Optimization of In Vitro Transcription by Design of Experiment to Achieve High Self-Amplifying RNA Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Animals
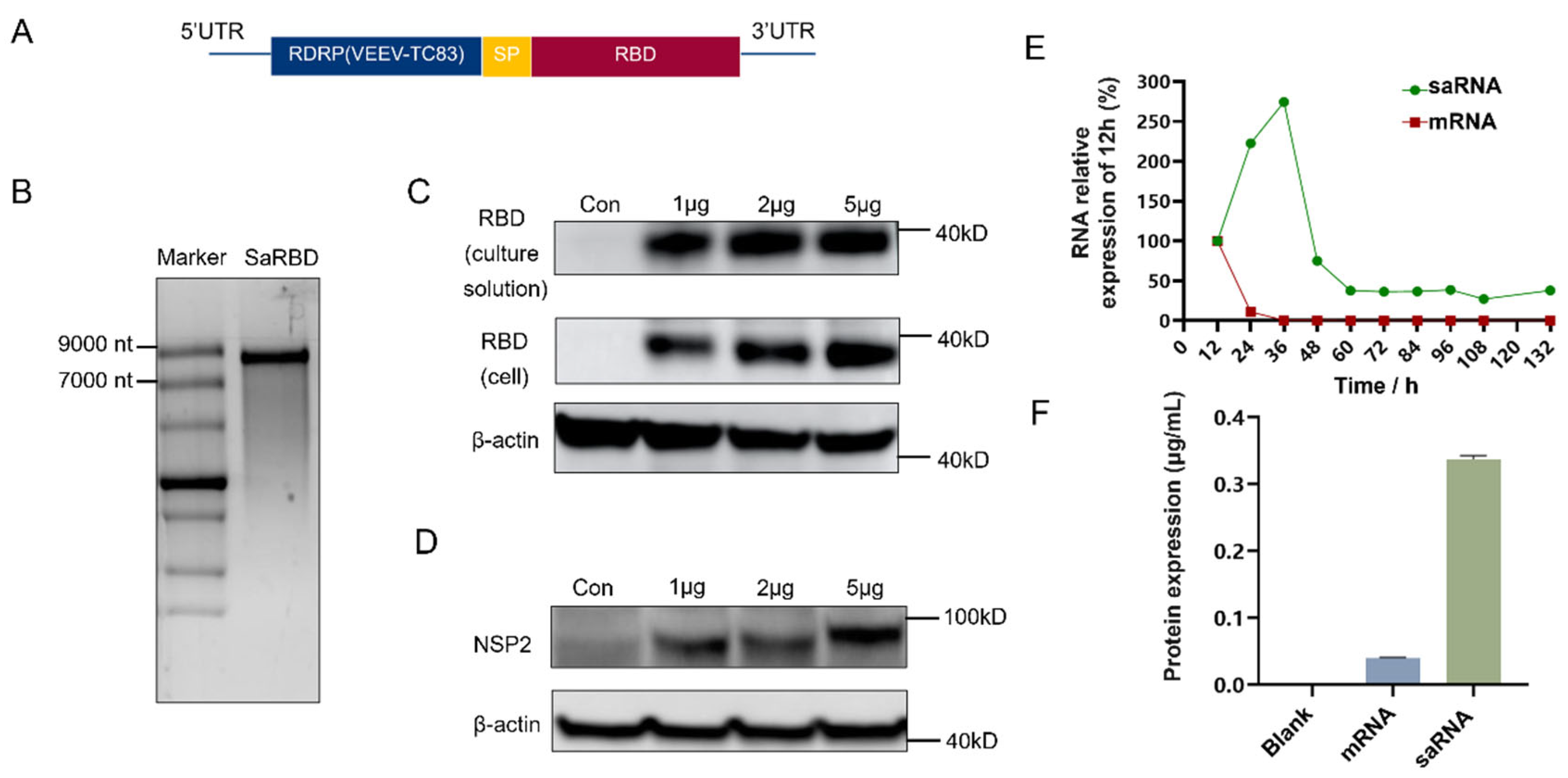
2.2. Design and Construction of saRNA Vaccine
2.3. Synthesis of saRNA
2.4. Detection of Protein Expression by Western Blot
2.5. Detection of the Potein Expression by ELISA
2.6. Detection of Target RNA Relative Content Using qPCR
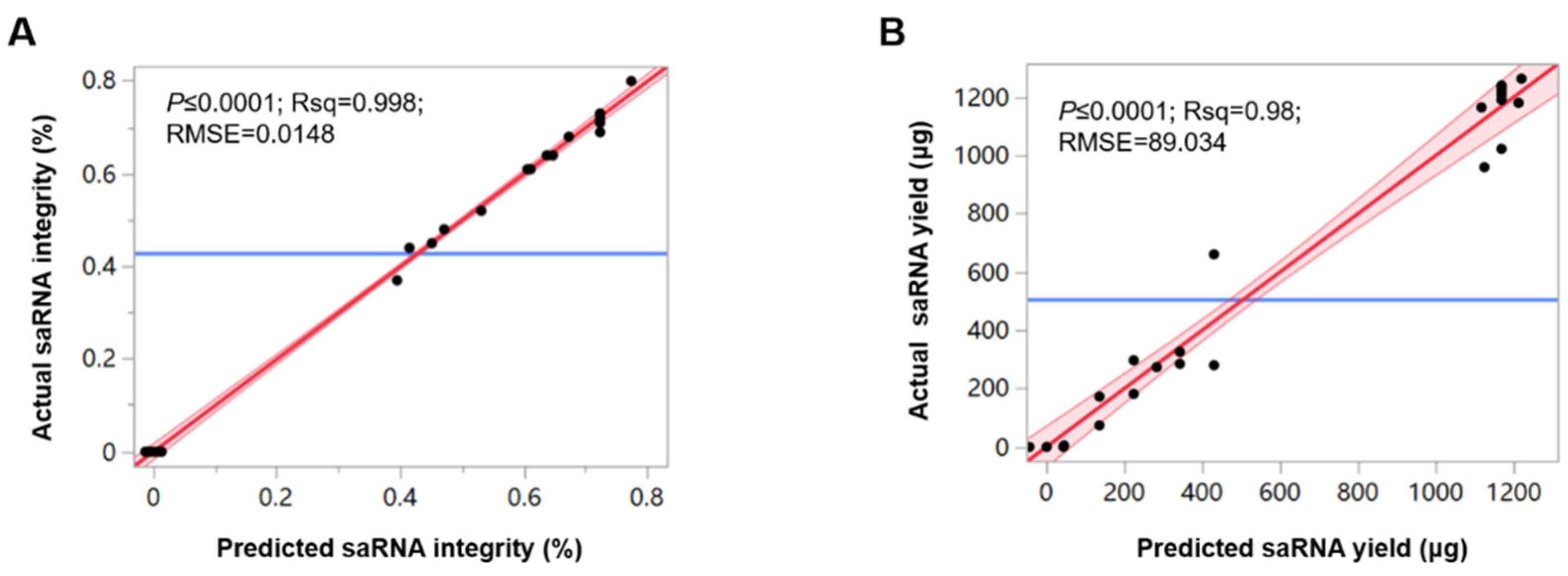
2.7. DoE Experimental Design and Data Analysis
2.8. Detection of saRNA Integrity and Yield
2.9. Preparation of saRNA Vaccines
2.10. Immunization Sample Collection and Processing
2.11. ELISA for Spike-Specific IgG
2.12. Pseudovirus Neutralization Assay
2.13. Enzyme-Linked Immunospot (ELISpot) Assay for IFN-γ
2.14. Data Processing
3. Results
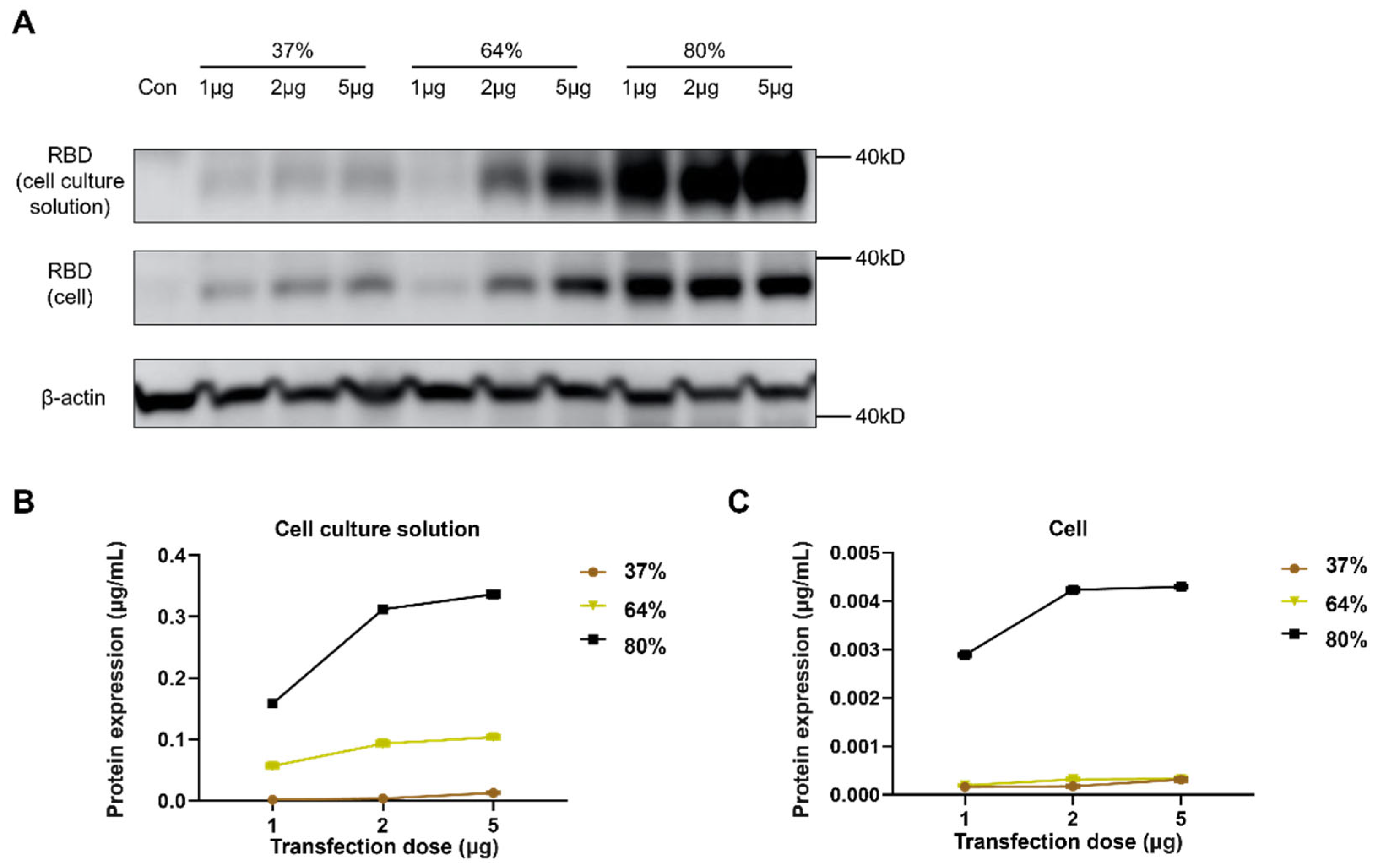
3.1. Construction of saRNA Encoding RBD
3.2. DoE-Designed IVT Experiments
3.3. Main Effects and Interaction Effects of Different IVT Reaction Parameters on Integrity and Yield
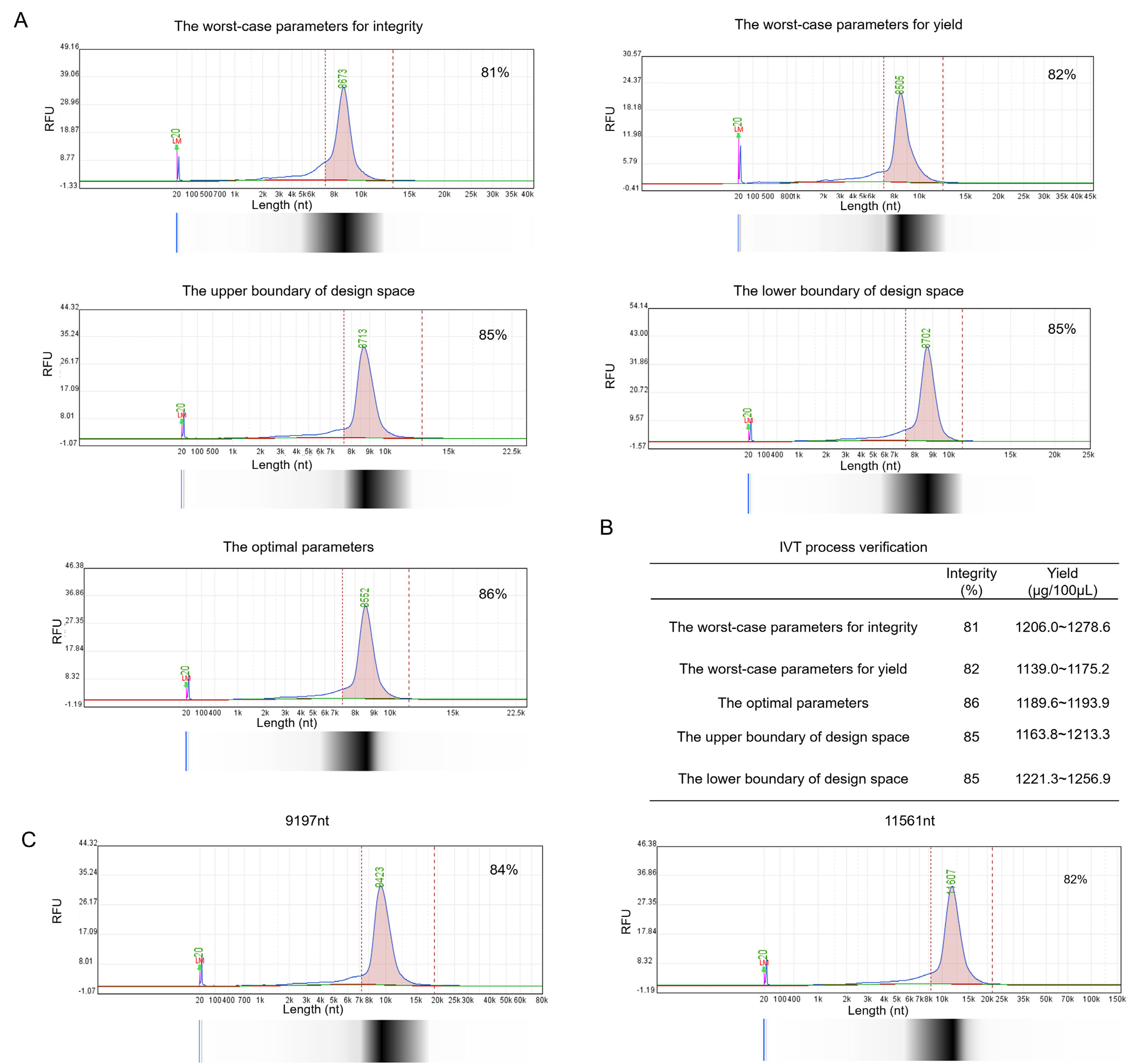
3.4. The Optimal IVT System and Multivariate Design Space
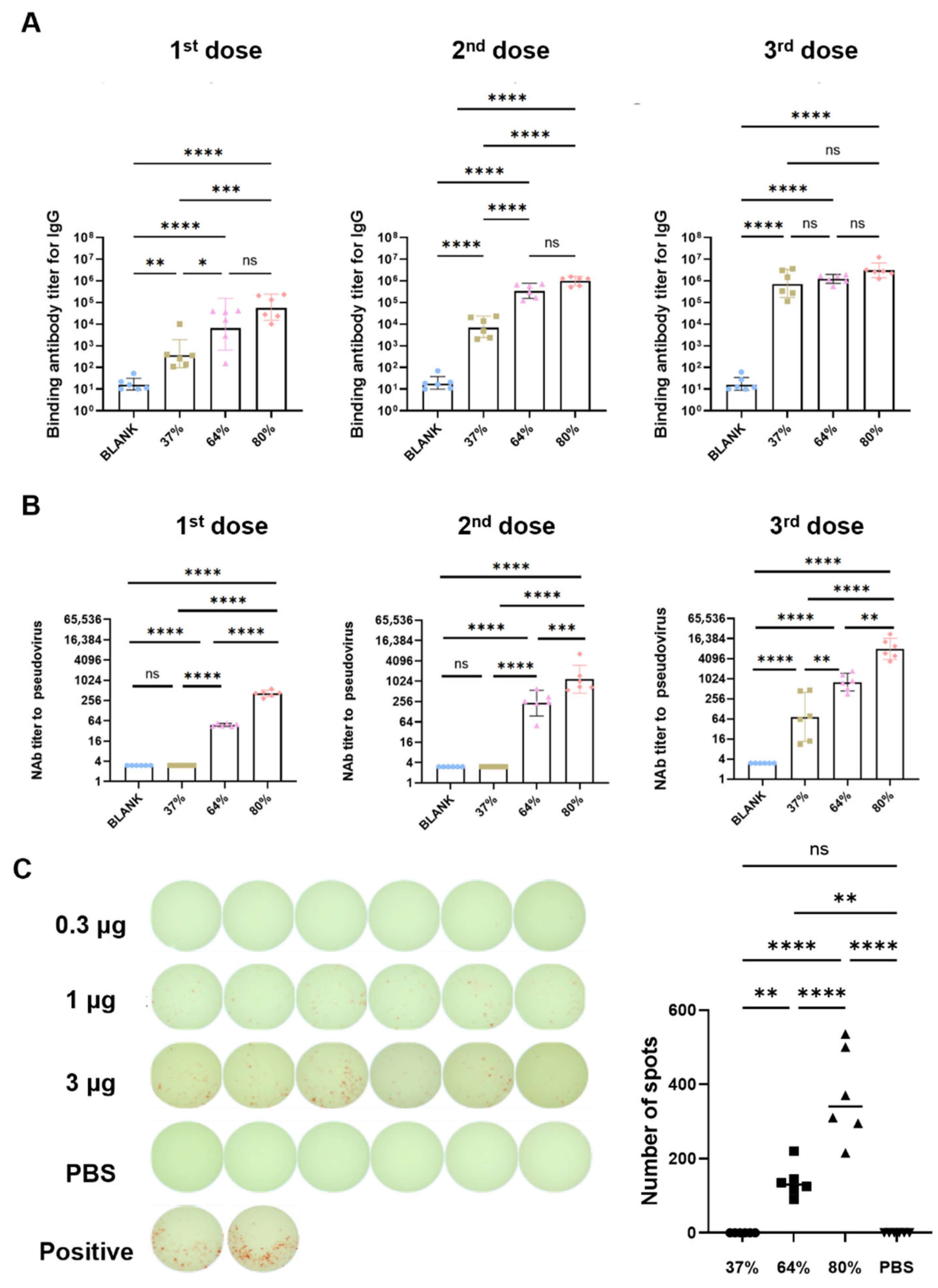
3.5. Immunogenicity of Sa-RBD Vaccines with Different Integrities in Murine Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef]
- Schmidt, C.; Schnierle, B.S. Self-Amplifying RNA Vaccine Candidates: Alternative Platforms for mRNA Vaccine Development. Pathogens 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Wayne, C.J.; Blakney, A.K. Self-amplifying RNA COVID-19 vaccine. Cell 2024, 187, 1822. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Kostaive: EPAR—Medicine Overview. 12 February 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kostaive (accessed on 14 October 2025).
- Casmil, I.C.; Jin, J.; Won, E.J.; Huang, C.; Liao, S.; Cha-Molstad, H.; Blakney, A.K. The advent of clinical self-amplifying RNA vaccines. Mol. Ther. 2025, 33, 2565–2582. [Google Scholar] [CrossRef]
- Pourseif, M.M.; Masoudi-Sobhanzadeh, Y.; Azari, E.; Parvizpour, S.; Barar, J.; Ansari, R.; Omidi, Y. Self-amplifying mRNA vaccines: Mode of action, design, development and optimization. Drug Discov. Today 2022, 27, 103341. [Google Scholar] [CrossRef]
- Pietilä, M.K.; Hellström, K.; Ahola, T. Alphavirus polymerase and RNA replication. Virus Res. 2017, 234, 44–57. [Google Scholar] [CrossRef]
- Rubach, J.K.; Wasik, B.R.; Rupp, J.C.; Kuhn, R.J.; Hardy, R.W.; Smith, J.L. Characterization of purified Sindbis virus nsP4 RNA-dependent RNA polymerase activity in vitro. Virology 2009, 384, 201–208. [Google Scholar] [CrossRef]
- Das, P.K.; Merits, A.; Lulla, A. Functional cross-talk between distant domains of chikungunya virus non-structural protein 2 is decisive for its RNA-modulating activity. J. Biol. Chem. 2014, 289, 5635–5653. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Dousis, A.; Ravichandran, K.; Hobert, E.M.; Moore, M.J.; Rabideau, A.E. An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts. Nat. Biotechnol. 2023, 41, 560–568. [Google Scholar] [CrossRef]
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Pharmaceutical Development Q8 (R2). 2009. Available online: https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf (accessed on 20 June 2025).
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Quality Risk Management Q9 (R1). 2020. Available online: https://database.ich.org/sites/default/files/Q9-R1_Concept%20Paper_2020_1113.pdf (accessed on 20 June 2025).
- VanDuyse, S.A.; Fulford, M.J.; Bartlett, M.G. ICH Q10 Pharmaceutical Quality System Guidance: Understanding Its Impact on Pharmaceutical Quality. AAPS J. 2021, 23, 117. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Pharmaceutical Current Good Manufacturing Practices for the 21st Century—A Risk-Based Approach, Final Report. 2004. Available online: https://www.fda.gov/media/77391/download (accessed on 20 June 2025).
- Mu’azzam, K.; da Silva, F.V.S.; Murtagh, J.; Gallagher, M.J.S. A roadmap for model-based bioprocess development. Biotechnol. Adv. 2024, 73, 108378. [Google Scholar] [CrossRef]
- Alt, N.; Zhang, T.Y.; Motchnik, P.; Taticek, R.; Quarmby, V.; Schlothauer, T.; Beck, H.; Emrich, T.; Harris, R.J. Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biologicals 2016, 44, 291–305. [Google Scholar] [CrossRef]
- Politis, S.N.; Colombo, P.; Colombo, G.; MRekkas, D. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Hakemeyer, C.; McKnight, N.; John, R.S.; Meier, S.; Trexler-Schmidt, M.; Kelley, B.; Zettl, F.; Puskeiler, R.; Kleinjans, A.; Lim, F.; et al. Process characterization and Design Space definition. Biologicals 2016, 44, 306–318. [Google Scholar] [CrossRef]
- Popova, P.G.; Lagace, M.A.; Tang, G.; Blakney, A.K. Effect of in vitro transcription conditions on yield of high quality messenger and self-amplifying RNA. Eur. J. Pharm. Biopharm. 2024, 198, 114247. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.E.; Kirsch, J.R.; Kenney, D.; Chavez, E.; Shih, T.Y.; Douam, F.; Wong, W.W.; Grinstaff, M.W. Complete substitution with modified nucleotides suppresses the early interferon response and increases the potency of self-amplifying RNA. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cui, L.; Ma, R.; Cai, J.; Guo, C.; Chen, Z.; Yao, L.; Wang, Y.; Fan, R.; Wang, X.; Shi, Y. RNA modifications: Importance in immune cell biology and related diseases. Signal Transduct. Target. Ther. 2022, 7, 334. [Google Scholar] [CrossRef]
- Vallet, T.; Vignuzzi, M. Self-Amplifying RNA: Advantages and Challenges of a Versatile Platform for Vaccine Development. Viruses 2025, 17, 566. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- de Haro, C.; Méndez, R.; Santoyo, J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996, 10, 1378–1387. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Bouton, C.R.; Hu, K.; Samnuan, K.; Shattock, R.J. Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA. Mol. Ther. 2021, 29, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.R.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.F.; Moreno, A.C.R.; Aps, L.R.M.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of the Quality, Safety and Efficacy of Messenger RNA Vaccines for the Prevention of Infectious Diseases: Regulatory Considerations. 2021. Available online: https://www.who.int/publications/m/item/evaluation-of-the-quality-safety-and-efficacy-of-messenger-rna-vaccines-for-the-prevention-of-infectious-diseases-regulatory-considerations (accessed on 20 June 2025).
- United States Pharmacopeia. Analytical Procedures for mRNA Vaccine Quality. April 2023. Available online: https://www.usp.org/sites/default/files/usp/document/our-work/biologics/documents/vaccine-mrna-guidelines-2.pdf (accessed on 20 June 2025).
- Kern, J.A.; Davis, R.H. Application of solution equilibrium analysis to in vitro RNA transcription. Biotechnol. Prog. 1997, 13, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.A.; Davis, R.H. Application of a fed-batch system to produce RNA by in vitro transcription. Biotechnol. Prog. 1999, 15, 174–184. [Google Scholar] [CrossRef] [PubMed]
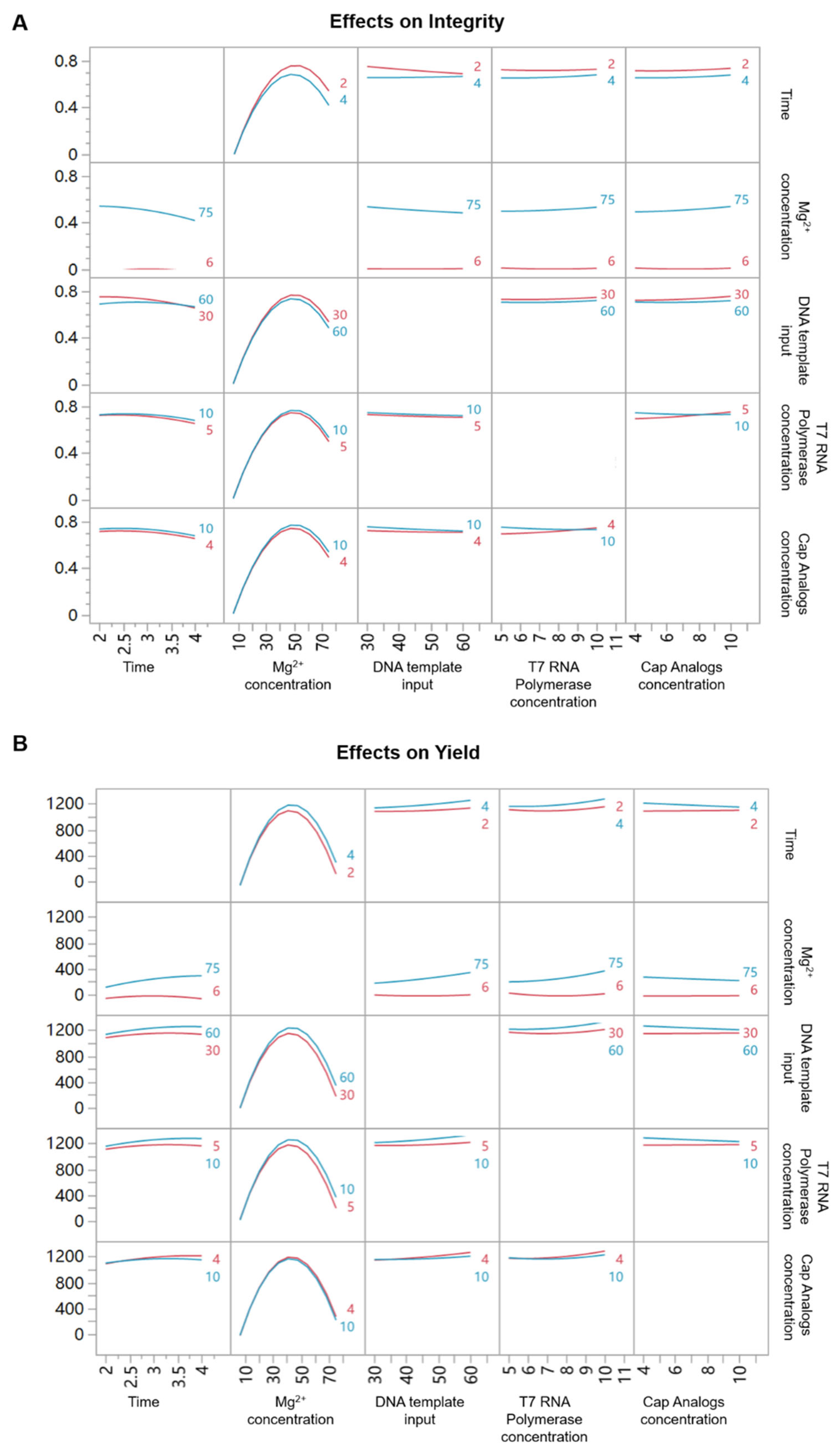
| Parameters and Interactions | LogWorth (Integrity) | LogWorth (Yield) |
|---|---|---|
| Mg2+ | 10.017 | 3.987 |
| Mg2+ × Mg2+ | 8.816 | 7.180 |
| Time × Mg2+ | 3.740 | 1.428 |
| Time | 3.685 | 1.430 |
| Cap Analog × T7 RNA polymerase | 2.328 | 0.418 |
| DNA template × Time | 2.328 | 0.418 |
| DNA template | 1.730 | 1.389 |
| DNA template × Mg2+ | 1.671 | 1.265 |
| Cap Analog × Mg2+ | 1.496 | 0.367 |
| Cap Analog | 1.483 | 0.313 |
| Time × Time | 1.308 | 0.392 |
| T7 RNA polymerase × Mg2+ | 1.137 | 1.402 |
| T7 RNA polymerase | 0.975 | 1.299 |
| T7 RNA polymerase × Time | 0.609 | 0.431 |
| DNA template × Cap Analog | 0.609 | 0.431 |
| T7 RNA polymerase × T7 RNA polymerase | 0.298 | 0.383 |
| Cap Analog × Cap Analog | 0.298 | 0.010 |
| DNA template × DNA template | 0.098 | 0.136 |
| DNA template × T7 RNA polymerase | 0.050 | 0.468 |
| Cap Analog × Time | 0.050 | 0.468 |
| IVT System | Value | |
|---|---|---|
| Parameters | DNA Template (ng/μL) | 60 |
| Mg2+ (mM) | 52 | |
| Time (h) | 2 | |
| Cap Analog (mM) | 10 | |
| T7 RNA Polymerase(U/μL) | 5 | |
| Yield | Mean (95% CI) (μg) | 1121.5 (1038.2, 1204.8) |
| Integrity | Mean (95% CI) (%) | 86.5 (84.4, 88.6) |
| Parameters | Upper Boundary | Lower Boundary |
|---|---|---|
| DNA Template (ng/μL) | 60 | 50 |
| Mg2+ (mM) | 55 | 50 |
| Time (h) | 2.2 | 2 |
| Cap Analog (mM) | 10 | 7 |
| T7 RNA Polymerase(U/μL) | 7.5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Wang, H.; Liu, G.; Li, K.; Zhang, X.; Song, L.; Gao, F.; Wu, X.; Wang, Q.; Liu, M.; et al. Optimization of In Vitro Transcription by Design of Experiment to Achieve High Self-Amplifying RNA Integrity. Vaccines 2025, 13, 1062. https://doi.org/10.3390/vaccines13101062
Hu C, Wang H, Liu G, Li K, Zhang X, Song L, Gao F, Wu X, Wang Q, Liu M, et al. Optimization of In Vitro Transcription by Design of Experiment to Achieve High Self-Amplifying RNA Integrity. Vaccines. 2025; 13(10):1062. https://doi.org/10.3390/vaccines13101062
Chicago/Turabian StyleHu, Chaoying, Haixin Wang, Guanxing Liu, Kelei Li, Xuanxuan Zhang, Lifang Song, Fan Gao, Xing Wu, Qian Wang, Mingchen Liu, and et al. 2025. "Optimization of In Vitro Transcription by Design of Experiment to Achieve High Self-Amplifying RNA Integrity" Vaccines 13, no. 10: 1062. https://doi.org/10.3390/vaccines13101062
APA StyleHu, C., Wang, H., Liu, G., Li, K., Zhang, X., Song, L., Gao, F., Wu, X., Wang, Q., Liu, M., Liu, J., Fu, Z., Ma, X., Xu, M., Mao, Q., Liang, Z., & He, Q. (2025). Optimization of In Vitro Transcription by Design of Experiment to Achieve High Self-Amplifying RNA Integrity. Vaccines, 13(10), 1062. https://doi.org/10.3390/vaccines13101062





