Third Booster Half Dose of ChAdOx1-nCov-19 Is Effective, Safe, and Induces Long-Duration Humoral and Cellular Immune Response to Omicron: 1-Year Follow-Up of Viana Study
Abstract
1. Introduction
2. Population, Materials and Methods
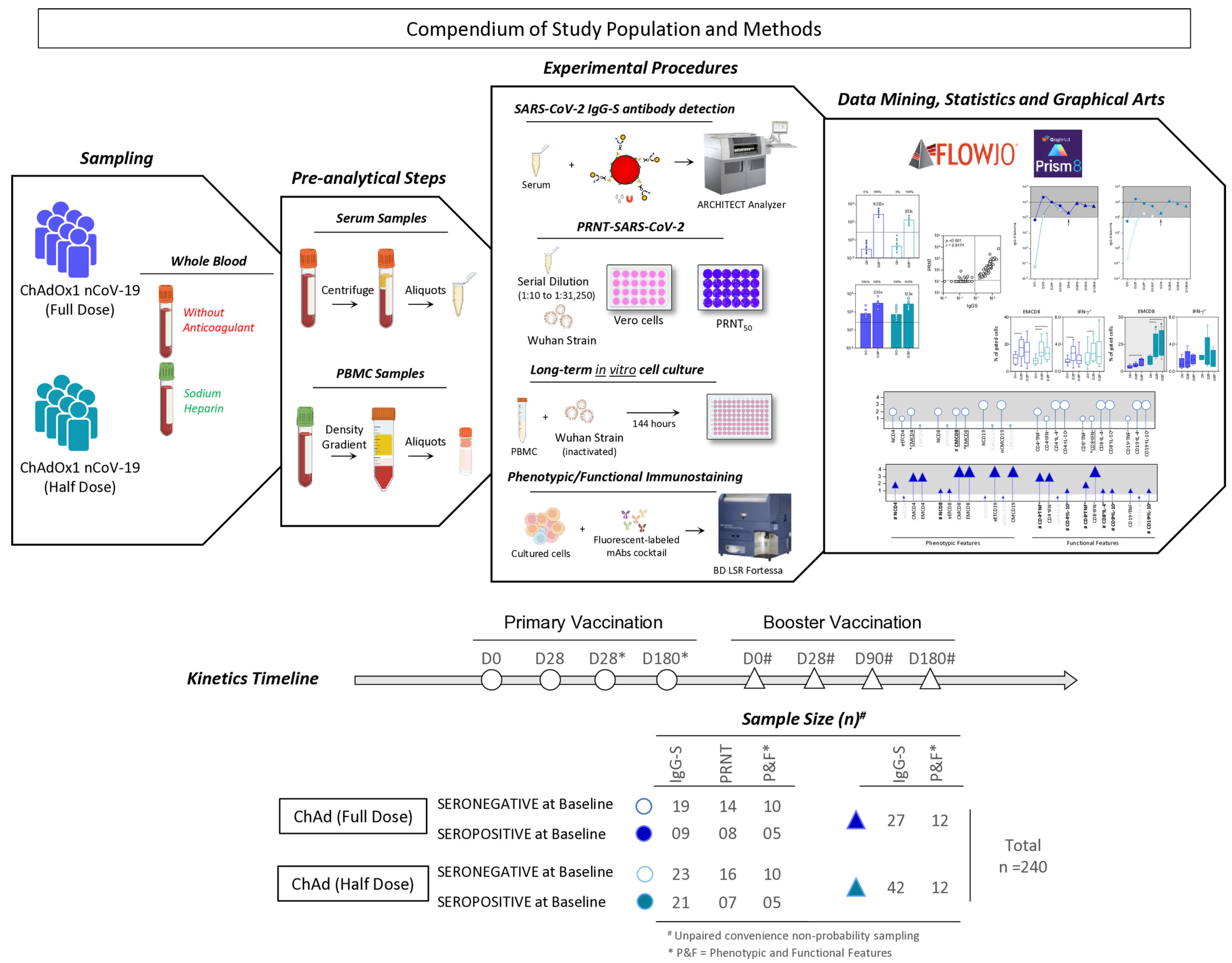
2.1. Study Population and Design
2.2. Biological Samples
2.3. Safety
2.4. Effectiveness
2.5. Immunogenicity
2.5.1. Detection of SARS-CoV-2 IgG-S Antibodies
2.5.2. SARS-CoV-2 Plaque Reduction Neutralization Test (PRNT)
2.5.3. Phenotypic and Functional Memory Cell Assays
2.6. Ethical Statements
2.7. Statistical Analysis
2.7.1. Effectiveness Outcomes
2.7.2. Immunogenicity Outcomes
3. Results
3.1. Safety Endpoint
3.2. Effectiveness Endpoint
3.3. SARS-CoV-2 IgG-S Reactivity and Neutralizing Antibody upon Primary COVID-19 Vaccination
3.4. Kinetics Timeline of IgG-S Reactivity Following Primary and Booster COVID-19 Vaccination
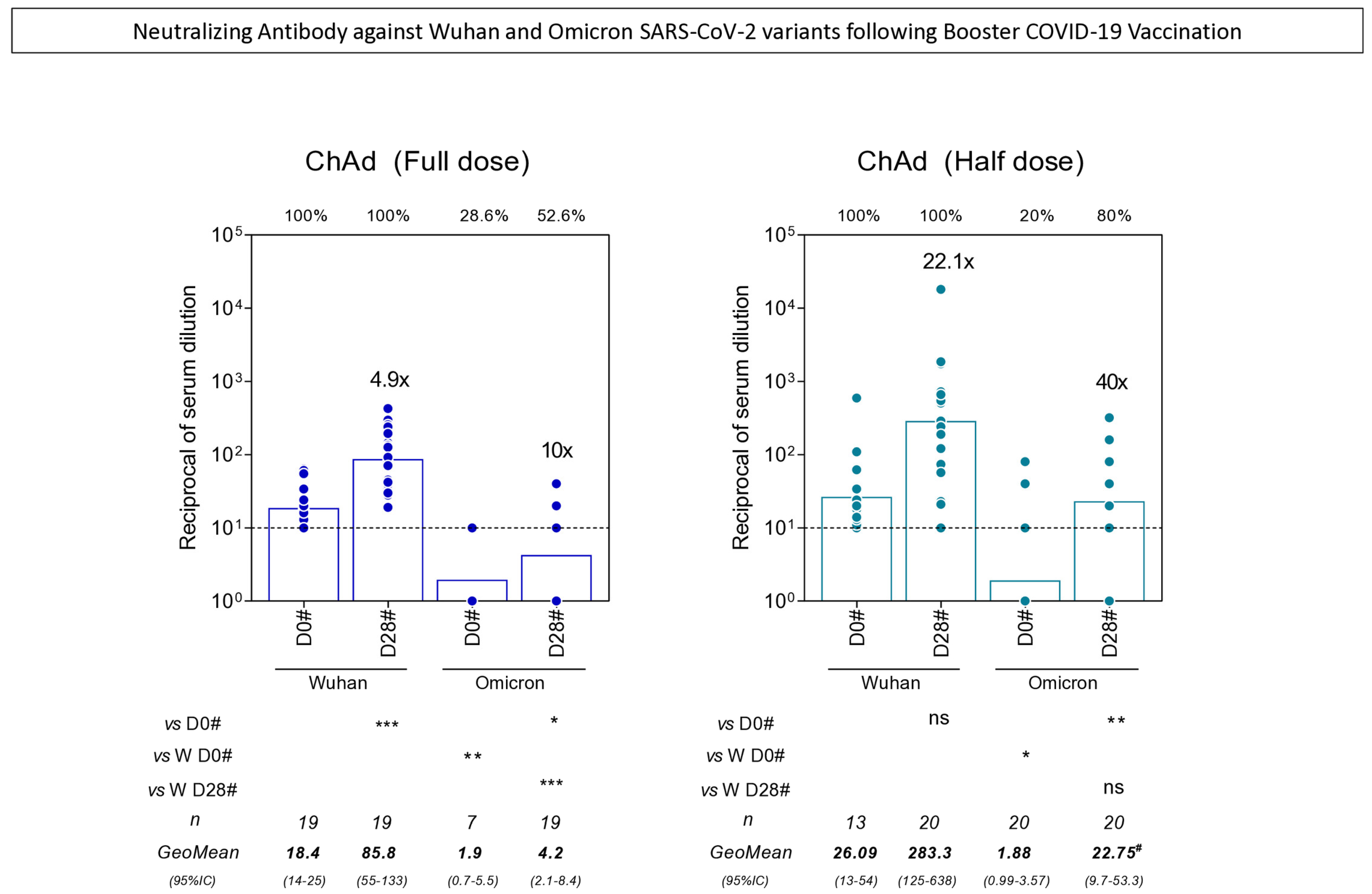
3.5. Neutralizing Antibody Against Wuhan and Omicron SARS-CoV-2 Variants Following Booster COVID-19 Vaccination
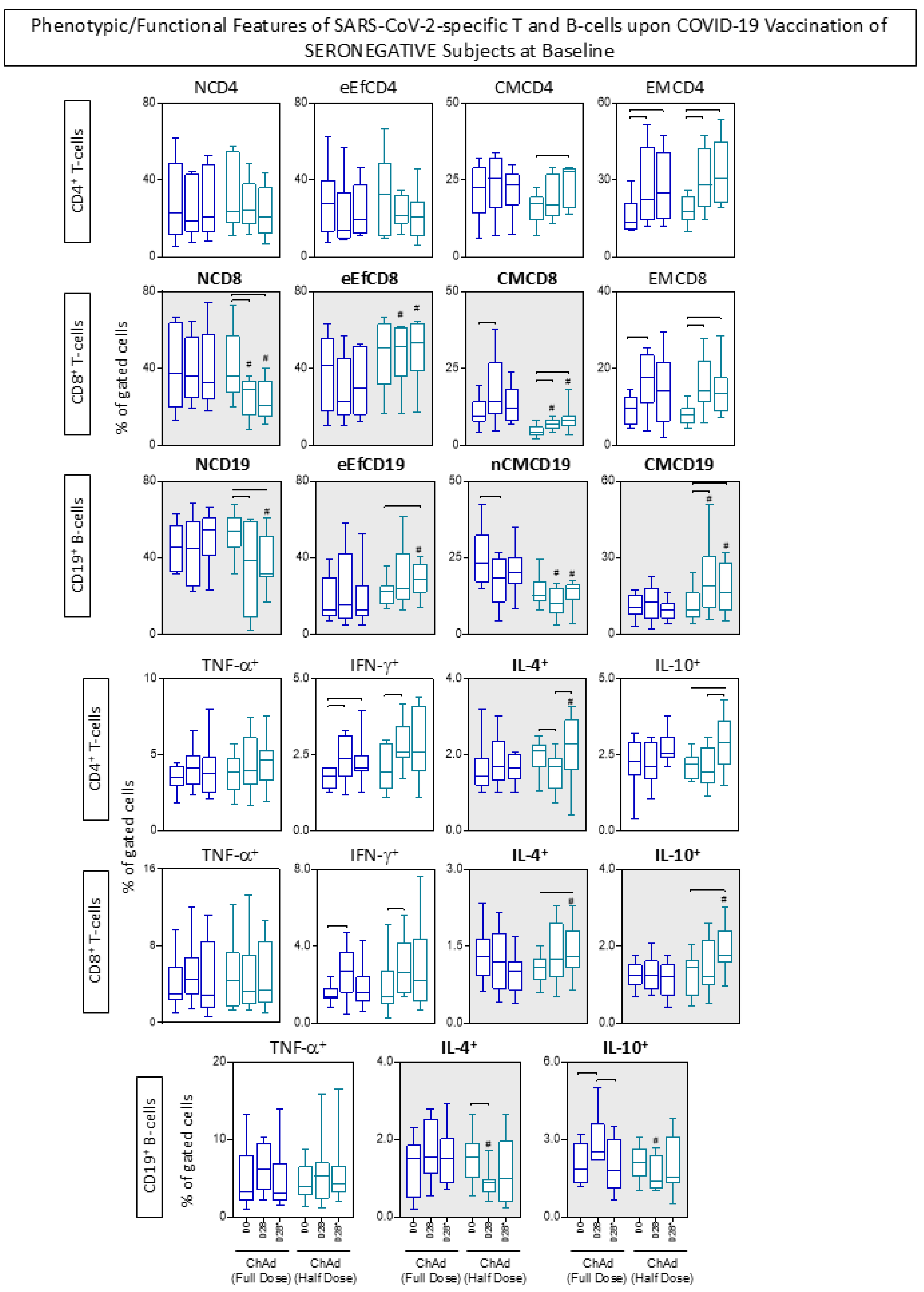
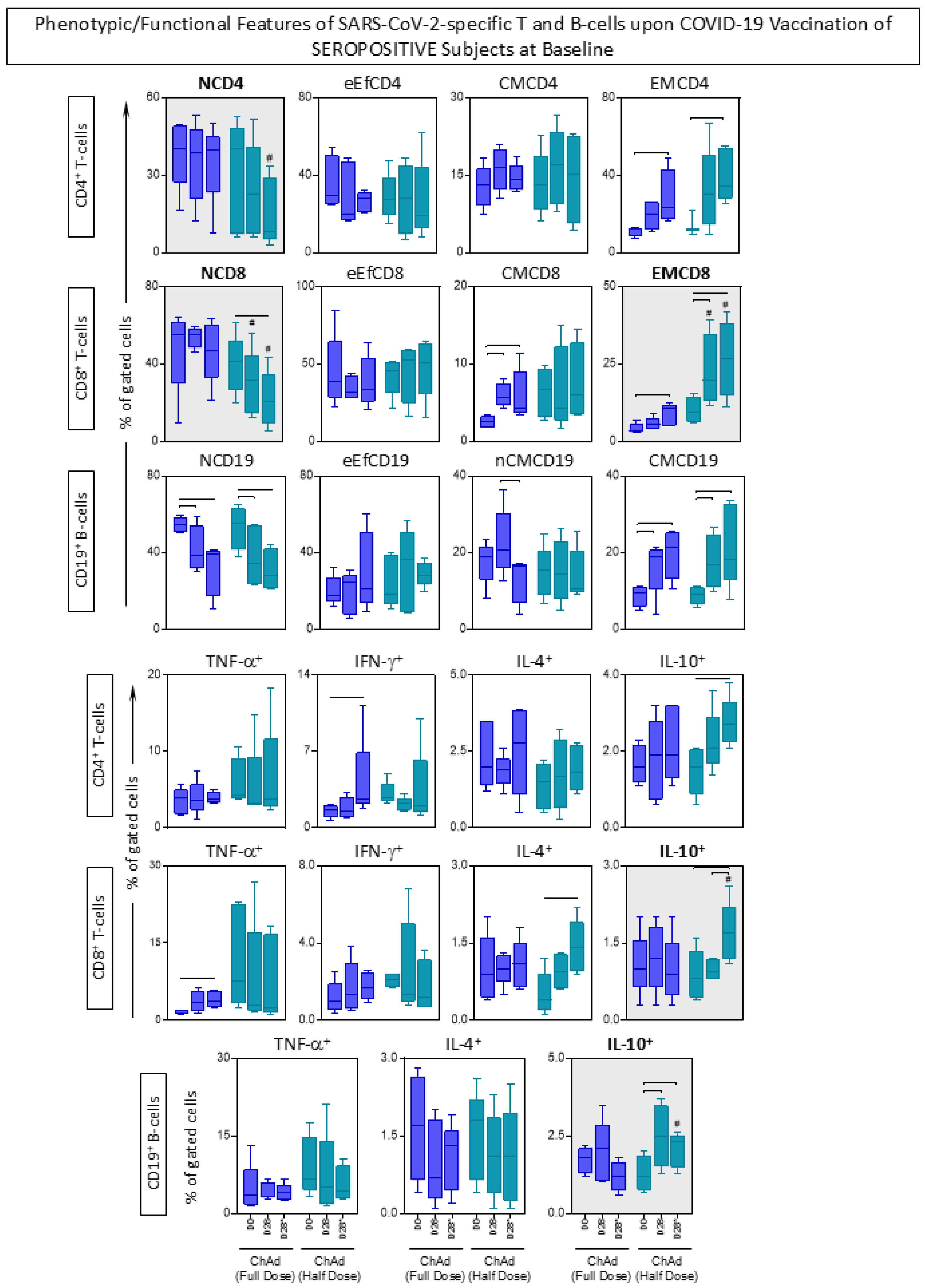
3.6. Phenotypic/Functional Features of SARS-CoV-2-Specific T and B-Cells upon COVID-19 Vaccination
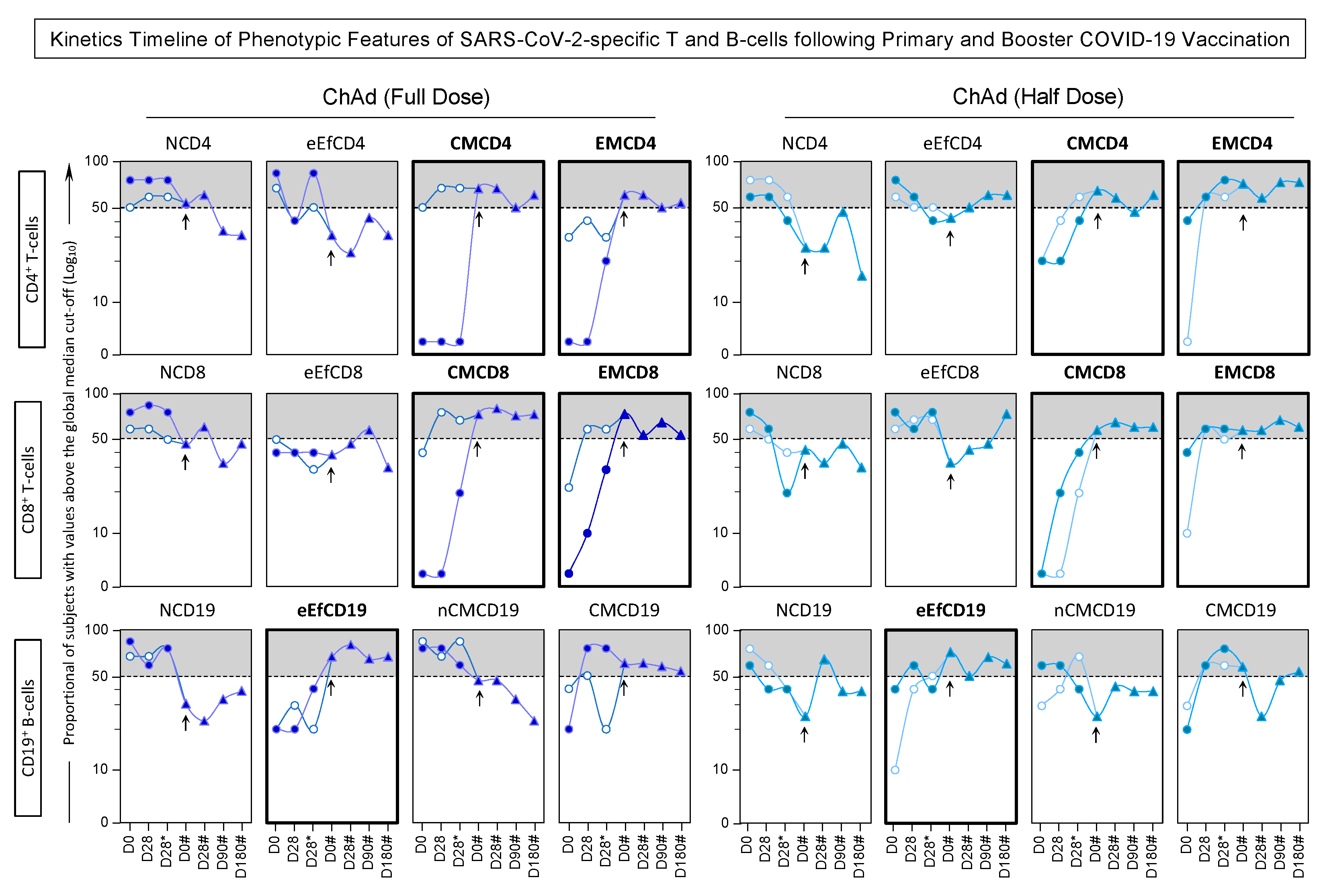
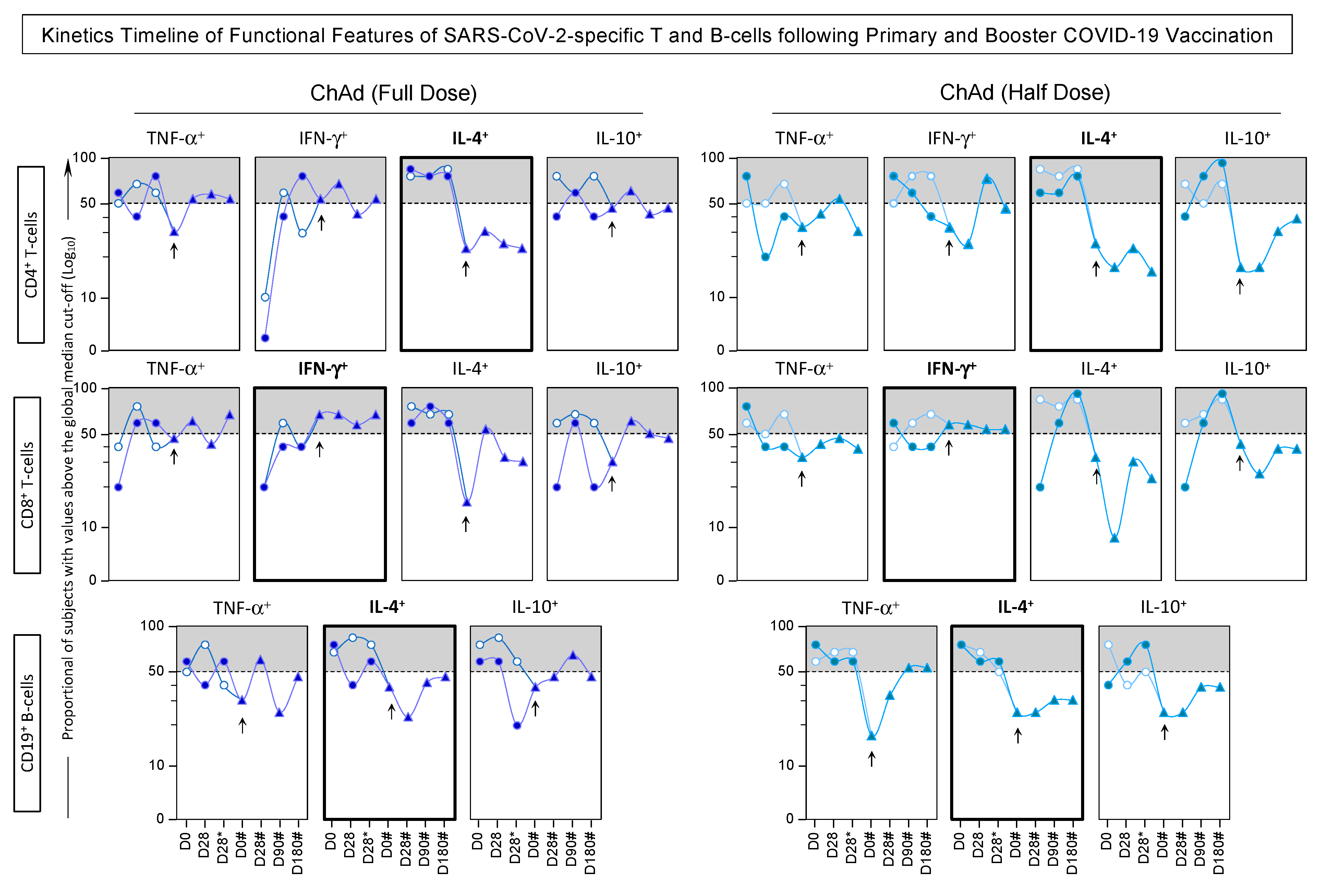
3.7. Kinetics Timeline of Phenotypic and Functional Features of SARS-CoV-2-Specific T and B-Cells Following Primary and Booster COVID-19 Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIH | Hospital Discharge Database (Autorização de Internação Hospitalar) |
| BAU/mL | Binding Antibody Units per Milliliter |
| BNT162b2 | Pfizer-BioNTech COVID-19 vaccine |
| BNT | BioNTech |
| BSL-3 | Biosafety Level 3 |
| CMCD4 | Central Memory CD4+ T-cells |
| CMCD8 | Central Memory CD8+ T-cells |
| CONEP | National Research Ethics Committee (Comissão Nacional de Ética em Pesquisa) |
| COVID-19 | Coronavirus Disease 2019 |
| ChAd Full Dose | Full Dose of ChAdOx1 nCoV-19 |
| ChAd Half Dose | Half Dose of ChAdOx1 nCoV-19 |
| ChAd | ChAdOx1 nCoV-19 Vaccine |
| CrI | Credibility Interval |
| DECF/UFES | Departamento de Ciências Fisiológicas, UFES |
| DEIS/UFES | Departamento de Educação Integrada em Saúde, UFES |
| EBSERH | Empresa Brasileira de Serviços Hospitalares |
| EMCD4 | Effector Memory CD4+ T-cells |
| EMCD8 | Effector Memory CD8+ T-cells |
| EMESCAM | Escola Superior de Ciências da Santa Casa de Misericórdia de Vitória |
| e-SUS VS | National Health Surveillance Database |
| eEfCD19 | Early Effector CD19+ B-cells |
| eEfCD4 | Early Effector CD4+ T-cells |
| eEfCD8 | Early Effector CD8+ T-cells |
| FD | Full Dose |
| FIOCRUZ-Minas | Fundação Oswaldo Cruz, Instituto René Rachou, Belo Horizonte, MG, Brazil |
| FIOCRUZ | Oswaldo Cruz Foundation (Fundação Oswaldo Cruz) |
| GAL | National Laboratory Management System |
| HD | Half Dose |
| HIV | Human Immunodeficiency Virus |
| HUCAM-UFES | University Hospital of the Federal University of Espírito Santo |
| IFN-γ | Interferon Gamma |
| IL-10 | Interleukin 10 |
| IL-4 | Interleukin 4 |
| IRR | René Rachou Institute (Instituto René Rachou) |
| IgG-S | Immunoglobulin G-Specific Antibodies to Spike protein |
| MCMC | Markov Chain Monte Carlo |
| NCD19 | Naïve CD19+ B-cells |
| NCD4 | Naïve CD4+ T-cells |
| NCD8 | Naïve CD8+ T-cells |
| NCMCD19 | Non-classical Memory CD19+ B-cells |
| PAHOERC | Pan American Health Organization Ethics Review Committee |
| PBMC | Peripheral Blood Mononuclear Cell |
| PRNT | Plaque Reduction Neutralization Test |
| PRNT50 | Neutralizing Antibody Titers (50%) |
| PRNT90 | Neutralizing Antibody Titers (90%) |
| PROCC | Programa de Computação Científica, Fundação Oswaldo Cruz |
| RNAm | mRNA (Messenger RNA) |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SESA | Secretaria de Estado da Saúde do Espírito Santo, Vitória, ES, Brazil |
| SIM | Mortality Information System (Sistema de Informações sobre Mortalidade) |
| TNF-α | Tumor Necrosis Factor Alpha |
| Tfh | Follicular Helper T-cells |
| WHO | World Health Organization |
References
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, tAAA021. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard > Cases. data.who.int. 2024. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 10 May 2025).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard > Deaths. data.who.int. 2024. Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 10 May 2025).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Wright, B.J.; Tideman, S.; Diaz, G.A.; French, T.; Parsons, G.T.; Robicsek, A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: A case-control study. Lancet Respir. Med. 2022, 10, 557–565. [Google Scholar] [CrossRef]
- Quan, N.K.; Anh, N.L.M.; Taylor-Robinson, A.W. The global COVID-19 vaccine surplus: Tackling expiring stockpiles. Infect. Dis. Poverty 2023, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard > Vaccines. data.who.int. 2024. Available online: https://data.who.int/dashboards/covid19/vaccines?n=o (accessed on 10 May 2025).
- Bown, C.P.; Bollyky, T.J. How COVID-19 vaccine supply chains emerged in the midst of a pandemic. World Econ. 2022, 45, 468–522. [Google Scholar] [CrossRef]
- Cano-Marin, E.; Ribeiro-Soriano, D.; Mardani, A.; González-Tejero, C. Exploring the challenges of the COVID-19 vaccine supply chain using social media analytics: A global perspective. Sustain. Technol. Entrep. 2023, 2, 100047. [Google Scholar] [CrossRef]
- Golan, Y.; Prahl, M.; Cassidy, A.G.; Gay, C.; Wu, A.H.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; Basilio, E.; Chidboy, M.A.; et al. COVID-19 mRNA Vaccination in Lactation: Assessment of Adverse Events and Vaccine Related Antibodies in Mother-Infant Dyads. Front. Immunol. 2021, 12, 777103. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Ali, Y.; Khan, H.U. IoT platforms assessment methodology for COVID-19 vaccine logistics and transportation: A multi-methods decision making model. Sci. Rep. 2023, 13, 17575. [Google Scholar] [CrossRef]
- Su, Y.; Li, Y.; Zhang, S.; Chen, X. How can COVID-19 vaccines benefit people? A study based on the theory of the commons. Hum. Vaccin. Immunother. 2023, 19, 2225991. [Google Scholar] [CrossRef]
- Batmunkh, T.; Moore, K.A.; Thomson, H.; Altangerel, B.; Amraa, O.; Avaa, N.; Batbayar, L.; Batsukh, K.; Bright, K.; Burentogtokh, T.; et al. Immunogenicity, safety, and reactogenicity of a half- versus full-dose BNT162b2 (Pfizer-BioNTech) booster following a two-dose ChAdOx1 nCoV-19, BBIBP-CorV, or Gam-COVID-Vac priming schedule in Mongolia: A randomised, controlled, non-inferiority trial. Lancet Reg. Health West. Pac. 2023, 42, 100953. [Google Scholar] [CrossRef] [PubMed]
- Cowling, B.J.; Lim, W.W.; Cobey, S. Fractionation of COVID-19 vaccine doses could extend limited supplies and reduce mortality. Nat. Med. 2021, 27, 1321–1323. [Google Scholar] [CrossRef]
- Puthanakit, T.; Chantasrisawad, N.; Yoohat, K.; Nantanee, R.; Sophonphan, J.; Meepuksom, T.; Sodsai, P.; Phanthanawiboon, S.; Jantarabenjakul, W.; Hirankarn, N.; et al. Immunogenicity of a Fractional Dose of mRNA BNT162b2 COVID-19 Vaccine for Primary Series and Booster Vaccination among Healthy Adolescents. Vaccines 2022, 10, 1646. [Google Scholar] [CrossRef] [PubMed]
- Więcek, W.; Ahuja, A.; Chaudhuri, E.; Kremer, M.; Simoes Gomes, A.; Snyder, C.M.; Tabarrok, A.; Tan, B.J. Testing fractional doses of COVID-19 vaccines. Proc. Natl. Acad. Sci. USA 2022, 119, e2116932119. [Google Scholar] [CrossRef] [PubMed]
- Lukaszuk, K.; Podolak, A.; Malinowska, P.; Lukaszuk, J.; Jakiel, G. Cross-Reactivity between Half Doses of Pfizer and AstraZeneca Vaccines-A Preliminary Study. Vaccines 2022, 10, 521. [Google Scholar] [CrossRef]
- Nantanee, R.; Jantarabenjakul, W.; Jaru-Ampornpan, P.; Sodsai, P.; Himananto, O.; Athipunjapong, J.; Sophonphan, J.; Nanthapisal, S.; Hirankarn, N.; Puthanakit, T. A Randomized Clinical Trial of a Fractional Low Dose of BNT162b2 Booster in Adults Following AZD1222. Vaccines 2022, 10, 914. [Google Scholar] [CrossRef]
- Fadlyana, E.; Setiabudi, D.; Kartasasmita, C.B.; Putri, N.D.; Hadinegoro, S.R.; Mulholland, K.; Sofiatin, Y.; Suryadinata, H.; Hartantri, Y.; Sukandar, H.; et al. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: A randomised, observer-masked, controlled trial in Indonesia. Lancet Infect. Dis. 2023, 23, 545–555. [Google Scholar]
- Valim, V.; Martins-Filho, O.A.; Gouvea, M.D.P.G.; Camacho, L.A.B.; Villela, D.A.M.; de Lima, S.M.B.; Azevedo, A.S.; Neto, L.F.P.; Domingues, C.M.A.S.; de Medeiros Junior, N.F.; et al. Effectiveness, Safety, and Immunogenicity of Half Dose ChAdOx1 nCoV-19 COVID-19 Vaccine: Viana Project. Front. Immunol. 2022, 13, 966416. [Google Scholar] [CrossRef]
- Galvão-Lima, L.J.; de Medeiros Júnior, N.F.; Jesus, G.S.; Morais, A.H.F.; Caldeira-Silva, G.J.P.; Queiroz Dos Santos, J.P.; Rocha, M.; Marques Dos Santos, M.; Freire, P.A.; Silva, R.D.; et al. Half Dose ChAdOx1 nCoV-19 Vaccine Was Equivalent to Full Doses to Reduce Moderate and Severe COVID-19 Cases. IJID Reg. 2023, 9, 88–94. [Google Scholar] [CrossRef]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 Serological Tests: Towards Harmonization of Anti-Spike Assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef]
- Meschi, S.; Matusali, G.; Colavita, F.; Lapa, D.; Bordi, L.; Puro, V.; Leoni, B.D.; Galli, C.; Capobianchi, M.R.; Castilletti, C.; et al. Predicting the Protective Humoral Response to a SARS-CoV-2 mRNA Vaccine. Clin. Chem. Lab. Med. 2021, 59, 2010–2018. [Google Scholar] [CrossRef]
- Yang, B.; Huang, X.; Gao, H.; Leung, N.H.; Tsang, T.K.; Cowling, B.J. Immunogenicity, Efficacy, and Safety of SARS-CoV-2 Vaccine Dose Fractionation: A Systematic Review and Meta-Analysis. BMC Med. 2022, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Nanthapisal, S.; Puthanakit, T.; Jaru-Ampornpan, P.; Nantanee, R.; Sodsai, P.; Himananto, O.; Sophonphan, J.; Suchartlikitwong, P.; Hiransuthikul, N.; Angkasekwinai, P.; et al. A Randomized Clinical Trial of a Booster Dose with Low versus Standard Dose of AZD1222 in Adults after Two Doses of Inactivated Vaccines. Vaccine 2022, 40, 2551–2560. [Google Scholar] [CrossRef]
- Roozen, G.V.T.; Prins, M.L.M.; Prins, C.; Janse, J.J.; de Gruyter, H.L.M.; Pothast, C.R.; Huisman, W.; Koopman, J.P.R.; Lamers, O.A.C.; Kuijer, M.; et al. Intradermal Delivery of the Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine: Safety and Immunogenicity of a Fractional Booster Dose. Clin. Microbiol. Infect. 2024, 30, 930–936. [Google Scholar] [CrossRef]
- Farhang-Sardroodi, S.; Korosec, C.S.; Gholami, S.; Craig, M.; Moyles, I.R.; Ghaemi, M.S.; Ooi, H.K.; Heffernan, J.M. Analysis of Host Immunological Response of Adenovirus-Based COVID-19 Vaccines. Vaccines 2021, 9, 861. [Google Scholar] [CrossRef]
- Matrajt, L.; Eaton, J.; Leung, T.; Dimitrov, D.; Schiffer, J.T.; Swan, D.A.; Janes, H. Optimizing Vaccine Allocation for COVID-19 Vaccines Shows the Potential Role of Single-Dose Vaccination. Nat. Commun. 2021, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). AstraZeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets [Internet]; EMA: Amsterdam, The Netherlands, 2021; Available online: https://www.ema.europa.eu/en/news (accessed on 19 June 2025).
- Krammer, F. A Correlate of Protection for SARS-CoV-2 Vaccines Is Urgently Needed. Nat. Med. 2021, 27, 1147–1148. [Google Scholar] [CrossRef]
- Bayart, J.L.; Douxfils, J.; Gillot, C.; David, C.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Gerin, V.; et al. Waning of IgG, Total and Neutralizing Antibodies 6 Months Post-Vaccination with BNT162b2 in Healthcare Workers. Vaccines 2021, 9, 1092. [Google Scholar] [CrossRef]
- Gouvea, M.D.P.G.; Machado, K.L.L.L.; de Oliveira, Y.G.P.; Moulaz, I.R.; Henriques, A.G.; Gouveia, T.M.; Thompson, B.P.; Lança, K.E.M.; de Souza Ramos, S.; Lacerda, G.C.C.; et al. Timeline Kinetics of Protective Immunity to SARS-CoV-2 upon Primary Vaccination and Humoral Response to Variants after Booster Dose. Vaccine 2023, 41, 6514–6528. [Google Scholar] [CrossRef]
- Riou, C.; Bhiman, J.N.; Ganga, Y.; Sawry, S.; Ayres, F.; Baguma, R.; Balla, S.R.; Benede, N.; Bernstein, M.; Besethi, A.S.; et al. Safety and Immunogenicity of Booster Vaccination and Fractional Dosing with Ad26.COV2.S or BNT162b2 in Ad26.COV2.S-Vaccinated Participants. PLoS Glob. Public Health 2024, 4, e0002703. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.M.; Chang, S.C.; Cheng, H.Y.; Shih, S.R.; Lien, C.E. Durability and Immunogenicity of Neutralizing Antibodies Response Against Omicron Variants After Three Doses of Subunit SARS-CoV-2 Vaccine MVC-COV1901: An Extension to an Open-Label, Dose-Escalation Phase 1 Study. Infect. Dis. Ther. 2022, 11, 1493–1504. [Google Scholar] [CrossRef]
- L’Huillier, A.G.; Meyer, B.; Andrey, D.O.; Arm-Vernez, I.; Baggio, S.; Didierlaurent, A.; Eberhardt, C.S.; Eckerle, I.; Grasset-Salomon, C.; Huttner, A.; et al. Antibody Persistence in the First 6 Months Following SARS-CoV-2 Infection Among Hospital Workers: A Prospective Longitudinal Study. Clin. Microbiol. Infect. 2021, 27, 784.e1–784.e8. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, M.; Bellali, H.; Gdoura, M.; Zamali, I.; Kallala, O.; Ben Hmid, A.; Hamdi, W.; Ayari, H.; Fares, H.; Mechri, K.; et al. Humoral and Cellular Immunogenicity of Six Different Vaccines Against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa). Vaccines 2022, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Rha, M.S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-Specific T Cell Memory Is Sustained in COVID-19 Convalescent Patients for 10 Months with Successful Development of Stem Cell-like Memory T Cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Lu, Z.; Laing, E.D.; Pena DaMata, J.; Pohida, K.; Tso, M.S.; Samuels, E.C.; Epsi, N.J.; Dorjbal, B.; Lake, C.; Richard, S.A.; et al. Durability of SARS-CoV-2–Specific T-Cell Responses at 12 Months Postinfection. J. Infect. Dis. 2021, 224, 2010–2019. [Google Scholar] [CrossRef]
- Kang, C.K.; Kim, M.; Lee, S.; Kim, G.; Choe, P.G.; Park, W.B.; Kim, N.J.; Lee, C.H.; Kim, I.S.; Jung, K.; et al. Longitudinal Analysis of Human Memory T-Cell Response According to the Severity of Illness up to 8 Months After SARS-CoV-2 Infection. J. Infect. Dis. 2021, 224, 39–48. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Abdul Quadeer, A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust Against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Verheul, M.K.; Nijhof, K.H.; de Zeeuw-Brouwer, M.L.; Duijm, G.; Ten Hulscher, H.; de Rond, L.; Beckers, L.; Eggink, D.; van Tol, S.; Reimerink, J.; et al. Booster Immunization Improves Memory B Cell Responses in Older Adults Unresponsive to Primary SARS-CoV-2 Immunization. Vaccines 2023, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Bladh, O.; Marking, U.; Havervall, S.; Norin, N.G.; Aguilera, K.; Hober, S.; Smed-Sörensen, A.; Gordon, M.; Blom, K.; Åberg, M.; et al. Mucosal immune responses following a fourth SARS-CoV-2 vaccine dose. Lancet Microbe 2023, 4, e488. [Google Scholar] [CrossRef] [PubMed]
- Tinessia, A.; Clark, K.; Randell, M.; Leask, J.; King, C. Strategies to Address COVID-19 Vaccine Hesitancy in First Nations Peoples: A Systematic Review. Glob. Health Action 2024, 17, 2384497. [Google Scholar] [CrossRef]
- Nnaji, C.A.; Shey, M.S.; Adetokunboh, O.O.; Wiysonge, C.S. Immunogenicity and Safety of Fractional Dose Yellow Fever Vaccination: A Systematic Review and Meta-Analysis. Vaccine 2020, 38, 1291–1301. [Google Scholar] [CrossRef] [PubMed]





| ChAdOx1 Full Dose | ChAdOx1 Half Dose | ||||
|---|---|---|---|---|---|
| Age (years) | Sex | New cases/total person-year | Rate (cases/1000 person-year) | New cases/total person-year | Rate (cases/1000 person-year) |
| 18–29 | F | 3/4261 | 257.0 | 35/63,530 | 201.1 |
| 18–29 | M | 0/2680 | 0.0 | 17/58,208 | 106.6 |
| 30–39 | F | 8/7244 | 403.1 | 35/57,585 | 221.8 |
| 30–39 | M | 4/6701 | 217.9 | 27/49,872 | 197.6 |
| 40–49 | F | 7/9425 | 271.1 | 26/42,877 | 221.3 |
| 40–49 | M | 2/8621 | 84.7 | 13/49,274 | 96.3 |
| All | 24/38,932 | 225 | 153/321,346 | 173.8 | |
| Effect | beta | −0.05 (95% CrI: −0.24–0.15) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros-Junior, N.F.d.; Gouvea, M.d.P.G.; Camacho, L.A.B.; Villela, D.A.M.; Lima, S.M.B.d.; Schwarcz, W.D.; Azevedo, A.S.; Neto, L.F.P.; Domingues, C.M.A.S.; Fantoni, R.N.d.S.; et al. Third Booster Half Dose of ChAdOx1-nCov-19 Is Effective, Safe, and Induces Long-Duration Humoral and Cellular Immune Response to Omicron: 1-Year Follow-Up of Viana Study. Vaccines 2025, 13, 1113. https://doi.org/10.3390/vaccines13111113
Medeiros-Junior NFd, Gouvea MdPG, Camacho LAB, Villela DAM, Lima SMBd, Schwarcz WD, Azevedo AS, Neto LFP, Domingues CMAS, Fantoni RNdS, et al. Third Booster Half Dose of ChAdOx1-nCov-19 Is Effective, Safe, and Induces Long-Duration Humoral and Cellular Immune Response to Omicron: 1-Year Follow-Up of Viana Study. Vaccines. 2025; 13(11):1113. https://doi.org/10.3390/vaccines13111113
Chicago/Turabian StyleMedeiros-Junior, Nésio Fernandes de, Maria da Penha Gomes Gouvea, Luiz Antônio Bastos Camacho, Daniel Antunes Maciel Villela, Sheila Maria Barbosa de Lima, Waleska Dias Schwarcz, Adriana Souza Azevedo, Lauro Ferreira Pinto Neto, Carla Magda Allan Santos Domingues, Rosilene Nilo dos Santos Fantoni, and et al. 2025. "Third Booster Half Dose of ChAdOx1-nCov-19 Is Effective, Safe, and Induces Long-Duration Humoral and Cellular Immune Response to Omicron: 1-Year Follow-Up of Viana Study" Vaccines 13, no. 11: 1113. https://doi.org/10.3390/vaccines13111113
APA StyleMedeiros-Junior, N. F. d., Gouvea, M. d. P. G., Camacho, L. A. B., Villela, D. A. M., Lima, S. M. B. d., Schwarcz, W. D., Azevedo, A. S., Neto, L. F. P., Domingues, C. M. A. S., Fantoni, R. N. d. S., Forechi, L., Ruchdeschel, T., Albertino, L. F., Pereira, M., Rizzi, R. B., Muniz, S. M., Santos, H. C. d., de Oliveira Roza, T. L., Oliveira, Y. G. P. d., ... Valim, V. (2025). Third Booster Half Dose of ChAdOx1-nCov-19 Is Effective, Safe, and Induces Long-Duration Humoral and Cellular Immune Response to Omicron: 1-Year Follow-Up of Viana Study. Vaccines, 13(11), 1113. https://doi.org/10.3390/vaccines13111113









