Serological Response to COVID-19 Vaccination in Saudi Arabia: A Comparative Study of IgG and Neutralising Antibodies Across Vaccine Platforms
Abstract
1. Introduction
2. Methodology
2.1. Ethical Approval
2.2. Participant Recruitment and Sample Collection
2.3. IgG & Neutralising Antibody Assays
2.4. Statistical Analysis
3. Results
3.1. Overall IgG Response Across Time Points
3.2. Impact of Demographic Factors (Co-Variates)
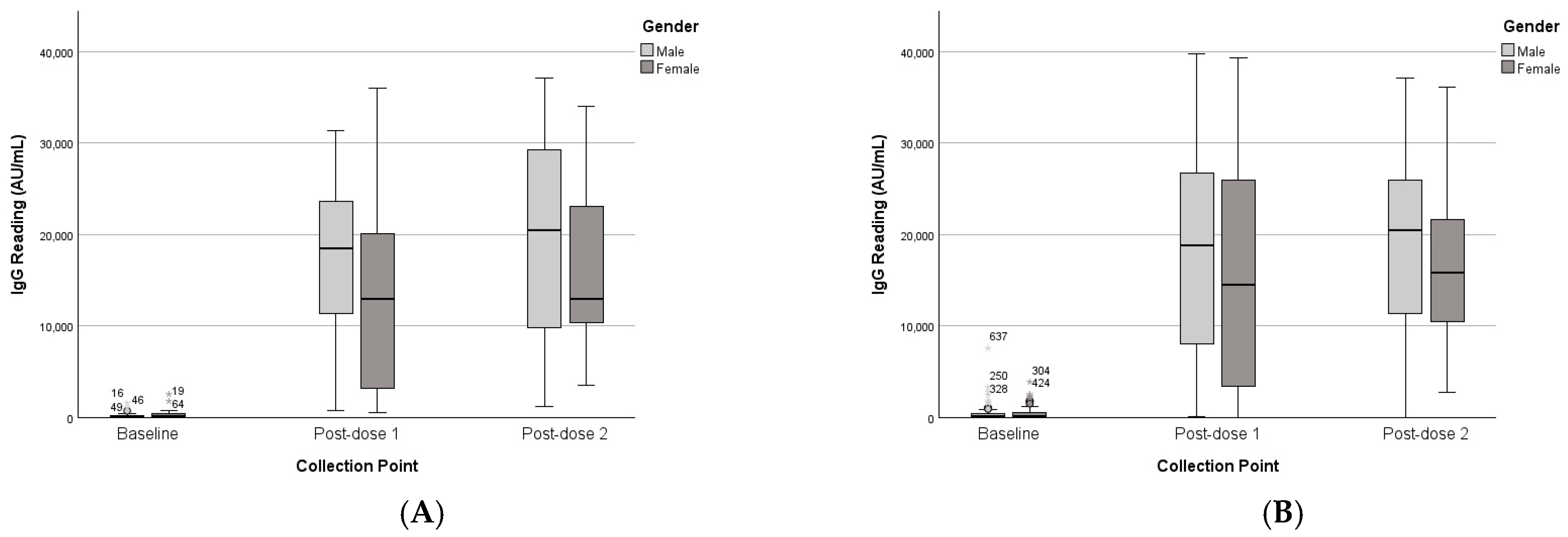
3.2.1. Gender
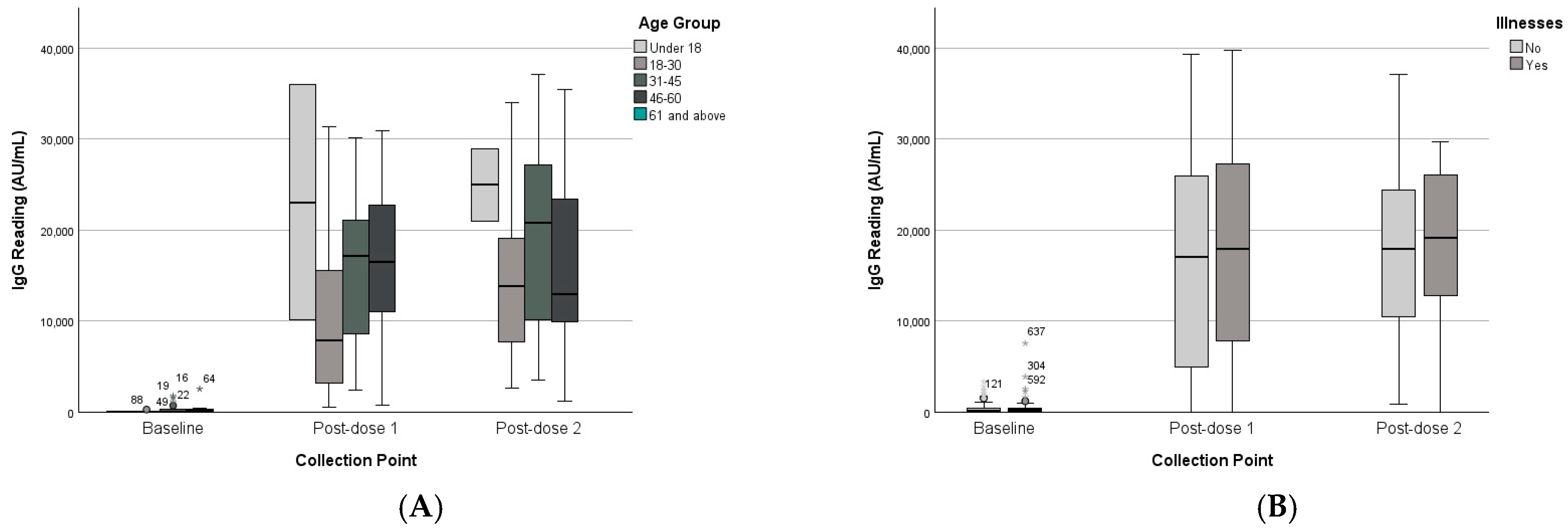
3.2.2. Age Groups
3.2.3. Chronic Disease Status
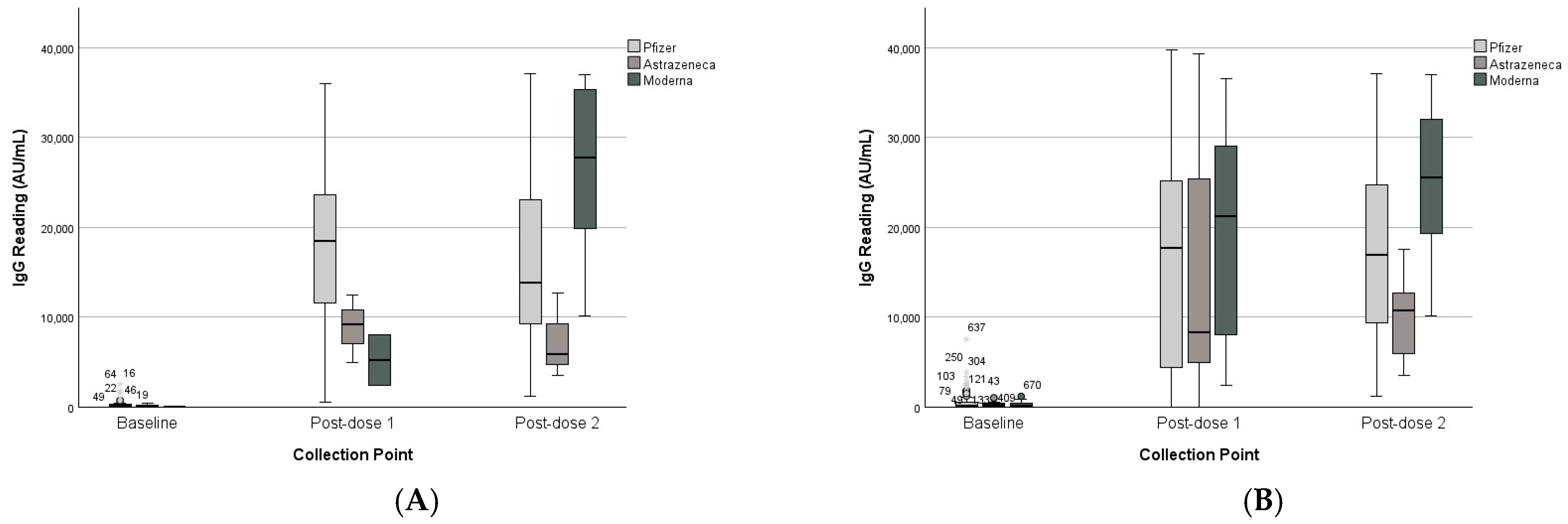
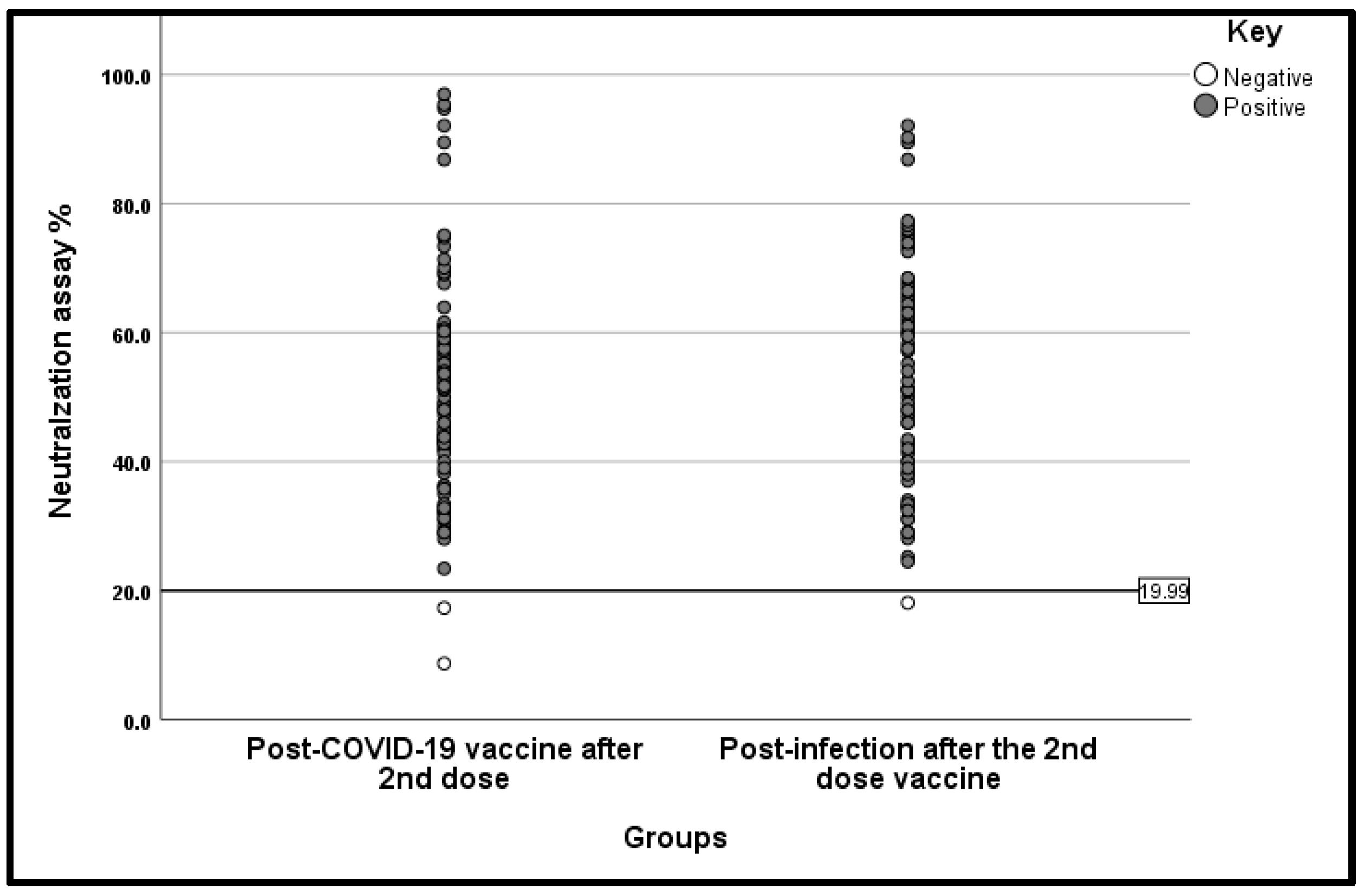
3.3. Vaccine Platform Comparison
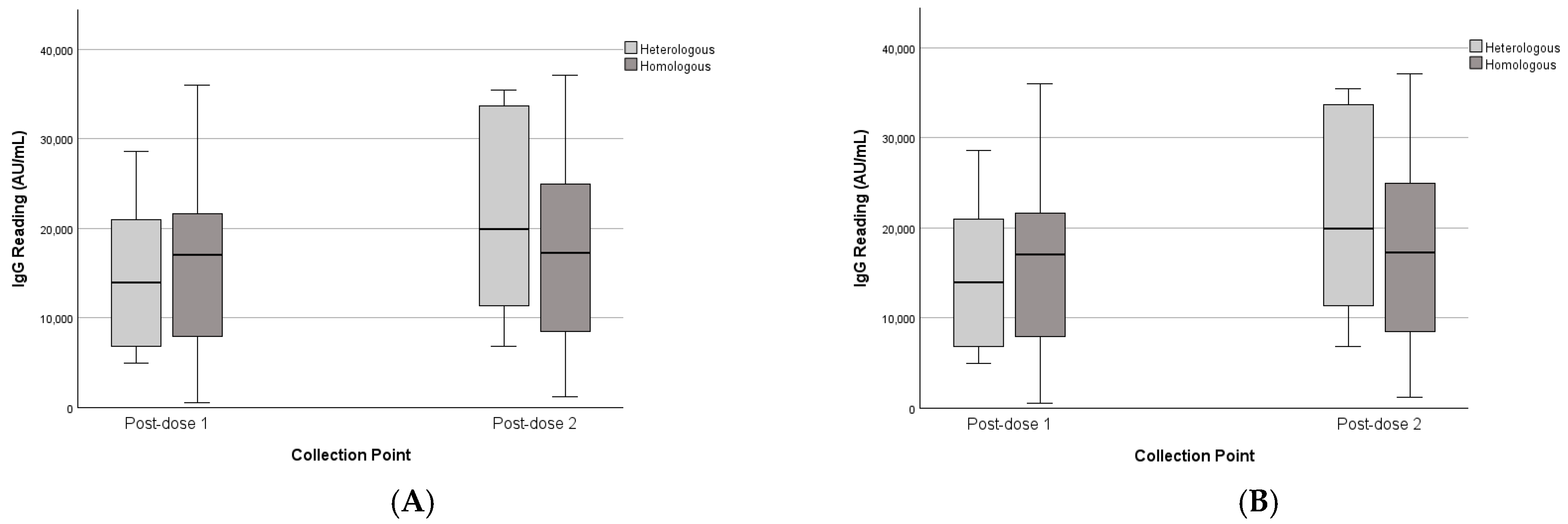
3.4. Heterologous vs. Homologous Schedules
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID-19 Public Health Emergency of International Concern (PHEIC). 2020. Available online: https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (accessed on 1 November 2024).
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. Atenei Parm. 2020, 91, 157. [Google Scholar]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Al Eissa, M.M.; Almsned, F.; AlQurashi, R.; Alsanosi, S.M.; Alshanberi, A.M.; A Alsaieedi, A.; Alkharji, R.R.; Halawani, A.J.; Alsaieedi, A.A.; Saleh, N.M. Perceptions of Saudis Toward Participating in the COVID-19 Convalescent Plasma Clinical Trial. Cureus 2023, 15, e48879. [Google Scholar] [CrossRef]
- WHO. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. 2019. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 26 November 2023).
- U.S Food & Drug. Pfizer-BioNTech COVID-19 Vaccines. 2021. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccines (accessed on 14 September 2023).
- SPA. SFDA Approves Registration of Pfizer-BioNTech COVID-19 Vaccine The official Saudi Press Agency. 2020. Available online: https://www.sfda.gov.sa/en/released-vaccines (accessed on 22 July 2023).
- AstraZeneca. AstraZeneca Welcomes Court Ruling on Supply of Its COVID-19 Vaccine to Europe; AstraZeneca: Cambridge, UK, 2021. [Google Scholar]
- SPA. SFDA Approves Importing, Using Oxford-AstraZeneca COVID-19 Vaccine. 2021. Available online: https://www.sfda.gov.sa/en/released-vaccines (accessed on 23 July 2024).
- CDC. Stay Up to Date with COVID-19 Vaccines Including Boosters. 2023. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html (accessed on 2 July 2024).
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Awadalla, M.; AlRawi, H.Z.; Henawi, R.A.; Barnawi, F.; Alkadi, H.; Alyami, A.; Alsughayir, A.; Alsaif, A.S.; Mubarak, A.; Alturaiki, W.; et al. Humoral and cellular immune durability of different COVID-19 vaccine platforms following homologous/heterologous boosters: One-year post vaccination. Front Immunol. 2025, 16, 1526444. [Google Scholar] [CrossRef]
- Bhadauria, D.S.; Katiyar, H.; Goel, A.; Tiwari, P.; Kishore, R.V.K.; Aggarwal, A.; Verma, A.; Khetan, D.; Kaul, A.; Yachha, M.; et al. Antibody Response to ChAdOx1 nCoV-19 (AZD1222) Vaccine in Kidney Transplant Recipients. Vaccines 2022, 10, 1693. [Google Scholar] [CrossRef]
- Choe, P.G.; Perera, R.; Park, W.B.; Song, K.H.; Bang, J.H.; Kim, E.S.; Bin Kim, H.; Ko, L.W.R.; Park, S.W.; Kim, N.-J.; et al. MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg. Infect. Dis. 2017, 23, 1079. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Alghnam, S.; Algaissi, A.; Albalawi, H.; Alenazi, M.W.; Albargawi, A.M.; Alharbi, A.G.; Alhazmi, A.; Al Qarni, A.; Alfarhan, A.; et al. Nationwide Seroprevalence of SARS-CoV-2 in Saudi Arabia. J. Infect. Public Health 2021, 14, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.; Lee, J.A.; Lee, S.J.; Lee, K.H.; Kim, J.H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Yeom, J.-S.; et al. Immunogenicity Differences of the ChAdOx1 nCoV-19 Vaccine According to Pre-Existing Adenovirus Immunity. Vaccines 2023, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; O’connell, S.E.; Schmidt, S.D.; O’dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef]
- Mubarak, A.; Almutairi, S.; Al-Dhabbah, A.D.; Aldabas, S.Y.; Bhat, R.; Alqoufail, M.M.; Abdel-Maksoud, M.A.; Almanaa, T.N.; Farrag, M.A.; Alturaiki, W. Durability of SARS-CoV-2 Specific IgG Antibody Responses Following Two Doses of Match and Mixed COVID-19 Vaccines Regimens in Saudi Population. Infect. Drug Resist. 2022, 15, 3791–3800. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; A Triccas, J.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [PubMed]
- WHO. Interim Statement on COVID-19 Vaccines in the Context of the Circulation of the Omicron SARS-CoV-2 Variant from the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC); WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Collier, A.R.Y.; Miller, J.; Hachmann, N.P.; McMahan, K.; Liu, J.; Bondzie, E.A.; Gallup, L.; Rowe, M.; Schonberg, E.; Thai, S.; et al. Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters. N. Engl. J. Med. 2023, 388, 565–567. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against COVID-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef] [PubMed]



| Female (n = 110, 46.6%) | Male (n = 126, 53.4%) | Total (n = 236, 100%) | |
|---|---|---|---|
| Age group | |||
| Under 18 | 9 (3.8%) | 6 (3%) | 15 (6.3%) |
| 18–30 | 35 (14.8%) | 35 (14.8%) | 70(29.7%) |
| 31–45 | 49 (20.8%) | 58 (24.6%) | 107 (45.3%) |
| 46–60 | 15 (6.4%) | 18 (7.6%) | 33 (14%) |
| 61 and above | 0 (0%) | 5 (2.1%) | 5 (2.1%) |
| Unknown | 2 (0.8%) | 4 (1.7%) | 6 (2.5%) |
| Does the participant have a chronic illness? | |||
| Yes | 21 (8.9%) | 24 (10.2%) | 45 (19.1%) |
| No | 89 (37.7%) | 102 (43.2%) | 191 (80.9%) |
| Type of chronic illness | |||
| Dementia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Allergy | 2 (0.8%) | 1 (0.4%) | 3 (1.3%) |
| Blood Disorders | 2 (0.8%) | 0 (0%) | 2 (0.8%) |
| Diabetes | 2 (0.8%) | 6 (2.5%) | 8 (3.4%) |
| HTN | 3 (1.3%) | 7 (3%) | 10 (4.2%) |
| HIT | 1 (0.4%) | 0 (0%) | 1 (0.4%) |
| Cholesterol | 0 (0%) | 4 (1.7%) | 4 (1.7%) |
| Stroke | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Cancer | 3 (1.3%) | 1 (0.4%) | 4 (1.7%) |
| Renal disease | 0 (0%) | 3 (1.3%) | 3 (1.3%) |
| Heart problems | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Rhesus disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Respiratory failure | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Asthma | 2 (0.8%) | 2 (0.8%) | 4 (1.7%) |
| Other | 11 (4.7%) | 7 (3%) | 18 (7.6%) |
| Type of Vaccine in the First Dose | |||
| Pfizer | 97 (41.1%) | 101 (42.8%) | 198 (83.9%) |
| AstraZeneca | 8 (3.4%) | 9 (3.8%) | 17 (7.2%) |
| Moderna | 5 (2.1%) | 16 (6.8%) | 21 (8.9%) |
| Type of Vaccine in the Second Dose | |||
| Pfizer | 48 (20.3%) | 55 (23.3%) | 103 (43.6%) |
| AstraZeneca | 2 (0.8%) | 4 (1.7%) | 6 (2.5%) |
| Moderna | 17 (7.2%) | 30 (12.7%) | 47 (19.9%) |
| Unknown | 43 (18.2%) | 37 (15.7%) | 80 (33.9%) |
| Heterologous vs. homologous schedules | |||
| Heterologous | 2 (0.8%) | 6 (2.5%) | 8 (3.4%) |
| Homologous | 10 (4.2%) | 14 (5.9%) | 24 (10.2%) |
| Unknown | 98 (41.5%) | 106 (44.9%) | 204 (86.4%) |
| Baseline (i.e., before 1st dose) | |||
| Positive | 80 (33.9%) | 98 (41.5%) | 178 (75.4%) |
| Negative | 30 (12.7%) | 28 (11.9%) | 58 (24.6%) |
| Post-dose 1 (i.e., after 20 days from 1st dose) | |||
| Positive | 93 (39.4%) | 102 (43.2%) | 195 (82.6%) |
| Negative | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unknown | 17 (7.2%) | 24 (10.2%) | 41 (17.4%) |
| Post-dose 2 (i.e., after 20 days from 2nd dose) | |||
| Positive | 30 (12.7%) | 47 (19.9%) | 77 (32.6%) |
| Negative | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unknown | 80 (33.9%) | 79 (33.5%) | 159 (67.4%) |
| Paired Sample | Unpaired Sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Dose 1 | Post-Dose 2 | Baseline | Post-Dose 1 | Post-Dose 2 | ||||||||
| Variable | Values | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Collection Point | Baseline | 350 | 567 | - | - | - | - | 435 | 757 | - | - | - | - |
| Post-dose 1 | - | - | 16,350 | 9827 | - | - | - | - | 17,019 | 12,009 | - | - | |
| Post-dose 2 | - | - | - | - | 18,605 | 10,680 | - | - | - | - | 18,140 | 9617 | |
| Gender | Male | 276 | 388 | 18,001 | 8800 | 19,994 | 11,490 | 429 | 838 | 18,206 | 11,363 | 19,163 | 10,099 |
| Female | 480 | 796 | 13,429 | 11,190 | 16,146 | 8967 | 443 | 657 | 15,717 | 12,613 | 16,539 | 8732 | |
| Age Group | Under 18 | 49 | 55 | 23,073 | 18,296 | 24,966 | 5673 | 382 | 713 | 13,707 | 12,482 | 24,141 | 11,000 |
| 18–30 | 94 | 120 | 11,734 | 12,366 | 15,467 | 12,105 | 459 | 1039 | 16,848 | 13,302 | 14,534 | 10,930 | |
| 31–45 | 394 | 571 | 16,393 | 8625 | 20,167 | 11,088 | 418 | 535 | 16,037 | 11,027 | 18,595 | 9324 | |
| 46–60 | 417 | 724 | 16,479 | 9950 | 16,162 | 10,764 | 548 | 813 | 21,664 | 11,314 | 17,292 | 9088 | |
| 61 and above | - | - | - | - | - | - | 137 | 194 | 19,908 | 28,018 | 26,504 | 3132 | |
| Unknown | 745 | 0 | 23,837 | 0 | 21,882 | 0 | 242 | 265 | 21,898 | 6021 | 21,882 | 0 | |
| Chronic Illness | No | 302 | 460 | 15,968 | 10,600 | 18,468 | 11,438 | 379 | 546 | 16,757 | 11,994 | 18,176 | 9699 |
| Yes | 587 | 971 | 18,259 | 4366 | 19,289 | 6241 | 674 | 1305 | 18,177 | 12,178 | 17,994 | 9596 | |
| Type of vaccine (post-dose 1) | Pfizer | 386 | 601 | 17,788 | 9761 | - | - | 460 | 813 | 16,885 | 11,850 | - | - |
| AstraZeneca | 204 | 186 | 8916 | 3761 | - | - | 328 | 311 | 14,589 | 14,079 | - | - | |
| Moderna | 7 | 10 | 5217 | 3986 | - | - | 286 | 341 | 20,126 | 11,864 | - | - | |
| Type of vaccine (post-dose 2) | Pfizer | - | - | 17,077 | 10,527 | 16,780 | 10,493 | - | - | 16,901 | 11,878 | 17,381 | 9942 |
| AstraZeneca | - | - | 12,139 | 2764 | 7365 | 4742 | - | - | 10,950 | 3279 | 10,088 | 5554 | |
| Moderna | - | - | 13,471 | 9294 | 26,657 | 9715 | 19,143 | 12,626 | 25,426 | 8475 | |||
| Unknown | 515 | 740 | 21,450 | 10,150 | 20,506 | 5835 | - | - | 15,967 | 12,078 | 17,059 | 8729 | |
| Heterologous vs. homologous | Heterologous | - | - | 14,630 | 8575 | 21,516 | 11,471 | - | - | 14,630 | 8575 | 21,516 | 11,471 |
| Homologous | - | - | 16,073 | 10,272 | 17,317 | 11,125 | - | - | 16,073 | 10,272 | 17,317 | 11,125 | |
| Unknown | - | - | 21,450 | 10,150 | 20,506 | 5835 | - | - | 17,276 | 12,412 | 17,979 | 8464 | |
| F | df | Error df | p-Value | |
|---|---|---|---|---|
| Collection Point | 75 | 2 | 34 | <0.001 ** |
| Gender | 2.10 | 1 | 34 | 0.157 |
| Gender × Collection Point | 1.26 | 2 | 33 | 0.297 |
| Age Group | 0.88 | 3 | 31 | 0.463 |
| Age Group × Collection Point | 0.54 | 6 | 60 | 0.774 |
| Chronic Illness | 0.20 | 1 | 34 | 0.654 |
| Chronic Illness × Collection Point | 0.10 | 2 | 33 | 0.905 |
| Type of vaccine (post-dose 1) | 2.8 | 2 | 33 | 0.074 |
| Type of vaccine (post-dose 1) × Collection Point | 2.60 | 2 | 33 | 0.090 |
| Type of vaccine (post-dose 2) | 1.62 | 2 | 29 | 0.215 |
| Type of vaccine (post-dose 2) × Collection Point | 5.67 | 2 | 29 | 0.008 ** |
| Heterologous vs. homologous | 0.15 | 1 | 30 | 0.703 |
| Heterologous vs. homologous × Collection Point | 1.36 | 1 | 30 | 0.252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlEissa, M.M.; Alsaieedi, A.A.; Alduaiji, R.; Almsned, F.; AlDossary, Y.; Saleh, N.; AlQurashi, R.A.; Hawsa, E.A.; Ben Shaded, M.b.; Alshehri, A.M.; et al. Serological Response to COVID-19 Vaccination in Saudi Arabia: A Comparative Study of IgG and Neutralising Antibodies Across Vaccine Platforms. Vaccines 2025, 13, 1042. https://doi.org/10.3390/vaccines13101042
AlEissa MM, Alsaieedi AA, Alduaiji R, Almsned F, AlDossary Y, Saleh N, AlQurashi RA, Hawsa EA, Ben Shaded Mb, Alshehri AM, et al. Serological Response to COVID-19 Vaccination in Saudi Arabia: A Comparative Study of IgG and Neutralising Antibodies Across Vaccine Platforms. Vaccines. 2025; 13(10):1042. https://doi.org/10.3390/vaccines13101042
Chicago/Turabian StyleAlEissa, Mariam M., Ahdab A. Alsaieedi, Reema Alduaiji, Fahad Almsned, Yousif AlDossary, Nada Saleh, Raghad A. AlQurashi, Esraa A. Hawsa, Muath b Ben Shaded, Amer M. Alshehri, and et al. 2025. "Serological Response to COVID-19 Vaccination in Saudi Arabia: A Comparative Study of IgG and Neutralising Antibodies Across Vaccine Platforms" Vaccines 13, no. 10: 1042. https://doi.org/10.3390/vaccines13101042
APA StyleAlEissa, M. M., Alsaieedi, A. A., Alduaiji, R., Almsned, F., AlDossary, Y., Saleh, N., AlQurashi, R. A., Hawsa, E. A., Ben Shaded, M. b., Alshehri, A. M., Khojah, O. T., Abu Sarhan, E. Y., Alonazi, H. H., Nouh, W. A., AlAnazi, K. H., Almudrra, S. S., AlAbdulkareem, K. I., AlJurayyan, A., & Asiri, A. M. (2025). Serological Response to COVID-19 Vaccination in Saudi Arabia: A Comparative Study of IgG and Neutralising Antibodies Across Vaccine Platforms. Vaccines, 13(10), 1042. https://doi.org/10.3390/vaccines13101042






