Intracellular Parasitic Infections Caused by Plasmodium falciparum, Leishmania spp., Toxoplasma gondii, Echinococcus multilocularis, Among Key Pathogens: Global Burden, Transmission Dynamics, and Vaccine Advances—A Narrative Review with Contextual Insights from Armenia
Abstract
1. Introduction
Methodology
2. Global Overview of Intracellular Parasitic Infections: Classification, Transmission and Immune Evasion
2.1. Classification of Intracellular Parasitic Infections
- (i)
- Protozoa (Eukaryotes);
- (ii)
- Apicomplexa: Plasmodium, Toxoplasma, Cryptosporidium;
- (iii)
- Kinetoplastida: Leishmania, Trypanosoma;
- (iv)
- Microsporidia: Spore-forming unicellular parasites (e.g., Enterocytozoon bieneusi);
- (v)
- (i)
- Direct (monoxenous) (single host): Parasites live their whole lives in one host organism without needing an intermediate host. Some examples are Cryptosporidium spp., Giardia lamblia, and Entamoeba histolytica.
- (ii)
- Indirect (heteroxenous) (Multi-host): Parasites need more than one host to finish their life cycle. Plasmodium spp. (human + mosquito), Leishmania spp. (human/animal + sandfly), Toxoplasma gondii (cat + intermediate hosts), and Echinococcus multilocularis (dog/fox + rodent/human) are some examples [55,56].
| Parasite | Type | Obligate/ Facultative | Definitive Host(s) | Intermediate Host(s) | Transmission/Vector | Ref. |
|---|---|---|---|---|---|---|
| Plasmodium falciparum | Protozoa (Apicomplexa) | Obligate | Anopheles mosquitoes (the sexual cycle takes place in them) | Humans (hepatocytes, RBCs) | Mosquito bite (sporozoite) | [52,54] |
| Toxoplasma gondii | Protozoa (Apicomplexa) | Obligate | Felidae family (e.g., domestic and wild cats) | Humans, many warm-blooded animals | Oral (oocysts, tissue cysts) | [46,53] |
| Leishmania donovani | Protozoa (Kinetoplastida) | Obligate/ Facultative * | Humans | Sand flies (Phlebotomus, Lutzomyia); reservoirs: canines, rodents, mammals | Sandfly bite (promastigote) | [49,52] |
| Trypanosoma cruzi | Protozoa (Kinetoplastida) | Obligate/ Facultative * | Humans, animals (various cells) | Not applicable in the same way as the definitive host | Triatomine bugs (Triatoma, Rhodnius, Panstrongylus); infection via feces at bite site/mucosa. | [48,51] |
| Cryptosporidium parvum | Protozoa (Apicomplexa) | Obligate | Humans, cattle (sexual stage) | Humans, cattle (asexual stage) | Fecal–oral (water, food, contact); flies may act as mechanical vectors | [45,51] |
| Echinococcus multilocularis | Cestode (tapeworm, helminth) | Obligate | Canids (dogs, foxes) | Rodents, humans (accidental) | Fecal–oral (egg ingestion) | [51,57] |
2.2. Modes of Transmission in Intracellular Parasitic Infections
2.2.1. Vector-Borne Transmission
- (i)
- malaria (Plasmodium falciparum, P. vivax, P. ovale, P. malariae, P. knowlesi)—spread by Anopheles mosquitoes and causing hundreds of thousands of deaths each year.
- (ii)
- Leishmaniasis (Leishmania spp.): transmitted by phlebotomine sandflies; it is still one of the most neglected tropical diseases with a high rate of illness and death.
- (iii)
- Chagas disease (Trypanosoma cruzi): spread by triatomine bugs; mostly affects Latin America but is being reported more and more in places where it is not common.
- (iv)
- African trypanosomiasis, also known as sleeping sickness (Trypanosoma brucei gambiense, T. b. rhodesiense): transmitted by tsetse flies (Glossina spp.); causes severe neurological disease if untreated.
- (v)
- Babesiosis (Babesia microti, B. divergens), transmitted by ixodid ticks; significant in humans and animals, particularly in temperate regions.
- (vi)
- Theileriosis (Theileria parva, T. annulata), transmitted by ticks (Rhipicephalus, Hyalomma), primarily affects livestock but is significant in veterinary parasitology. Filarial infections, such as lymphatic filariasis caused by Wuchereria bancrofti, Brugia malayi, and Onchocerca volvulus, are transmitted by mosquitoes (Culex, Anopheles, Aedes) or blackflies (Simulium spp.) and are significant contributors to chronic morbidity in endemic areas. These vector-borne infections are difficult to control because vectors are ecologically resilient, so we need both medical treatments and integrated vector management strategies [62,63,64,65,66,67,68].
2.2.2. Fecal–Oral Transmission
2.2.3. Vertical Transmission
2.2.4. Direct Contact and Sexual Transmission
2.2.5. Environmental and Foodborne Routes
3. Advances in Diagnostics, Control Strategies, and Vaccine Development for Intracellular Parasites
3.1. Advances in Diagnostics
3.2. Control Strategies
3.3. Vaccine Development
3.3.1. Plasmodium falciparum (Malaria)
3.3.2. Leishmania spp. (Leishmaniasis)
3.3.3. Toxoplasma gondii
3.3.4. Echinococcus multilocularis
3.3.5. Cryptosporidium spp.
4. Intracellular Parasitic Infections in Armenia: Epidemiological Trends, Diagnostic Gaps, and Future Directions Within a ‘One Health’ Framework
- (i)
- establishment of systematic surveillance programs for waterborne, vector-borne, and zoonotic parasites.
- (ii)
- expansion of molecular diagnostic infrastructure to improve early detection and case confirmation.
- (iii)
- awareness and training among healthcare professionals to reduce underreporting and misdiagnosis.
- (iv)
- participation in international research collaborations on parasite epidemiology, molecular typing, and vaccine development.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Alveolar Echinococcosis |
| bp | Base Pair (unit of DNA length) |
| CD | Chagas Disease (context-specific abbreviation) |
| DALY | Disability-Adjusted Life Year |
| DF | Degrees of Freedom (in statistical tests) |
| DNA | Deoxyribonucleic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IFAT | Immunofluorescent Antibody Test |
| IFN-γ | Interferon-gamma |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IRS | Indoor Residual Spraying |
| IVM | Integrated Vector Management |
| ITS1-PCR-RFLP | Internal Transcribed Spacer 1-PCR-Restriction Fragment Length Polymorphism |
| ITNs | Insecticide-Treated Nets |
| kDNA | Kinetoplast DNA |
| LLINs | Long-Lasting Insecticidal Nets |
| mRNA | Messenger Ribonucleic Acid |
| mZN | Modified Ziehl–Neelsen (staining) |
| NTDs | Neglected Tropical Diseases |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
| rDNA | Ribosomal DNA |
| RE gene | Repetitive Element gene (used in T. gondii PCR assays) |
| RIDASCREEN® | Brand name for a commercial ELISA kit |
| RNA | Ribonucleic Acid |
| TNF-α | Tumor Necrosis Factor-alpha |
| VL | Visceral Leishmaniasis |
| WHO | World Health Organization |
| χ2 | Chi-square (statistical test symbol) |
References
- Osier, F.; Uzonna, J.E. Editorial: Regulation of Immunity to Parasitic Infections Endemic to Africa. Front. Immunol. 2020, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jiang, N.; Farr, L.; Ngobeni, R.; Moonah, S. Parasite-Produced MIF Cytokine: Role in Immune Evasion, Invasion, and Pathogenesis. Front. Immunol. 2019, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Islam, M.M.; Singh, R.; Mkhize-Kwitshana, Z.L. Demographic Profile of HIV and Helminth-Coinfected Adults in KwaZulu-Natal, South Africa. S. Afr. J. Infect. Dis. 2023, 38, 466. [Google Scholar] [CrossRef]
- Lukeš, J.; Mauricio, I.L.; Schönian, G.; Dujardin, J.; Soteriadou, K.; Dedet, J.; Kuhls, K.; Tintaya, K.W.Q.; Jirků, M.; Chocholová, E.; et al. Evolutionary and Geographical History of the Leishmania donovani Complex with a Revision of Current Taxonomy. Proc. Natl. Acad. Sci. USA 2007, 104, 9375–9380. [Google Scholar] [CrossRef] [PubMed]
- Maurice, A.P.; Jenkin, A.; Norton, R.E.; Hamilton, A.; Ho, Y. Epidemiology of Parasitic Diseases; Springer: Cham, Switzerland, 2020; pp. 3–21. [Google Scholar] [CrossRef]
- Torgerson, P.R.; de Silva, N.R.; Fèvre, E.M.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Sripa, B.; Gargouri, N.; Willingham, A.L.; Stein, C. The Global Burden of Foodborne Parasitic Diseases: An Update. Trends Parasitol. 2014, 30, 20–26. [Google Scholar] [CrossRef]
- Kaminsky, R.; Mäser, P. Global Impact of Parasitic Infections and the Importance of Parasite Control. Front. Parasitol. 2025, 4, 1546195. [Google Scholar] [CrossRef]
- Van de Vuurst, P.; Escobar, L.E. Climate Change and Infectious Disease: A Review of Evidence and Research Trends. Infect. Dis. Poverty 2023, 12, 51. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, H.; Ren, N.; Qadeer, A.; Tabios, I.K.B.; Fontanilla, I.K.C.; Leonardo, L.R.; Sripa, B.; Cheng, G. The Global Prevalence of and Factors Associated with Parasitic Coinfection in People Living with Viruses: A Systematic Review and Meta-Analysis. Pathogens 2025, 14, 534. [Google Scholar] [CrossRef]
- Taye, B.; Desta, K.; Ejigu, S.; Dori, G.U. The Magnitude and Risk Factors of Intestinal Parasitic Infection in Relation to Human Immunodeficiency Virus Infection and Immune Status at ALERT Hospital, Addis Ababa, Ethiopia. Parasitol. Int. 2014, 63, 550–556. [Google Scholar] [CrossRef]
- Brown, M.; Mawa, P.A.; Kaleebu, P.; Elliott, A.M. Helminths and HIV Infection: Epidemiological Observations on Immunological Hypotheses. Parasite Immunol. 2006, 28, 613–623. [Google Scholar] [CrossRef]
- Chulanetra, M.; Chaicumpa, W. Revisiting the Mechanisms of Immune Evasion Employed by Human Parasites. Front. Cell Infect. Microbiol. 2021, 11, 702125. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Ajibola, O.; Gulumbe, B.H.; Eze, A.A.; Obishakin, E. Tools for Detection of Schistosomiasis in Resource Limited Settings. Med. Sci. 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Fasogbon, I.V.; Ondari, E.N.; Tusubira, D.; Ogbonnia, E.C.; Ashley, J.; Sasikumar, S.; Aja, P.M. A Critical Review of the Limitations of Current Diagnostic Techniques for Schistosomiasis. All Life 2024, 17, 2379305. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.A.; Mkrtchyan, A.F.; Saghyan, A.S.; Palumbo, R.; Belter, A.; Hayriyan, L.A.; Simonyan, H.; Roviello, V.; Roviello, G.N. Spectroscopic and SEM evidences for G4-DNA binding by a synthetic alkyne-containing amino acid with anticancer activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117884. [Google Scholar] [CrossRef]
- Roviello, V.; Musumeci, D.; Mokhir, A.; Roviello, G.N. Evidence of protein binding by a nucleopeptide based on a thymine-decorated L-diaminopropanoic acid through CD and in silico studies. Curr. Med. Chem. 2021, 28, 5004–5015. [Google Scholar] [CrossRef]
- Marzano, M.; Falanga, A.P.; Marasco, D.; Borbone, N.; D’Errico, S.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Evaluation of an Analogue of the Marine ε-PLL Peptide as a Ligand of G-quadruplex DNA Structures. Mar. Drugs 2020, 18, 49. [Google Scholar] [CrossRef]
- Roviello, G.N.; Ricci, A.; Bucci, E.M.; Pedone, C. Synthesis, biological evaluation and supramolecular assembly of novel analogues of peptidyl nucleosides. Mol. BioSyst. 2011, 7, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, A.S.; Karapetyan, L.T.; Mkhitaryan, A.V.; Stepanyan, L.A.; Sargsyan, T.H.; Danghyan, Y.M.; Sargsyan, A.V.; Oganezova, G.G.; Hovhannisyan, N.A. Modeling, Synthesis and In Vitro Testing of Peptides Based on Unusual Amino Acids as Potential Antibacterial Agents. Biomed. Khim. 2024, 70, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.N.; Musumeci, D.; Bucci, E.M.; Pedone, C. Evidences for supramolecular organization of nucleopeptides: Synthesis, spectroscopic and biological studies of a novel dithymine L-serine tetrapeptide. Mol. BioSyst. 2011, 7, 1181–1189. [Google Scholar] [CrossRef]
- Roviello, G.; Musumeci, D.; Pedone, C.; Bucci, E.M. Synthesis, characterization and hybridization studies of an alternate nucleo-ε/γ-peptide: Complexes formation with natural nucleic acids. Amino Acids 2010, 38, 103–111. [Google Scholar] [CrossRef]
- Musumeci, D.; Oliviero, G.; Roviello, G.N.; Bucci, E.M.; Piccialli, G. G-quadruplex-forming oligonucleotide conjugated to magnetic nanoparticles: Synthesis, characterization, and enzymatic stability assays. Bioconjug. Chem. 2012, 23, 382–391. [Google Scholar] [CrossRef]
- Roviello, G.N.; Di Gaetano, S.; Capasso, D.; Cesarani, A.; Bucci, E.M.; Pedone, C. Synthesis, spectroscopic studies and biological activity of a novel nucleopeptide with Moloney murine leukemia virus reverse transcriptase inhibitory activity. Amino Acids 2010, 38, 1489–1496. [Google Scholar] [CrossRef]
- Liu, L.X.; Weller, P.F. Antiparasitic drugs. N. Engl. J. Med. 1996, 334, 1178–1184. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Koger-Pease, C.; Perera, D.J.; Ndao, M. Recent Advances in the Development of Adenovirus-Vectored Vaccines for Parasitic Infections. Pharmaceuticals 2023, 16, 334. [Google Scholar] [CrossRef]
- Chu, K.B.; Quan, F.S. Virus-Like Particle Vaccines Against Respiratory Viruses and Protozoan Parasites. Curr. Top. Microbiol. Immunol. 2021, 433, 77–106. [Google Scholar] [CrossRef]
- Sukiasyan, A.; Keshishyan, A.; Manukyan, D.; Melik-Andreasyan, G.; Atshemyan, L.; Apresyan, H.; Strelkova, M.; Frohme, M.; Cortes, S.; Kuhls, K. Re-Emerging Foci of Visceral Leishmaniasis in Armenia—First Molecular Diagnosis of Clinical Samples. Parasitology 2019, 146, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; Oliveira, D.S.d.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.d.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens 2024, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Kuhls, K.; Moskalenko, O.; Sukiasyan, A.; Manukyan, D.; Melik-Andreasyan, G.; Atshemyan, L.; Apresyan, H.; Strelkova, M.; Jaeschke, A.; Wieland, R.; et al. Microsatellite-based molecular epidemiology of Leishmania infantum in Armenia reveals re-emerging endemic foci of visceral leishmaniasis in Armenia and pilot risk assessment by ecological niche modeling. PLoS Negl. Trop. Dis. 2021, 15, e0009288. [Google Scholar] [CrossRef] [PubMed]
- Paronyan, L.; Apresyan, H.; Galstyan, A.; Keshishyan, A.; Vanyan, A. Trends in visceral leishmaniasis in Armenia 1999–2014. In International Conference on Emerging Infectious Diseases 2015 poster and oral presen-tation abstacts. Emerg. Inf. Dis. 2015, 396. Available online: http://www.cdc.gov/EID/pdfs/ICEID2015.pdf (accessed on 20 October 2025).
- Aghayan, S.A.; Grigoryan, G.; Gevorgyan, H.; Harutyunyan, T.; Rukhkyan, M.; Muradyan, V.; Karadjian, G.; Marsot, M.; Moutailler, S.; Pollet, T. Diversity and Distribution of Bacterial and Parasitic Tick-Borne Pathogens in Armenia, Transcaucasia. Iran. J. Public Health 2024, 53, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Manucharyan, A.; Achenbach, J.; Paronyan, L.; Avetisyan, L.; Danielyan, R.; Melik-Andreasyan, G. Gamasid Ticks as Vectors of Tularemia in the Southeast of Armenia. Vector Borne Zoonotic Dis. 2023, 23, 284–290. [Google Scholar] [CrossRef]
- Avetisian, L.M. Sovremennoe sostoianie épidemiologicheskogo nadzora za vazhneĭshimi parazitozami v respublike Armeniia [Epidemiological Surveillance of Parasitic Diseases in the Republic of Armenia]. Med. Parazitol. 2004, 1, 21–24. (In Russian) [Google Scholar]
- Manukyan, A.; Grigoryan, G.; Gevorgyan, K.; Melik-Andreasyan, G.; Keshishyan, A.; Sahakyan, G. The Potential Risk Factors of Cystic Echinococcosis in Armenia. Iran. J. Parasitol. 2025, 20, 322–324. [Google Scholar] [CrossRef]
- Manukyan, A.; Avetisyan, L.; Sahakyan, G.; Paez Jimenez, A.; Paronyan, L.; Gevorgyan, K.; Vanyan, A. Epidemiological Surveillance of Human Alveolar Echinococcosis in the Republic of Armenia from 2008 to 2020: A Review. Parasite Epidemiol. Control 2022, 17, e00246. [Google Scholar] [CrossRef]
- Turki, H.; Shamseddin, J.; Pournasrolla, N.; Kahrizi, S.; Kahrizi, S.; Kazemi, E. Molecular and cellular aspects of medical parasitology: Insights into pathogenesis and host interactions. Cell. Mol. Biomed. Rep. 2025, 5, 253–276. [Google Scholar] [CrossRef]
- Bargieri, D.; Lagal, V.; Andenmatten, N.; Tardieux, I.; Meissner, M.; Ménard, R. Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum. PLoS Pathog. 2014, 10, e1004273. [Google Scholar] [CrossRef]
- Silva, M.T. Classical Labeling of Bacterial Pathogens According to Their Lifestyle in the Host: Inconsistencies and Alternatives. Front. Microbiol. 2012, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Sojkova, P.B.; Butenko, A.; Richtova, J.; Fiala, I.; Obornik, M.; Lukes, J. Inside the Host: Understanding the Evolutionary Trajectories of Intracellular Parasitism. Annu. Rev. Microbiol. 2024, 78, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T.; Silva Pestana, N.T. The In Vivo Extracellular Life of Facultative Intracellular Bacterial Parasites: Role in Pathogenesis. Immunobiology 2013, 218, 325–337. [Google Scholar] [CrossRef]
- Rodriguez, A.; Suo, X.; Liu, D. Classification of Medically Important Parasites. In Molecular Medical Microbiology, 3rd ed.; Tang, Y.W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 3, pp. 2907–2919. [Google Scholar] [CrossRef]
- van Dooren, G.G.; McConville, M.J. Nutrient Acquisition by Intracellular Parasitic Protists. Cell Host Microbe 2025, 33, 854–868. [Google Scholar] [CrossRef]
- Mead, J.R. Early Immune and Host Cell Responses to Cryptosporidium Infection. Front. Parasitol. 2023, 2, 1113950. [Google Scholar] [CrossRef]
- Wang, Z.X.; Jiao, W.J.; Yang, Y.; Liu, H.-L.; Wang, H.-L. Role of Inflammasomes in Toxoplasma and Plasmodium Infections. Parasites Vectors 2024, 17, 466. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.F.; Nájera, C.A.; Meneghelli, I.; Bahia, D. The Parasitic Intracellular Lifestyle of Trypanosomatids: Parasitophorous Vacuole Development and Survival. Front. Cell Dev. Biol. 2020, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Epting, C.L.; Coates, B.M.; Engman, D.M. Molecular Mechanisms of Host Cell Invasion by Trypanosoma cruzi. Exp. Parasitol. 2010, 126, 283–291. [Google Scholar] [CrossRef][Green Version]
- Silva-Moreira, A.L.; Serravite, A.M.; Rios-Barros, L.V.; de Menezes, J.P.B.; Horta, M.F.; Castro-Gomes, T. New Insights into the Life Cycle, Host Cell Tropism, and Infection Amplification of Leishmania spp. Infect. Immun. 2025, 93, e0012325. [Google Scholar] [CrossRef]
- Shadduck, J.A.; Orenstein, J.M. Comparative Pathology of Microsporidiosis. Arch. Pathol. Lab. Med. 1993, 117, 1215–1219. [Google Scholar] [PubMed]
- Bonazzi, M. Editorial: Microbiology and Pathogenesis of Chlamydia, Coxiella, and Rickettsia. Front. Cell. Infect. Microbiol. 2024, 14, 1445682. [Google Scholar] [CrossRef]
- Gunn, A.; Pitt, S.J. Parasitology: An Integrated Approach; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Hill, D.E.; Chirukandoth, S.; Dubey, J.P. Biology and Epidemiology of Toxoplasma gondii in Man and Animals. Anim. Health Res. Rev. 2005, 6, 41–61. [Google Scholar] [CrossRef]
- Ramasamy, R. Zoonotic Malaria—Global Overview and Research and Policy Needs. Front. Public Health 2014, 2, 123. [Google Scholar] [CrossRef]
- Auld, S.K.; Tinsley, M.C. The Evolutionary Ecology of Complex Lifecycle Parasites: Linking Phenomena with Mechanisms. Heredity 2015, 114, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kara, R. “Parasitic Disease”. Encyclopedia Britannica, 21 April 2025. Available online: https://www.britannica.com/science/parasitic-disease (accessed on 12 July 2025).
- Tamarozzi, F.; Deplazes, P.; Casulli, A. Reinventing the Wheel of Echinococcus granulosus sensu lato Transmission to Humans. Trends Parasitol. 2020, 36, 427–434. [Google Scholar] [CrossRef]
- Theel, E.S.; Pritt, B.S. Parasites. Microbiol. Spectr. 2016, 4, 411–466. [Google Scholar] [CrossRef]
- Walker, D.M.; Oghumu, S.; Gupta, G.; McGwire, B.S.; Drew, M.E.; Satoskar, A.R. Mechanisms of Cellular Invasion by Intracellular Parasites. Cell. Mol. Life Sci. 2014, 71, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- WHO. Fact Sheet: Vector-Borne Diseases. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 10 October 2025).
- Breedlove, B. Deadly, Dangerous, and Decorative Creatures. Emerg. Infect. Dis. 2022, 28, 495–496. [Google Scholar] [CrossRef]
- Chala, B.; Hamde, F. Emerging and Re-Emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef]
- Carbone, G.; De Bona, A.; Septelici, D.; Cipri, A.; Nobilio, A.; Esposito, S. Beyond Mosquitoes: A Review of Pediatric Vector-Borne Diseases Excluding Malaria and Arboviral Infections. Pathogens 2025, 14, 553. [Google Scholar] [CrossRef]
- Obradović, Z.; Smjecanin, E.; Pindzo, E.; Omerović, H.; Cibo, N. A literature review on vector borne diseases. Int. J. Med. Rev. Case Rep. 2022, 6, 27–34. [Google Scholar] [CrossRef]
- Ouologuem, D.T.; Dara, A.; Kone, A.; Ouattara, A.; Djimde, A.A. Plasmodium falciparum Development from Gametocyte to Oocyst: Insight from Functional Studies. Microorganisms 2023, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Mehraban, N.; Ayodele, O.; Tshitenge, S.K.; Knox, R.; Mullaj, E.; Nandzo, A.; El-Samman, A.; Nesshwat, S.; et al. Epidemiology of Zoonotic Diseases in the United States: A Comprehensive Review. J. Infect. Dis. Epidemiol. 2016, 3, 21. [Google Scholar] [CrossRef]
- Ruybal-Pesántez, S.; Sáenz, F.E.; Deed, S.L.; Johnson, E.K.; Larremore, D.B.; Vera-Arias, C.A.; Tiedje, K.E.; Day, K.P. Molecular Epidemiology of Continued Plasmodium falciparum Disease Transmission after an Outbreak in Ecuador. Front. Trop. Dis. 2023, 4, 1085862. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Biswas, S.; Bisht, K.; Sharma, A. The Threat of Increased Transmission of Non-knowlesi Zoonotic Malaria in Humans: A Systematic Review. Parasitology 2023, 150, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of Transmission and Sources of Infection in Outbreaks of Human Toxoplasmosis. Emerg. Infect. Dis. 2019, 25, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–564. [Google Scholar] [CrossRef]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental Transmission of Toxoplasma gondii: Oocysts in Water, Soil and Food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Razakandrainibe, R.; Ortega, Y.; La Carbona, S. Editorial: Cryptosporidium, Giardia, Cyclospora, and Toxoplasma—Insights into Their Transmission. Front. Cell. Infect. Microbiol. 2023, 13, 1175108. [Google Scholar] [CrossRef]
- Bouzid, M.; Hunter, P.R.; Chalmers, R.M.; Tyler, K.M. Cryptosporidium Pathogenicity and Virulence. Clin. Microbiol. Rev. 2013, 26, 115–134. [Google Scholar] [CrossRef]
- Peng, X.; Murphy, T.; Holden, N.M. Evaluation of the Effect of Temperature on the Die-Off Rate for Cryptosporidium parvum Oocysts in Water, Soils, and Feces. Appl. Environ. Microbiol. 2008, 74, 7101–7107. [Google Scholar] [CrossRef]
- Kubina, S.; Costa, D.; Cazeaux, C.; Villena, I.; Favennec, L.; Razakandrainibe, R.; La Carbona, S. Persistence and Survival of Cryptosporidium parvum Oocysts on Lamb’s Lettuce Leaves during Plant Growth and in Washing Conditions of Minimally-Processed Salads. Int. J. Food Microbiol. 2023, 388, 110085. [Google Scholar] [CrossRef]
- Checkley, W.; White, A.C.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.-M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- McManus, D.P.; Gray, D.J.; Zhang, W.; Yang, Y. Diagnosis, Treatment, and Management of Echinococcosis. BMJ 2012, 344, e3866. [Google Scholar] [CrossRef]
- Schneider, C.; Kratzer, W.; Binzberger, A.; Schlingeloff, P.; Baumann, S.; Romig, T.; Schmidberger, J. Echinococcus multilocularis and other zoonotic helminths in red foxes (Vulpes vulpes) from a southern German hotspot for human alveolar echinococcosis. Parasites Vectors 2023, 16, 425. [Google Scholar] [CrossRef]
- McCluskey, J.M.; Sato, A.I. Vertical Transplacental Infections. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Carlier, Y.; Truyens, C.; Deloron, P.; Peyron, F. Congenital parasitic infections: A review. Acta Trop. 2012, 121, 55–70. [Google Scholar] [CrossRef]
- da Silva, R.J.; Cabo, L.F.; Boyle, J.P. Teratogenic parasites: Disease mechanisms and emerging study models. Trends Parasitol. 2024, 40, 1159–1172. [Google Scholar] [CrossRef]
- Cevallos, A.M.; Hernández, R. Chagas’ disease: Pregnancy and congenital transmission. Biomed Res. Int. 2014, 2014, 401864. [Google Scholar] [CrossRef] [PubMed]
- Faral-Tello, P.; Pagotto, R.; Bollati-Fogolín, M.; Francia, M.E. Modeling the human placental barrier to understand Toxoplasma gondii’s vertical transmission. Front. Cell. Infect. Microbiol. 2023, 13, 1130901. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Gad, N.; Koren, G. Toxoplasmosis and pregnancy. Can. Fam. Physician 2014, 60, 334–336. [Google Scholar] [PubMed]
- Avelino, M.M.; Amaral, W.N.; Rodrigues, I.M.; Rassi, A.R.; Gomes, M.B.; Costa, T.L.; Castro, A.M. Congenital toxoplasmosis and prenatal care state programs. BMC Infect. Dis. 2014, 14, 33. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Kurniawan, S.C.; Widodo, A.; Effendi, M.H.; Hasib, A.; Silaen, O.S.M.; Ramandinianto, S.C.; Moses, I.B.; Riwu, K.H.P.; Afnani, D.A. A comprehensive review of toxoplasmosis: Serious threat to human health. Open Public Health J. 2024, 17, e0202094637. [Google Scholar] [CrossRef]
- Bua, J.; Volta, B.J.; Velazquez, E.B.; Ruiz, A.M.; De Rissio, A.M.; Cardoni, R.L. Vertical transmission of Trypanosoma cruzi infection: Quantification of parasite burden in mothers and their children by parasite DNA amplification. Trans. R. Trop. Med. Hyg. 2012, 106, 623–628. [Google Scholar] [CrossRef]
- Herrera Choque, A.G.; Cuna, W.R.; Gabrielli, S.; Mattiucci, S.; Passera, R.; Rodriguez, C. Parasitic Effects on the Congenital Transmission of Trypanosoma cruzi in Mother–Newborn Pairs. Microorganisms 2024, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Kemmerling, U.; Osuna, A.; Schijman, A.G.; Truyens, C. Congenital transmission of Trypanosoma cruzi: A review about the interactions between the parasite, the placenta, the maternal and the fetal/neonatal immune responses. Front. Microbiol. 2019, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, T.M.; Patiño, L.H.; Ramírez, J.D. Understanding Host–Pathogen Interactions in Congenital Chagas Disease Through Transcriptomic Approaches. Pathogens 2025, 14, 106. [Google Scholar] [CrossRef]
- Minwuyelet, A.; Yewhalaw, D.; Siferih, M.; Atenafu, G. Current update on malaria in pregnancy: A systematic review. Trop. Dis. Travel Med. Vaccines 2025, 11, 14. [Google Scholar] [CrossRef]
- Yeika, E.V.; Schlemmer, B.; Abanda, M.H.; Tolefac, P.N. Congenital Plasmodium falciparum malaria: A report of three cases. Clin. Pediatr. Res. 2017, 1, 21–24. [Google Scholar] [CrossRef]
- Omidiji, M.O.; Lesi, F.E.A.; Esezobor, C.; Fajolu, I.B.; Oyibo, W.A.; Daramola, A. Prevalence of congenital malaria in an urban and a semirural area in Lagos: A two-centre cross-sectional study. Sci. Rep. 2025, 15, 10709. [Google Scholar] [CrossRef]
- Donahoe, S.L.; Lindsay, S.A.; Krockenberger, M.; Phalen, D.; Šlapeta, J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int. J. Parasitol. Parasites Wildl. 2015, 4, 216–238. [Google Scholar] [CrossRef]
- Rojas-Pirela, M.; Medina, L.; Rojas, M.V.; Liempi, A.I.; Castillo, C.; Pérez-Pérez, E.; Guerrero-Muñoz, J.; Araneda, S.; Kemmerling, U. Congenital transmission of apicomplexan parasites: A review. Front. Microbiol. 2021, 12, 751648. [Google Scholar] [CrossRef]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F. Dardé M Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D. Q Fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef]
- Pexara, A.; Solomakos, N.; Govaris, A. Q fever and prevalence of Coxiella burnetii in milk. Trends Food Sci. Technol. 2018, 7, 65–72. [Google Scholar] [CrossRef]
- Ullah, H.; Qadeer, A.; Rashid, M.; Rashid, M.I.; Cheng, G. Recent advances in nucleic acid-based methods for detection of helminth infections and the perspective of biosensors for future development. Parasitology 2020, 147, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Baltrušis, P.; Höglund, J. Digital PCR: Modern solution to parasite diagnostics and population trait genetics. Parasites Vectors 2023, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Choi, M.K.; Choe, Y.L.; Lim, C.S. Development of a Rapid Malaria LAMP-MS Assay for Diagnosis of Malaria Infections. Sci. Rep. 2025, 15, 8547. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Piubelli, C.; Perandin, F.; Bisoffi, Z. Digital PCR: A new technology for diagnosis of parasitic infections. Clin. Microbiol. Infect. 2019, 12, 1510–1516. [Google Scholar] [CrossRef]
- Agut, H.; Gautheret-Dejean, A. Serologic Testing for Acute and Chronic Infection. Perspect. Med. Virol. 2006, 12, 91–103. [Google Scholar] [CrossRef]
- Iatta, R.; Carbonara, M.; Morea, A.; Trerotoli, P.; Benelli, G.; Nachum-Biala, Y.; Mendoza-Roldan, J.A.; Cavalera, M.A.; Baneth, G.; Bandi, C.; et al. Assessment of the Diagnostic Performance of Serological Tests in Areas Where Leishmania infantum and Leishmania tarentolae Occur in Sympatry. Parasites Vectors 2023, 16, 352. [Google Scholar] [CrossRef]
- Fitri, L.E.; Widaningrum, T.; Endharti, A.T.; Prabowo, M.H.; Winaris, N.; Nugraha, R.Y.B. Malaria Diagnostic Update: From Conventional to Advanced Method. J. Clin. Lab. Anal. 2022, 36, e24314. [Google Scholar] [CrossRef]
- McCaffery, J.N.; Nace, D.; Herman, C.; Singh, B.; Sompwe, E.M.; Nkoli, P.M.; Ngoyi, D.M.; Kahunu, G.M.; Halsey, E.S.; Rogier, E. Plasmodium falciparum pfhrp2 and pfhrp3 gene deletions among patients in the DRC enrolled from 2017 to 2018. Sci. Rep. 2021, 11, 22979. [Google Scholar] [CrossRef]
- Krueger, T.; Ikegbunam, M.; Lissom, A.; Sandri, T.L.; Ntabi, J.D.M.; Djontu, J.C.; Baina, M.T.; Lontchi, R.A.L.; Maloum, M.; Ella, G.Z.; et al. Low Prevalence of Plasmodium falciparum Histidine-Rich Protein 2 and 3 Gene Deletions—A Multiregional Study in Central and West Africa. Pathogens 2023, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Makarani, N.; Bharadava, K.; Kaushik, A.; Dave, A.; Gangawane, A.K.; Kaushal, R.S. Leishmaniasis: A Multifaceted Approach to Diagnosis, Maladies, Drug Repurposing and Way Forward. Microbe 2025, 6, 100239. [Google Scholar] [CrossRef]
- Robert, M.G.; Brenier-Pinchart, M.P.; Garnaud, C.; Fricker-Hidalgo, H.; Pelloux, H. Molecular diagnosis of toxoplasmosis: Recent advances and a look to the future. Expert Rev. Anti-Infect. Ther. 2021, 19, 1529–1542. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.J.; Park, H. Trend in serological and molecular diagnostic methods for Toxoplasma gondii infection. Eur. J. Med. Res. 2024, 29, 520. [Google Scholar] [CrossRef]
- Switaj, K.; Master, A.; Skrzypczak, M.; Zaborowski, P. Recent trends in molecular diagnostics for Toxoplasma gondii infections. Clin. Microbiol. Infect. 2005, 11, 170–176. [Google Scholar] [CrossRef]
- Fadel, E.F.; El-Hady, H.A.; Ahmed, A.M.; Tolba, M.E.M. Molecular Diagnosis of Human Toxoplasmosis: The State of the Art. J. Parasit. Dis. 2024, 48, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-time PCR applications for diagnosis of leishmaniasis. Parasites Vectors 2018, 11, 273. [Google Scholar] [CrossRef]
- Cardoso, L.; Mendão, C.; Madeira de Carvalho, L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal—A national serological study. Parasites Vectors 2012, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.R.; Schoone, G.; Versteeg, I.; Gomez, M.A.; Diro, E.; Mori, Y.; Perlee, D.; Downing, T.; Saravia, N.; Assaye, A.; et al. Development and Evaluation of a Novel Loop-Mediated Isothermal Amplification Assay for Diagnosis of Cutaneous and Visceral Leishmaniasis. J. Clin. Microbiol. 2018, 56, e00386-18. [Google Scholar] [CrossRef]
- Van der Auwera, G.; Dujardin, J.C. Species Typing in Dermal Leishmaniasis. Clin. Microbiol. Rev. 2015, 28, 265–294. [Google Scholar] [CrossRef]
- Hijjawi, N.; Kanani, K.A.; Rasheed, M.; Atoum, M.; Abdel-Dayem, M.; Irhimeh, M.R. Molecular Diagnosis and Identification of Leishmania Species in Jordan from Saved Dry Samples. Biomed Res. Int. 2016, 2016, 6871739. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; An, X.; Xu, D.; Cai, S.; Chu, C.; Liu, G. Advances in Novel Diagnostic Techniques for Alveolar Echinococcosis. Diagnostics 2025, 15, 585. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, H.; Chen, J.; Zhao, L.; Liu, Q. Circulating Free DNA as a Diagnostic Marker for Echinococcosis: A Systematic Review and Meta-Analysis. Front. Microbiol. 2024, 15, 1413532. [Google Scholar] [CrossRef]
- Otero-Abad, B.; Armua-Fernandez, M.T.; Deplazes, P.; Torgerson, P.R.; Hartnack, S. Latent Class Models for Echinococcus multilocularis Diagnosis in Foxes in Switzerland in the Absence of a Gold Standard. Parasites Vectors 2017, 10, 612. [Google Scholar] [CrossRef]
- World Health Organization. Global Vector Control Response 2017–2030; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241512978 (accessed on 13 August 2025).
- Rahi, M.; van den Berg, H.; Vythilingam, I.; Velayudhan, R. Insect Vectors on the Move. One Earth 2024, 7, 535–536. [Google Scholar] [CrossRef]
- Tourapi, C.; Tsioutis, C. Circular Policy: A New Approach to Vector and Vector-Borne Diseases’ Management in Line with the Global Vector Control Response (2017–2030). Trop. Med. Infect. Dis. 2022, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, J.; Zhang, W.; Chen, E.; Gao, Y.; Sun, J.; Huang, W.; Xia, J.; Zeng, W.; Guo, J.; et al. Sustainable Control and Integrated Management through a One Health Approach to Mitigate Vector-Borne Disease. One Health 2025, 20, 101018. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Dhiman, R.C.; Kitron, U.; Scott, T.W.; van den Berg, H.; Lindsay, S.W. Benefit of Insecticide-Treated Nets, Curtains and Screening on Vector-Borne Diseases, Excluding Malaria: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2014, 8, e3228. [Google Scholar] [CrossRef] [PubMed]
- Pryce, J.; Richardson, M.; Lengeler, C. Insecticide-Treated Nets for Preventing Malaria. Cochrane Database Syst. Rev. 2018, 11, CD000363. [Google Scholar] [CrossRef]
- Lengeler, C. Insecticide-Treated Bed Nets and Curtains for Preventing Malaria. Cochrane Database Syst. Rev. 2004, 2, CD000363. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Malaria Vaccine Implementation Programme; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/feature-stories/detail/malaria-vaccine-implementation-programme (accessed on 14 August 2025).
- Ballou, W.R.; Cahill, C.P. Two Decades of Commitment to Malaria Vaccine Development: GlaxoSmithKline Biologicals. Am. J. Trop. Med. Hyg. 2007, 77 (Suppl. 6), 289–295. [Google Scholar] [CrossRef]
- Egbewande, O.M. The RTS, S Malaria Vaccine: Journey from Conception to Recommendation. Public Health Pract. 2022, 4, 100283. [Google Scholar] [CrossRef]
- Vanderberg, J.P. Reflections on Early Malaria Vaccine Studies, the First Successful Human Malaria Vaccination, and Beyond. Vaccine 2009, 27, 2–9. [Google Scholar] [CrossRef][Green Version]
- Casares, S.; Brumeanu, T.-D.; Richie, T.L. The RTS, S Malaria Vaccine. Vaccine 2010, 28, 4880–4894. [Google Scholar] [CrossRef]
- Sinnis, P.; Fidock, D.A. The RTS, S Vaccine—A Chance to Regain the Upper Hand against Malaria? Cell 2022, 185, 750–754. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk. 2021. Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (accessed on 17 June 2025).
- Feehan, J.; Plebanski, M.; Apostolopoulos, V. Recent Perspectives in Clinical Development of Malaria Vaccines. Nat. Commun. 2025, 16, 3565. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.R.; Voss, W.N.; Bowyer, G.; Rush, S.A.; Spencer, A.J.; Bellamy, D.; Ulaszewska, M.; Goike, J.; Gregory, S.; King, C.R.; et al. Repertoire, function, and structure of serological antibodies induced by the R21/Matrix-M Malaria Vaccine. J. Exp. Med. 2025, 222, e20241908. [Google Scholar] [CrossRef]
- Osoro, C.B.; Ochodo, E.; Kwambai, T.K.; Otieno, J.A.; Were, L.; Sagam, C.K.; Owino, E.J.; Kariuki, S.; Ter Kuile, F.O.; Hill, J. Policy Uptake and Implementation of the RTS, S/AS01 Malaria Vaccine in Sub-Saharan African countries: Status Two Years Following the WHO Recommendation. BMJ Glob. Health 2024, 9, e014719. [Google Scholar] [CrossRef]
- Ouattara, A.; Laurens, M.B. Vaccines against Malaria. Clin. Infect. Dis. 2015, 60, 930–936. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). WHO Recommends R21/Matrix-M Vaccine for Malaria Prevention in Updated Advice on Immunization. 2023. Available online: https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization (accessed on 19 October 2025).
- Asante, K.P.; Mathanga, D.P.; Milligan, P.; Akech, S.; Oduro, A.; Mwapasa, V.; Moore, K.A.; Kwambai, T.K.; Hamel, M.J.; Gyan, T.; et al. Feasibility, Safety, and Impact of the RTS,S/AS01E Malaria Vaccine When Implemented through National Immunisation Programmes: Evaluation of Cluster-Randomised Introduction of the Vaccine in Ghana, Kenya, and Malawi. Lancet 2024, 403, 1660–1670. [Google Scholar] [CrossRef]
- Sallam, M.; Al-Khatib, A.O.; Al-Mahzoum, K.S.; Abdelaziz, D.H.; Sallam, M. Current Developments in Malaria Vaccination: A Concise Review on Implementation, Challenges, and Future Directions. Clin. Pharmacol. 2025, 17, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Ilani, P.; Nyarko, P.B.; Camara, A.; Amenga-Etego, L.N.; Aniweh, Y. PfRH5 Vaccine: From the Bench to the Vial. NPJ Vaccines 2025, 10, 82. [Google Scholar] [CrossRef]
- van der Boor, S.C.; Smit, M.J.; van Beek, S.W.; Ramjith, J.; Teelen, K.; van de Vegte-Bolmer, M.; van Gemert, G.-J.; Pickkers, P.; Wu, Y.; Locke, E.; et al. Safety, Tolerability, and Plasmodium falciparum Transmission-Reducing Activity of Monoclonal Antibody TB31F: A Single-Centre, Open-Label, First-in-Human, Dose-Escalation, Phase 1 Trial in Healthy Malaria-Naive Adults. Lancet Infect. Dis. 2022, 22, 1596–1605. [Google Scholar] [CrossRef]
- Karmakar, S.; Ismail, N.; Oliveira, F.; Oristian, J.; Zhang, W.W.; Kaviraj, S.; Singh, K.P.; Mondal, A.; Das, S.; Pandey, K.; et al. Preclinical validation of a live attenuated dermotropic Leishmania vaccine against vector transmitted fatal visceral leishmaniasis. Commun. Biol. 2021, 4, 929. [Google Scholar] [CrossRef]
- Karmakar, S.; Volpedo, G.; Zhang, W.W.; Lypaczewski, P.; Ismail, N.; Oliveira, F.; Oristian, J.; Meneses, C.; Gannavaram, S.; Kamhawi, S.; et al. Centrin-deficient Leishmania mexicana confers protection against Old World visceral leishmaniasis. NPJ Vaccines 2022, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Rabienia, M.; Roudbari, Z.; Ghanbariasad, A.; Farjadfar, A.; Mortazavidehkordi, N. Expression and immunogenicity evaluation of a novel Lentiviral multi-epitope vaccine against Leishmania major in BALB/c mice. Trop. Dis. Travel. Med. Vaccines. 2025, 11, 17. [Google Scholar] [CrossRef]
- Lim, L.Z.; Ee, S.; Fu, J.; Tan, Y.; He, C.Y.; Song, J. Kinetoplastid membrane protein-11 adopts a four-helix bundle fold in DPC micelle. FEBS Lett. 2017, 591, 3793–3804. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Li, X.; Zhu, W.; Zhao, M.; Li, J.; Liu, X.; Cao, L.; Li, S.; Zhang, S.; et al. Structural analyses of a dominant Cryptosporidium parvum epitope presented by H-2Kb offer new options to combat cryptosporidiosis. mBio 2023, 14, e02666-22. [Google Scholar] [CrossRef]
- Younis, B.M.; Osman, M.; Khalil, E.A.; Santoro, F.; Furini, S.; Wiggins, R.; Keding, A.; Carraro, M.; Musa, A.E.; Abdarahaman, M.A.; et al. Safety and Immunogenicity of ChAd63-KH Vaccine in Post-Kala-Azar Dermal Leishmaniasis Patients in Sudan. Mol. Ther. 2021, 29, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Bhattacharya, P.; Gannavaram, S.; Pacheco-Fernandez, T.; Oljuskin, T.; Dey, R.; Satoskar, A.R.; Nakhasi, H.L. The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates. Pathogens 2022, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Dinc, R. Leishmania Vaccines: The Current Situation with Its Promising Aspect for the Future. Korean J. Parasitol. 2022, 60, 379–391. [Google Scholar] [CrossRef]
- Oliveira, D.S.d.; Zaldívar, M.F.; Gonçalves, A.A.M.; Resende, L.A.; Mariano, R.M.d.S.; Pereira, D.F.S.; Conrado, I.d.S.S.; Costa, M.A.F.; Lair, D.F.; Vilas-Boas, D.F.; et al. New Approaches to the Prevention of Visceral Leishmaniasis: A Review of Recent Patents of Potential Candidates for a Chimeric Protein Vaccine. Vaccines 2024, 12, 271. [Google Scholar] [CrossRef]
- Petitdidier, E.; Pagniez, J.; Pissarra, J.; Holzmuller, P.; Papierok, G.; Vincendeau, P.; Lemesre, J.-L.; Bras-Gonçalves, R. Peptide-based vaccine successfully induces protective immunity against canine visceral leishmaniasis. NPJ Vaccines 2019, 4, 49. [Google Scholar] [CrossRef]
- Regina-Silva, S.; Feres, A.M.L.T.; França-Silva, J.C.; Dias, E.S.; Michalsky, É.M.; de Andrade, H.M.; Coelho, E.A.F.; Ribeiro, G.M.; Fernandes, A.P.; Machado-Coelho, G.L.L. Field Randomized Trial to Evaluate the Efficacy of the Leish-Tec® Vaccine against Canine Visceral Leishmaniasis in an Endemic Area of Brazil. Vaccine 2016, 34, 2233–2239. [Google Scholar] [CrossRef]
- Fernández Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañón, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A Large-Scale Field Randomized Trial Demonstrates Safety and Efficacy of the Vaccine LetiFend® against Canine Leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Toepp, A.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Leal-Lima, A.; Anderson, B.; Parrish, M.; Anderson, M.; Fowler, H.; et al. Randomized, Controlled, Double-Blinded Field Trial to Assess Leishmania Vaccine Effectiveness as Immunotherapy for Canine Leishmaniosis. Vaccine 2018, 36, 6433–6441. [Google Scholar] [CrossRef] [PubMed]
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef] [PubMed]
- de Souza Testasicca, M.C.; dos Santos, M.S.; Marques Machado, L.; Vieira Serufo, A.; Doro, D.; Avelar, D.; Leonardi Tibúrcio, A.M.; de Freitas Abrantes, C.; Machado-Coelho, G.L.L.; Grimaldi, G., Jr.; et al. Antibody responses induced by Leish-Tec®, an A2-based vaccine for visceral leishmaniasis, in a heterogeneous canine population. Vet. Parasitol. 2014, 204, 169–176. [Google Scholar] [CrossRef]
- Kaye, P.M.; Matlashewski, G.; Mohan, S.; Le Rutte, E.; Mondal, D.; Khamesipour, A.; Malvolti, S. Vaccine value profile for leishmaniasis. Vaccine 2023, 41 (Suppl. 2), S153–S175. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Wu, H.; Qiu, H.; Sun, Y. T-Cell Epitope-Based Vaccines: A Promising Strategy for Prevention of Infectious Diseases. Vaccines 2024, 12, 1181. [Google Scholar] [CrossRef]
- Leite, J.C.; Gonçalves, A.A.M.; de Oliveira, D.S.; Resende, L.A.; Boas, D.F.V.; Ribeiro, H.S.; Pereira, D.F.S.; da Silva, A.V.; Mariano, R.M.d.S.; Reis, P.C.C.; et al. Transmission-Blocking Vaccines for Canine Visceral Leishmaniasis: New Progress and Yet New Challenges. Vaccines 2023, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Vale, D.L.; Freitas, C.S.; Martins, V.T.; Moreira, G.J.L.; Machado, A.S.; Ramos, F.F.; Pereira, I.A.G.; Bandeira, R.S.; de Jesus, M.M.; Tavares, G.S.V.; et al. Efficacy of an Immunotherapy Combining Immunogenic Chimeric Protein Plus Adjuvant and Amphotericin B against Murine Visceral Leishmaniasis. Biology 2023, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.F.; Freire, M.L.; Murta, S.M.F.; Oliveira, E. Efficacy of vaccines based on chimeric or multiepitope antigens for protection against visceral leishmaniasis: A systematic review. PLoS Negl. Trop. Dis. 2024, 18, e0012757. [Google Scholar] [CrossRef]
- Boussoffara, T.; Labidi, I.; Trimèche, M.; Chelbi, I.; Dachraoui, K.; Msallem, N.; Abdo Saghir Abbas, M.; Cherni, S.; Singh, K.P.; Kaviraj, S.; et al. LmCen−/−-Based Vaccine Is Protective against Canine Visceral Leishmaniasis Following Three Natural Exposures in Tunisia. NPJ Vaccines 2025, 10, 31. [Google Scholar] [CrossRef]
- Oliveira, F.; Rowton, E.; Aslan, H.; Gomes, R.; Castrovinci, P.A.; Alvarenga, P.H.; Abdeladhim, M.; Teixeira, C.; Meneses, C.; Kleeman, L.T.; et al. A sand fly salivary protein Vaccine shows efficacy against vector-transmitted cutaneous Leishmaniasis in Nonhuman primates. Sci. Transl. Med. 2015, 7, 290ra90. [Google Scholar] [CrossRef]
- Martiniano de Pádua, J.A.; Ribeiro, D.; de Aguilar, V.F.F.; Melo, T.F.; Bueno, L.L.; Grossi de Oliveira, A.L.; Fujiwara, R.T.; Keller, K.M. Overview of Commercial Vaccines Against Canine Visceral Leishmaniasis: Current Landscape and Future Directions. Pathogens 2025, 14, 970. [Google Scholar] [CrossRef]
- Ayala, A.; Llanes, A.; Lleonart, R.; Restrepo, C.M. Advances in Leishmania Vaccines: Current Development and Future Prospects. Pathogens 2024, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Jones, M.K.; Gordon, C.A.; Arganda, A.E.; Cai, P.; Al-Wassiti, H.; Pouton, C.W.; McManus, D.P. The mRNA vaccine technology era and the future control of parasitic infections. Clin. Microbiol. Rev. 2022, 35, e00241-21. [Google Scholar] [CrossRef]
- Wang, C.; Fu, S.; Yu, X.; Zhou, H.; Zhang, F.; Song, L.; Zhao, J.; Yang, Y.; Du, J.; Luo, Q.; et al. Toxoplasma WH3 Δrop18 Acts as a Live Attenuated Vaccine against Acute and Chronic Toxoplasmosis. NPJ Vaccines 2024, 9, 996. [Google Scholar] [CrossRef]
- Peng, G.H.; Yuan, Z.G.; Zhou, D.H.; He, X.H.; Liu, M.M.; Yan, C.; Yin, C.C.; He, Y.; Lin, R.Q.; Zhu, X.Q. Toxoplasma gondii Microneme Protein 6 (MIC6) Is a Potential Vaccine Candidate against Toxoplasmosis in Mice. Vaccine 2009, 27, 6570–6574. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Li, S.; Zheng, B. A novel Toxoplasma gondii TGGT1_316290 mRNA-LNP vaccine elicits protective immune response against toxoplasmosis in mice. Front. Microbiol. 2023, 14, 1145114. [Google Scholar] [CrossRef]
- Qi, W.; Yu, Y.; Yang, C.; Wang, X.J.; Jiang, Y.; Yu, Z. Nanospheres as the delivery vehicle: Novel application of Toxoplasma gondii ribosomal protein S2 in PLGA and chitosan nanospheres against acute toxoplasmosis. Front. Immunol. 2024, 15, 1475280. [Google Scholar] [CrossRef]
- Chu, K.-B.; Quan, F.-S. Advances in Toxoplasma gondii Vaccines: Current Strategies and Challenges for Vaccine Development. Vaccines 2021, 9, 413. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Gauci, C.G.; Jenkins, D.J.; Lightowlers, M.W. Protection against Cystic Echinococcosis in Sheep Using an Escherichia coli—Expressed Recombinant Antigen (EG95) as a Bacterin. Parasitology 2023, 150, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Kouguchi, H.; Matsumoto, J.; Katoh, Y.; Oku, Y.; Suzuki, T.; Yagi, K. The Vaccination Potential of EMY162 Antigen against Echinococcus multilocularis Infection. Biochem. Biophys. Res. Commun. 2007, 363, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Tzipori, S.; Widmer, G. A Hundred-Year Retrospective on Cryptosporidiosis. Trends Parasitol. 2008, 24, 184–189. [Google Scholar] [CrossRef]
- Mead, J.R. Prospects for Immunotherapy and Vaccines against Cryptosporidium. Hum. Vaccines Immunother. 2014, 10, 1505–1513. [Google Scholar] [CrossRef]
- MSD Animal Health. Bovilis Cryptium eu Approval Press Release. 2023. Available online: https://www.msd-animal-health.com/2023/11/29/msd-animal-health-receives-eu-approval-of-bovilis-cryptium/ (accessed on 19 October 2025).
- DairyProducer.com. New Vaccine Targets Cryptosporidiosis in Calves. 2025. Available online: https://www.dairyproducer.com/new-vaccine-targets-cryptosporidiosis-in-calves-offering-early-life-protection/ (accessed on 17 June 2025).
- Innes, E.A.; Chalmers, R.M.; Wells, B.; Pawlowic, M.C. A One Health Approach to Tackle Cryptosporidiosis. Trends Parasitol. 2020, 36, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; He, Y.; Li, Y.; Yin, Z.; Shi, J.; Tian, T.; Shang, K.; Shi, H.; Zhang, F.; Wen, H. Design of a Novel EmTSP-3 and EmTIP Based Multi-Epitope Vaccine against Echinococcus multilocularis Infection. Front. Immunol. 2024, 15, 1425603. [Google Scholar] [CrossRef]
- Lundström-Stadelmann, B.; Rostami, A.; Frey, C.F.; Torgerson, P.R.; Riahi, S.M.; Bagheri, K.; Kaethner, M.; Lachenmayer, A.; Beldi, G.; Gasser, R.B.; et al. Human Alveolar Echinococcosis—Global, Regional, and National Annual Incidence and Prevalence Rates. Clin. Microbiol. Infect. 2025, 31, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45, Erratum in Lancet 2015, 386, 30. https://doi.org/10.1016/S0140-6736(15)60721-8. [Google Scholar]
- Chen, J.; Wang, Q.; He, X.; Yang, B. Malaria Vaccines: Current Achievements and Path Forward. Vaccines 2025, 13, 542. [Google Scholar] [CrossRef]
- Mordmüller, B.; Sulyok, Z.; Sulyok, M.; Molnar, Z.; Lalremruata, A.; Calle, C.L.; Bayon, P.G.; Esen, M.; Gmeiner, M.; Held, J.; et al. A PfSPZ vaccine immunization regimen equally protective against homologous and heterologous controlled human malaria infection. NPJ Vaccines 2022, 7, 100. [Google Scholar] [CrossRef]
- Martorell, S.; Ligda, P.; Rai, S.; Alward, L.; Berish, R.; Weber, A.; Isaacson, W.; Millership, J.; King, V.; Pardali, D.; et al. Efficacy of a Candidate Vaccine against Leishmania infantum on Naturally Exposed Dogs to Sand Flies. Vaccine 2025, 63, 127646. [Google Scholar] [CrossRef]
- European Medicines Agency. CaniLeish. EMA—European Public Assessment Report (Veterinary). Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/canileish (accessed on 6 October 2025).
- Moreno, J. Assessment of Vaccine-Induced Immunity against Canine Visceral Leishmaniasis. Front. Vet. Sci. 2019, 6, 168. [Google Scholar] [CrossRef]
- Valadares, D.G.; Kontowicz, E.; Tang, S.; Toepp, A.; Lima, A.; Larson, M.; Grinnage-Pulley, T.; Scorza, B.; Pessoa-Pereira, D.; Oleson, J.; et al. LeishTec vaccination disrupts vertical transmission of Leishmania infantum. PLoS Negl. Trop. Dis. 2025, 19, e0013320. [Google Scholar] [CrossRef] [PubMed]
- Coler, R.N.; Duthie, M.S.; Hofmeyer, K.A.; Guderian, J.; Jayashankar, L.; Vergara, J.; Rolf, T.; Misquith, A.; Laurance, J.D.; Raman, V.S.; et al. From Mouse to Man: Safety, Immunogenicity and Efficacy of a Candidate Leishmaniasis Vaccine LEISH-F3+GLA-SE. Clin. Transl. Immunol. 2015, 4, e35. [Google Scholar] [CrossRef]
- Mo, A.X.; Pesce, J.; Hall, B.F. Visceral Leishmaniasis Control and Elimination: Is There a Role for Vaccines in Achieving Regional and Global Goals? Am. J. Trop. Med. Hyg. 2016, 95, 514–521. [Google Scholar] [CrossRef][Green Version]
- Shital, S.; Madan, E.; Selvapandiyan, A.; Ganguly, N.K. An update on recombinant vaccines against leishmaniasis. Indian J. Med. Res. 2024, 160, 323–337. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, T.; Joshi, J. Immunogenicity and protective efficacy of DNA vaccine against visceral leishmaniasis in BALB/c mice. J. Biomed. Res. 2016, 30, 304–313. [Google Scholar] [CrossRef]
- Bavarsad Ahmadpour, N.; Dalimi, A.; Pirestani, M. Evaluation of a Novel Multi-Epitope Peptide Vaccine Candidate from LACK, LeIF, GP63, SMT Antigens of Leishmania major in BALB/c Mice. Arch. Razi Inst. 2022, 77, 2223–2233. [Google Scholar] [CrossRef]
- Li, X.; Yuan, W.; He, T.; Guo, R.; Du, X.; He, Y.; Li, X.; El-Ashram, S.; Al-Olayan, E.M.; Yang, N.; et al. Boosting Mouse Defense against Lethal Toxoplasma gondii Infection with Full-Length and Soluble SAG1 Recombinant Protein. Vaccines 2023, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Paronyan, L.; Babayan, L.; Vardanyan, H.; Manucharyan, A.; Papapostolou, K.M.; Balaska, S.; Vontas, J.; Mavridis, K. Molecular monitoring of insecticide resistance in major disease vectors in Armenia. Parasites Vectors 2024, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Quan, F.-S. Efficacy of Heterologous Vaccination Using Virus-Like Particles and Vaccinia Virus Containing MIC8 and AMA1 Proteins of Toxoplasma gondii. Vaccines 2025, 13, 862. [Google Scholar] [CrossRef]
- Zhu, L.; Lei, Z.; Xia, X.; Zhang, Y.; Chen, Y.; Wang, B.; Li, J.; Li, G.; Yang, G.; Cao, G.; et al. Yeast Shells Encapsulating Adjuvant AS04 as an Antigen Delivery System for a Novel Vaccine against Toxoplasma gondii. ACS Appl. Mater. Interfaces 2021, 13, 40415–40428. [Google Scholar] [CrossRef]
- Fang, Y.N.; Zhou, P.; Qi, W.Y.; Yu, Y.L.; Wang, X.J.; Jiang, Y.C.; Zhang, L.; Yu, Y.L.; Wang, J.D.; Yu, Z.Q.; et al. Research on inner membrane complex protein 1: A novel nanovaccines against Toxoplasma gondii. BMC Vet. Res. 2025, 21, 521. [Google Scholar] [CrossRef]
- El Bissati, K.; Zhou, Y.; Paulillo, S.M.; Raman, S.K.; Karch, C.P.; Reed, S.; Estes, A.; Estes, A.; Lykins, J.; Burkhard, P.; et al. Engineering and characterization of a novel Self Assembling Protein for Toxoplasma peptide vaccine in HLA-A11:01, HLA-A02:01 and HLA-B*07:02 transgenic mice. Sci. Rep. 2020, 10, 16984. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; Shamsinia, S.; Nourmohammadi, H.; Majidiani, H.; Fatollahzadeh, M.; Nemati, T.; Irannejad, H.; Nouri, H.R.; Ghasemi, E.; Shams, M. Development of a chimeric vaccine candidate based on Toxoplasma gondii major surface antigen 1 and apicoplast proteins using comprehensive immunoinformatics approaches. Eur. J. Pharm. Sci. 2021, 162, 105837. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Hashemi, S.; Siamian, D.; Asghari, A.; Fathollahzadeh, M.; Majidiani, H.; Shahraki, I.; Safari, M.H. Discovery of novel vaccine candidates based on the immunogenic epitopes derived from Toxoplasma membrane proteins. Clin. Exp. Vaccine Res. 2025, 14, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Teh-Poot, C.F.; Alfaro-Chacón, A.; Pech-Pisté, L.M.; Rosado-Vallado, M.E.; Asojo, O.A.; Villanueva-Lizama, L.E.; Dumonteil, E.; Cruz-Chan, J.V. Immunogenicity of Trypanosoma cruzi Multi-Epitope Recombinant Protein as an Antigen Candidate for Chagas Disease Vaccine in Humans. Pathogens 2025, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Aboelsoued, D.; Abdullah, H.H.A.M.; Megeed, K.N.A.; Hassan, S.E.; Toaleb, N.I. Evaluation of a Vaccine Candidate Isolated from Cryptosporidium parvum Oocyst in Mice. Vet. World 2022, 15, 2772–2784. [Google Scholar] [CrossRef]
- Zhou, P.; Zhou, Z.; Huayu, M.; Wang, L.; Feng, L.; Xiao, Y.; Dai, Y.; Xin, M.; Tang, F.; Li, R. A multi-epitope vaccine GILE against Echinococcus multilocularis infection in mice. Front. Immunol. 2023, 13, 1091004. [Google Scholar] [CrossRef]
- Aghayan, S.A.; Asikyan, M.V.; Shcherbakov, O.; Ghazaryan, A.; Hayrapetyan, T.; Malkhasyan, A.; Gevorgyan, H.; Makarikov, A.; Kornienko, S.; Daryani, A. Toxoplasma gondii in rodents and shrews in Armenia, Transcaucasia. Int. J. Parasitol. Parasites Wildl. 2024, 25, 100987. [Google Scholar] [CrossRef]
- Shcherbakov, O.V.; Aghayan, S.A.; Gevorgyan, H.S.; Abgaryan, T.A.; Gevorgyan, R.H.; Jiménez-Meléndez, A.; Robertson, L.J. Preliminary investigations of parasite contamination of water sources in Armenia. Food Waterborne Parasitol. 2024, 34, e00221. [Google Scholar] [CrossRef]
- Naghashyan, N.Z.; Grigoryan, L.H.; Mkrtchyan, A.R.; Hakobyan, A.R. Cryptosporidiosis of farm animals in the Republic of Armenia. SM J. Infect. Dis. 2018, 3, 1011s. [Google Scholar]
- Manukyan, A.; Avetisyan, L.; Sahakyan, G.; Paez, A.; Paronyan, L.; Vanyan, A. Epidemiology of Human Alveolar Echinococcosis in Armenia. Eur. J. Public Health 2021, 31 (Suppl. 3), ckab165.240. [Google Scholar] [CrossRef]
- Manukyan, A. Young Age of Alveolar Echinococcosis Patient in Armenia: A Case Report. Iran. J. Parasitol. 2023, 18, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Strelkova, M.V.; Ponirovsky, E.N.; Morozov, E.N.; Zhirenkina, E.N.; Razakov, S.A.; Kovalenko, D.A.; Schnur, L.F.; Schönian, G. A Narrative Review of Visceral Leishmaniasis in Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, the Crimean Peninsula and Southern Russia. Parasites Vectors 2015, 8, 330. [Google Scholar] [CrossRef]
- Rossia, A.L.; Chuelov, S.B.; Kondratenko, N.V.; Tselovalnikova, E.V.; Tebenkov, A.V.; Kolyagina, S.V.; Sokolova, N.A.; Nepokulchitskaya, N.V. A case of visceral leishmaniasis imported from Armenia. Child. Infect. 2023, 22, 66–70. (In Russian) [Google Scholar] [CrossRef]
- Gevorgyan RG (2023) Epizootological situation on toxoplasmosis among livestock animals in the Tavush region of Armenia. In Collection of Scientific Articles adapted from the International Scientific Conference: Theory and Practice of Parasitic Disease Control, 24th ed.; Nauka (Science): Moscow, Russia, 2023; pp. 142–145. [CrossRef]
- Hovsepyan, A.; Voskanyan, K.; Semerjian, S.; Alexanian, Y. Some results of epidemiological and epizootological investigations of toxoplasmosis in Armenian SSR. Med. Sci. Armen. 1990, 30, 350–353. Available online: https://arar.sci.am/dlibra/publication/100535/edition/91408/content?ref=struct (accessed on 19 October 2025).
- Aghayan, S.A.; Asikyan, M.; Raković, M.; Stanković, D.; Fadeev, I.V.; Gevorgyan, H.; Shcherbakov, O.; Arakelyan, M.; Aghababyan, K.; Pagheh, A.S.; et al. Molecular Detection of Toxoplasma gondii (Chromista: Apicomplexa) in the Blood of Passerines (Aves: Passeriformes) in South-Eastern Armenia. Zoologia 2024, 41, e24016. [Google Scholar] [CrossRef]
- Janibekyan, Z.; Amiryan, S.; Antonyan, R. Prevalence of toxoplasmposis in women of childbearing age in Armenia. Med. Sci. Armen. 2002, 42, 97–99. Available online: https://arar.sci.am/dlibra/publication/101631/edition/92440/content (accessed on 19 October 2025).
- Nasir, S.M.I.; Amarasekara, S.; Wickremasinghe, R.; Fernando, D.; Udagama, P. Prevention of Re-Establishment of Malaria: Historical Perspective and Future Prospects. Malar. J. 2020, 19, 452. [Google Scholar] [CrossRef]
- Davidyants, V.; Avetisyan, L.; Ejov, M. Malaria In Armenia: Achievements And Priorities. New Armen. Med. J. 2008, 2, 5–24. Available online: https://ysmu.am/v2/wp-content/uploads/2023/09/1-Davidyants-Eng.pdf (accessed on 19 October 2025).
- Davidyants, V.A.; Kondrashin, A.V.; Vanyan, A.V.; Morozova, L.F.; Turbabina, N.A.; Stepanova, E.V.; Maksimova, M.S.; Morozov, E.N. Role of Malaria Partners in Malaria Elimination in Armenia. Malar. J. 2019, 18, 178. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Record: Progress towards malaria elimination. Wkly. Epidemiol. Rec. 2017, 92, 573–577. Available online: https://iris.who.int/server/api/core/bitstreams/c7ebad07-b940-413f-8847-fb9b00cf302f/content (accessed on 19 October 2025).
- World Health Organization. World Malaria Report 2023: Tracking Progress and Gaps in the Global Response to Malaria. 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 19 October 2025).
- World Health Organization. Countries and Territories Certified Malaria-Free by WHO; WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/teams/global-malaria-programme/elimination/countries-and-territories-certified-malaria-free-by-who (accessed on 19 October 2025).
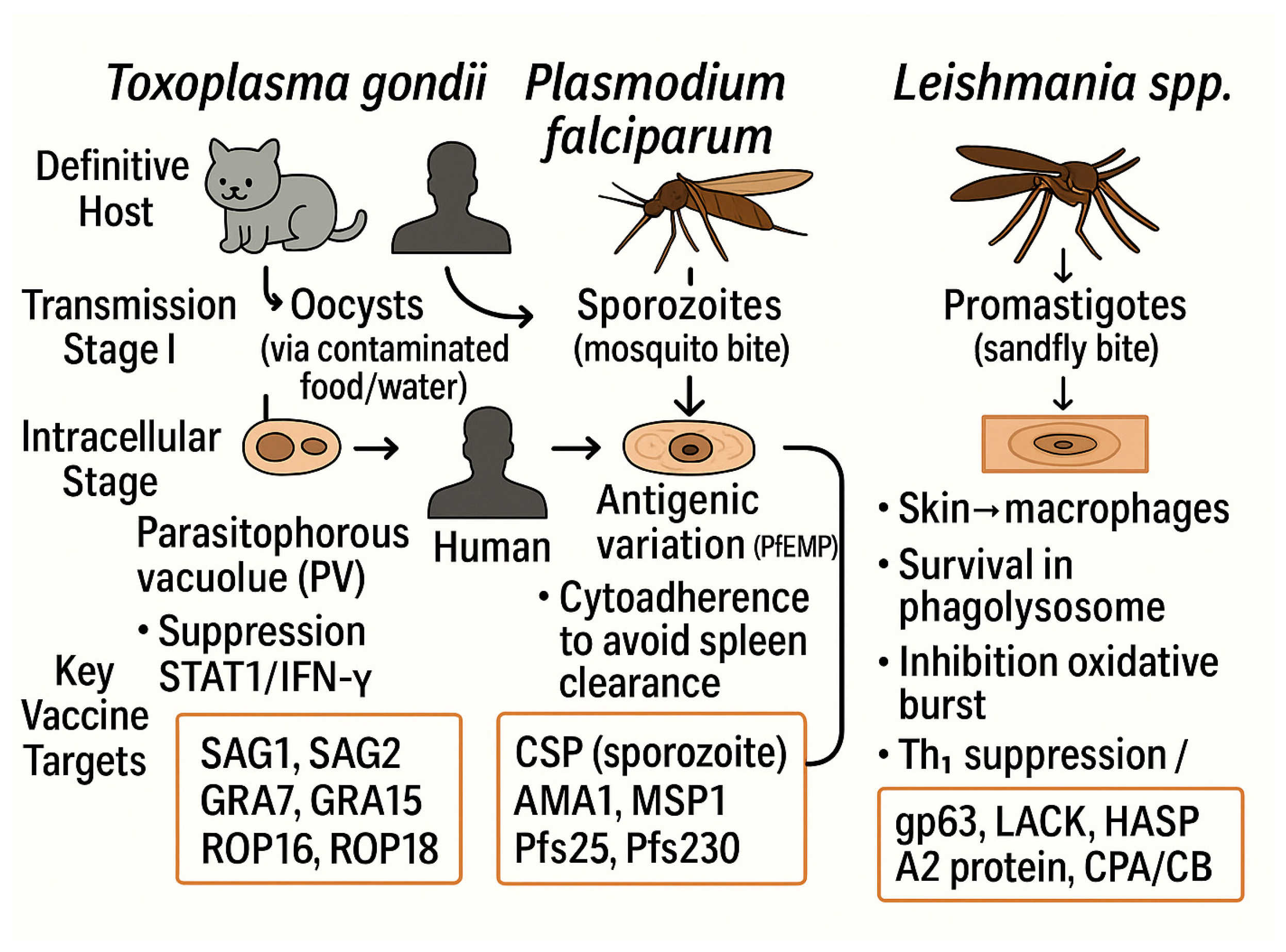
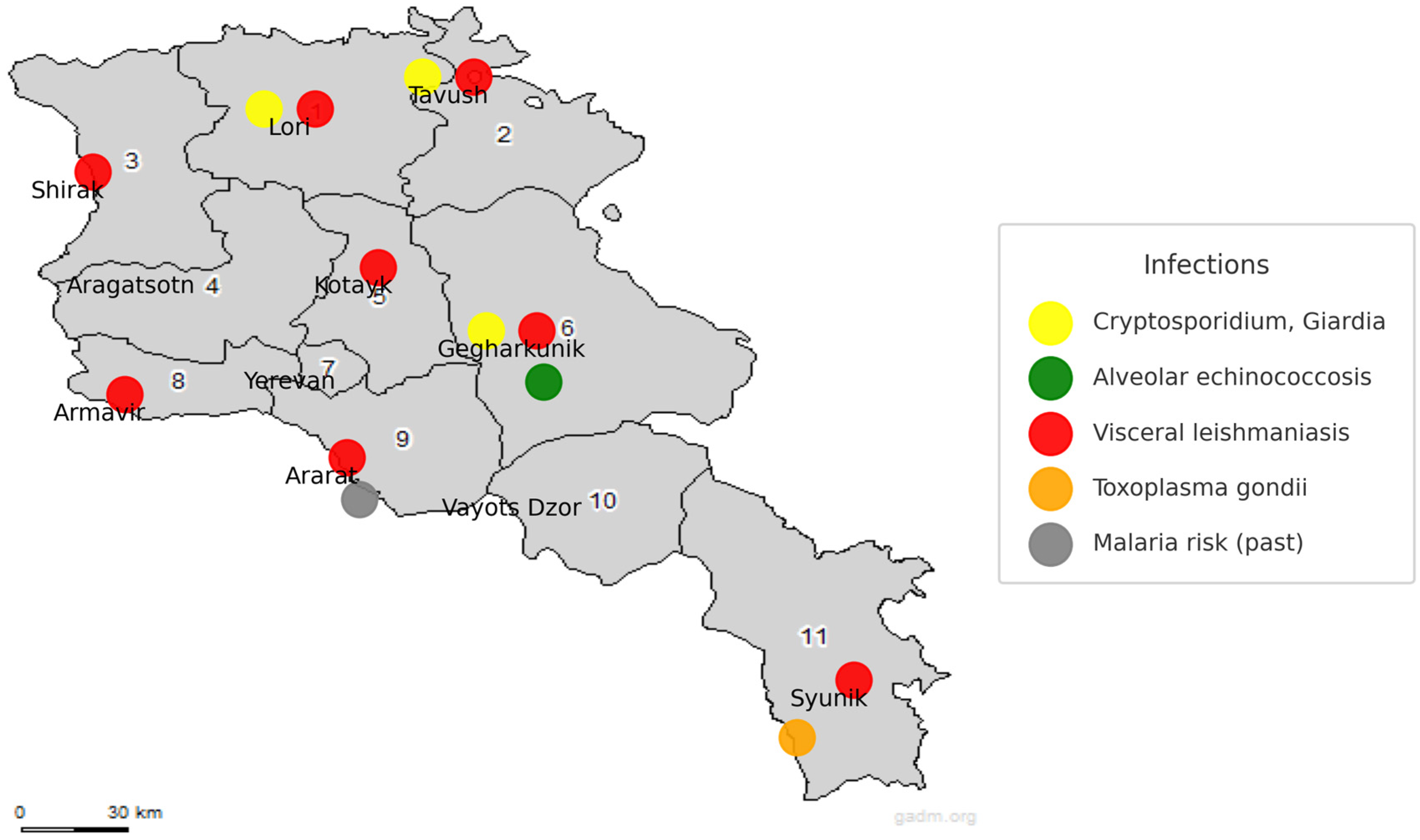
| Parasite | Vaccine/Candidate | Platform Adjuvant | Antigen Target | Stage Status | Geography | Efficacy | Ref. |
|---|---|---|---|---|---|---|---|
| Plasmodium falciparum | RTS,S/AS01 (Mosquirix) | Protein subunit + AS01 | CSP | Phase IV licensed | Sub-Saharan Africa | ~30–50% | [187] |
| Plasmodium falciparum | R21/Matrix-M | Protein subunit + Matrix-M | CSP | Phase III Advanced trial | West Africa | >75% | [188] |
| Plasmodium falciparum | PfSPZ Vaccine (Sanaria) | Live attenuated sporozoites | Whole sporozoites | Phase II/III Experimental | Africa, USA | High (under study) | [189] |
| Leishmania spp. | Letifend® (dogs) | Recombinant protein | Q proteins (L. infantum) | Licensed (vet) Veterinary | Europe | Protective (dogs) | [190] |
| Leishmania spp. | CaniLeish® (dogs) | Subunit protein vaccine | Excreted secreted protein from L. infantum | Licensed veterinary vaccine | Europe | 68.4%. ~3.6-fold reduced risk of disease | [191] |
| Leishmania spp. | LeishTec® | Recombinant protein vaccine (Quil A/saponin) | rA2 (amastigote antigen A2) | Veterinary licensed | Brazil (endemic zones | 80.8% (seroconverted dogs) | [192,193] |
| Leishmania spp. | LEISH-F3 + GLA-SE | Recombinant fusion protein GLA-SE (TLR4 agonist) | NH + SMT fusion | Phase I/II clinical trials | Safe, immunogenic; induced Th1 cytokines; efficacy | [194,195] | |
| Leishmania spp. | ChAd63 KH | Viral vector vaccine (chimpanzee adenovirus) | KMP-11 + HASPB (KH antigen) | Phase I clinical trial | UK, Sudan | Safe, strong CD8+ responses; efficacy under evaluation | [196] |
| Leishmania spp. | DNA vaccine (gp63 + Hsp70) | DNA plasmid | gp63 + Hsp70 | Pre-clinical/experimental | Mouse models | Protective immunity, reduced parasite loads | [197] |
| Leishmania spp. | Multi-epitope peptide vaccine (LACK, LeIF, GP63, SMT) | Synthetic peptides in various formulations | LACK, LeIF, GP63, SMT | Pre-clinical/experimental | Mouse models (India, Iran) | Th1-biased immunity; protection in mice | [198] |
| Toxoplasma gondii | rSAG1 | Recombinant protein | SAG1 | Preclinical Experimental | Mouse | increased survival | [199] |
| T. gondii | MIC8 + AMA1 heterologous prime/boost | Prime: recombinant vaccinia virus (rVV), Boost: virus-like particles (VLPs) | MIC8 + AMA1 proteins | Preclinical (mouse, BALB/c)—heterologous (rVV + VLP) regimen yields better reduction in brain cysts than VLP + VLP | Preclinical (mouse, BALB/c) | Significant reduction in brain cyst burden compared to homologous VLP + VLP | [200] |
| T. gondii | MIC8 + AMA1 heterologous prime/boost | DNA/protein prime boost varies | MIC8, AMA1 | Preclinical (mice) | Lab studies | Protective, experimental | [201] |
| T. gondii | GP–AS04–TE | Glucan particle delivery AS04 (MPL + alum) | T. gondii lysate | Preclinical | Lab studies | Improved survival | [202] |
| T. gondii | pVAX1-TgIMC1 nanosphere DNA vaccine | DNA nanosphere Encapsulated delivery | IMC1 protein | Preclinical | Lab studies | Partial protection | [203] |
| T. gondii | SAPN with multi-epitope peptides | Self-assembling protein nanoparticle None/experimental | GRA7 + others | Preclinical | Lab studies | Protective immune response | [204] |
| T. gondii | Chimeric multi-epitope vaccine | DNA/protein varies | SAG1 + apicoplast proteins | Preclinical | Lab studies | Reduced parasite load | [205] |
| T. gondii | Multi-epitope membrane protein vaccine | DNA/protein varies | Predicted membrane epitopes | Preclinical | Lab studies | In silico + mouse testing | [206] |
| Trypanosoma cruzi | multi-epitope recombinant protein | Recombinant chimeric protein (with adjuvant, unspecified) | Trivalent antigen (multi-epitope) | Preclinical Experimental | Animals (mice, others) | Reduced parasite load | [207] |
| Cryptosporidium parvum | Oocyst antigen | Recombinant/purified proteins | Oocyst antigens | Preclinical Experimental | Mice | Reduced shedding | [208] |
| Echinococcus multilocularis | GILE vaccine | Multi-epitope recombinant | EMY162, LAP, GLUT1 | Preclinical Experimental | Mice | Reduced cysts | [209] |
| Pathogen/Disease | Type | Affected Populations/Reservoirs | Prevalence/Incidence | Diagnostic Methods | Timeline | Key Insights/Notes | Ref. |
|---|---|---|---|---|---|---|---|
| Cryptosporidium spp. | Waterborne protozoa | Humans, livestock (sheep, cattle, pigs), environment | Humans: 7% (mZN), 17% (ELISA) in 2011 Animals: Up to 50% in pigs Environment: Widespread (2019–2024) | mZN staining, ELISA (RIDASCREEN®), qPCR | 2006–2024 | Environmental contamination peaks in autumn; high parasite loads in river sediment; linked to untreated sewage (e.g., Aghstev River). | [212] |
| Giardia spp. | Waterborne protozoa | Humans (limited data), environmental samples | 4/5 samples positive (2019–2024) | qPCR (environment), no recent human/animal data | 2019–2024 | High levels in sediment imply long-term contamination; no animal infection data available; human prevalence remains unknown. | [212] |
| Echinococcus multilocularis (AE) | Zoonotic helminth | Humans (esp. rural areas), likely wild canids | 11 confirmed cases (2008–2020); peak incidence: 0.1/100,000 in 2017 | Tissue biopsy, medical imaging | 2008–2020 | Previously thought non-endemic; mainly affects younger adults; comparable to endemic European countries; poor early diagnosis. | [37,214] |
| Leishmania infantum (VL) | Vector-borne protozoa | Humans, sandfly vector | 167 cases by 2019 (since 1999) | Microscopy, PCR, sequencing (ITS1-PCR-RFLP) | 1999–2019+ | Re-emerged in 1999 after 30 years; 8/11 regions affected; likely underreported due to diagnostic and awareness gaps. | [29,30] |
| Toxoplasma gondii | Food-/zoonotic protozoa | Birds (migratory), small mammals (rodents, shrews), possibly humans | Birds: up to 36% (PCR); Mammals: 10.9% positive | PCR (RE gene, 529 bp fragment) | 2013–2024 | Migratory birds aid spread; infection in mammals confirms environmental maintenance; human exposure likely but no national survey exists. | [96,97,98,99] |
| Plasmodium spp. (malaria) | Vector-borne protozoa | Humans (previously), Anopheles mosquitoes | 1156 cases by 1998; last local case: 2005 | Microscopy, epidemiological surveillance | 1920s–2011 | Eradicated by 1963; resurgence in 1990s due to instability; certified malaria-free in 2011 but remains at risk due to ecological and vector presence. | [223,226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargsyan, T.; Stepanyan, L.; Tsaturyan, A.; Palumbo, R.; Vicidomini, C.; Roviello, G.N. Intracellular Parasitic Infections Caused by Plasmodium falciparum, Leishmania spp., Toxoplasma gondii, Echinococcus multilocularis, Among Key Pathogens: Global Burden, Transmission Dynamics, and Vaccine Advances—A Narrative Review with Contextual Insights from Armenia. Vaccines 2025, 13, 1082. https://doi.org/10.3390/vaccines13111082
Sargsyan T, Stepanyan L, Tsaturyan A, Palumbo R, Vicidomini C, Roviello GN. Intracellular Parasitic Infections Caused by Plasmodium falciparum, Leishmania spp., Toxoplasma gondii, Echinococcus multilocularis, Among Key Pathogens: Global Burden, Transmission Dynamics, and Vaccine Advances—A Narrative Review with Contextual Insights from Armenia. Vaccines. 2025; 13(11):1082. https://doi.org/10.3390/vaccines13111082
Chicago/Turabian StyleSargsyan, Tatevik, Lala Stepanyan, Avetis Tsaturyan, Rosanna Palumbo, Caterina Vicidomini, and Giovanni N. Roviello. 2025. "Intracellular Parasitic Infections Caused by Plasmodium falciparum, Leishmania spp., Toxoplasma gondii, Echinococcus multilocularis, Among Key Pathogens: Global Burden, Transmission Dynamics, and Vaccine Advances—A Narrative Review with Contextual Insights from Armenia" Vaccines 13, no. 11: 1082. https://doi.org/10.3390/vaccines13111082
APA StyleSargsyan, T., Stepanyan, L., Tsaturyan, A., Palumbo, R., Vicidomini, C., & Roviello, G. N. (2025). Intracellular Parasitic Infections Caused by Plasmodium falciparum, Leishmania spp., Toxoplasma gondii, Echinococcus multilocularis, Among Key Pathogens: Global Burden, Transmission Dynamics, and Vaccine Advances—A Narrative Review with Contextual Insights from Armenia. Vaccines, 13(11), 1082. https://doi.org/10.3390/vaccines13111082









