Genetic Divergence of H1N1pdm09 in Saudi Arabia: Unveiling a Novel N-Glycosylation Site and Its Role in Vaccine Mismatch
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patient Selection and Clinical Samples
2.3. Identification, Typing, and Sequencing of Influenza a Virus
2.3.1. Identification and Typing
2.3.2. Sequencing and Editing of A/H1N1 pdm09 Strains
2.4. Sequence and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Identifying and Typing of the Influenza a Virus
3.2. Analysis of Nucleotide and Amino Acid Sequences of the HA and NA Genes in A/H1N1pdm09 Subtype Study Strains
3.3. Analysis of N and O Glycosylation Sites
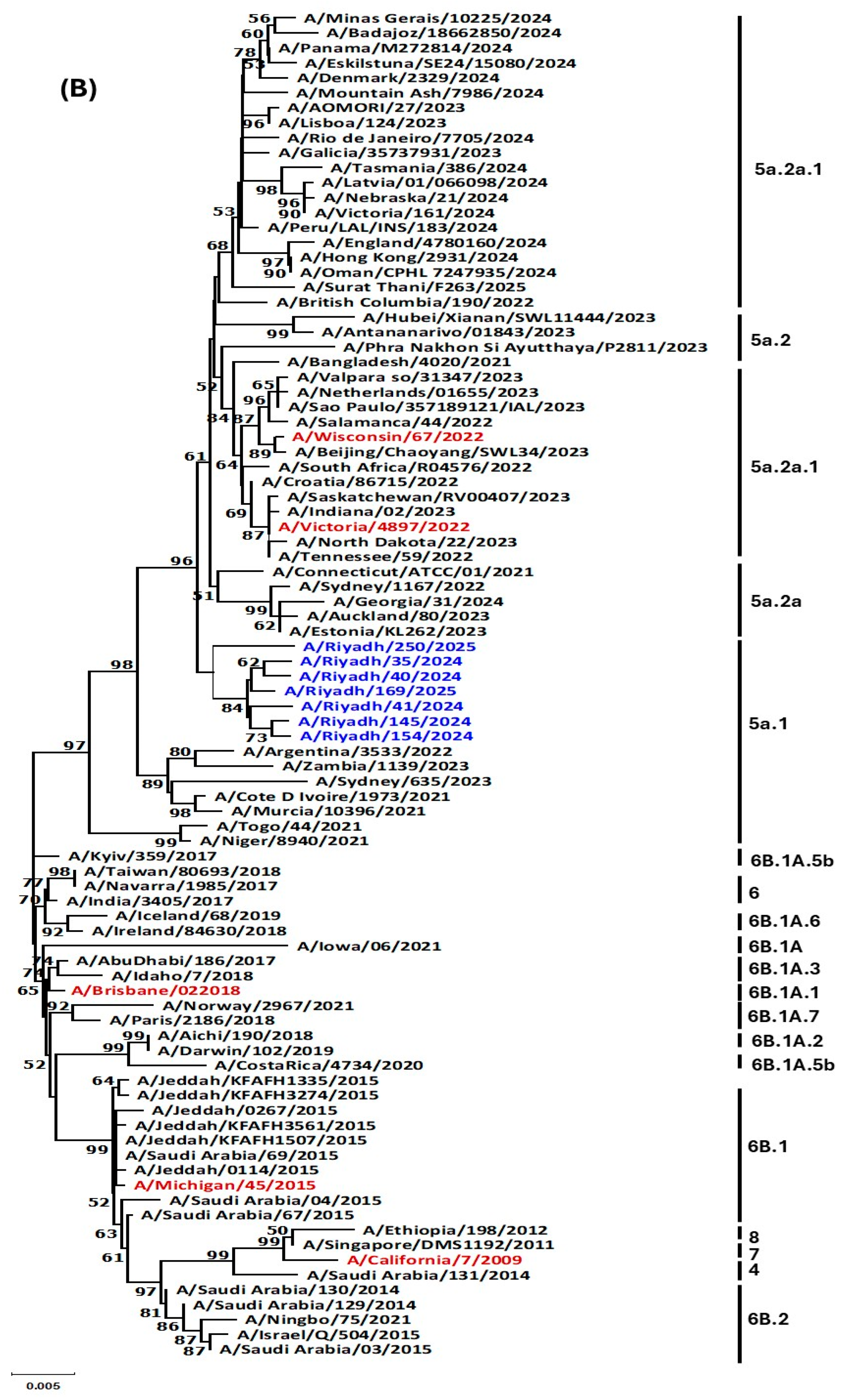
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lafond, K. Estimating the Disease Burden of Influenza from Global Surveillance Data: A Meta-Analysis and Case Study from Indonesia; Tampere University: Tampere, Finland, 2024. [Google Scholar]
- Who, C.C. WHO Information for the Molecular Detection of Influenza Viruses; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Scalera, N.M.; Mossad, S.B. The first pandemic of the 21st century: Review of the 2009 pandemic variant influenza A (H1N1) virus. Postgrad. Med. 2009, 121, 43–47. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Xue, J.; Xiang, Z.; Guo, J.; Zhan, L.; Wei, Q.; Kong, Q. Flu-CED: A comparative transcriptomics database of influenza virus-infected human and animal models. Anim. Models Exp. Med. 2024, 7, 881–892. [Google Scholar] [CrossRef]
- Foni, E.; Chiapponi, C.; Baioni, L.; Zanni, I.; Merenda, M.; Rosignoli, C.; Kyriakis, C.S.; Luini, M.V.; Mandola, M.L.; Bolzoni, L. Influenza D in Italy: Towards a better understanding of an emerging viral infection in swine. Sci. Rep. 2017, 7, 11660. [Google Scholar] [CrossRef]
- Delia, A.; Affinati, B.; Varman, M.; Chatterjee, A. Influenza update. In Viral, Parasitic, Bacterial, And Fungal Infections; Elsevier: Amsterdam, The Netherlands, 2023; pp. 161–166. [Google Scholar]
- Tyrrell, C.S.; Allen, J.L.Y.; Gkrania-Klotsas, E. Influenza: Epidemiology and hospital management. Medicine 2021, 49, 797–804. [Google Scholar] [CrossRef]
- AlMazroa, M.A.; Memish, Z.A.; AlWadey, A.M. Pandemic influenza A (H1N1) in Saudi Arabia: Description of the first one hundred cases. Ann. Saudi Med. 2010, 30, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Ginex, T.; Luque, F.J. Searching for effective antiviral small molecules against influenza A virus: A patent review. Expert Opin. Ther. Pat. 2021, 31, 53–66. [Google Scholar] [PubMed]
- Oladejo, B.O.; Adeboboye, C.F. Influenza Viruses: Targetting Conserved Viral Ha-Stem, Matrix and Nucleo-Proteins to Disarm a Resilient and Recurring Pandemic. In RNA Viruses Infection; IntechOpen: London, UK, 2022. [Google Scholar]
- Moore, K.A.; Ostrowsky, J.T.; Kraigsley, A.M.; Mehr, A.J.; Bresee, J.S.; Friede, M.H.; Gellin, B.G.; Golding, J.P.; Hart, P.J.; Moen, A. A Research and Development (R&D) roadmap for influenza vaccines: Looking toward the future. Vaccine 2021, 39, 6573–6584. [Google Scholar] [CrossRef]
- Agha, A.; Alrawi, A.; Munayco, C.V.; AlAyed, M.S.; Al-Hakami, M.; Korairi, H.; Bella, A. Characteristics of patients hospitalized with 2009 H1N1 influenza in a tertiary care hospital in southern Saudi Arabia. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012002. [Google Scholar] [CrossRef] [PubMed]
- Uthman, N.A.; Sohrab, S.S.; Kamal, I.H.; Farraj, S.A.; Masri, B.E.; Ashshi, A.M.; Kumosani, T.A.; Azhar, E.I. Genetic diversity of the pandemic influenza A (H1N1) virus in Saudi Arabia. J. Infect. Dev. Ctries. 2014, 8, 1563–1573. [Google Scholar] [CrossRef]
- Al Shammari, B.R. Sequence and Phylogenetic Analysis of Influenza Virus (H1N1pdm2009) Circulating in Riyadh, Saudi Arabia. J. Pure Appl. Microbiol. 2024, 18, 2380. [Google Scholar] [CrossRef]
- Naeem, A.; Elbakkouri, K.; Alfaiz, A.; Hamed, M.E.; Alsaran, H.; AlOtaiby, S.; Enani, M.; Alosaimi, B. Antigenic drift of hemagglutinin and neuraminidase in seasonal H1N1 influenza viruses from Saudi Arabia in 2014 to 2015. J. Med. Virol. 2020, 92, 3016–3027. [Google Scholar]
- Alshammari, T.M.; Yusuff, K.B.; Aziz, M.M.; Subaie, G.M. Healthcare professionals’ knowledge, attitude and acceptance of influenza vaccination in Saudi Arabia: A multicenter cross-sectional study. BMC Health Serv. Res. 2019, 19, 1–10. [Google Scholar]
- Dudin, G.A.; Aziz, I.M.; Alzayed, R.M.; Ahmed, A.; Hussain, T.; Somily, A.M.; Alsaadi, M.M.; Almajhdi, F.N. Genetic diversity and evolutionary kinetics of influenza A virus H3N2 subtypes circulating in Riyadh, Saudi Arabia. Vaccines 2023, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Jung, E.; Brunak, S. Prediction of N-Glycosylation Sites in Human Proteins; ResearchGate: Berlin, Germany, 2004. [Google Scholar]
- Julenius, K.; Mølgaard, A.; Gupta, R.; Brunak, S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 2005, 15, 153–164. [Google Scholar] [PubMed]
- Benkouiten, S.; Al-Tawfiq, J.A.; Memish, Z.A.; Albarrak, A.; Gautret, P. Clinical respiratory infections and pneumonia during the Hajj pilgrimage: A systematic review. Travel Med. Infect. Dis. 2019, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Alrezaihi, A.; Amin, D.; AlRagi, N.; Alhatlani, B.; Binjomah, A.; Aleisa, K.; Dong, X.; Hiscox, J.A.; Aljabr, W. Molecular surveillance of influenza A virus in Saudi Arabia: Whole-genome sequencing and metagenomic approaches. Microbiol. Spectr. 2024, 12, e00665-24. [Google Scholar] [CrossRef]
- Elhakim, M.; Rasooly, M.H.; Fahim, M.; Ali, S.S.; Haddad, N.; Cherkaoui, I.; Hjaija, D.; Nadeem, S.; Assiri, A.; Aljifri, A. Epidemiology of severe cases of influenza and other acute respiratory infections in the Eastern Mediterranean Region, July 2016 to June 2018. J. Infect. Public Health 2020, 13, 423–429. [Google Scholar]
- Al-Dorzi, H.M.; Alsafwani, Z.A.; Alsalahi, E.; Aljulayfi, A.S.; Alshaer, R.; Alanazi, S.; Aldossari, M.A.; Alsahoo, D.A.; Khan, R. Patients with influenza admitted to a tertiary-care hospital in Riyadh between 2018 and 2022: Characteristics, outcomes and factors associated with ICU admission and mortality. BMC Pulm. Med. 2024, 24, 464. [Google Scholar] [CrossRef]
- Al-Baadani, A.M.; Elzein, F.E.; Alhemyadi, S.A.; Khan, O.A.; Albenmousa, A.H.; Idrees, M.M. Characteristics and outcome of viral pneumonia caused by influenza and Middle East respiratory syndrome-coronavirus infections: A 4-year experience from a tertiary care center. Ann. Thorac. Med. 2019, 14, 179–185. [Google Scholar] [CrossRef]
- Nelson, M.I.; Edelman, L.; Spiro, D.J.; Boyne, A.R.; Bera, J.; Halpin, R.; Sengamalay, N.; Ghedin, E.; Miller, M.A.; Simonsen, L. Correction: Molecular Epidemiology of A/H3N2 and A/H1N1 Influenza Virus during a Single Epidemic Season in the United States. PLoS Pathog. 2008, 4, e1000133. [Google Scholar] [CrossRef]
- Mauskopf, J.; Klesse, M.; Lee, S.; Herrera-Taracena, G. The burden of influenza complications in different high-risk groups: A targeted literature review. J. Med. Econ. 2013, 16, 264–277. [Google Scholar] [CrossRef]
- Zhang, A.J.; To, K.K.; Tse, H.; Chan, K.-H.; Guo, K.-Y.; Li, C.; Hung, I.F.; Chan, J.F.; Chen, H.; Tam, S. High incidence of severe influenza among individuals over 50 years of age. Clin. Vaccine Immunol. 2011, 18, 1918–1924. [Google Scholar] [CrossRef]
- Awadalla, M.E.; Alkadi, H.; Alarjani, M.; Al-Anazi, A.E.; Ibrahim, M.A.; ALOhali, T.A.; Enani, M.; Alturaiki, W.; Alosaimi, B. Moderately low effectiveness of the influenza quadrivalent vaccine: Potential mismatch between circulating strains and vaccine strains. Vaccines 2023, 11, 1050. [Google Scholar] [CrossRef]
- Althaqafi, A.; Farahat, F.; Alsaedi, A.; Alshamrani, M.; Alsaeed, M.S.; AlhajHussein, B.; El-Kafrawy, S.A.; Azhar, E.I. Molecular detection of influenza A and B viruses in four consecutive influenza seasons 2015–16 to 2018–19 in a tertiary center in Western Saudi Arabia. J. Epidemiol. Glob. Health 2021, 11, 208–215. [Google Scholar] [CrossRef]
- Samal, S.K. Structural vaccinology approaches to enhance efficacy, stability, and delivery of protective antigens. In Reverse Vaccinology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 217–235. [Google Scholar]
- Adeola, O.; Olugasa, B.; Emikpe, B. Molecular detection of influenza A (H1N1) pdm09 viruses with M genes from human pandemic strains among Nigerian pigs, 2013–2015: Implications and associated risk factors. Epidemiol. Infect. 2017, 145, 3345–3360. [Google Scholar] [CrossRef]
- Azbazdar, M.E.; Dikmenogullari, M.; Kavalci, Z.; Koçer, Z.A. Genetic Evolution of the Hemagglutinin Genes of Seasonal Influenza A Viruses in Türkiye Between 2017 and 2023. Influenza Other Respir. Viruses 2025, 19, e70134. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Pybus, O.G.; Nelson, M.I.; Viboud, C.; Taubenberger, J.K.; Holmes, E.C. The genomic and epidemiological dynamics of human influenza A virus. Nature 2008, 453, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Simonsen, L.; Viboud, C.; Miller, M.A.; Taylor, J.; George, K.S.; Griesemer, S.B.; Ghedin, E.; Sengamalay, N.A.; Spiro, D.J. Stochastic processes are key determinants of short-term evolution in influenza a virus. PLoS Pathog. 2006, 2, e125. [Google Scholar] [CrossRef]
- Viboud, C.; Alonso, W.J.; Simonsen, L. Influenza in tropical regions. PLoS Med. 2006, 3, e89. [Google Scholar] [CrossRef]
- Nelson, M.I.; Simonsen, L.; Viboud, C.; Miller, M.A.; Holmes, E.C. Phylogenetic analysis reveals the global migration of seasonal influenza A viruses. PLoS Pathog. 2007, 3, e131. [Google Scholar] [CrossRef]
- Russell, C.A. The Global Circulation of Seasonal Influenza A. Geophys. Res. Lett 2007, 34, 1–8. [Google Scholar] [CrossRef]
- Holmes, E.C.; Ghedin, E.; Miller, N.; Taylor, J.; Bao, Y.; St George, K.; Grenfell, B.T.; Salzberg, S.L.; Fraser, C.M.; Lipman, D.J. Whole-genome analysis of human influenza A virus reveals multiple persistent lineages and reassortment among recent H3N2 viruses. PLoS Biol. 2005, 3, e300. [Google Scholar] [CrossRef]
- Hashem, A.M.; Azhar, E.I.; Shalhoub, S.; Abujamel, T.S.; Othman, N.A.; Al Zahrani, A.B.; Abdullah, H.M.; Al-Alawi, M.M.; Sindi, A.A. Genetic characterization and diversity of circulating influenza A/H1N1pdm09 viruses isolated in Jeddah, Saudi Arabia between 2014 and 2015. Arch. Virol. 2018, 163, 1219–1230. [Google Scholar] [CrossRef]
- Mokalla, V.R.; Gundarapu, S.; Kaushik, R.S.; Rajput, M.; Tummala, H. Influenza Vaccines: Current Status, Adjuvant Strategies, and Efficacy. Vaccines 2025, 13, 962. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Palese, P. Is a universal influenza virus vaccine possible? Annu. Rev. Med. 2020, 71, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, W.K.; Azziz-Baumgartner, E.; Bashir, U.; Cox, N.J.; Fasce, R.; Giovanni, M.; Grohmann, G.; Huang, S.; Katz, J.; Mironenko, A. Strengthening the influenza vaccine virus selection and development process: Report of the 3rd WHO Informal Consultation for Improving Influenza Vaccine Virus Selection held at WHO headquarters, Geneva, Switzerland, 1–3 April 2014. Vaccine 2015, 33, 4368–4382. [Google Scholar] [CrossRef]
- Xing, L.; Chen, Y.; Chen, B.; Bu, L.; Liu, Y.; Zeng, Z.; Guan, W.; Chen, Q.; Lin, Y.; Qin, K. Antigenic drift of the hemagglutinin from an influenza A (H1N1) pdm09 clinical isolate increases its pathogenicity in vitro. Virol. Sin. 2021, 36, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef]
- Mair, C.M.; Ludwig, K.; Herrmann, A.; Sieben, C. Receptor binding and pH stability—How influenza A virus hemagglutinin affects host-specific virus infection. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1153–1168. [Google Scholar] [CrossRef]
- Tzarum, N.; De Vries, R.P.; Zhu, X.; Yu, W.; McBride, R.; Paulson, J.C.; Wilson, I.A. Structure and receptor binding of the hemagglutinin from a human H6N1 influenza virus. Cell Host Microbe 2015, 17, 369–376. [Google Scholar] [CrossRef]
- Raymond, D.D.; Bajic, G.; Ferdman, J.; Suphaphiphat, P.; Settembre, E.C.; Moody, M.A.; Schmidt, A.G.; Harrison, S.C. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl. Acad. Sci. USA 2018, 115, 168–173. [Google Scholar] [CrossRef] [PubMed]


| Type/Subtype | Gene | Primer Name | Sequence 5′-3′ | Product Size (bp) | Ref. | |
|---|---|---|---|---|---|---|
| Used primers for detection | influenza A virus | M30F2/08 | ATGAGYCTTYTAACCGAGGTCGAAACG | 244 | [2] | |
| M264R3/08 | TGGACAAANCGTCTACGCTGCAG | |||||
| Used primers for typing | (A/H1N1) Pdm09 | HKU-SWF | TGAGCTCAGTGTCATCATTTGA | 174 | [2] | |
| HKU-SWR | TGCTGAGCTTTGGGTATGAA | |||||
| A/H3N2 | H3A1F6 | AAGCAGGGGATAATTCTATTAACC | 1127 | |||
| H3A1R1 | GTCTATCATTCCCTCCCAACCATT | |||||
| Used primers for sequencing | A/H1N1 pdm09 | HA | H1-F1 | AGCAAAAGCAGGGGAAAATAAAAGC | 1264 | |
| H1-R1 | CCTACTGCTGTGAACTGTGTATTC | |||||
| H1-F2 | GGGAGAATGAACTATTACTGG | 979 | [2] | |||
| H1-R2 | AGTAGAAACAAGGGTGTTTTT | |||||
| A/H1N1 pdm09 | NA | N1-F1 | AGCAAAAGCAGGAGTTTAAAATG | 1099 | ||
| N1-R1 | CCTATCCAAACACCATTGCCGTAT | |||||
| N1-F2 | GGAATGCAGAACCTTCTTCTTGAC | 1073 | ||||
| N1-R2 | ATATGGTCTCGTATTAGTAGAAACAAGGAGTTTTTT |
| No. of Samples n (%) | Positive for Influenza A Virus n (%) | Positive for | |||
| A/H1N1pdm09 n (%) | A/H3N2 n (%) | ||||
| Total | 363 | 110 (30.3) | 68 (61.8) | 42 (38.2) | |
| Year | 2024 | 166 (45.7) | 47 (28.3) | 38 (80.8) | 9 (19.2) |
| 2025 | 197 (54.3) | 63 (31.9) | 30 (47.6) | 33 (52.4) | |
| Gender | Male | 176 (48.4) | 45 (25.5) | 29 (64.4) | 16 (35.5) |
| Female | 187 (51.5) | 65 (34.7) a | 39 (60) a | 26 (39.4) | |
| Age in years | 0–4 | 121 (33.3) | 27 (22.3) | 13 (48.1) | 14 (51.8) |
| 5–14 | 88 (24.2) | 29 (32.9) | 20 (68.9) | 9 (31.0) | |
| 15–64 | 101 (27.8) | 33 (32.6) b | 16 (48.4) | 17 (51.5) b | |
| ≥65 | 53 (14.6) | 21 (39.6) | 19 (90.4) | 2 (9.5) | |
| 130-Loop | 190-Helix | 220-Loop | ||||||||||||||||||
| Mutation sites | 134 | 135 | 136 | 138 | 188 | 189 | 190 | 191 | 192 | 193 | 194 | 195 | 221 | 222 | 223 | 224 | 225 | 226 | 227 | 228 |
| A/California/7/2009 | F | P | K | S | K | E | V | L | V | L | W | G | S | R | Y | S | K | K | F | K |
| A/Wisconsin/67/2022 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Victoria/4897/2022 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Riyadh/35/2024 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Riyadh/40/2024 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Riyadh/41/2024 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Riyadh/145/2024 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Riyadh/154/2024 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Riyadh/169/2025 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| A/Riyadh/250/2025 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulgader, S.A.; Abdulwahed, A.M.; Almuqrin, A.M.; Aziz, I.M.; Alkubaisi, N.A.; Aljowaie, R.M.; Farrag, M.A.; Alhetheel, A.F.; Abdulmanea, A.A.; Alanazi, F.N.; et al. Genetic Divergence of H1N1pdm09 in Saudi Arabia: Unveiling a Novel N-Glycosylation Site and Its Role in Vaccine Mismatch. Vaccines 2025, 13, 1111. https://doi.org/10.3390/vaccines13111111
Abdulgader SA, Abdulwahed AM, Almuqrin AM, Aziz IM, Alkubaisi NA, Aljowaie RM, Farrag MA, Alhetheel AF, Abdulmanea AA, Alanazi FN, et al. Genetic Divergence of H1N1pdm09 in Saudi Arabia: Unveiling a Novel N-Glycosylation Site and Its Role in Vaccine Mismatch. Vaccines. 2025; 13(11):1111. https://doi.org/10.3390/vaccines13111111
Chicago/Turabian StyleAbdulgader, Shatha Ata, Abdulhadi M. Abdulwahed, Abdulaziz M. Almuqrin, Ibrahim M. Aziz, Noorah A. Alkubaisi, Reem M. Aljowaie, Mohamed A. Farrag, Abdulkarim F. Alhetheel, Adel A. Abdulmanea, Fatimah N. Alanazi, and et al. 2025. "Genetic Divergence of H1N1pdm09 in Saudi Arabia: Unveiling a Novel N-Glycosylation Site and Its Role in Vaccine Mismatch" Vaccines 13, no. 11: 1111. https://doi.org/10.3390/vaccines13111111
APA StyleAbdulgader, S. A., Abdulwahed, A. M., Almuqrin, A. M., Aziz, I. M., Alkubaisi, N. A., Aljowaie, R. M., Farrag, M. A., Alhetheel, A. F., Abdulmanea, A. A., Alanazi, F. N., Alsaleh, A. N., & Almajhdi, F. N. (2025). Genetic Divergence of H1N1pdm09 in Saudi Arabia: Unveiling a Novel N-Glycosylation Site and Its Role in Vaccine Mismatch. Vaccines, 13(11), 1111. https://doi.org/10.3390/vaccines13111111





