a built-in, multi-component adjuvant system, effectively obviating the need for the synthetic adjuvants (e.g., alum, MF59, AS01) required in classical vaccine formulations [59]. It is important to emphasize that different ablation modalities yield distinct “DAMP signatures”—varying in the relative amounts and timing of these signals—which profoundly influence the magnitude, quality, and durability of the downstream adaptive immune response [26].
2.3. Anatomical Re-Engineering: Lymph Nodes and Tertiary Lymphoid Structures as Priming Hubs
Following ablation, the released antigens and DAMPs are transported via lymphatic drainage to the tumor-draining lymph nodes (TDLNs), which are the classical and primary sites for the initiation of adaptive immune responses [60]. Here, resident and newly arrived dendritic cells present tumor antigens to naïve T cells, leading to their activation, clonal expansion, and differentiation into effector T cells [61].
However, a more recently appreciated, and potentially equally important, mechanism is ablation’s ability to remodel the local tumor microenvironment to support the de novo formation and maturation of tertiary lymphoid structures (TLSs) [62]. TLSs are ectopic lymphoid aggregates that develop in non-lymphoid organs at sites of chronic inflammation, including cancer [63]. They are highly organized structures that recapitulate the architecture and functionality of secondary lymphoid organs, containing distinct T-cell zones, B-cell follicles, germinal centers, follicular dendritic cell networks, and high endothelial venules (HEVs) that facilitate lymphocyte entry [64].
The presence of mature, well-organized TLS within the TME is consistently associated with a favorable prognosis and improved responses to ICIs across multiple cancer types, including melanoma, lung cancer, and sarcoma [65,66,67,68,69]. They are thought to function as local “immune hubs” or “booster stations,” enabling ongoing antigen presentation, T-cell priming, B-cell activation, and antibody class switching directly at the tumor site [65,66,67,68,69,70]. This creates a sustained inflammatory microenvironment that can counteract local immunosuppression [70,71].
Spatial transcriptomics and single-cell immune profiling have revealed that TLS-rich tumors harbor expanded clones of T and B cells and show enriched signatures for IFN-γ signaling and germinal center reactions [72,73]. The post-ablation induction of TLSs can therefore function as a persistent local site for immune education and amplification, fostering durable T-cell/B-cell collaboration and maintaining immune pressure on the tumor [72,74]. Recent technological advances in spatial immune repertoire mapping, such as Slide-TCR-seq and CODEX, provide powerful tools to assess TLS maturation after ablation quantitatively and to track the spatial dynamics of epitope spreading and clonal expansion within these structures [75,76].
2.4. Systemic Manifestation: The Abscopal Effect and Beyond
Preclinical evidence robustly demonstrates that ablation can generate a wave of tumor-specific CD8+ T cells that enter the circulation, traffic to distant, non-treated metastatic lesions, and mediate their regression [77,78]. The defining feature of any successful vaccine is the establishment of systemic immunity that can survey and protect the entire organism. The immune basis of this effect is confirmed by experiments where depletion of CD8+ T cells, but not CD4+ T cells, completely abrogates the anti-tumor response at secondary sites [79,80].
While initially and most famously described in patients receiving radiotherapy, bona fide abscopal-like responses have now been reported following RFA, cryoablation, and IRE, particularly when these modalities are combined with ICIs [81]. Although the incidence of dramatic abscopal effects remains relatively low in retrospective analyses, these case reports and small series provide crucial proof-of-principle that ablation can indeed function as an effective in situ vaccine in humans [82]. The paramount challenge for the field is to transform these serendipitous observations into predictable and reproducible therapeutic outcomes through rational combination strategies, optimized treatment parameters, and patient selection based on biomarkers, including emerging predictors of innate immune resistance.
3. Determinants of Vaccinal Efficacy: A Vaccinologist’s Perspective
Although tumor ablation holds clear theoretical potential as an in situ vaccine, its immunologic outcome is not guaranteed and is highly context-dependent [83]. Not every ablation procedure results in productive systemic immunity; in some cases, it may even reinforce tolerance or accelerate immunosuppression by releasing inhibitory factors [10,84]. As with classical vaccinology, efficacy depends on the careful optimization of multiple interacting parameters [84].
3.1. Adjuvant Quality: Decoding the “DAMP Signature”
In vaccinology, the choice of adjuvant is paramount and tailored to induce a specific immune response (e.g., Th1 vs. Th2) [85,86]. Similarly, tumor ablation generates endogenous adjuvants in the form of DAMPs. Still, the profile, or “signature,” of these signals varies dramatically depending on the physical modality used and its specific technical parameters [87].
- Thermal Ablation (RFA/MWA): These techniques induce rapid coagulative necrosis through high-temperature heating, resulting in the release of large quantities of tumor antigens [88]. However, extreme heat can also cause widespread protein denaturation, potentially destroying conformational epitopes and reducing antigenic fidelity [89]. Thermal ablation produces abundant ATP, HMGB1, and reactive oxygen species (ROS), which are potently inflammatory [90]. A significant limitation is the frequent creation of a perfused, sublethal peripheral rim due to heat-sink effects from adjacent blood vessels [91]. This rim of stressed but viable tissue often becomes a potent source of immunosuppressive cytokines, such as IL-6, VEGF, and HGF, which promote angiogenesis and recruit myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) [92,93]. Thus, while thermal ablation generates strong adjuvanticity, it carries a concomitant risk of inducing counter-regulatory immunosuppressive mechanisms [94].
- Cryoablation: This modality utilizes freeze–thaw cycles to cause necrotic cell death. A key advantage is that it preserves the structural integrity of proteins and lipids, thereby maintaining antigenic fidelity and enabling T-cell receptors (TCRs) and B-cell receptors (BCRs) to recognize conformational determinants [95]. The necrotic death mode triggers a robust inflammatory response characterized by dense neutrophilic infiltration and the release of pro-inflammatory cytokines such as TNF-α and IL-1β. Clinical studies in breast and prostate cancer have shown that cryoablation is particularly effective at generating measurable systemic T-cell responses, especially when combined with checkpoint blockade [96]. A rare but serious risk is cryoshock, a systemic inflammatory response syndrome characterized by coagulopathy and multi-organ failure [97].
- Irreversible Electroporation (IRE): A non-thermal modality that uses high-voltage electrical pulses to create permanent nanoscale defects in cell membranes, leading to apoptosis and secondary necrosis. Its unique advantage is that it spares the extracellular matrix, blood vessels, and nerves, thereby preserving the native tissue scaffolding and facilitating the subsequent infiltration of immune cells into the ablation zone [98]. IRE is a particularly potent activator of the cGAS-STING axis, making it highly effective at inducing strong IFN-I–driven immune priming [99]. However, the potency of this response can be limited in tumors with intrinsic suppression of the cGAS-STING pathway, a phenomenon recently documented in specific cancer subtypes [100]. The precision of the electric field is critical; incomplete electroporation can result in zones of non-immunogenic apoptosis, undermining the vaccinal effect [101,102].
- High-Intensity Focused Ultrasound (HIFU): HIFU induces mechanical and thermal stress through acoustic cavitation and heating, generating DAMPs and exposing new epitopes. Its completely non-invasive nature is a major advantage [103]. However, its reproducibility and efficacy are highly dependent on technical factors such as acoustic windows, lesion depth, and local hemodynamics. While still largely experimental, early clinical trials in patients with hepatic metastases suggest that HIFU can synergize with ICIs to enhance T-cell infiltration and systemic cytokine responses [104].
A comparative summary of modality-specific immunologic features is provided in Table 2.

Table 2.
Immunologic profiles of ablation modalities.
3.2. Antigen Dose and the Imperative of Completeness
In classical vaccines, antigen dose and formulation are critical determinants of immunogenicity. Suboptimal doses may fail to prime the immune system effectively, while excessive doses can paradoxically lead to T-cell exhaustion and tolerance. Analogous principles apply directly to ablation-based in situ vaccination.
- Incomplete Ablation: The presence of residual viable tumor tissue is perhaps the single greatest impediment to successful in situ vaccination [105]. This residual tissue often resides in a hypoxic, nutrient-deprived state that sustains a profoundly immunosuppressive niche, dominated by regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and the secretion of inhibitory cytokines like VEGF, TGF-β, and IL-10 [106]. This microenvironment can actively trap infiltrating effector T cells, induce their functional exhaustion, and prevent the establishment of systemic immunity [107]. In clinical practice, incomplete ablation has been associated with accelerated local progression and increased metastatic spread in diseases like hepatocellular carcinoma, underscoring the danger of creating an immunosuppressive “escape niche” [108].
- Complete Ablation: Ensuring the complete destruction of the target lesion is paramount. It maximizes the antigenic payload delivered to the immune system while simultaneously eliminating a major source of immunosuppressive cells and factors. Preclinical studies consistently demonstrate that complete ablation results in significantly stronger and more durable systemic T-cell responses compared to partial ablation [108]. In HCC, the completeness of ablation is a well-established key predictor of recurrence-free survival, and this benefit appears magnified when ablation is combined with adjuvant ICI therapy [107].
- Antigen Integrity: It is not only the quantity of antigen that matters, but also its quality. As mentioned, thermal denaturation induced by RFA/MWA can reduce the recognition of protein epitopes by TCRs and BCRs [108]. In contrast, modalities such as cryoablation and IRE better preserve the native conformation of antigens, potentially enhancing their immunogenicity. This is analogous to the difference between a whole-cell inactivated vaccine and a denatured protein subunit [109].
Therefore, procedural optimization—utilizing advanced intraprocedural imaging (e.g., contrast-enhanced ultrasound, CT perfusion), meticulous energy delivery mapping, and real-time thermal monitoring—is essential not only for achieving local oncologic control but also for maximizing immunologic efficacy. In this refined view, the interventional oncologist acts as an immune engineer, carefully sculpting the immune response through precise tissue destruction.
3.3. Route and Delivery: The Biophysical Encoding of an Immune Response
In vaccinology, the route of administration (intramuscular, subcutaneous, intradermal, mucosal) profoundly shapes the quality of the immune response by influencing antigen persistence, drainage to lymphoid organs, and the involvement of local tissue-resident immune cells [110]. For ablation, the equivalent of “route” is the biophysical nature and spatial geometry of the injury it creates.
- The concentric thermal gradients characteristic of RFA/MWA generate distinct zones: a central necrotic core surrounded by a transition zone of stressed but viable cells [111]. This peripheral rim often secretes pro-angiogenic and immunosuppressive cytokines, analogous to a poorly adjuvanted vaccine that risks inducing tolerance rather than productive immunity [108].
- The freeze–thaw cycles of cryoablation promote necrotic death with excellent antigen preservation, akin to a whole-cell inactivated vaccine [112].
- The electric-field permeabilization of IRE produces a more homogeneous zone of apoptosis and necrosis while preserving the stromal architecture, analogous to a well-formulated subunit vaccine co-delivered with a potent STING-activating adjuvant [113].
- The acoustic cavitation and mechanical effects of HIFU can increase membrane permeability and expose new epitopes, resembling a vaccine delivered via nanoparticle or liposome technology [114].
Furthermore, the anatomical site of the tumor (e.g., hepatic vs. pulmonary vs. cutaneous) intrinsically influences the immune outcome due to differences in lymphatic drainage, baseline immune cell populations, and the organ-specific metabolic and immunologic environment [115]. For instance, liver ablation occurs in an organ rich in inherent tolerogenic signals (e.g., IL-10, TGF-β from Kupffer cells), which may dampen systemic priming unless counterbalanced by powerful adjuvant signals from the ablation itself or from combination therapy [116]. In contrast, ablation in the lung or skin—organs with robust lymphoid tissues and a predisposition towards immune surveillance—may be more conducive to the generation of potent effector and tissue-resident memory T-cell responses, akin to the advantages of mucosal vaccination routes [117].
3.4. Prime–Boost Scheduling: Countering Adaptive Resistance
Ablation functions most effectively as a potent priming event, generating an initial wave of antigen release and triggering the expansion of tumor-specific T-cell clones. However, tumors and their microenvironment rapidly adapt to this immune attack through a multitude of resistance mechanisms, collectively known as adaptive resistance [118,119].
These include the following:
- Rapid upregulation of PD-L1 on tumor cells and infiltrating myeloid cells.
- Recruitment and expansion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs).
- Induction of T-cell exhaustion phenotypes characterized by upregulation of multiple inhibitory receptors (e.g., PD-1, TIM-3, LAG-3).
- Secretion of inhibitory cytokines (TGF-β, IL-10).
To sustain and amplify the initial immune response, pharmacologic boosting is essential. The timing of this boost, as in classical vaccinology, is critical.
- Neoadjuvant Approach: Administering ICIs before ablation is a strategy to “precondition” or “pre-inflame” the TME. By blocking inhibitory pathways upfront, neoadjuvant ICIs can reduce baseline immunosuppressive tone, ensuring that ablation-induced antigen release occurs in a more permissive, receptive immune environment. This strategy has shown remarkable success in the surgical neoadjuvant setting for melanoma and NSCLC [120].
- Concurrent Approach: Delivering ICIs simultaneously with ablation aims to capture the peak of innate immune activation and antigen release [121]. The goal is to prevent the immediate exhaustion of newly primed T cells by blocking PD-1/PD-L1 interactions at the moment they are first engaged, potentially leading to synergistic enhancement of CD8+ T cell expansion and function.
- Adjuvant Approach: Administering ICIs after ablation is designed to consolidate the immune response and prevent relapse. This approach protects the primed T-cell population from becoming exhausted as they encounter residual micrometastatic disease [122]. The phase III IMbrave050 trial in HCC, which demonstrated a significant improvement in recurrence-free survival with adjuvant atezolizumab/bevacizumab following ablation or resection, provides the strongest clinical validation for this sequencing strategy [123].
The question of optimal timing directly parallels classical prime–boost vaccination schedules, in which the interval and order of vaccinations strongly influence the balance between generating effector cells, promoting memory formation, and preventing exhaustion. Rational design of ablation–ICI regimens will require prospective, randomized trials specifically designed to test these sequencing windows [124,125].
3.5. Host and Tumor Microenvironmental Factors: The Biological Soil
Beyond procedure-specific variables, the host’s biological context plays a decisive role in determining the outcome of in situ vaccination.
- Baseline Immune Contexture: The pre-existing state of the TME, as classified by the “immunoscore,” is highly predictive. Tumors with a pre-existing immune infiltrate (“hot” or “immune-inflamed” tumors) are significantly more likely to mount a robust systemic response post-ablation. In contrast, immune-desert (no infiltrate) or immune-excluded (infiltrate restricted to the stroma) tumors may require additional interventions (e.g., innate agonists, cytokines) to make the TME permissive before ablation can be effective [126].
- Organ-Specific Immunology: The liver, bone marrow, brain, and placenta are considered immunologically privileged sites, enriched in tolerogenic signals. Ablation in these locations may inherently require stronger adjuvant co-treatments to overcome the organ-imposed immune privilege [127].
- Systemic Host Factors: Comorbid conditions, concomitant medications (especially systemic corticosteroids and other immunosuppressants), age-related immunosenescence, and nutritional status can all blunt the immune response. Conversely, a diverse microbiome has been associated with improved responses to ICIs and may similarly modulate the adjuvanticity of ablation [128].
- Tumor Burden and Stage: Patients with low-volume, oligometastatic disease likely represent the ideal candidates for ablation-based vaccination [129]. The antigenic load from ablating a few lesions is sufficient to prime a response, yet the overall systemic immunosuppressive burden is not so entrenched as to be irreversible. In patients with high-volume disease, the sheer quantity of immunosuppressive factors may overwhelm any nascent immune response generated by ablating a single site [130].
4. Translational Strategies: Engineering a Reproducible In Situ Vaccine
The translation of tumor ablation from a cytoreductive procedure into a reliable systemic immunotherapy platform requires moving beyond opportunistic observations. In vaccinology, reproducibility is achieved through rigorous antigen standardization, controlled adjuvant delivery, and standardized prime–boost schedules. By applying these same principles, ablation can be developed into a precision in situ vaccination platform.
4.1. Pursuing Immune-Complete Ablation
The first and most critical determinant of immunologic efficacy is procedural completeness. Even minute volumes of residual tumor can act as immunosuppressive hubs, secreting VEGF, IL-10, and TGF-β, while attracting Tregs and MDSCs [131].
Technological innovations are increasingly focused on ensuring “immune-complete ablation,” moving beyond visual inspection to software-based and fusion imaging techniques that provide objective, reproducible confirmation of adequate margins, which is critical for robust trial outcomes [123,132,133].
- Intraprocedural imaging and margin assessment: Advanced techniques like contrast-enhanced ultrasound (CEUS), perfusion CT, and MR thermometry can detect sublethal zones. Furthermore, the integration of CT-CT or US-MR fusion imaging and software-based volumetric analysis of the ablation zone relative to the tumor provides a more objective assessment of the minimal ablative margin (MAM) than visual inspection alone, and it directly correlates with improved local control and potentially superior immunologic outcomes [123,132,133].
- Thermal Dose Mapping: Sophisticated software algorithms now calculate the precise thermal profile of a lesion based on energy input and local tissue properties, enabling optimized and personalized energy delivery to eliminate marginal tumor cells [134].
- AI-Assisted Navigation: Machine learning algorithms are being integrated into image-guided ablation systems to improve targeting accuracy, predict heat-sink effects, and plan optimal probe trajectories, particularly in complex anatomical locations near major vessels or ducts [135].
These tools, while developed for oncologic efficacy, should also be regarded as essential immune-quality controls. Optimizing ablation is not just about preventing local recurrence but about maximizing the vaccine-like potential of the procedure.
4.2. Augmenting Endogenous Adjuvanticity
While DAMPs provide a natural adjuvant effect, their release is inconsistent and can be counterbalanced by the simultaneous release of tolerogenic cytokines. Rational augmentation strategies aim to boost immunogenicity and actively suppress inhibitory pathways [136].
- STING Agonists: Direct injection of synthetic cyclic dinucleotides (e.g., ADU-S100, MK-1454) into the ablation cavity (the central zone of complete coagulative necrosis created at the treatment site following ablation) or peri-ablation zone can profoundly prolong and amplify local IFN-I production, enhancing DC licensing and T-cell priming. This is particularly relevant for tumors with intrinsic cGAS-STING suppression [137,138]. Numerous early-phase trials are testing this combination in pancreatic cancer, melanoma, and lymphoma.
- TLR Agonists: Local administration of TLR agonists, such as CpG oligodeoxynucleotides (TLR9) or poly(I:C) (TLR3), has been shown in murine models to synergize powerfully with cryoablation, leading to enhanced CD8+ T-cell expansion and abscopal effects [136].
- Oncolytic Viruses: Local instillation of oncolytic viruses (e.g., talimogene laherparepvec, T-VEC) after ablation can serve a dual purpose: they selectively infect and kill remaining tumor cells, and their viral pathogen-associated molecular patterns (PAMPs) provide strong adjuvant signals. Viruses engineered to express GM-CSF can further enhance local DC recruitment and activation [137].
- Nanoparticle-Based Adjuvants: The development of injectable, thermoresponsive biomaterials and liposomes represents a cutting-edge approach. These can be designed to release immunostimulatory payloads (e.g., cytokines, agonists) in a sustained manner within the ablation cavity, effectively creating a long-lasting “immune niche” that prolongs immune activation beyond the acute phase of cell death [138].
These strategies reflect the core vaccinology principle that an antigen without adequate adjuvant signaling is rarely sufficient for productive immunity. By combining ablation with rational adjuvant engineering, the goal of reproducible systemic immunity becomes increasingly feasible.
4.3. A Biomarker Framework for Vaccination Efficacy
Biomarkers are the essential bridge between mechanism and clinical translation. The objective is to develop immune equivalents of vaccine titers, enabling standardized assessment, patient stratification, and regulatory approval.
A consolidated overview of candidate biomarkers and their rationale for ablation-induced in situ vaccination is presented in Table 3.

Table 3.
Candidate biomarkers for ablation-induced in situ vaccination.
4.3.1. Pharmacodynamic Biomarkers (Measure the “Activity” of the Vaccine)
- Serum HMGB1, ATP (24–72 h post-ablation): Quantifies the acute release of key DAMPs and serves as a direct measure of ICD magnitude [139].
- IFN-I Signatures (ISG15, IFIT1, MX1, IFI44; Day 3–10): A peripheral blood mononuclear cell (PBMC) transcriptional score or serum protein level of IFN-α/β can serve as a robust readout of systemic innate immune activation, a critical step for T-cell priming [140].
- Serum IL-6, VEGF: These can serve as negative pharmacodynamic biomarkers, indicating the presence of sublethal injury and a shift towards an immunosuppressive, pro-angiogenic bias [141].
4.3.2. Immunological Biomarkers (Measure the “Response” to the Vaccine)
- TLS Detection and Scoring (Weeks 4–6 post-ablation):Multiplex immunohistochemistry (IHC) and spatial transcriptomics (e.g., GeoMx, CosMx) should be performed approximately 4–6 weeks after ablation (relative to Day 0) to quantify TLS architecture, cellular composition, and maturation state (e.g., presence of germinal centers). Mature TLSs observed at this post-ablation stage are a strong positive predictor of durable immune response [142].
- TCR Repertoire Analysis (Weeks 2–4 post-ablation):High-throughput TCR sequencing (TCR-Seq) of peripheral blood or tumor tissue obtained 2–4 weeks after ablation (relative to Day 0) allows tracking of clonal expansion and the breadth of epitope spreading. An increase in T-cell clonality and richness during this early adaptive-response window post-ablation is a favorable immunologic sign [143].
- Functional T-cell Assays (Weeks 4–6 post-ablation):Ex vivo stimulation of PBMCs with tumor lysate or peptide pools performed approximately 4–6 weeks after ablation (relative to Day 0) can quantify the frequency and polyfunctionality (production of IFN-γ, TNF-α, IL-2) of tumor-reactive T cells [144].
4.3.3. Tumor-Derived Biomarkers
- ctDNA Clearance (2–6 weeks post-ablation, relative to Day 0): The reduction and eventual clearance of circulating tumor DNA (ctDNA) within 2–6 weeks after ablation (Day 0) represents a highly promising, non-invasive biomarker for predicting systemic tumor control and recurrence-free survival in colorectal cancer, hepatocellular carcinoma (HCC), and other malignancies [145]. This interval reflects the expected window of early immune-mediated clearance of minimal residual disease.
- Tumor-Derived Exosomes (1–4 weeks post-ablation, relative to Day 0): Shifts in the protein or RNA cargo of tumor-derived exosomes isolated from plasma approximately 1–4 weeks after ablation (Day 0) can provide an early readout of tumor immunogenicity changes and stress-response signaling [146]. Such changes frequently precede measurable clinical or radiologic responses.
- Tumor-Intrinsic Biomarkers (Baseline/pre-ablation; Day −7 to 0): Assessment of tumor expression of innate-immune suppressors, such as RECQL4 or TRIM6, should be performed before ablation (Day −7 to Day 0) to identify patients who may be intrinsically resistant to ablation-induced STING activation and who could benefit from combination therapy with specific innate agonists [147].
Collectively, these biomarkers form the backbone of a vaccinology-inspired monitoring framework that can guide therapy and assess efficacy.
4.4. Standardized Assay Timepoints for Cross-Trial Interpretation
Without harmonized timing, biomarker data remain incomparable across trials and institutions. To ensure consistency, all hours, days, and months in this framework are referenced to the day of ablation (Day 0), which serves as the procedural baseline for immunologic and biomarker assessments. We propose the following standardized blood and tissue sampling schedule:
- Baseline (Pre-ablation; Day −7 to 0): Comprehensive immune profiling (flow cytometry), circulating tumor DNA (ctDNA) measurement, serum cytokines, and tumor biopsy for immune contexture and innate immune suppressor status (e.g., RECQL4, TRIM6).
- 24–72 h post-ablation (relative to Day 0): Peak release of damage-associated molecular patterns (DAMPs), including serum HMGB1 and ATP, accompanied by early cytokine changes (IL-6, VEGF, IL-10).
- Day 7–10 post-ablation: Period of peak innate immune activation characterized by elevated type I interferon (IFN-I) transcriptional signatures in peripheral blood mononuclear cells (PBMCs) and serum CXCL10.
- Week 2–4 post-ablation: Early adaptive immune-response phase, reflected by expansion of tumor-specific T-cell clones (TCR clonality in blood) and initial ctDNA kinetics.
- Week 4–6 post-ablation: Established adaptive immune response and early memory formation, assessed by comprehensive immune profiling, functional T-cell assays, and ctDNA clearance.
- Follow-up at 3, 6, and 12 months post-ablation: Long-term immune-memory and surveillance phase, including serial ctDNA monitoring and delayed assessment of tertiary lymphoid structure (TLS) formation on imaging if recurrence occurs.
This structured design mirrors vaccine immunogenicity studies, which measure antibody titers and T-cell assays at specific intervals post-vaccination [148]. Standardized immunologic windows will dramatically accelerate cross-trial interpretability and facilitate meta-analyses.
4.5. Clinical Trial Design: From Exploration to Confirmation
To date, most studies combining ablation with immunotherapy are exploratory, single-arm, or retrospective. A definitive roadmap for robust clinical translation must include:
4.5.1. Endpoint Reformation
Moving beyond local control, trials should prioritize non-index lesion endpoints, using immune-modified criteria such as irRECIST to capture abscopal effects. Recurrence-free survival (RFS) and ctDNA clearance are highly relevant surrogate endpoints. Local control should be rigorously assessed using standardized volumetric software to confirm ablation completeness, a factor now shown in randomized trials to be critical for outcomes [149,150,151].
4.5.2. Mandatory Biomarker Integration
Pre-specified biomarker panels should be a mandatory component of trial design, not an exploratory add-on. This includes not only serum and immune biomarkers but also tumor-intrinsic biomarkers of immune competence, such as the expression of proteins that regulate key pathways, like cGAS-STING (e.g., RECQL4, TRIM6), which may stratify patients who are likely to need additional innate immune agonists [152,153].
4.5.3. ICI Timing Randomization
Phase II trials should randomize patients to neoadjuvant, concurrent, or adjuvant ICI arms to definitively resolve the question of optimal sequencing.
4.5.4. Window-of-Opportunity Cohorts
Embedding small, intensive biomarker-monitoring cohorts within larger trials enables rapid qualification of predictive and pharmacodynamic biomarkers, including novel imaging biomarkers such as LAG-3 PET to track immune cell infiltration [154].
4.5.5. Multimodal Combinations
Exploring triple combinations—e.g., ablation + radiotherapy (for enhanced antigen release) + ICI, or ablation + oncolytic virus + TLR agonist—will be necessary to overcome resistance in “cold” tumors, particularly those with biomarkers indicating suppressed innate immunity [155,156].
Such rigorously designed trials are essential to determine whether ablation can consistently be transformed from a local tool into a vaccine-like systemic therapeutic modality.
5. Clinical Applications and Trials
Building on the immunologic principles outlined in the preceding sections, this chapter discusses how tumor ablation can be integrated with systemic immunotherapies to optimize both local control and systemic antitumor immunity. The goal is to translate the in situ vaccination effect into consistent clinical benefit by combining ablation with immune-checkpoint inhibitors, cytokine modulators, and pattern-recognition receptor agonists. Various temporal and mechanistic strategies—such as neoadjuvant, concurrent, and adjuvant approaches—are explored, together with emerging clinical trial frameworks that aim to standardize these interventions. Collectively, these approaches represent the next stage in the evolution of ablation from a local procedure to a reproducible immunotherapeutic platform.
5.1. Clinical Translation Across Tumor Types
The clinical trial landscape is rapidly expanding (Figure 1).
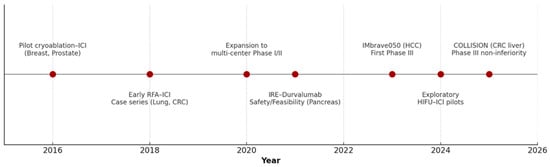
Figure 1.
Timeline of Clinical Development of In Situ Vaccination by Tumor Ablation from 2015 to 2025.
Table 4 summarizes selected key trials investigating this paradigm. Notable trends include the dominance of HCC trials (reflecting its global burden and the synergy between local and systemic therapy), the prominence of cryoablation in breast and prostate cancer, and the exploration of novel adjuvants (TLR agonists, STING agonists) in early-phase studies.

Table 4.
Clinical trials investigating ablation as in situ vaccination (2022–2025).
5.2. Safety and Nuanced Patient Selection
While generally well-tolerated, ablation carries modality-specific risks that intersect with immunologic goals [157,158].
5.2.1. Cryoablation
The robust inflammatory response is immunologically beneficial but carries a rare risk of cryoshock syndrome, a systemic inflammatory response with coagulopathy and multi-organ failure. Meticulous technique and complete freeze–thaw cycles mitigate this risk [159,160].
5.2.2. RFA/MWA
Risks include pain, post-ablation syndrome (fever, malaise), and incomplete ablation due to the heat-sink effect. The sublethal rim can be a source of immunosuppression [161,162].
5.2.3. IRE
Requires general anesthesia and precise ECG synchronization to avoid potentially fatal arrhythmias. Its non-thermal, matrix-sparing nature is a significant immunologic advantage [163,164].
5.2.4. HIFU
Non-invasive, but can be limited by bone or air interposition [165]. Risks include skin burns and cavitation-induced damage to adjacent structures [166].
Patient selection must consider both oncologic and immunologic factors. Key contraindications include active uncontrolled infections, ongoing flares of severe autoimmune disease requiring immunosuppression, and concurrent use of high-dose systemic corticosteroids. Ideal candidates are likely those with oligometastatic or low-volume metastatic disease, good performance status, and evidence of a competent immune system [167].
6. Limitations and Outlook
Despite the compelling rationale and encouraging early data, several significant limitations currently hinder the reproducibility and broad adoption of ablation as a reliable in situ vaccination strategy.
6.1. Biological and Mechanistic Heterogeneity
The immune consequences of ablation are not uniform. Identical procedures can yield opposite outcomes depending on factors such as tumor histology, genetic makeup, and baseline immune contexture [168]. For instance, while cryoablation preserves antigenicity, it can also release tolerogenic extracellular vesicles in some contexts [169]. Similarly, the ability to form functional TLS post-ablation is highly variable. Furthermore, tumor-intrinsic suppressors of immune sensor pathways have emerged as key predictive biomarkers. For example, high expression of RECQL4 in HCC or TRIM6 in microsatellite-stable gastric cancer potently suppresses the cGAS-STING pathway, leading to poor T-cell infiltration and likely blunting the response to ablation-driven vaccination without additional targeted modulation [138].
6.2. Clinical and Technical Variability
A major obstacle is the lack of procedural standardization. Energy delivery parameters, device manufacturers, operator skill, and lesion characteristics (size, location) vary immensely across studies. This heterogeneity makes cross-trial comparisons and meta-analyses exceedingly difficult, slowing down the generation of high-level evidence needed for guidelines [83].
6.3. Biomarker Validation and Access
While promising biomarkers exist, none have been prospectively validated or approved for clinical use. Liquid biopsies (ctDNA) are scalable but require standardization. Advanced spatial profiling techniques (multiplex IHC, spatial transcriptomics) provide deep mechanistic insights but are costly, complex, and not widely available, limiting their use in multicenter trials [170].
6.4. Trial Design Gaps
Many existing studies are limited by their single-arm, exploratory nature. Common shortcomings include the following:
- Lack of randomization for ICI timing.
- Absence of control arms (ablation alone vs. ablation + ICI).
- Underpowered sample sizes for assessing systemic efficacy.
- Inconsistent use of immune-related response criteria.
- Inadequate integration of correlative biomarker studies.
7. Conclusions
Tumor ablation is evolving from a local cytoreductive technique into a reproducible in situ vaccination platform; by ensuring procedural completeness, integrating immune checkpoint blockade or innate agonists—particularly in settings with cGAS–STING pathway suppression such as RECQL4-high HCC or TRIM6-high MSS gastric cancer—and embedding a robust biomarker framework, the abscopal effect can be transformed from a rare anecdote into predictable systemic immunity, positioning ablation as a cornerstone of next-generation cancer immunotherapy.
A graphical summary of the mechanistic and translational concepts discussed in this review is presented in Figure 2, highlighting the sequential immunological events and key therapeutic intervention points.
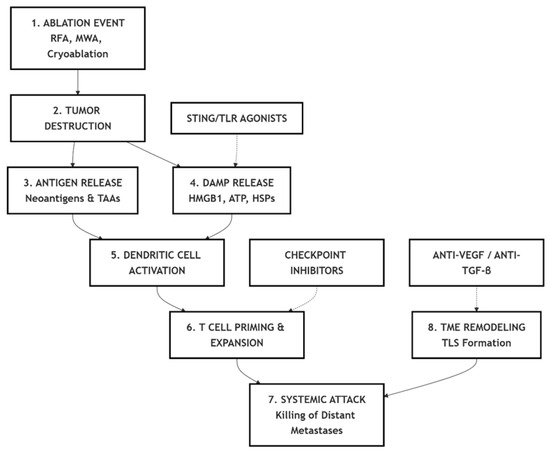
Figure 2.
Summary schematic of the immunological cascade and therapeutic modulation in in situ vaccination induced by tumor ablation. RFA—Radiofrequency ablation; MWA—Microwave ablation; TAA—Tumor-associated antigen; DAMP—Damage-associated molecular pattern; HMGB1—High-mobility group box 1; ATP—Adenosine triphosphate; HSP—Heat shock protein; TME—Tumor microenvironment; TLS—Tertiary lymphoid structure; STING—Stimulator of interferon genes; TLR—Toll-like receptor; VEGF—Vascular endothelial growth factor; TGF-β—Transforming growth factor beta.
Author Contributions
Conceptualization, E.M. and T.C.; methodology, E.M. and T.C.; software, E.M.; validation, E.M. and T.C.; formal analysis, E.M. and T.C.; investigation, E.M. and T.C.; resources, E.M. and T.C.; data curation, E.M. and T.C.; writing—original draft preparation, E.M.; writing—review and editing, E.M. and T.C.; visualization, E.M.; project administration, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 4-1BB | CD137 co-stimulatory receptor |
| AI | Artificial intelligence |
| APC | Antigen-presenting cell |
| ATP | Adenosine triphosphate |
| BCR | B-cell receptor |
| CAR-T | Chimeric antigen receptor T cells |
| cDC1 | Conventional type 1 dendritic cell |
| CEA | Carcinoembryonic antigen |
| CEUS | Contrast-enhanced ultrasound |
| CD4+ | Cluster of Differentiation 4–positive T cell (helper T cell) |
| CD8+ | Cluster of Differentiation 8–positive T cell (cytotoxic T cell) |
| cGAMP | Cyclic GMP–AMP |
| cGAS | Cyclic GMP–AMP synthase |
| CODEX | CO-Detection by indEXing (multiplex imaging platform) |
| CosMx | Spatial molecular imaging platform (NanoString) |
| CRC | Colorectal cancer |
| ctDNA | Circulating tumor DNA |
| CTCs | Circulating tumor cells |
| CT | Computed tomography |
| CTLA-4 | Cytotoxic T-lymphocyte–associated protein 4 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DAMP(s) | Damage-associated molecular pattern(s) |
| DC | Dendritic cell |
| DNA | Deoxyribonucleic acid |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| HEV(s) | High endothelial venule(s) |
| HGF | Hepatocyte growth factor |
| HIFU | High-intensity focused ultrasound |
| HMGB1 | High-mobility group box 1 |
| HCC | Hepatocellular carcinoma |
| ICI(s) | Immune checkpoint inhibitor(s) |
| ICD | Immunogenic cell death |
| IFI44 | Interferon-induced protein 44 |
| IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 |
| IFN-I | Type I interferon |
| IFN-γ | Interferon gamma |
| IHC | Immunohistochemistry |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IMbrave050 | Phase III trial of adjuvant atezolizumab plus bevacizumab after resection or ablation in HCC |
| IRE | Irreversible electroporation |
| irRECIST | Immune-related Response Evaluation Criteria in Solid Tumors |
| ISG15 | Interferon-stimulated gene 15 |
| LAG-3 | Lymphocyte-activation gene 3 |
| MAM | Minimal ablative margin |
| MR | Magnetic resonance (imaging) |
| MX1 | Myxovirus resistance protein 1 |
| MDSC(s) | Myeloid-derived suppressor cell(s) |
| MUC1 | Mucin 1 |
| MWA | Microwave ablation |
| NR | Not reported |
| NSCLC | Non-small-cell lung cancer |
| NY-ESO-1 | New York esophageal squamous cell carcinoma-1 antigen |
| OX40 | TNFRSF4 co-stimulatory receptor |
| PAMP(s) | Pathogen-associated molecular pattern(s) |
| PBMC(s) | Peripheral blood mononuclear cell(s) |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PET | Positron emission tomography |
| P2RX7 | Purinergic receptor P2X7 |
| P2Y2 | Purinergic receptor P2Y2 |
| PRR(s) | Pattern-recognition receptor(s) |
| RFA | Radiofrequency ablation |
| RFS | Recurrence-free survival |
| RCC | Renal cell carcinoma |
| RECQL4 | RecQ like helicase 4 |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SII | Systemic immune-inflammation index |
| Slide-TCR-seq | Spatial T-cell receptor sequencing on tissue slides |
| STING | Stimulator of interferon genes |
| TAA(s) | Tumor-associated antigen(s) |
| TCR | T-cell receptor |
| TCR-seq | T-cell receptor sequencing |
| TDLN(s) | Tumor-draining lymph node(s) |
| TGF-β | Transforming growth factor beta |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TLS | Tertiary lymphoid structure(s) |
| TLR | Toll-like receptor |
| TLR3 | Toll-like receptor 3 |
| TLR4 | Toll-like receptor 4 |
| TLR9 | Toll-like receptor 9 |
| TMB | Tumor mutational burden |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor alpha |
| TRIM6 | Tripartite motif-containing protein 6 |
| Treg(s) | Regulatory T cell(s) |
| US | Ultrasound |
| VEGF | Vascular endothelial growth factor |
References
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging concepts for immune checkpoint blockade–based combination therapies. Cancer Cell 2018, 34, 690–691. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.H.; Choi, B.I.; de Baère, T.; Dodd, G.D., 3rd; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef]
- Keisari, Y.; Kelson, I. Tumour ablation induced anti-tumour immunity: Destruction of the tumour in situ with the aim to evoke a robust anti-tumour immune response. Cancer Metastasis Rev. 2023, 42, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Kepp, O.; Galluzzi, L.; Martins, I.; Schlemmer, F.; Adjemian, S.; Michaud, M.; Sukkurwala, A.Q.; Menger, L.; Zitvogel, L.; Kroemer, G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011, 30, 61–69. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842, Erratum in Immunity 2015, 42, 199. [Google Scholar] [CrossRef] [PubMed]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Demaria, O.; De Gassart, A.; Coso, S.; Gestermann, N.; Di Domizio, J.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518. [Google Scholar] [CrossRef]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; De Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Nahm, W.J.; Sakunchotpanit, G.; Nambudiri, V.E. Abscopal Effects and Immunomodulation in Skin Cancer Therapy. Am. J. Clin. Dermatol. 2025, 26, 555–585. [Google Scholar] [CrossRef]
- Dromi, S.A.; Walsh, M.P.; Herby, S.; Traughber, B.; Xie, J.; Sharma, K.V.; Sekhar, K.P.; Luk, A.; Liewehr, D.J.; Dreher, M.R.; et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology 2009, 251, 58–66. [Google Scholar] [CrossRef]
- den Brok, M.H.; Sutmuller, R.P.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.; Boerman, O.C.; Figdor, C.G.; Ruers, T.J.; Adema, G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer 2006, 95, 896–905. [Google Scholar] [CrossRef]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamai, T.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn. Interv. Imaging 2017, 98, 651–659. [Google Scholar] [CrossRef]
- Thim, E.A.; Kitelinger, L.E.; Rivera-Escalera, F.; Mathew, A.S.; Elliott, M.R.; Bullock, T.N.J.; Price, R.J. Focused ultrasound ablation of melanoma with boiling histotripsy yields abscopal tumor control and antigen-dependent dendritic cell activation. Theranostics 2024, 14, 1647–1661. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Zitvogel, L. Abscopal but desirable: The contribution of immune responses to the efficacy of radiotherapy. Oncoimmunology 2012, 1, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Ma, Y.; Baracco, E.E.; Sistigu, A.; Enot, D.P.; Pietrocola, F.; Yang, H.; Adjemian, S.; Chaba, K.; Semeraro, M.; et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015, 350, 972–978. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221, Erratum in Nature 2018, 555, 402. [Google Scholar] [CrossRef]
- Jaime-Sanchez, P.; Uranga-Murillo, I.; Aguilo, N.; Khouili, S.C.; Arias, M.A.; Sancho, D.; Pardo, J. Cell death induced by cytotoxic CD8+ T cells is immunogenic and primes caspase-3-dependent spread immunity against endogenous tumor antigens. J. Immunother. Cancer 2020, 8, e000528. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Cancer vaccines. BMJ 2015, 350, h988. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.M.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef]
- Yadav, M.; Jhunjhunwala, S.; Phung, Q.T.; Lupardus, P.; Tanguay, J.; Bumbaca, S.; Franci, C.; Cheung, T.K.; Fritsche, J.; Weinschenk, T.; et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014, 515, 572–576. [Google Scholar] [CrossRef]
- Linnemann, C.; van Buuren, M.M.; Bies, L.; Verdegaal, E.M.; Schotte, R.; Calis, J.J.; Behjati, S.; Velds, A.; Hilkmann, H.; Atmioui, D.E.; et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 2015, 21, 81–85. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Zhang, A.; He, K.; Liu, P.; Xu, L.X. Cryo-thermal therapy elicits potent anti-tumor immunity by inducing extracellular Hsp70-dependent MDSC differentiation. Sci. Rep. 2016, 6, 27136. [Google Scholar] [CrossRef]
- Wang, M.; Duan, Y.; Yang, M.; Guo, Y.; Li, F.; Wang, J.; Si, T. The analysis of immunogenic cell death induced by ablation at different temperatures in hepatocellular carcinoma cells. Front. Cell Dev. Biol. 2023, 11, 1146195. [Google Scholar] [CrossRef]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Martins, I.; Wang, Y.; Michaud, M.; Ma, Y.; Sukkurwala, A.Q.; Shen, S.; Kepp, O.; Métivier, D.; Galluzzi, L.; Perfettini, J.L.; et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014, 21, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Binder, R.J.; Ramalingam, T.; Srivastava, P.K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001, 14, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Aymeric, L.; Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Martins, I.; Kroemer, G.; Smyth, M.J.; Zitvogel, L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010, 70, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef]
- Rivas-Yáñez, E.; Barrera-Avalos, C.; Parra-Tello, B.; Briceño, P.; Rosemblatt, M.V.; Saavedra-Almarza, J.; Rosemblatt, M.; Acuña-Castillo, C.; Bono, M.R.; Sauma, D. P2X7 Receptor at the Crossroads of T Cell Fate. Int. J. Mol. Sci. 2020, 21, 4937. [Google Scholar] [CrossRef]
- Grassi, F.; De Ponte Conti, B. The P2X7 Receptor in Tumor Immunity. Front. Cell Dev. Biol. 2021, 9, 694831. [Google Scholar] [CrossRef]
- Yamazaki, T.; Hannani, D.; Poirier-Colame, V.; Ladoire, S.; Locher, C.; Sistigu, A.; Prada, N.; Adjemian, S.; Catani, J.P.; Freudenberg, M.; et al. Defective immunogenic cell death of HMGB1-deficient tumors: Compensatory therapy with TLR4 agonists. Cell Death Differ. 2014, 21, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Klarquist, J.; Hennies, C.M.; Lehn, M.A.; Reboulet, R.A.; Feau, S.; Janssen, E.M. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J. Immunol. 2014, 193, 6124–6134. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Chung, J.H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp. Mol. Med. 2023, 55, 510–519. [Google Scholar] [CrossRef]
- Korabecna, M.; Zinkova, A.; Brynychova, I.; Chylikova, B.; Prikryl, P.; Sedova, L.; Neuzil, P.; Seda, O. Cell-free DNA in plasma as an essential immune system regulator. Sci. Rep. 2020, 10, 17478. [Google Scholar] [CrossRef]
- Stejskal, P.; Goodarzi, H.; Srovnal, J.; Hajdúch, M.; van ‘t Veer, L.J.; Magbanua, M.J.M. Circulating tumor nucleic acids: Biology, release mechanisms, and clinical relevance. Mol. Cancer 2023, 22, 15. [Google Scholar] [CrossRef]
- Yang, H.; Lee, W.S.; Kong, S.J.; Kim, C.G.; Kim, J.H.; Chang, S.K.; Kim, S.; Kim, G.; Chon, H.J.; Kim, C. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J. Clin. Investig. 2019, 129, 4350–4364. [Google Scholar] [CrossRef]
- Lv, H.; Zong, Q.; Chen, C.; Lv, G.; Xiang, W.; Xing, F.; Jiang, G.; Yan, B.; Sun, X.; Ma, Y.; et al. TET2-mediated tumor cGAS triggers endothelial STING activation to regulate vasculature remodeling and anti-tumor immunity in liver cancer. Nat. Commun. 2024, 15, 6. [Google Scholar] [CrossRef]
- Moriya, T.; Kitagawa, K.; Hayakawa, Y.; Hemmi, H.; Kaisho, T.; Ueha, S.; Ikebuchi, R.; Yasuda, I.; Nakanishi, Y.; Honda, T.; et al. Immunogenic tumor cell death promotes dendritic cell migration and inhibits tumor growth via enhanced T cell immunity. iScience 2021, 24, 102424. [Google Scholar] [CrossRef] [PubMed]
- Wissniowski, T.T.; Hänsler, J.; Neureiter, D.; Frieser, M.; Schaber, S.; Esslinger, B.; Voll, R.; Strobel, D.; Hahn, E.G.; Schuppan, D. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003, 63, 6496–6500. [Google Scholar] [PubMed]
- du Bois, H.; Heim, T.A.; Lund, A.W. Tumor-draining lymph nodes: At the crossroads of metastasis and immunity. Sci. Immunol. 2021, 6, eabg3551. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Fu, H.; Li, J.; Xu, L.; Wang, G.; Wu, H. Peritumoral Tertiary Lymphoid Structures Correlate with Protective Immunity and Improved Prognosis in Patients with Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 648812. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L.; et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Figenschau, S.L.; Fismen, S.; Fenton, K.A.; Fenton, C.; Mortensen, E.S. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer 2015, 15, 101. [Google Scholar] [CrossRef]
- Vanhersecke, L.; Brunet, M.; Guégan, J.P.; Rey, C.; Bougouin, A.; Cousin, S.; Moulec, S.L.; Besse, B.; Loriot, Y.; Larroquette, M.; et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2021, 2, 794–802. [Google Scholar] [CrossRef]
- Su, X.; Kang, D.; Wang, J.; Li, L.; Huang, R.; Zou, Z. Tertiary lymphoid structures associated with improved survival and enhanced antitumor immunity in acral melanoma. npj Precis. Oncol. 2025, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Yan, G.; Zhang, G. Tertiary lymphoid structures in cancer: Maturation and induction. Front. Immunol. 2024, 15, 1369626. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Meylan, M.; Petitprez, F.; Sun, C.M.; Italiano, A.; Sautès-Fridman, C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 2022, 19, 441–457. [Google Scholar] [CrossRef]
- JGunderson, A.; Rajamanickam, V.; Bui, C.; Bernard, B.; Pucilowska, J.; Ballesteros-Merino, C.; Schmidt, M.; McCarty, K.; Philips, M.; Piening, B.; et al. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology 2021, 10, 1900635. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.J.; Zhang, B.; Lin, W.P.; Li, S.J.; Xiong, D.; Wang, Q.; Wang, W.D.; Yang, Q.C.; Huang, C.F.; et al. Mature tertiary lymphoid structures evoke intra-tumoral T and B cell responses via progenitor exhausted CD4+ T cells in head and neck cancer. Nat. Commun. 2025, 16, 4228. [Google Scholar] [CrossRef]
- Fan, X.; Feng, D.; Wei, D.; Li, A.; Wei, F.; Deng, S.; Shen, M.; Qin, C.; Yu, Y.; Liang, L. Characterizing tertiary lymphoid structures associated single-cell atlas in breast cancer patients. Cancer Cell Int. 2025, 25, 12. [Google Scholar] [CrossRef]
- Meylan, M.; Petitprez, F.; Becht, E.; Bougoüin, A.; Pupier, G.; Calvez, A.; Giglioli, I.; Verkarre, V.; Lacroix, G.; Verneau, J.; et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 2022, 55, 527–541.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Iorgulescu, J.B.; Li, S.; Borji, M.; Barrera-Lopez, I.A.; Shanmugam, V.; Lyu, H.; Morriss, J.W.; Garcia, Z.N.; Murray, E.; et al. Spatial maps of T cell receptors and transcriptomes reveal distinct immune niches and interactions in the adaptive immune response. Immunity 2022, 55, 1940–1952.e5. [Google Scholar] [CrossRef]
- Kuswanto, W.; Nolan, G.; Lu, G. Highly multiplexed spatial profiling with CODEX: Bioinformatic analysis and application in human disease. Semin. Immunopathol. 2023, 45, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Vardam, T.D.; Appenheimer, M.M.; Eng, K.H.; Gollnick, S.O.; Muhitch, J.B.; Evans, S.S. In situ thermal ablation augments antitumor efficacy of adoptive T cell therapy. Int. J. Hyperth. 2019, 36 (Suppl. S1), 22–36. [Google Scholar] [CrossRef] [PubMed]
- Hoover, A.R.; Kaabinejadian, S.; Krawic, J.R.; Sun, X.H.; Naqash, A.R.; Yin, Q.; Yang, X.; Christopher Garcia, K.; Davis, M.M.; Hildebrand, W.H.; et al. Localized ablative immunotherapy drives de novo CD8+ T-cell responses to poorly immunogenic tumors. J. Immunother. Cancer 2022, 10, e004973. [Google Scholar] [CrossRef]
- Park, S.S.; Dong, H.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, Z.; Lei, K.; Liao, J.; Peng, Z.; Lin, M.; Liang, P.; Yu, J.; Peng, S.; Chen, S.; et al. Irreversible electroporation induces CD8+ T cell immune response against post-ablation hepatocellular carcinoma growth. Cancer Lett. 2021, 503, 1–10. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. The abscopal effect 67 years later: From a side story to center stage. Br. J. Radiol. 2020, 93, 20200042. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.R.; Shen, Y.; Qian, D.C.; Buchwald, Z.S. The Abscopal Effect: A Review of Pre-Clinical and Clinical Advances. Int. J. Mol. Sci. 2021, 22, 11061. [Google Scholar] [CrossRef]
- Kim, N.J.; Yoon, J.H.; Tuomi, A.C.; Lee, J.; Kim, D. In-situ tumor vaccination by percutaneous ablative therapy and its synergy with immunotherapeutics: An update on combination therapy. Front. Immunol. 2023, 14, 1118845. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Soullié, T.; van Nimwegen, M.; Willart, M.A.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008, 205, 869–882. [Google Scholar] [CrossRef]
- Zheng, S.; Ma, X.; Zhang, L.; Gunn, L.; Smith, M.T.; Wiemels, J.L.; Leung, K.; Buffler, P.A.; Wiencke, J.K. Hypermethylation of the 5′ CpG island of the FHIT gene is associated with hyperdiploid and translocation-negative subtypes of pediatric leukemia. Cancer Res. 2004, 64, 2000–2006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leuchte, K.; Staib, E.; Thelen, M.; Gödel, P.; Lechner, A.; Zentis, P.; Garcia-Marquez, M.; Waldschmidt, D.; Datta, R.R.; Wahba, R.; et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol. Immunother. 2021, 70, 893–907. [Google Scholar] [CrossRef]
- Yin, L.; Li, X.Y.; Zhu, L.L.; Chen, G.L.; Xiang, Z.; Wang, Q.Q.; Bi, J.W.; Wang, Q. Clinical application status and prospect of the combined anti-tumor strategy of ablation and immunotherapy. Front. Immunol. 2022, 13, 965120. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Zhang, W.; Xu, Y.; Zhang, Q.; Xiong, S.; Tang, H.; Luo, B. HMGB1 released from dead tumor cells after insufficient radiofrequency ablation promotes progression of HCC residual tumor via ERK1/2 pathway. Int. J. Hyperth. 2023, 40, 2174709. [Google Scholar] [CrossRef]
- Neizert, C.A.; Do, H.N.C.; Zibell, M.; Rieder, C.; Sinden, D.; Niehues, S.M.; Vahldiek, J.L.; Lehmann, K.S.; Poch, F.G.M. Three-dimensional assessment of vascular cooling effects on hepatic microwave ablation in a standardized ex vivo model. Sci. Rep. 2022, 12, 17061. [Google Scholar] [CrossRef] [PubMed]
- Velez, E.; Goldberg, S.N.; Kumar, G.; Wang, Y.; Gourevitch, S.; Sosna, J.; Moon, T.; Brace, C.L.; Ahmed, M. Hepatic Thermal Ablation: Effect of Device and Heating Parameters on Local Tissue Reactions and Distant Tumor Growth. Radiology 2016, 281, 782–792. [Google Scholar] [CrossRef]
- Markezana, A.; Goldberg, S.N.; Kumar, G.; Zorde-Khvalevsky, E.; Gourevtich, S.; Rozenblum, N.; Galun, E.; Ahmed, M. Incomplete thermal ablation of tumors promotes increased tumorigenesis. Int. J. Hyperth. 2021, 38, 263–272. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457. [Google Scholar] [CrossRef]
- Aarts, B.M.; Klompenhouwer, E.G.; Rice, S.L.; Imani, F.; Baetens, T.; Bex, A.; Horenblas, S.; Kok, M.; Haanen, J.B.A.G.; Beets-Tan, R.G.H.; et al. Cryoablation and immunotherapy: An overview of evidence on its synergy. Insights Imaging 2019, 10, 53. [Google Scholar] [CrossRef]
- McArthur, H.L.; Diab, A.; Page, D.B.; Yuan, J.; Solomon, S.B.; Sacchini, V.; Comstock, C.; Durack, J.C.; Maybody, M.; Sung, J.; et al. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin. Cancer Res. 2016, 22, 5729–5737. [Google Scholar] [CrossRef]
- Ní Eochagáin, A. Cryoshock following cryoablation for hepatocellular carcinoma. J. Clin. Anesth. 2022, 77, 110641. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; van Tilborg, A.A.; Vieveen, J.M.; Bouwman, A.R.; Meijer, S.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: A systematic review of safety and efficacy. J. Vasc. Interv. Radiol. 2014, 25, 997–1011, quiz 1011. [Google Scholar] [CrossRef]
- Alonso-González, R.; Abadal Villayandre, J.M.; Gálvez Gonzalez, E.; Álvarez Perez, M.J.; Méndez Alonso, S.; de Gregorio Ariza, M.A. Irreversible electroporation: Beyond the limits of tumor ablation. Radiologia 2024, 66, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Konno, H.; Ahn, J.; Barber, G.N. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates with Tumorigenesis. Cell Rep. 2016, 14, 282–297. [Google Scholar] [CrossRef]
- Vogel, J.A.; van Veldhuisen, E.; Alles, L.K.; Busch, O.R.; Dijk, F.; van Gulik, T.M.; Huijzer, G.M.; Besselink, M.G.; van Lienden, K.P.; Verheij, J. Time-Dependent Impact of Irreversible Electroporation on Pathology and Ablation Size in the Porcine Liver: A 24-Hour Experimental Study. Technol. Cancer Res. Treat. 2019, 18, 1533033819876899. [Google Scholar] [CrossRef] [PubMed]
- Polajzer, T.; Jarm, T.; Miklavcic, D. Analysis of damage-associated molecular pattern molecules due to electroporation of cells in vitro. Radiol. Oncol. 2020, 54, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Yang, X.; Liao, Y.; Fan, L.; Lin, B.; Li, J.; Wu, D.; Liao, D.; Yuan, L.; Liu, J.; Gao, F.; et al. High-intensity focused ultrasound ablation combined with immunotherapy for treating liver metastases: A prospective non-randomized trial. PLoS ONE 2024, 19, e0306595. [Google Scholar] [CrossRef]
- Hammerich, L.; Binder, A.; Brody, J.D. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol. Oncol. 2015, 9, 1966–1981. [Google Scholar] [CrossRef]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Yao, C.; Dong, S.; Gao, J.; Ke, S.; Zhu, R.; Huang, S.; Wang, S.; Xu, L.; et al. Progression of hepatocellular carcinoma after radiofrequency ablation: Current status of research. Front. Oncol. 2022, 12, 1032746. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Ding, N.; Zhang, Y.; Zhu, Y.; Dong, S.; Wang, X.; Peng, C.; Zhou, C.; Zhou, L.; et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 2019, 10, 5421. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Xia, L.; Sun, Z.; Chan, K.M.; Bernards, R.; Qin, W.; Chen, J.; Xia, Q.; Jin, H. Hepatocellular carcinoma: Signaling pathways and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 35. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Tchitchek, N.; Joly, C.; Rodriguez Pozo, A.; Stimmer, L.; Langlois, S.; Hocini, H.; Gosse, L.; Pejoski, D.; Cosma, A.; et al. Vaccine Inoculation Route Modulates Early Immunity and Consequently Antigen-Specific Immune Response. Front. Immunol. 2021, 12, 645210. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, B.; Qu, Y.; Sun, W.; Liang, M.; Mao, X.; Jiang, Y.; Wang, J.; Tang, X.; Pan, H.; et al. Comparison of local effects and systemic T-cell responses in patients with breast cancer treated by radiofrequency ablation versus microwave ablation. Cancer Cell Int. 2025, 25, 261. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, L.; Zhang, J.; Zhang, X. Progress in the cryoablation and cryoimmunotherapy for tumor. Front. Immunol. 2023, 14, 1094009. [Google Scholar] [CrossRef]
- Mercadal, B.; Beitel-White, N.; Aycock, K.N.; Castellví, Q.; Davalos, R.V.; Ivorra, A. Dynamics of cell death after conventional IRE and H-FIRE treatments. Ann. Biomed. Eng. 2020, 48, 1451–1462. [Google Scholar] [CrossRef]
- Yildirim, A.; Shi, D.; Roy, S.; Blum, N.T.; Chattaraj, R.; Cha, J.N.; Goodwin, A.P. Nanoparticle-Mediated Acoustic Cavitation Enables High Intensity Focused Ultrasound Ablation Without Tissue Heating. ACS Appl Mater Interfaces 2018, 10, 36786–36795. [Google Scholar] [CrossRef]
- Salmon, H.; Remark, R.; Gnjatic, S.; Merad, M. Host tissue determinants of tumour immunity. Nat. Rev. Cancer 2019, 19, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Cheon, I.S.; Son, Y.M.; Sun, J. Tissue-resident memory T cells and lung immunopathology. Immunol. Rev. 2023, 316, 63–83. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, Y.; Li, C.Y. DNA repair at the crossroads of tumor immunogenicity and cancer therapy: Harnessing innate and adaptive immune pathways for improved therapeutic outcomes. DNA Repair 2025, 152, 103874. [Google Scholar] [CrossRef] [PubMed]
- Toghraie, F.S.; Bayat, M.; Hosseini, M.S.; Ramezani, A. Tumor-infiltrating myeloid cells: Mechanisms, functional significance, and targeting in cancer therapy. Cell. Oncol. 2025, 48, 559–590. [Google Scholar] [CrossRef]
- Liang, W.; Cai, K.; Chen, C.; Chen, H.; Chen, Q.; Fu, J.; Hu, J.; Jiang, T.; Jiao, W.; Li, S.; et al. Expert consensus on neoadjuvant immunotherapy for non-small cell lung cancer. Transl. Lung Cancer Res. 2020, 9, 2696–2715. [Google Scholar] [CrossRef] [PubMed]
- van den Bijgaart, R.J.E.; Schuurmans, F.; Fütterer, J.J.; Verheij, M.; Cornelissen, L.A.M.; Adema, G.J. Immune modulation plus tumor ablation: Adjuvants and antibodies to prime and boost anti-tumor immunity in situ. Front. Immunol. 2021, 12, 617365. [Google Scholar] [CrossRef]
- Tang, L.; Wang, D.; Hu, T.; Lin, X.; Wu, S. Current applications of tumor local ablation combined with immune checkpoint inhibitors in breast cancer treatment. Cancer Drug Resist. 2024, 7, 33. [Google Scholar] [CrossRef]
- Qin, S.; Chen, M.; Cheng, A.L.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef]
- Choi, N.; Shin, D.Y.; Kim, H.J.; Moon, U.Y.; Baek, K.H.; Jeong, H.S. Postoperative anti-PD-1 antibody treatment to reduce recurrence in a cancer ablation surgical wound. J. Surg. Res. 2018, 221, 95–103. [Google Scholar] [CrossRef]
- Natalini, A.; Simonetti, S.; Favaretto, G.; Lucantonio, L.; Peruzzi, G.; Muñoz-Ruiz, M.; Kelly, G.; Contino, A.M.; Sbrocchi, R.; Battella, S.; et al. Improved memory CD8 T cell response to delayed vaccine boost is associated with a distinct molecular signature. Front. Immunol. 2023, 14, 1043631. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Shen, L.; Yao, Y.; Xia, W.; Ni, C. Tumor battlefield within inflamed, excluded or desert immune phenotypes: The mechanisms and strategies. Exp. Hematol. Oncol. 2024, 13, 80. [Google Scholar] [CrossRef]
- Galassi, C.; Musella, M.; Manduca, N.; Maccafeo, E.; Sistigu, A. The immune privilege of cancer stem cells: A key to understanding tumor immune escape and therapy failure. Cells 2021, 10, 2361. [Google Scholar] [CrossRef] [PubMed]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and immune system decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef]
- Mazzola, R.; Jereczek-Fossa, B.A.; Franceschini, D.; Tubin, S.; Filippi, A.R.; Tolia, M.; Lancia, A.; Minniti, G.; Corradini, S.; Arcangeli, S.; et al. Oligometastasis and local ablation in the era of systemic targeted and immunotherapy. Radiat. Oncol. 2020, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Ye, X. Potential biomarkers for predicting immune response and outcomes in lung cancer patients undergoing thermal ablation. Front. Immunol. 2023, 14, 1268331. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Elkord, E. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- van der Lei, S.; Puijk, R.S.; Dijkstra, M.; Schulz, H.H.; Vos, D.J.W.; De Vries, J.J.J.; Scheffer, H.J.; Lissenberg-Witte, B.I.; Aldrighetti, L.; Arntz, M.; et al. Thermal ablation versus surgical resection of small-size colorectal liver metastases (COLLISION): An international, randomised, controlled, phase 3 non-inferiority trial. Lancet Oncol. 2025, 26, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, K.H.M.; Jenniskens, S.F.M.; van den Boezem, P.B.; Tjwa, E.T.T.L.; de Wilt, J.H.W.; Fütterer, J.J.; Stommel, M.W.J.; Overduin, C.G. CT-guided Thermal Ablation of Liver Tumors Using Intraprocedural CT-CT Fusion for Applicator Position and Ablation Completeness Assessment: A Single-Center Comparative Analysis. Cardiovasc. Interv. Radiol. 2025, 48, 1327–1338. [Google Scholar] [CrossRef]
- Xu, Y.; Long, S.; Yang, Y.; Zhou, F.; Dong, N.; Yan, K.; Wang, B.; Zeng, Y.; Du, N.; Li, X.; et al. Mathematical simulation of temperature distribution in tumor tissue and surrounding healthy tissue treated by laser combined with indocyanine green. Theor. Biol. Med. Model. 2019, 16, 12. [Google Scholar] [CrossRef]
- Matsui, Y.; Ueda, D.; Fujita, S.; Fushimi, Y.; Tsuboyama, T.; Kamagata, K.; Ito, R.; Yanagawa, M.; Yamada, A.; Kawamura, M.; et al. Applications of artificial intelligence in interventional oncology: An up-to-date review of the literature. Jpn. J. Radiol. 2025, 43, 164–176. [Google Scholar] [CrossRef]
- Skalickova, M.; Hadrava Vanova, K.; Uher, O.; Leischner Fialova, J.; Petrlakova, K.; Masarik, M.; Kejík, Z.; Martasek, P.; Pacak, K.; Jakubek, M. Injecting hope: The potential of intratumoral immunotherapy for locally advanced and metastatic cancer. Front. Immunol. 2025, 15, 1479483. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Y.K.; Chen, X.Y.; Chen, S.S.; Yu, X.R.; Li, H.W.; Lu, L.G.; Chen, M.H. Targeting cGAS-STING: Modulating the immune landscape of hepatic diseases. Front. Immunol. 2025, 16, 1498323. [Google Scholar] [CrossRef]
- Niu, Y.; Ding, C.; Wang, Q.; Yin, J.; Li, L.; Liu, W.; Wang, X.; Huang, L. TRIM6 ablation reverses ICB resistance in MSS gastric cancer by unleashing cGAS-STING-dependent antitumor immunity. J. Exp. Clin. Cancer Res. 2025, 44, 242. [Google Scholar] [CrossRef]
- Eek Mariampillai, A.; Hauge, S.; Kongsrud, K.; Syljuåsen, R.G. Immunogenic cell death after combined treatment with radiation and ATR inhibitors is dually regulated by apoptotic caspases. Front. Immunol. 2023, 14, 1138920. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wei, Z.; Ye, X. Immunostimulatory effects of thermal ablation: Challenges and future prospects. J. Cancer Res. Ther. 2024, 20, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.M.; Klausen, T.W.; Davidsen, U.H.; Johnsen, H.E. Early changes in serum IL-6 and VEGF levels predict clinical outcome following first-line therapy in aggressive non-Hodgkin’s lymphoma. Ann. Hematol. 2005, 84, 510–516. [Google Scholar] [CrossRef]
- Yang, M.; Che, Y.; Li, K.; Fang, Z.; Li, S.; Wang, M.; Zhang, Y.; Xu, Z.; Luo, L.; Wu, C.; et al. Detection and quantitative analysis of tumor-associated tertiary lymphoid structures. J. Zhejiang Univ. Sci. B 2023, 24, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; He, Y.Z.; Yang, Y.; Pang, M.; Zheng, G.P.; Wang, H.L. Exploring the potential of the TCR repertoire as a tumor biomarker. Oncol. Lett. 2024, 28, 413. [Google Scholar] [CrossRef]
- Singh, S.K.; Meyering, M.; Ramwadhdoebe, T.H.; Stynenbosch, L.F.; Redeker, A.; Kuppen, P.J.; Melief, C.J.; Welters, M.J.; van der Burg, S.H. The simultaneous ex vivo detection of low-frequency antigen-specific CD4+ and CD8+ T-cell responses using overlapping peptide pools. Cancer Immunol. Immunother. 2012, 61, 1953–1963. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loszko, A.; Byrne, M.M.; Jimenez-Soto, C.; Tomiyama, K.; Bekki, Y.; Hernandez-Alejandro, R. Updates on liquid biopsy and ctDNA in transplant oncology. Cancers 2025, 17, 1930. [Google Scholar] [CrossRef]
- Javdani-Mallak, A.; Mowla, S.J.; Alibolandi, M. Tumor-derived exosomes and their application in cancer treatment. J. Transl. Med. 2025, 23, 751. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, Q.Y.; Huang, W.J.; Feng, S.; Tan, Y.L.; Li, L.L.; Xie, X.T.; Li, Q.Y.; Huang, S.H.; Mao, C.Z.; et al. DNMT inhibition epigenetically restores the cGAS-STING pathway and activates RIG-I/MDA5-MAVS to enhance antitumor immunity. Acta Pharmacol. Sin. 2025. [Google Scholar] [CrossRef]
- Lei, W.; Zhou, K.; Lei, Y.; Li, Q.; Zhu, H. Cancer vaccines: Platforms and current progress. Mol. Biomed. 2025, 6, 3. [Google Scholar] [CrossRef]
- Paolucci, I.; Albuquerque Marques Silva, J.; Lin, Y.M.; Laimer, G.; Cignini, V.; Menchini, F.; Meira, M.; Shieh, A.; O’Connor, C.; Jones, K.A.; et al. Identification of A0 minimum ablative margins for colorectal liver metastases: Multicentre, retrospective study using deformable CT registration and artificial intelligence-based autosegmentation. Br. J. Surg. 2024, 111, znae165. [Google Scholar] [CrossRef]
- Hansen, I.S.; Lappen, K.R.; Brudvik, K.W.; Røsok, B.I.; Björk, I.; Østergaard, D.; Andersen, M.H.; Pelanis, E.; Espinoza, A.W.; Halvorsen, P.S.; et al. A Randomized Controlled Trial of Resection Versus Thermal Ablation of Colorectal Cancer Liver Metastases (New Comet): Study Protocol. Ann. Surg. Oncol. 2025, 32, 9146–9153. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Xu, Y.; Zeng, Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Zhang, Y.; Wang, S.; Li, Z.; Zheng, D.; Hsu, S.; Zhou, J.; Fan, J.; Chen, Z.; Xia, X.; et al. RECQL4 Inhibits Radiation-Induced Tumor Immune Awakening via Suppressing the cGAS-STING Pathway in Hepatocellular Carcinoma. Adv. Sci. 2024, 11, e2308009. [Google Scholar] [CrossRef]
- Kanoda, R.; Nakajima, S.; Fukai, S.; Saito, M.; Saito, K.; Suzuki, H.; Kikuchi, T.; Nirei, A.; Okayama, H.; Mimura, K.; et al. High levels of tumor cell-intrinsic STING signaling are associated with increased infiltration of CD8+ T cells in dMMR/MSI-H gastric cancer. Sci. Rep. 2024, 14, 20859. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Gao, Y.; Sun, B.; Guo, Y.; Jin, Z.; Hao, N.; Jiang, D.; Zheng, C.; Li, X.; Chen, Q. Combination immunotherapy targeting LAG-3, PD-1 and STING suppresses hepatocellular carcinoma as monitored by LAG-3 targeted PET imaging. Biomark. Res. 2025, 13, 102. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, H.; Fang, Z.; Wu, Y.; Jiao, J.; Fang, W.; Wu, Y.; Lang, Y.; Chen, N.; Zhong, Z.; et al. Enhanced abscopal anti-tumor response via a triple combination of thermal ablation, IL-21, and PD-1 inhibition therapy. Cancer Immunol. Immunother. 2024, 73, 138. [Google Scholar] [CrossRef]
- Shou, J.; Mo, F.; Zhang, S.; Lu, L.; Han, N.; Liu, L.; Qiu, M.; Li, H.; Han, W.; Ma, D.; et al. Combination treatment of radiofrequency ablation and peptide neoantigen vaccination: Promising modality for future cancer immunotherapy. Front. Immunol. 2022, 13, 1000681. [Google Scholar] [CrossRef]
- Testoni, S.G.G.; Minici, C.; Benetti, E.; Clemente, F.; Boselli, D.; Sciorati, C.; De Monte, L.; Petrone, M.C.; Enderle, M.; Linzenbold, W.; et al. Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 3704. [Google Scholar] [CrossRef]
- Mehta, A.; Oklu, R.; Sheth, R.A. Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response? Gastroenterol. Res. Pract. 2016, 2016, 9251375. [Google Scholar] [CrossRef]
- Erinjeri, J.P.; Clark, T.W. Cryoablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21 (Suppl. S8), S187–S191. [Google Scholar] [CrossRef]
- Kolck, J.; Schulze, D.; Brönnimann, M.; Fürstner, M.; Fehrenbach, U.; Collettini, F.; Gebauer, B.; Auer, T.A. Percutaneous Cryoablation in the Liver: A Meta-Analysis and Review of Safety with a Focus on Incidence of Cryoshock and Major Complications. Cardiovasc. Interv. Radiol. 2024, 47, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Ringe, K.I.; Lutat, C.; Rieder, C.; Schenk, A.; Wacker, F.; Raatschen, H.J. Experimental Evaluation of the Heat Sink Effect in Hepatic Microwave Ablation. PLoS ONE 2015, 10, e0134301. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, T.; Ouyang, Y.; Rao, W.; Liu, K.; Zheng, J.; Lv, F.; Shi, Y.; Wang, F.; Liu, D.; et al. Radiofrequency ablation plays double role in immunosuppression and activation of PBMCs in recurrent hepatocellular carcinoma. Front. Immunol. 2024, 15, 1339213. [Google Scholar] [CrossRef]
- Aycock, K.N.; Davalos, R.V. Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology. Bioelectricity 2019, 1, 214–234. [Google Scholar] [CrossRef]
- O’Neill, C.H. Martin RCG2nd Cardiac synchronization arrhythmia during irreversible electroporation. J. Surg. Oncol. 2020, 122, 407–411. [Google Scholar] [CrossRef]
- Elhelf, I.A.S.; Albahar, H.; Shah, U.; Oto, A.; Cressman, E.; Almekkawy, M. High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagn. Interv. Imaging 2018, 99, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Xu, G.L.; Gu, M.F.; Luo, G.Y.; Rong, Z.; Wu, P.H.; Xia, J.C. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J. Gastroenterol. 2007, 13, 2747–2751. [Google Scholar] [CrossRef]
- Sehmbi, A.S.; Froghi, S.; Oliveira de Andrade, M.; Saffari, N.; Fuller, B.; Quaglia, A.; Davidson, B. Systematic review of the role of high intensity focused ultrasound (HIFU) in treating malignant lesions of the hepatobiliary system. HPB 2021, 23, 187–196. [Google Scholar] [CrossRef]
- Kuang, M. Editorial: Tumor ablation and immunity. Front. Immunol. 2024, 14, 1291918. [Google Scholar] [CrossRef]
- Regen-Tuero, H.C.; Ward, R.C.; Sikov, W.M.; Littrup, P.J. Cryoablation and immunotherapy for breast cancer: Overview and rationale for combined therapy. Radiol. Imaging Cancer 2021, 3, e200134. [Google Scholar] [CrossRef] [PubMed]
- Akturk, G.; Parra, E.R.; Gjini, E.; Lako, A.; Lee, J.J.; Neuberg, D.; Zhang, J.; Yao, S.; Laface, I.; Rogic, A.; et al. Multiplex Tissue Imaging Harmonization: A Multicenter Experience from CIMAC-CIDC Immuno-Oncology Biomarkers Network. Clin. Cancer Res. 2021, 27, 5072–5083. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).