Production of Clinical-Grade SARS-CoV-2 Spike Ferritin Nanoparticle Protein Immunogen by Transient Transfection
Abstract
1. Introduction
2. Materials and Methods
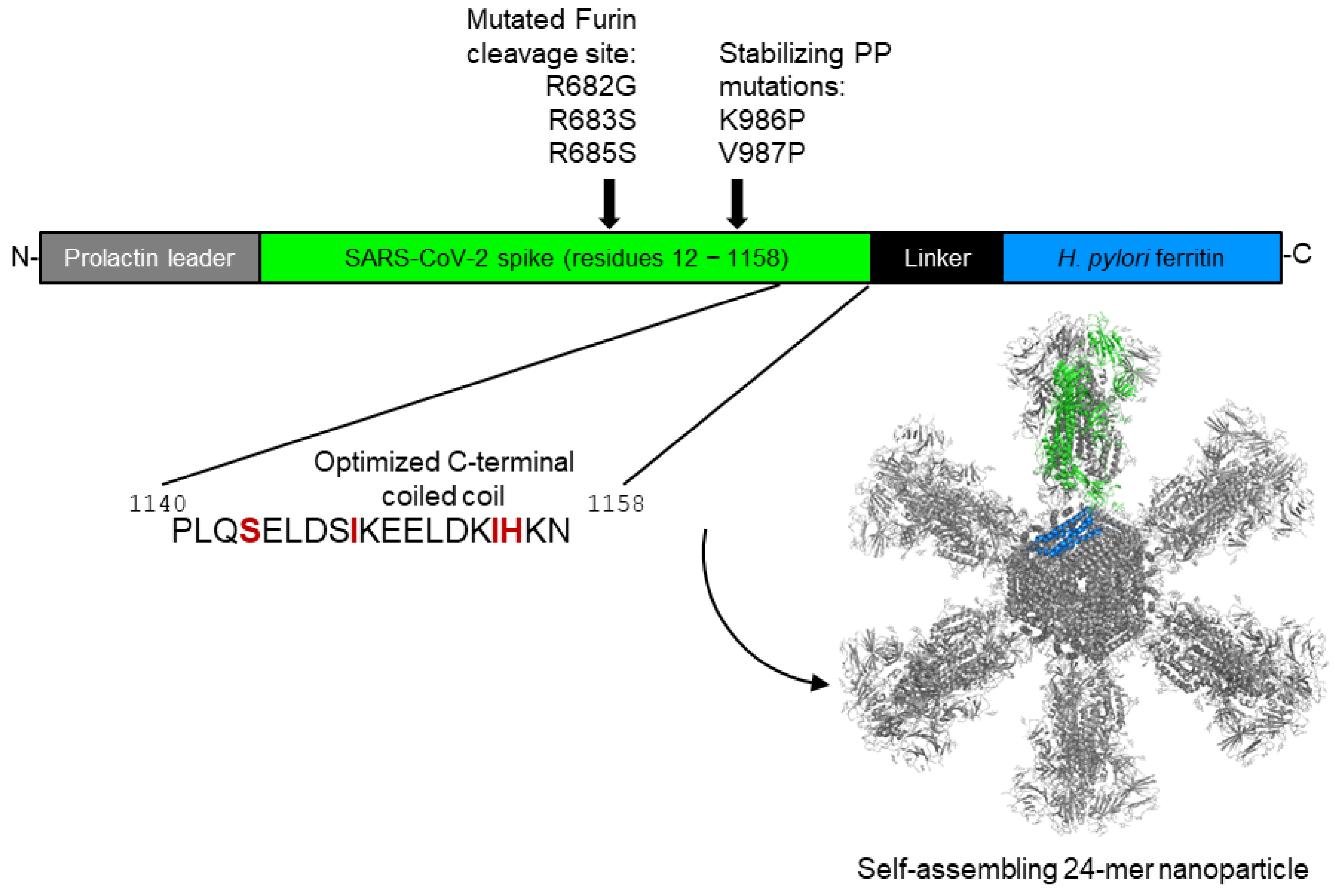
2.1. SpFN Construct Design, Gene Synthesis, and Cloning
2.2. SpFN Plasmid Preparation for CGMP Transfection
2.3. SpFN CGMP Drug Substance and Drug Product Production
2.3.1. Cell Growth, Transfection, and Harvest
2.3.2. Bulk Drug Substance Production: Concentration, Diafiltration, Column Chromatography, and Filtration
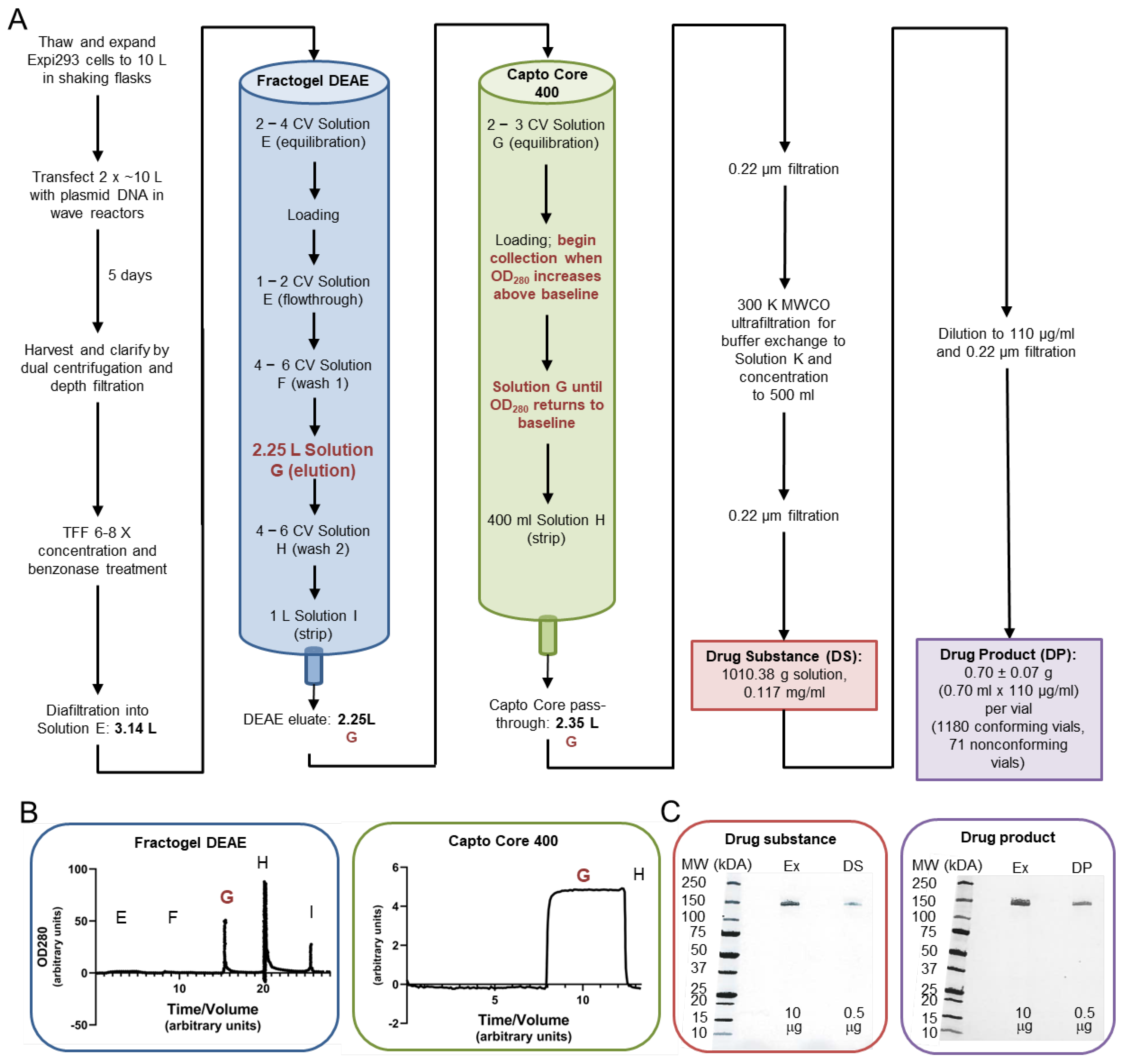
2.3.3. Formulation and Vialing of SpFN Drug Product
2.3.4. Production of Development Grade and Reference Standard Material
2.4. SpFN Drug Product Characterization
2.5. Pre-Clinical Product Testing
2.5.1. Immunogen–Adjuvant Preparation
2.5.2. Immunization of Mice with SpFN Adjuvanted with ALFQ
2.5.3. SARS-CoV-2 and SARS-CoV-1 Pseudovirus Neutralization Analysis
3. Results
3.1. SpFN Bulk and Vialed Product Concentration and Identity Determinations
3.2. SpFN Particle Formation and SpFN/ALFQ Liposome Formation Assessment by Negative-Stain EM
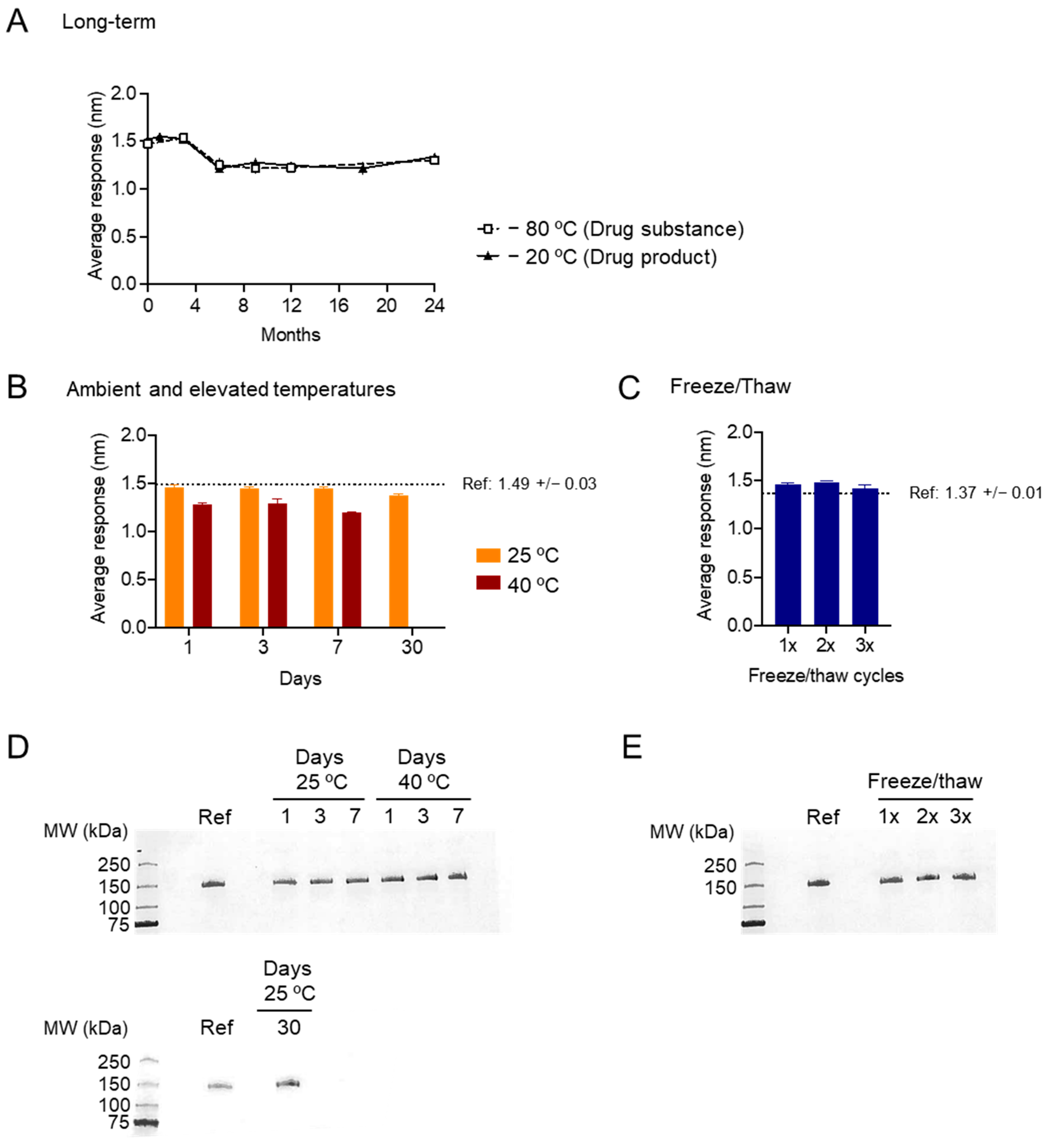
3.3. DS and DP Stability Analyses by BLI and SDS-PAGE
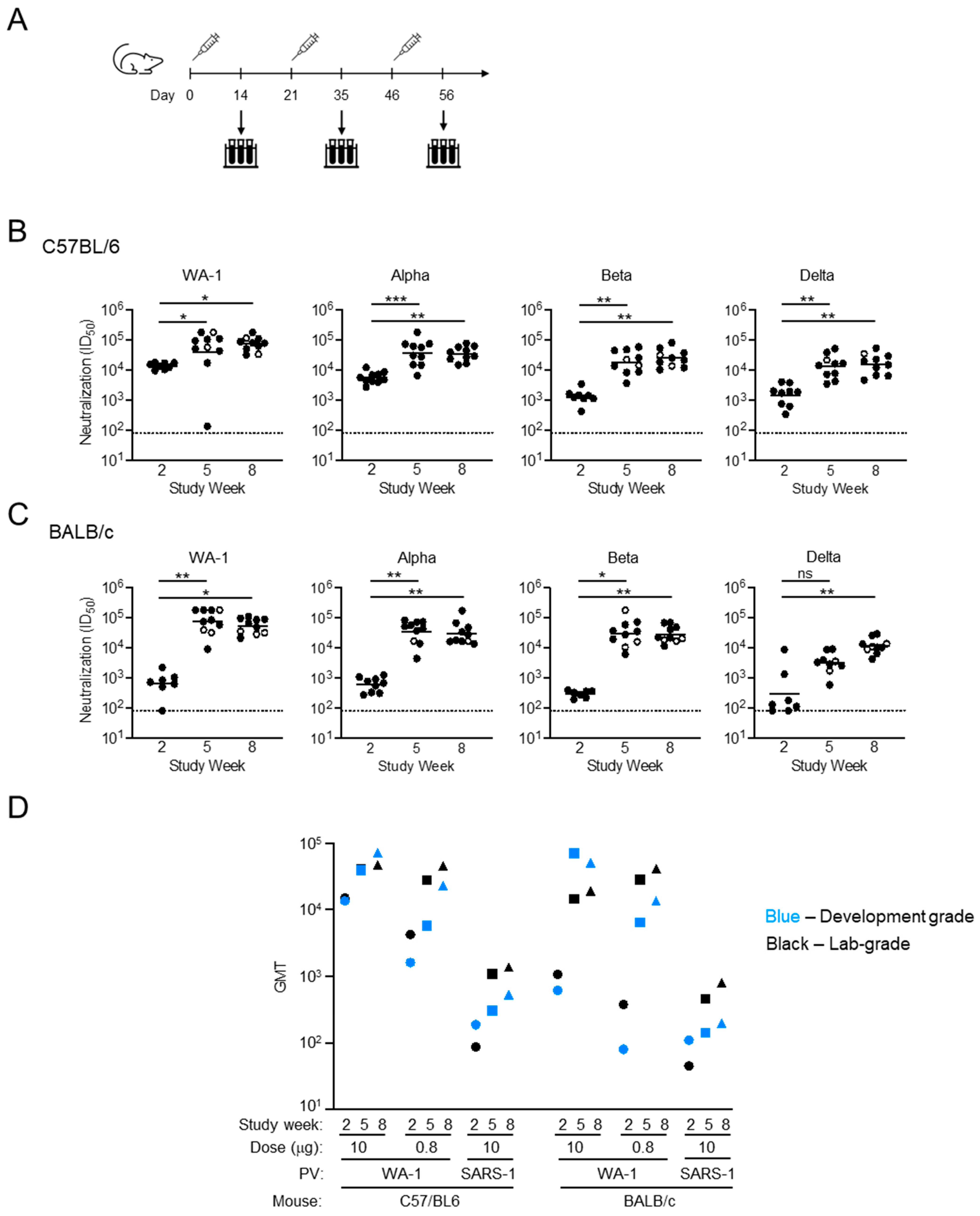
3.4. In Vivo Assessment of Development-Grade SpFN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | acquired immunodeficiency syndrome |
| ALFQ | Army Liposome Formulation containing Quillaja saponaria saponin fraction 21 |
| ANOVA | Analysis of Variance |
| CFU | Colony-Forming Units |
| CGMP | Current Good Manufacturing Practice |
| COVID-19 | Coronavirus Disease 2019 |
| CV | column volume |
| DEAE | diethylaminoethyl |
| DLS | Dynamic Light Scattering |
| DNA | deoxyribonucleic acid |
| DP | drug product |
| DS | drug substance |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EQAPOL | External Quality Assurance Program Oversite Laboratory |
| Expi | expression improvement |
| FDA | Food and Drug Administration |
| GMT | geometric mean titer |
| HEK | human embryonic kidney |
| HIC | hydrophobic interaction chromatography |
| IMAC | immobilized metal affinity chromatography |
| mRNA | messenger ribonucleic acid |
| MWCO | molecular weight cut-off |
| NIAID | National Institute of Allergy and Infectious Disease |
| NIH | National Institutes of Health |
| QC | quality control |
| qPCR | quantitative polymerase chain reaction |
| qSEC | Quantitative Size Exclusion Chromatography |
| RBD | receptor-binding domain |
| S-2P | spike with two prolines at amino acid positions 986 and987 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2` |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| SNACs | SARS-CoV-2 Neutralizing Assay Concordance Survey |
| SpFN | Spike Ferritin Nanoparticle |
| US | United States |
| USP | United States Pharmacopeia |
| VNAR | single-chain variable domain of new antigen receptor |
| VOC | variants of concern |
References
- Graepel, K.W.; Kochhar, S.; Clayton, E.W.; Edwards, K.E. Balancing Expediency and Scientific Rigor in Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Development. J. Infect. Dis. 2020, 222, 180–182. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Davis, J.P. Experience with hepatitis A and B vaccines. Am. J. Med. 2005, 118 (Suppl. S10A), 7S–15S. [Google Scholar] [CrossRef]
- Mast, E.E.; Margolis, H.S.; Fiore, A.E.; Brink, E.W.; Goldstein, S.T.; Wang, S.A.; Moyer, L.A.; Bell, B.P.; Alter, M.J.; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR Recomm. Rep. 2005, 54, 1–31. [Google Scholar]
- Sanchez-Martinez, A.; Moore, T.; Freitas, T.S.; Benzaken, T.R.; O’Hagan, S.; Millar, E.; Groves, H.E.; Drysdale, S.B.; Broadbent, L. Recent advances in the prevention and treatment of respiratory syncytial virus disease. J. Gen. Virol. 2025, 106, 002095. [Google Scholar] [CrossRef]
- Rappuoli, R.; Alter, G.; Pulendran, B. Transforming vaccinology. Cell 2024, 187, 5171–5194. [Google Scholar] [CrossRef] [PubMed]
- Sankhala, R.S.; Lal, K.G.; Jensen, J.L.; Dussupt, V.; Mendez-Rivera, L.; Bai, H.; Wieczorek, L.; Mayer, S.V.; Zemil, M.; Wagner, D.A.; et al. Diverse array of neutralizing antibodies elicited upon Spike Ferritin Nanoparticle vaccination in rhesus macaques. Nat. Commun. 2024, 15, 200. [Google Scholar] [CrossRef]
- Walls, A.C.; Miranda, M.C.; Schafer, A.; Pham, M.N.; Greaney, A.; Arunachalam, P.S.; Navarro, M.J.; Tortorici, M.A.; Rogers, K.; O’Connor, M.A.; et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. Cell 2021, 184, 5432–5447.e16. [Google Scholar] [CrossRef]
- Janes, H.; Fisher, L.H.; Kee, J.J.; Parameswaran, L.; Goepfert, P.A.; Falsey, A.R.; Ludwig, J.; Magaret, C.A.; Gilbert, P.B.; Kublin, J.G.; et al. Association Between SARS-CoV-2 Viral Load and COVID-19 Vaccination in 4 Phase 3 Trials. J. Infect. Dis. 2024, 230, 1384–1389. [Google Scholar] [CrossRef]
- Marchese, A.M.; Rousculp, M.; Macbeth, J.; Beyhaghi, H.; Seet, B.T.; Toback, S. The Novavax Heterologous Coronavirus Disease 2019 Booster Demonstrates Lower Reactogenicity Than Messenger RNA: A Targeted Review. J. Infect. Dis. 2024, 230, e496–e502. [Google Scholar] [CrossRef]
- Medicines & Healthcare Products Regulatory Agency. Public Assessment Report for Nuvaxovid Dispersion for Injection; Medicines & Healthcare Products Regulatory Agency: London, UK, 2022. [Google Scholar]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Galvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.A.; Galloway, J.; et al. Safety and Efficacy of the NVX-CoV2373 Coronavirus Disease 2019 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin. Infect. Dis. 2023, 76, 398–407. [Google Scholar] [CrossRef]
- Graham, B.S.; Gilman, M.S.A.; McLellan, J.S. Structure-Based Vaccine Antigen Design. Annu. Rev. Med. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Karlsson Hedestam, G.B.; Guenaga, J.; Corcoran, M.; Wyatt, R.T. Evolution of B cell analysis and Env trimer redesign. Immunol. Rev. 2017, 275, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Krarup, A.; Truan, D.; Furmanova-Hollenstein, P.; Bogaert, L.; Bouchier, P.; Bisschop, I.J.M.; Widjojoatmodjo, M.N.; Zahn, R.; Schuitemaker, H.; McLellan, J.S.; et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 2015, 6, 8143. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 2020, 369, 1501–1505. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Andrews, S.F.; Cominsky, L.Y.; Shimberg, G.D.; Gillespie, R.A.; Gorman, J.; Raab, J.E.; Brand, J.; Creanga, A.; Gajjala, S.R.; Narpala, S.; et al. An influenza H1 hemagglutinin stem-only immunogen elicits a broadly cross-reactive B cell response in humans. Sci. Transl. Med. 2023, 15, eade4976. [Google Scholar] [CrossRef]
- Houser, K.V.; Chen, G.L.; Carter, C.; Crank, M.C.; Nguyen, T.A.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Gordon, I.J.; et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: A phase 1 trial. Nat. Med. 2022, 28, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Widge, A.T.; Hofstetter, A.R.; Houser, K.V.; Awan, S.F.; Chen, G.L.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Holman, L.A.; et al. An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in healthy adults. Sci. Transl. Med. 2023, 15, eade4790. [Google Scholar] [CrossRef]
- Joyce, M.G.; Chen, W.H.; Sankhala, R.S.; Hajduczki, A.; Thomas, P.V.; Choe, M.; Martinez, E.J.; Chang, W.C.; Peterson, C.E.; Morrison, E.B.; et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. Cell Rep. 2021, 37, 110143. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Thomas, P.V.; McMahan, K.; Jacob-Dolan, C.; Liu, J.; He, X.; Hope, D.; Martinez, E.J.; Chen, W.H.; Sciacca, M.; et al. Protection against SARS-CoV-2 Omicron BA.1 variant challenge in macaques by prime-boost vaccination with Ad26.COV2.S and SpFN. Sci. Adv. 2022, 8, eade4433. [Google Scholar] [CrossRef]
- Yu, J.; Thomas, P.V.; Sciacca, M.; Wu, C.; Liu, J.; He, X.; Miller, J.; Hachmann, N.P.; Surve, N.; McMahan, K.; et al. Ad26.COV2.S and SARS-CoV-2 spike protein ferritin nanoparticle vaccine protect against SARS-CoV-2 Omicron BA.5 challenge in macaques. Cell Rep. Med. 2023, 4, 101018. [Google Scholar] [CrossRef]
- Ober Shepherd, B.L.; Scott, P.T.; Hutter, J.N.; Lee, C.; McCauley, M.D.; Guzman, I.; Bryant, C.; McGuire, S.; Kennedy, J.; Chen, W.H.; et al. SARS-CoV-2 recombinant spike ferritin nanoparticle vaccine adjuvanted with Army Liposome Formulation containing monophosphoryl lipid A and QS-21: A phase 1, randomised, double-blind, placebo-controlled, first-in-human clinical trial. Lancet Microbe 2024, 5, e581–e593. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R.; Peachman, K.K.; Matyas, G.R.; Rao, M.; Beck, Z. Army Liposome Formulation (ALF) family of vaccine adjuvants. Expert Rev. Vaccines 2020, 19, 279–292. [Google Scholar] [CrossRef]
- Chen, W.H.; Hajduczki, A.; Martinez, E.J.; Bai, H.; Matz, H.; Hill, T.M.; Lewitus, E.; Chang, W.C.; Dawit, L.; Peterson, C.E.; et al. Shark nanobodies with potent SARS-CoV-2 neutralizing activity and broad sarbecovirus reactivity. Nat. Commun. 2023, 14, 580. [Google Scholar] [CrossRef]
- Piehler, B.; Nelson, E.K.; Eckels, J.; Ramsay, S.; Lum, K.; Wood, B.; Greene, K.M.; Gao, H.; Seaman, M.S.; Montefiori, D.C.; et al. LabKey Server NAb: A tool for analyzing, visualizing and sharing results from neutralizing antibody assays. BMC Immunol. 2011, 12, 33. [Google Scholar] [CrossRef]
- Johnston, S.C.; Ricks, K.M.; Lakhal-Naouar, I.; Jay, A.; Subra, C.; Raymond, J.L.; King, H.A.D.; Rossi, F.; Clements, T.L.; Fetterer, D.; et al. A SARS-CoV-2 Spike Ferritin Nanoparticle Vaccine Is Protective and Promotes a Strong Immunological Response in the Cynomolgus Macaque Coronavirus Disease 2019 (COVID-19) Model. Vaccines 2022, 10, 717. [Google Scholar] [CrossRef]
- Hutter, J.N.; Robben, P.M.; Lee, C.; Hamer, M.; Moon, J.E.; Merino, K.; Zhu, L.; Galli, H.; Quinn, X.; Brown, D.R.; et al. First-in-human assessment of safety and immunogenicity of low and high doses of Plasmodium falciparum malaria protein 013 (FMP013) administered intramuscularly with ALFQ adjuvant in healthy malaria-naive adults. Vaccine 2022, 40, 5781–5790. [Google Scholar] [CrossRef]
- Dey, A.K.; Cupo, A.; Ozorowski, G.; Sharma, V.K.; Behrens, A.J.; Go, E.P.; Ketas, T.J.; Yasmeen, A.; Klasse, P.J.; Sayeed, E.; et al. cGMP production and analysis of BG505 SOSIP.664, an extensively glycosylated, trimeric HIV-1 envelope glycoprotein vaccine candidate. Biotechnol. Bioeng. 2018, 115, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.G.; Tan, H.X.; Juno, J.A.; Esterbauer, R.; Ju, Y.; Jiang, W.; Wimmer, V.C.; Duckworth, B.C.; Groom, J.R.; Caruso, F.; et al. Self-assembling influenza nanoparticle vaccines drive extended germinal center activity and memory B cell maturation. JCI Insight 2020, 5, e136653. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, K.; Ozorowski, G.; Burger, J.A.; van Montfort, T.; Stunnenberg, M.; LaBranche, C.; Montefiori, D.C.; Moore, J.P.; Ward, A.B.; Sanders, R.W. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology 2015, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.J.; Bu, W.; Nguyen, L.A.; Batchelor, J.D.; Kim, J.; Pittaluga, S.; Fuller, J.R.; Nguyen, H.; Chou, T.H.; Cohen, J.I.; et al. A bivalent Epstein-Barr virus vaccine induces neutralizing antibodies that block infection and confer immunity in humanized mice. Sci. Transl. Med. 2022, 14, eabf3685. [Google Scholar] [CrossRef]
- Cibelli, N.; Arias, G.; Figur, M.; Khayat, S.S.; Leach, K.; Loukinov, I.; Shadrick, W.; Chuenchor, W.; Tsybovsky, Y.; Vaccine Production Program Analytical, D.; et al. Advances in purification of SARS-CoV-2 spike ectodomain protein using high-throughput screening and non-affinity methods. Sci. Rep. 2022, 12, 4458. [Google Scholar] [CrossRef]
- Gulla, K.; Cibelli, N.; Cooper, J.W.; Fuller, H.C.; Schneiderman, Z.; Witter, S.; Zhang, Y.; Changela, A.; Geng, H.; Hatcher, C.; et al. A non-affinity purification process for GMP production of prefusion-closed HIV-1 envelope trimers from clades A and C for clinical evaluation. Vaccine 2021, 39, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Vandersluis, M.; Stout, J.; Haupts, U.; Sanders, M.; Jacquemart, R. Affinity chromatography for vaccines manufacturing: Finally ready for prime time? Vaccine 2019, 37, 5491–5503. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Goepfert, P.A.; Fu, B.; Chabanon, A.L.; Bonaparte, M.I.; Davis, M.G.; Essink, B.J.; Frank, I.; Haney, O.; Janosczyk, H.; Keefer, M.C.; et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: Interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infect. Dis. 2021, 21, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Pavot, V.; Berry, C.; Kishko, M.; Anosova, N.G.; Huang, D.; Tibbitts, T.; Raillard, A.; Gautheron, S.; Gutzeit, C.; Koutsoukos, M.; et al. Protein-based SARS-CoV-2 spike vaccine booster increases cross-neutralization against SARS-CoV-2 variants of concern in non-human primates. Nat. Commun. 2022, 13, 1699. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Wei, J.; Kundu, R.T.; Adhikari, R.; Liu, Z.; Lee, J.; Versteeg, L.; Poveda, C.; Keegan, B.; Villar, M.J.; et al. Genetic modification to design a stable yeast-expressed recombinant SARS-CoV-2 receptor binding domain as a COVID-19 vaccine candidate. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129893. [Google Scholar] [CrossRef]
- Thuluva, S.; Paradkar, V.; Gunneri, S.R.; Yerroju, V.; Mogulla, R.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Medigeshi, G.; Singh, J.; et al. Evaluation of safety and immunogenicity of receptor-binding domain-based COVID-19 vaccine (Corbevax) to select the optimum formulation in open-label, multicentre, and randomised phase-1/2 and phase-2 clinical trials. eBioMedicine 2022, 83, 104217. [Google Scholar] [CrossRef]
- Thuluva, S.; Paradkar, V.; Gunneri, S.; Yerroju, V.; Mogulla, R.R.; Suneetha, P.V.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Adabala, S.; et al. Safety, tolerability and immunogenicity of Biological E’s CORBEVAX vaccine in children and adolescents: A prospective, randomised, double-blind, placebo controlled, phase-2/3 study. Vaccine 2022, 40, 7130–7140. [Google Scholar] [CrossRef]
- Pollet, J.; Strych, U.; Chen, W.H.; Versteeg, L.; Keegan, B.; Zhan, B.; Wei, J.; Liu, Z.; Lee, J.; Kundu, R.; et al. Receptor-binding domain recombinant protein on alum-CpG induces broad protection against SARS-CoV-2 variants of concern. Vaccine 2022, 40, 3655–3663. [Google Scholar] [CrossRef]
- Lee, J.; Liu, Z.; Chen, W.H.; Wei, J.; Kundu, R.; Adhikari, R.; Rivera, J.A.; Gillespie, P.M.; Strych, U.; Zhan, B.; et al. Process development and scale-up optimization of the SARS-CoV-2 receptor binding domain-based vaccine candidate, RBD219-N1C1. Appl. Microbiol. Biotechnol. 2021, 105, 4153–4165. [Google Scholar] [CrossRef]
- Martinez-Cano, D.; Ravichandran, R.; Le, H.; Wong, H.E.; Jagannathan, B.; Liu, E.J.; Bailey, W.; Yang, J.; Matthies, K.; Barkhordarian, H.; et al. Process development of a SARS-CoV-2 nanoparticle vaccine. Process Biochem. 2023, 129, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Fiala, B.; Schafer, A.; Wrenn, S.; Pham, M.N.; Murphy, M.; Tse, L.V.; Shehata, L.; O’Connor, M.A.; Chen, C.; et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382.e17. [Google Scholar] [CrossRef]
- Singh, P.; Matyas, G.R.; Anderson, A.; Beck, Z. Biophysical characterization of polydisperse liposomal adjuvant formulations. Biochem. Biophys. Res. Commun. 2020, 529, 362–365. [Google Scholar] [CrossRef]
- Tan, E.; Chin, C.S.H.; Lim, Z.F.S.; Ng, S.K. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front. Bioeng. Biotechnol. 2021, 9, 796991. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.J.; Kanekiyo, M.; Kong, W.P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Steger, K.; Brady, J.; Wang, W.; Duskin, M.; Donato, K.; Peshwa, M. CHO-S antibody titers >1 gram/liter using flow electroporation-mediated transient gene expression followed by rapid migration to high-yield stable cell lines. J. Biomol. Screen. 2015, 20, 545–551. [Google Scholar] [CrossRef]
- Chin, C.L.; Goh, J.B.; Srinivasan, H.; Liu, K.I.; Gowher, A.; Shanmugam, R.; Lim, H.L.; Choo, M.; Tang, W.Q.; Tan, A.H.; et al. A human expression system based on HEK293 for the stable production of recombinant erythropoietin. Sci. Rep. 2019, 9, 16768. [Google Scholar] [CrossRef] [PubMed]
- Pensiero, M.N. Accelerating the manufacturing of HIV Env protein vaccines for early phase clinical evaluation. NPJ Vaccines 2025, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Gustchina, E.; Guenaga, J.; Ayala, V.; Lee, W.H.; Ozorowski, G.; Whitney, S.; Wilson, R.; Baboo, S.; Diedrich, J.K.; et al. Accelerated cGMP production of near-native HIV-1 Env trimers following electroporation transfection and immunogenicity analysis. NPJ Vaccines 2025, 10, 198. [Google Scholar] [CrossRef]

| Buffer Name | Purpose | Composition |
|---|---|---|
| Solution A | 70% Ethanol for Sanitization | 70% ethanol |
| Solution B | 6 N Hydrochloric Acid (HCl) for pH Adjustment | 6 N HCl |
| Solution C | 1 N Sodium Hydroxide (NaOH) for pH Adjustment | 1 N NaOH |
| Solution D | Ultrafiltration System Cleaning Solution | 0.5 N NaOH |
| Solution E | 1st TFF Dialysis and Fractogel DEAE Equilibration Solution | 50 mM Tris, 50 mM NaCl, pH 8.0 |
| Solution F | Fractogel DEAE Wash Solution | 50 mM Tris, 120 mM NaCl, pH 8.0 |
| Solution G | Fractogel DEAE Elution Solution | 50 mM Tris, 200 mM NaCl, pH 8.0 |
| Solution H | Fractogel DEAE Column Solution | 50 mM Tris, 500 mM NaCl, pH 8.0 |
| Solution I | Fractogel DEAE Strip Solution | 50 mM Tris, 1 M NaCl, pH 8.0 |
| Solution J | Benzonase Treatment | 200 mM MgCl2 |
| Solution K | Final Formulation Buffer | 20 mM sodium phosphate, 100 mM NaCl, 5% Sucrose, 0.01% (w/v) Poloxamer 188, pH 7.2 |
| Solution L | Column Storage | 20% Ethanol |
| Test | Specification | DS Result | DP Result |
|---|---|---|---|
| Appearance, USP | Clear, colorless liquid with no turbidity and no visible particulates | Clear, colorless liquid with no turbidity and no visible particulates (PASS) | Clear, colorless liquid, with no turbidity and no visible particles (PASS) |
| pH, USP | 6.0–8.0 | pH @ 26 °C = 7.2 (PASS) | pH @ 26 °C = 7.3 (PASS) |
| Concentration by qSEC | >100 µg/mL | 117 µg/mL (PASS) | 104 µg/mL (PASS) |
| Purity by qSEC | ≥80% total area at a retention time of 3.8 min ± 10% | 92% area at a retention time of 3.8 min (PASS) | 91% area at a retention time of 3.8 min (PASS) |
| Endotoxin Content, USP | ≤10 EU/50 µg SpFN | 2.6 EU/50 µg SpFN (PASS) | 5.2 EU/50 µg SpFN (PASS) |
| Biolayer Interferometry Binding Assay | Report Result | % relative potency = 96.3% | % relative potency = 98.7% |
| SDS-PAGE | Comparable to Reference Standard QRS001 | Comparable to ref. std. QRS001 (PASS) | Comparable to ref. std. QRS001 (PASS) |
| Western Blot | Comparable to reference standard QRS001, positive for ShAb02 | Comparable to ref. std. QRS001, positive for ShAb02 (PASS) | Positive for ShAb02 (PASS) |
| Osmolarity | Report Result | 399 mOsm/kg | 396 mOsm/kg |
| Subvisible Particulate Matter by USP | Report Result | ≥10 µm = 963 ≥25 µm = 223 | Not tested |
| Product Particle Characterization by DLS | Report Result | Peak 1 = 98.8% at 35.26 d.nm Peak 2 = 1.2% at 3894 d.nm | Peak 1 = 96.8% at 35.44 d.nm Peak 2 = 3.2% at 1949 d.nm |
| Host Cell DNA by qPCR | Report Result | <4.27 × 104 fg/mL | Not required |
| Residual Benzoase by ELISA | Report Result | <0.195 ng/mL (below the limit of quantification) | Not required |
| Residual DNA for transforming Adenoviruses | Report Result | <2.11 × 103 copies/mL for both E1A and E1B | Not required |
| Residual pDNA by Kanamycin resistance marker | Report Result | None detected (below the limit of quantification) (PASS) | Not required |
| Bioburden, USP | <10 CFU/10 mL | TAMC = 0 CFU/10 mL TYMC = 0 CFU/10 mL (PASS) | Not required |
| Container Closure Integrity Test | No leaking containers | Not required | No leaking containers (PASS) |
| Sterility Qualification | No bacteriostasis or fungistasis | Not required | No bacteriostasis or fungistasis (PASS) |
| Sterility, USP | No growth | Not required | No growth (PASS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajduczki, A.; Chang, W.C.; De La Barrera, R.; Wood, J.F.; Chen, W.-H.; Martinez, E.J.; Jensen, J.L.; Sankhala, R.S.; Smith, C.; Anderson, A.; et al. Production of Clinical-Grade SARS-CoV-2 Spike Ferritin Nanoparticle Protein Immunogen by Transient Transfection. Vaccines 2025, 13, 1041. https://doi.org/10.3390/vaccines13101041
Hajduczki A, Chang WC, De La Barrera R, Wood JF, Chen W-H, Martinez EJ, Jensen JL, Sankhala RS, Smith C, Anderson A, et al. Production of Clinical-Grade SARS-CoV-2 Spike Ferritin Nanoparticle Protein Immunogen by Transient Transfection. Vaccines. 2025; 13(10):1041. https://doi.org/10.3390/vaccines13101041
Chicago/Turabian StyleHajduczki, Agnes, William C. Chang, Rafael De La Barrera, James F. Wood, Wei-Hung Chen, Elizabeth J. Martinez, Jaime L. Jensen, Rajeshwer S. Sankhala, Clayton Smith, Alexander Anderson, and et al. 2025. "Production of Clinical-Grade SARS-CoV-2 Spike Ferritin Nanoparticle Protein Immunogen by Transient Transfection" Vaccines 13, no. 10: 1041. https://doi.org/10.3390/vaccines13101041
APA StyleHajduczki, A., Chang, W. C., De La Barrera, R., Wood, J. F., Chen, W.-H., Martinez, E. J., Jensen, J. L., Sankhala, R. S., Smith, C., Anderson, A., Morrison, E. B., Peterson, C. E., Rees, P. A., Soman, S., Kuklis, C., Ahmed, A., King, J., Nasar, F., Corbitt, C., ... Zarling, S. (2025). Production of Clinical-Grade SARS-CoV-2 Spike Ferritin Nanoparticle Protein Immunogen by Transient Transfection. Vaccines, 13(10), 1041. https://doi.org/10.3390/vaccines13101041






