Real-World Effectiveness of Racotumomab as Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients
Abstract
1. Introduction
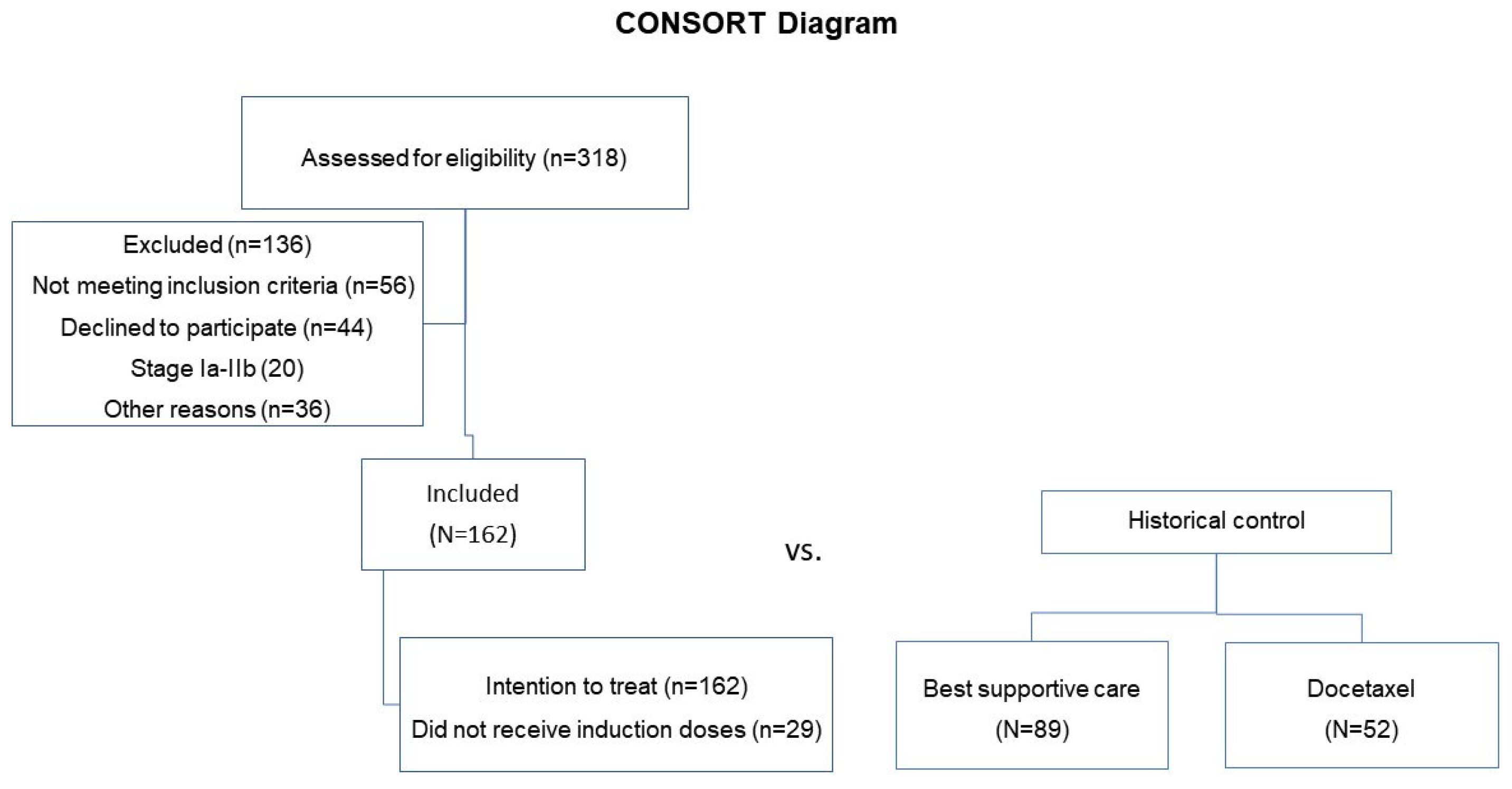
2. Materials and Methods
Statistical Analysis
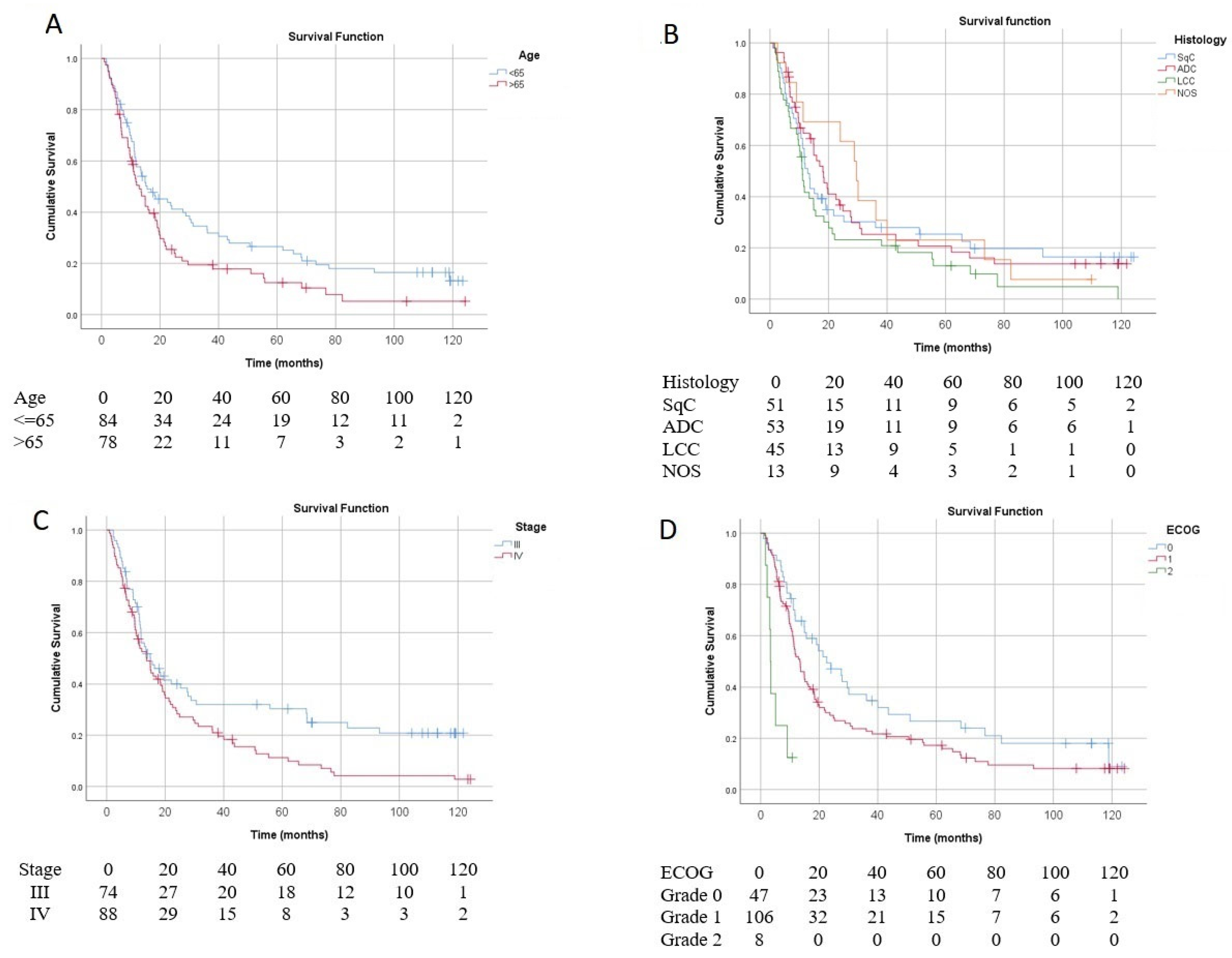
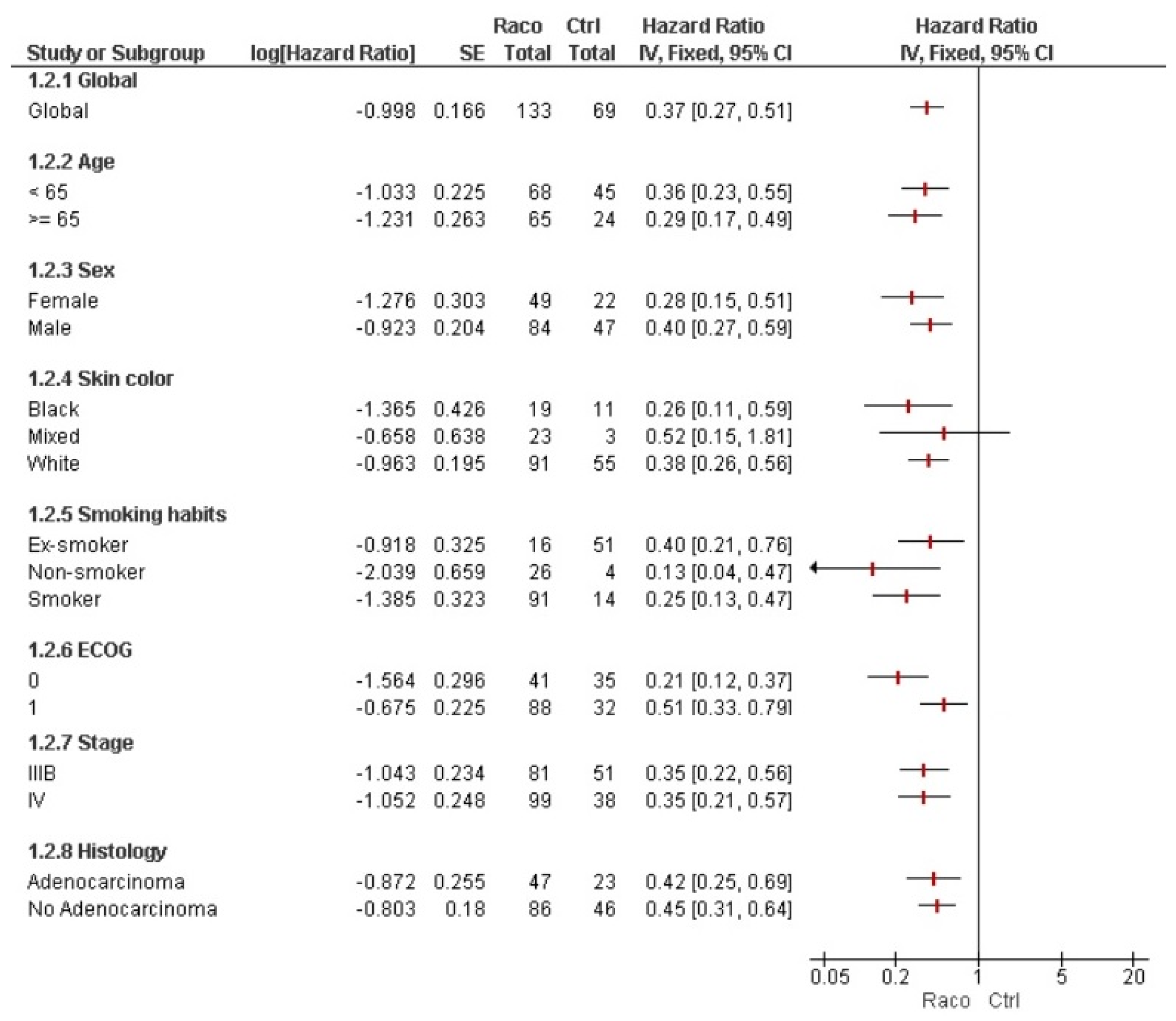
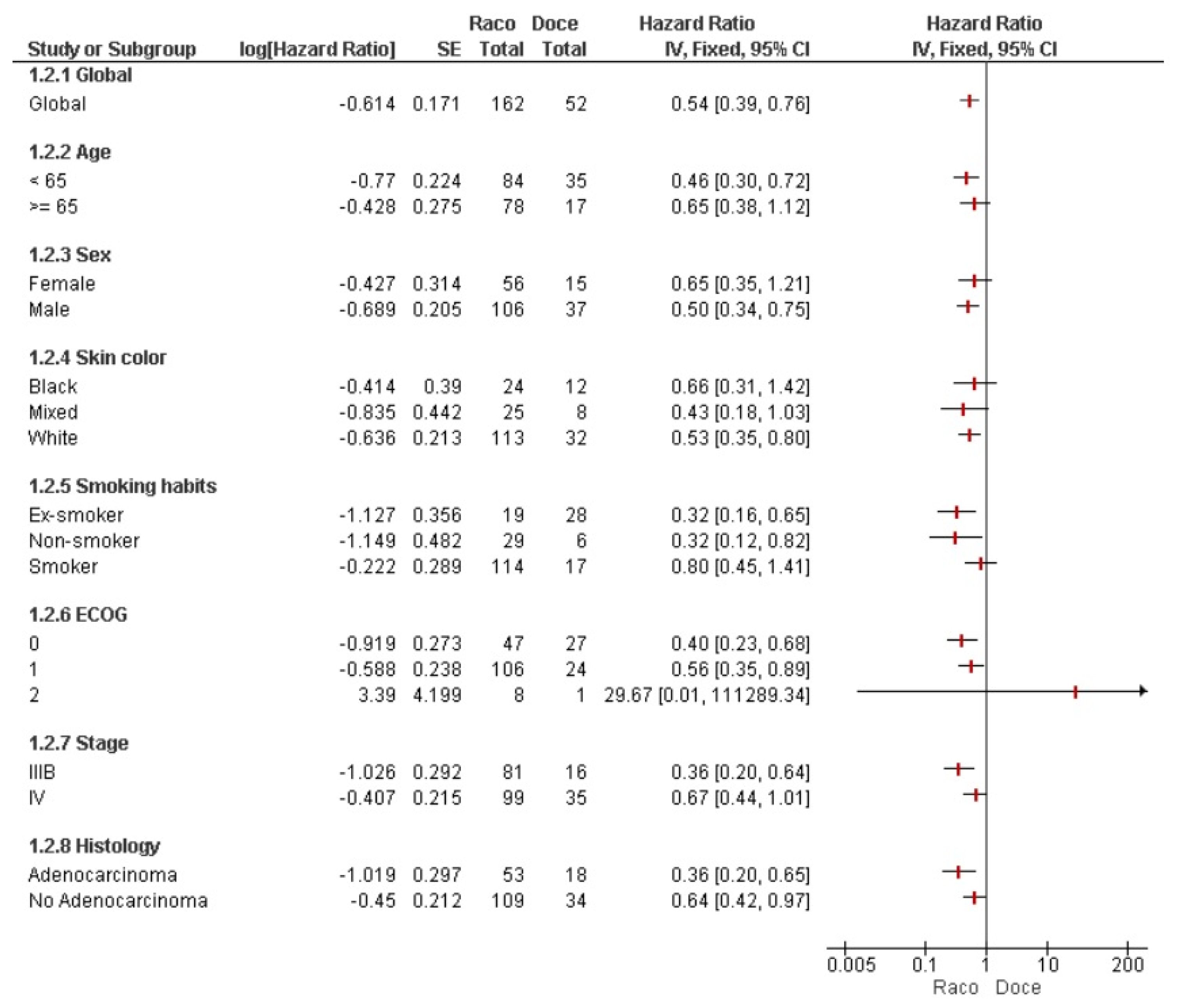
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MUC-1 | Mucin-1 |
| MAbs | monoclonal antibodies |
References
- Chen, P.; Liu, Y.; Wen, Y.; Zhou, C. Non-small cell lung cancer in China. Cancer Commun. 2022, 42, 937–970. [Google Scholar] [CrossRef]
- Flores, R.; Patel, P.; Alpert, N.; Pyenson, B.; Taioli, E. Association of stage shift and population mortality among patients with non–small cell lung cancer. JAMA Netw. Open 2021, 4, e2137508. [Google Scholar] [CrossRef]
- Wagle, N.S.; Nogueira, L.; Devasia, T.P.; Mariotto, A.B.; Yabroff, K.R.; Islami, F.; Jemal, A.; Alteri, R.; Ganz, P.A.; Siegel, R.L. Cancer treatment and survivorship statistics, 2025. CA Cancer J. Clin. 2025, 75, 308–340. [Google Scholar] [CrossRef]
- Guarga, L.; Ameijide, A.; Marcos-Gragera, R.; Carulla, M.; Delgadillo, J.; Borràs, J.M.; Galceran, J. Trends in lung cancer incidence by age, sex and histology from 2012 to 2025 in Catalonia (Spain). Sci. Rep. 2021, 11, 23274. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, X. Pembrolizumab provides long-term survival benefits in advanced non-small cell lung cancer: The 5-year outcomes of the KEYNOTE-024 trial. Thorac. Cancer 2021, 12, 3085–3087. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Liang, Q.; Jiang, X.; Ge, Y.; Jiang, Y.; Liu, L. Prognostic models for immunotherapy in non-small cell lung cancer: A comprehensive review. Heliyon 2024, 10, e29840. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, R.; Yin, R.; Wang, S.; Han, J.; Chen, R.; Fu, Z. Immunotherapy Improves the Survival of Stage 4 Non–Small Cell Lung Cancer Patients at the US Population Level: The Real-World Evidence. Clin. Respir. J. 2024, 18, e70000. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef]
- Rohilla, S.; Singh, M.; Alzarea, S.I.; Almalki, W.H.; Al-Abbasi, F.A.; Kazmi, I.; Afzal, O.; Altamimi, A.S.A.; Singh, S.K.; Chellappan, D.K.; et al. Recent Developments and Challenges in Molecular-Targeted Therapy of Non-Small-Cell Lung Cancer. J. Environ. Pathol. Toxicol. Oncol. 2023, 42, 27–50. [Google Scholar] [CrossRef]
- Mencoboni, M.; Ceppi, M.; Bruzzone, M.; Taveggia, P.; Cavo, A.; Scordamaglia, F.; Gualco, M.; Filiberti, R.A. Effectiveness and Safety of Immune Checkpoint Inhibitors for Patients with Advanced Non Small-Cell Lung Cancer in Real-World: Review and Meta-Analysis. Cancers 2021, 13, 1388. [Google Scholar] [CrossRef]
- Adotévi, O. Use of cancer vaccine after immunotherapy failure: A promising strategy for advanced NSCLC patients with secondary resistance to checkpoint inhibitors. Ann. Oncol. 2023, 34, 831–832. [Google Scholar] [CrossRef]
- Awad, M.M.; Govindan, R.; Balogh, K.N.; Spigel, D.R.; Garon, E.B.; Bushway, M.E.; Poran, A.; Sheen, J.H.; Kohler, V.; Esaulova, E. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell 2022, 40, 1010–1026.e11. [Google Scholar] [CrossRef]
- Evans, R.; Lee, K.; Wallace, P.K.; Reid, M.; Muhitch, J.; Dozier, A.; Mesa, C.; Luaces, P.L.; Santos-Morales, O.; Groman, A. Augmenting antibody response to EGF-depleting immunotherapy: Findings from a phase I trial of CIMAvax-EGF in combination with nivolumab in advanced stage NSCLC. Front. Oncol. 2022, 12, 958043. [Google Scholar] [CrossRef]
- Ramos, T.C.; Morales, O.S.; Dy, G.K.; Monzón, K.L.; Dávila, A.L. The position of EGF deprivation in the management of advanced non-small cell lung cancer. Front. Oncol. 2021, 11, 639745. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Jofré, M.; Rueda-Etxebarria, M.; Orillard, E.; Tejero, E.J.; Rueda, J.-R. Therapeutic vaccines for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2024, 3, CD013377. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Alfonso, M.; Lanne, B.; Karlsson, K.-A.; Carr, A.; Barroso, O.; Fernández, L.; Rengifo, E.; Lanio, M.; Alvarez, C. Generation of a murine monoclonal antibody specific for N-glycolylneuraminic acid-containing gangliosides that also recognizes sulfated glycolipids. Hybridoma 1995, 14, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Perez, A.; Hernandez, A.; Macias, A.; Alfonso, M.; Bombino, G.; Perez, R. Syngeneic anti-idiotypic monoclonal antibodies to an anti-NeuGc-containing ganglioside monoclonal antibody. Hybridoma 1998, 17, 527–534. [Google Scholar] [CrossRef]
- Vázquez, A.M.; Hernández, A.M.; Macías, A.; Montero, E.; Gómez, D.E.; Alonso, D.F.; Gabri, M.R.; Gómez, R.E. Racotumomab: An anti-idiotype vaccine related to N-glycolyl-containing gangliosides–preclinical and clinical data. Front. Oncol. 2012, 2, 150. [Google Scholar] [CrossRef]
- Jerne, N.K. Towards a network theory of the immune system. Ann. Immunol. 1974, 125, 373–389. [Google Scholar]
- Gajdosik, Z. Racotumomab-a novel anti-idiotype monoclonal antibody vaccine for the treatment of cancer. Drugs Today 2014, 50, 301–307. [Google Scholar] [CrossRef]
- Alfonso, S.; Valdés-Zayas, A.; Santiesteban, E.R.; Flores, Y.I.; Areces, F.; Hernández, M.; Viada, C.E.; Mendoza, I.C.; Guerra, P.P.; García, E. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non–small cell lung cancer patients. Clin. Cancer Res. 2014, 20, 3660–3671. [Google Scholar] [CrossRef]
- Hernández, A.M.; Vázquez, A.M. Racotumomab–alum vaccine for the treatment of non-small-cell lung cancer. Expert Rev. Vaccines 2015, 14, 9–20. [Google Scholar] [CrossRef]
- Hernandez, M.; Neninger, E.; Ortiz, R.; Camacho, K.; Amador, R.; Bello, L.; Flores, Y.; Acosta, S.; Pichs, G.; Cala, M. Switch maintenance therapy with racotumomab or nimotuzumab vs docetaxel for NSCLC patients. Ann. Oncol. 2016, 27, vi374. [Google Scholar] [CrossRef]
- Makady, A.; de Boer, A.; Hillege, H.; Klungel, O.; Goettsch, W. What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health 2017, 20, 858–865. [Google Scholar] [CrossRef]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Sok, M.; Zavrl, M.; Greif, B.; Srpčič, M. Objective assessment of WHO/ECOG performance status. Support. Care Cancer 2019, 27, 3793–3798. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Franklin, J.M.; Glynn, R.J.; Martin, D.; Schneeweiss, S. Evaluating the use of nonrandomized real-world data analyses for regulatory decision making. Clin. Pharmacol. Ther. 2019, 105, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Krengel, U.; Bousquet, P.A. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front. Immunol. 2014, 5, 325. [Google Scholar] [CrossRef] [PubMed]
- Birkle, S.; Zeng, G.; Gao, L.; Yu, R.; Aubry, J. Role of tumor-associated gangliosides in cancer progression. Biochimie 2003, 85, 455–463. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Mok, T.; Lopes, G.; Cho, B.; Kowalski, D.M.; Kasahara, K.; Wu, Y.-L.; de Castro, G., Jr.; Turna, H.; Cristescu, R.; Aurora-Garg, D. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: Pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann. Oncol. 2023, 34, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Parra, H.S.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Fidias, P.M.; Dakhil, S.R.; Lyss, A.P.; Loesch, D.M.; Waterhouse, D.M.; Bromund, J.L.; Chen, R.; Hristova-Kazmierski, M.; Treat, J.; Obasaju, C.K. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non–small-cell lung cancer. J. Clin. Oncol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Ciuleanu, T.; Brodowicz, T.; Zielinski, C.; Kim, J.H.; Krzakowski, M.; Laack, E.; Wu, Y.-L.; Bover, I.; Begbie, S.; Tzekova, V. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet 2009, 374, 1432–1440. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Ciuleanu, T.; Stelmakh, L.; Cicenas, S.; Szczésna, A.; Juhász, E.; Esteban, E.; Molinier, O.; Brugger, W.; Melezínek, I. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010, 11, 521–529. [Google Scholar] [CrossRef]
- Buccheri, G.; Ferrigno, D.; Tamburini, M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur. J. Cancer 1996, 32, 1135–1141. [Google Scholar] [CrossRef]
- O’Mahony, S.; Nathan, S.; Mohajer, R.; Bonomi, P.; Batus, M.; Fidler, M.J.; Wells, K.; Kern, N.; Sims, S.; Amin, D. Survival prediction in ambulatory patients with stage III/IV non-small cell lung cancer using the palliative performance scale, ECOG, and lung cancer symptom scale. Am. J. Hosp. Palliat. Care 2016, 33, 374–380. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, H.; Yue, L.; Zheng, W.; Pan, L.; Gao, F.; Liu, X. Immunosenescence and cancer: Opportunities and challenges. Medicine 2023, 102, e36045. [Google Scholar] [CrossRef]
- Pawelec, G. Immunosenescence and cancer. Biogerontology 2017, 18, 717–721. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-line immunotherapy for non–small-cell lung cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Meyer, M.-L.; Fitzgerald, B.G.; Paz-Ares, L.; Cappuzzo, F.; Jaenne, P.A.; Peters, S.; Hirsch, F.R. New promises and challenges in the treatment of advanced non-small-cell lung cancer. Lancet 2024, 404, 803–822. [Google Scholar] [CrossRef]
- Brito, A.B.C.; Camandaroba, M.P.G.; de Lima, V.C.C. Anti-PD1 versus anti-PD-L1 immunotherapy in first-line therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis. Thorac. Cancer 2021, 12, 1058–1066. [Google Scholar] [CrossRef]
- Sanchez, L.; Muchene, L.; Lorenzo-Luaces, P.; Viada, C.; Rodriguez, P.C.; Alfonso, S.; Crombet, T.; Neninger, E.; Shkedy, Z.; Lage, A. Differential effects of two therapeutic cancer vaccines on short-and long-term survival populations among patients with advanced lung cancer. Semin. Oncol. 2018, 45, 52–57. [Google Scholar] [CrossRef]
- Mazorra, Z.; Cáceres-Lavernia, H.H.; Nenínger-Vinageras, E.; Varona-Rodríguez, L.M.; Viada, C.E.; González, Z.; Rodríguez-Zhurbenko, N.; Thierry, A.-C.; Suarez-Formigo, G.M.; Ventura-Carmenate, Y. Treatment of Advanced NSCLC Patients with an Anti-Idiotypic NeuGcGM3-Based Vaccine: Immune Correlates in Long-Term Survivors. Biomedicines 2025, 13, 1122. [Google Scholar] [CrossRef]
| Demographic and Baseline Characteristics | N = 162 N (%) | |
|---|---|---|
| Age | Mean ± SD | 65 ± 10 |
| Median ± IQR | 65 ± 14 | |
| Minimum; Maximum | 32; 89 | |
| Sex | Female | 56 (34.6) |
| Male | 106 (65.4) | |
| Skin color | White | 113 (69.8) |
| Black | 24 (14.8) | |
| Mixed | 25 (15.4) | |
| Stage of the disease at diagnose | III (unresectable) | 74 (45.7) |
| IV | 88 (54.3) | |
| Histological type | Adenocarcinoma | 53 (32.7) |
| Large cell carcinoma | 45 (27.8) | |
| Squamous cell carcinoma | 51 (31.5) | |
| NOS | 13 (8) | |
| Clinical Status (ECOG) | 0 | 47 (29.2) |
| 1 | 106 (65.8) | |
| 2 | 8 (5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemán, S.A.; Lavernia, H.C.; Sosa, K.C.; Brooks, S.C.A.; Morales, O.S.; González, C.E.V.; Portales, M.C.; Concepción, M.T.; Pérez, L.M.; Benítez, L.C.; et al. Real-World Effectiveness of Racotumomab as Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Vaccines 2025, 13, 1035. https://doi.org/10.3390/vaccines13101035
Alemán SA, Lavernia HC, Sosa KC, Brooks SCA, Morales OS, González CEV, Portales MC, Concepción MT, Pérez LM, Benítez LC, et al. Real-World Effectiveness of Racotumomab as Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Vaccines. 2025; 13(10):1035. https://doi.org/10.3390/vaccines13101035
Chicago/Turabian StyleAlemán, Sailyn Alfonso, Haslen Cáceres Lavernia, Kirenia Camacho Sosa, Soraida C. Acosta Brooks, Orestes Santos Morales, Carmen E. Viada González, Meylán Cepeda Portales, Mayelín Troche Concepción, Loipa Medel Pérez, Leticia Cabrera Benítez, and et al. 2025. "Real-World Effectiveness of Racotumomab as Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients" Vaccines 13, no. 10: 1035. https://doi.org/10.3390/vaccines13101035
APA StyleAlemán, S. A., Lavernia, H. C., Sosa, K. C., Brooks, S. C. A., Morales, O. S., González, C. E. V., Portales, M. C., Concepción, M. T., Pérez, L. M., Benítez, L. C., Salmón, M. C. D., Iglesias, D. E., Suzarte, M. R., & Ramos, T. C. (2025). Real-World Effectiveness of Racotumomab as Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Vaccines, 13(10), 1035. https://doi.org/10.3390/vaccines13101035






