Safety Evaluation of Tetanus, Diphtheria, and Acellular Pertussis Vaccine (Tdap) During Pregnancy Among Vietnamese Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Setting and Duration
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.6. Sample Size
2.7. Statistical Analysis
2.8. Ethical Considerations
2.9. Data Collection Methods
3. Results
3.1. Demographic Characteristics of Pregnant Participants
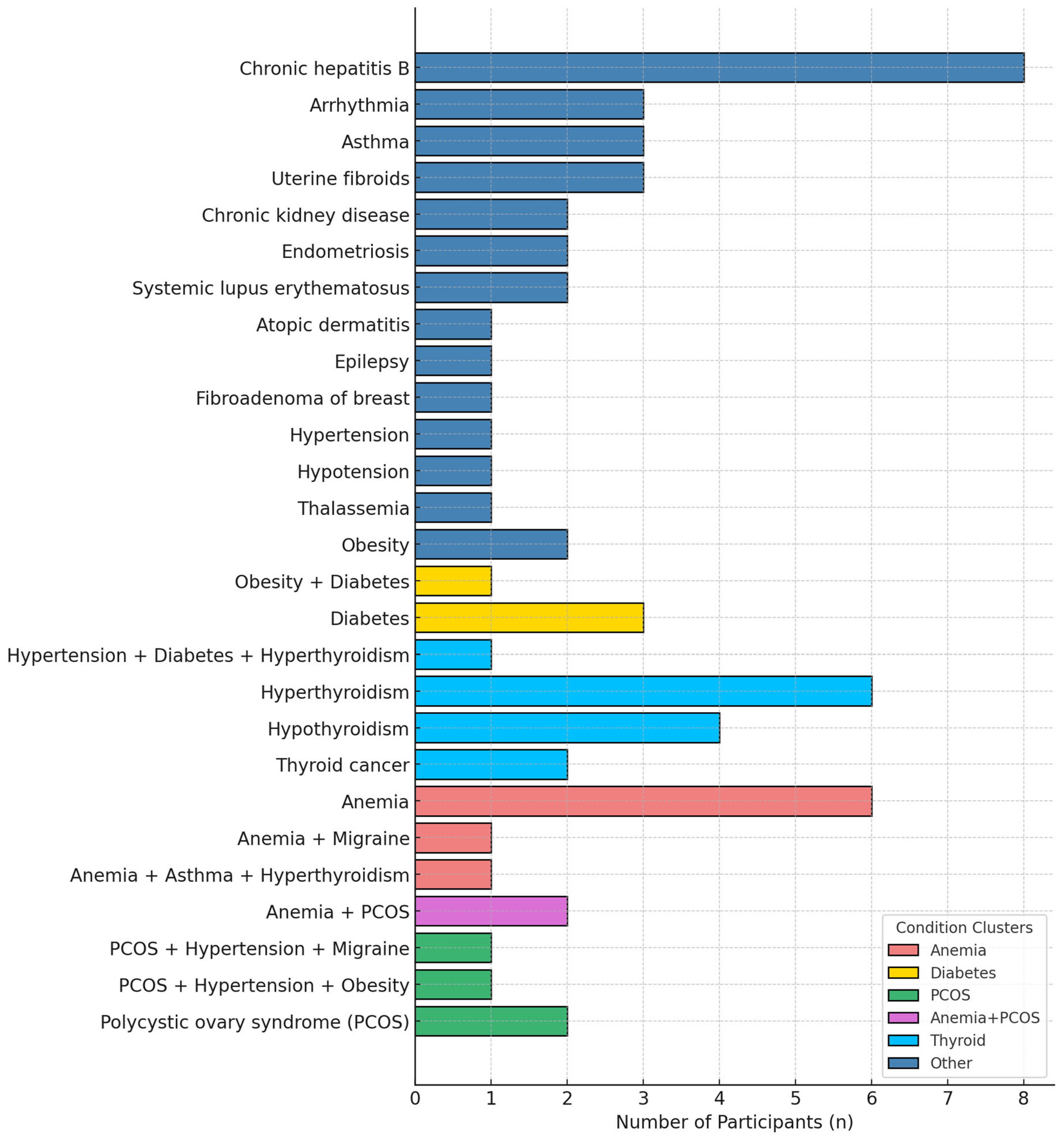
3.2. Maternal Comorbidities Before and During Pregnancy
3.2.1. Pre-Existing Conditions
3.2.2. Pregnancy-Related Conditions
3.3. Description of Adverse Event Following Immunization (AEFI) Following Tdap Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Pertussis—Number of Reported Cases. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/pertussis-number-of-reported-cases (accessed on 5 July 2025).
- Leontari, K.; Lianou, A.; Tsantes, A.G.; Filippatos, F.; Iliodromiti, Z.; Boutsikou, T.; Paliatsou, S.; Chaldoupis, A.E.; Ioannou, P.; Mpakosi, A.; et al. Pertussis in early infancy: Diagnostic challenges, disease burden, and public health implications amidst the 2024 resurgence, with emphasis on maternal vaccination strategies. Vaccines 2025, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.N.T.; Heininger, U.; Muloiwa, R.; von König, C.H.W.; Hozbor, D.; Ong-Lim, A.; Tan, T.Q.; Forsyth, K. Pertussis in southeast asia: Country-level burden and recommendations from the global pertussis initiative. IJID Reg. 2025, 14, 100559. [Google Scholar] [CrossRef] [PubMed]
- Hai, D.T. Tổng quan chẩn đoán và điều trị bệnh ho gà. Vietnam. J. Pediatr. 2021, 4, 10. [Google Scholar] [CrossRef]
- Pham, N.T.; Bui, Q.T.; Tran, D.M.; Larsson, M.; Pham, M.P.; Olson, L. Pertussis seasonal variation in northern vietnam: The evidence from a tertiary hospital. BMC Public Health 2024, 24, 286. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Leuridan, E.; Maertens, K.; Nguyen, T.D.; Hens, N.; Vu, N.H.; Caboré, R.N.; Duong, H.T.; Huygen, K.; Van Damme, P.; et al. Pertussis vaccination during pregnancy in vietnam: Results of a randomized controlled trial pertussis vaccination during pregnancy. Vaccine 2016, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; Hoang, T.T.; Nguyen, T.D.; Caboré, R.N.; Duong, T.H.; Huygen, K.; Hens, N.; Van Damme, P.; Dang, D.A.; Leuridan, E. The effect of maternal pertussis immunization on infant vaccine responses to a booster pertussis-containing vaccine in vietnam. Clin. Infect. Dis. 2016, 63, S197–S204. [Google Scholar] [CrossRef] [PubMed]
- Gattás, V.L.; Luna, E.J.A.; Sato, A.P.S.; Fernandes, E.G.; Vaz-de-Lima, L.R.A.; Sato, H.K.; Castilho, E.A. Adverse event occurrence following use of tetanus, diphtheria and acellular pertussis adsorbed vaccine-tdap-, são paulo, sp, brazil, 2015–2016. (Ocorrência de eventos adversos após o uso da vacina adsorvida difteria, tétano e pertussis (acelular)-dTpa-, São Paulo, SP, 2015–2016). Epidemiol. Serv. Saude 2020, 29, e2019280. [Google Scholar] [CrossRef] [PubMed]
- Jit, M.; Dang, T.T.; Friberg, I.; Hoang, V.M.; Huy, T.K.P.; Walker, N.; Nguyen, V.C.; Tran, N.D.; Toda, K.; Hutubessy, R.; et al. Thirty years of vaccination in vietnam: Impact and cost-effectiveness of the national expanded programme on immunization. Vaccine 2015, 33 (Suppl. 1), A233–A239. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Tran, N.T.; Cao, H.N. Knowledge, attitude and practices related to tetanus-diphtheria-pertussis maternal vaccination in pregnant women at university medical center of ho chi minh city. In 10th International Conference on the Development of Biomedical Engineering in Vietnam, Proceedings of the BME 2024, Phan Thiet, Vietnam, 25–27 July 2024; Vo, V.T., Nguyen, T.-H., Vong, B.L., Pham, T.T.H., Doan, N.H., Eds.; Springer: Cham, Switzerland, 2024; pp. 713–724. [Google Scholar]
- Bộ Y tế Việt Nam. Thông Tư Ban Hành Danh Mục bệnh Truyền Nhiễm, Đối Tượng và Phạm vi Phải sử Dụng vắc Xin, Sinh Phẩm y Tế Bắt Buộc. 2024. Available online: https://vbpl.vn/TW/Pages/vbpq-toanvan.aspx?ItemID=169843 (accessed on 17 July 2025).
- Amin, R. Barriers to tdap vaccination in pregnancy: Perspectives from obstetricians and patients. Int. J. Pregnancy Child Birth 2018, 4, 00077. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Tran, A.D. Safety of the combination vaccination with tetanus-diphtheria-pertussis vaccine and vaccine against covid-19 in pregnant women at university medical centre ho chi minh city. J. Control Vaccine Biol. 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Fortuna, L.; Chaithongwongwatthana, S.; Soonthornworasiri, N.; Spiegel, J.; Wijagkanalan, W.; Mansouri, S.; Biggelaar, A.; Pham, H. Enhanced post-licensure safety surveillance of a new recombinant acellular pertussis vaccine licensed as a monovalent (ap, pertagen®) and tetanus, reduced-dose diphtheria combination (tdap, boostagen®) vaccine for immunization of adolescents and adults in thailand. Vaccine 2020, 38, 8194–8199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, C.; Ye, C.; Zhu, J.; Shen, J.; Zhu, C.; Yang, P.; Liu, T.; Xu, Y. Surveillance for adverse events following immunization with dtap-containing combination vaccines in Linping, China, 2019–2022. Front. Public Health 2024, 12, 1278513. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.J.; Song, R.; Chen, J.; Kim, J.H.; Devadiga, R.; Kang, H.C. A 6-year prospective, observational, multi-center post-marketing surveillance of the safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (tdap) vaccine in Korea. J. Korean Med. Sci. 2019, 34, e105. [Google Scholar] [CrossRef] [PubMed]
- Petousis-Harris, H.; Walls, T.; Watson, D.; Paynter, J.; Graham, P.; Turner, N. Safety of tdap vaccine in pregnant women: An observational study. BMJ Open 2016, 6, e010911. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, L.; McCarthy, N.L.; Kharbanda, E.O.; Weintraub, E.S.; Vazquez-Benitez, G.; McNeil, M.M.; Li, R.; Klein, N.P.; Hambidge, S.J.; Naleway, A.L.; et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet. Gynecol. 2015, 126, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pertussis Vaccines: Who Position Paper, August 2015. Wkly. Epidemiol. Rec. 2015, 90, 433–460. Available online: https://www.who.int/publications/i/item/who-wer9035 (accessed on 17 July 2025).
- WHO. Vaccines Against Influenza Who Position Paper, May 2022. Wkly. Epidemiol. Rec. 2022, 97, 185–208. Available online: https://www.who.int/publications/i/item/who-wer9719 (accessed on 17 July 2025).
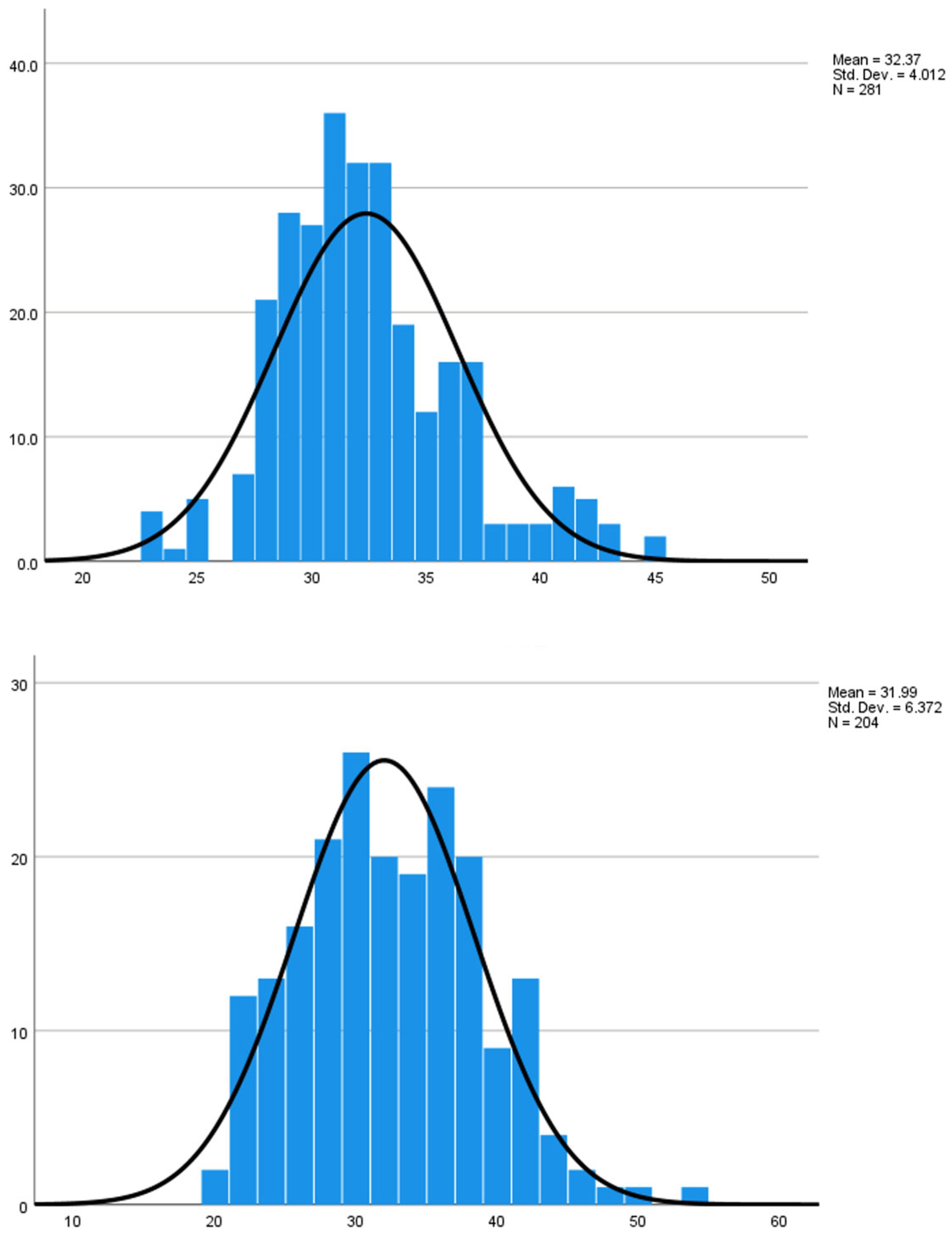

| Characteristics | Tdap (n = 281) | TT (n = 204) |
|---|---|---|
| Number of pregnancies | ||
| 1 | 164 (58.36%) | 90 (44.12%) |
| 2 | 103 (36.66%) | 79 (38.72%) |
| 3 | 14 (4.98%) | 34 (16.67%) |
| ≥4 | 0 (0%) | 1 (0.49%) |
| Number of fetuses | ||
| Singleton | 279 (99.29%) | 204 (100%) |
| Twin | 2 (0.71%) | 0 (0%) |
| Multiple (>2) | 0 (0%) | 0 (0%) |
| Maternal age, years (mean ± SD) | 32.37 ± 4.01 | 31.99 ± 6.37 |
| Type of Local AE | Reaction | Severity Grade | Number of Cases | Proportion (%) |
|---|---|---|---|---|
| Pain * | No | 0 | 158 | 56.23 |
| Yes (43.77%) | 1 | 66 | 23.49 | |
| 2 | 50 | 17.79 | ||
| 3 | 7 | 2.49 | ||
| Erythema | No | 0 | 270 | 96.09 |
| Yes (3.91%) | 1 | 9 | 3.20 | |
| 2 | 2 | 0.71 | ||
| Swelling | No | 0 | 270 | 96.09 |
| Yes (3.91%) | 1 | 8 | 2.85 | |
| 2 | 3 | 1.07 |
| Type of systemic AE | Reaction | Severity Grade | Number of Cases | Proportion (%) |
|---|---|---|---|---|
| Fatigue | No | 0 | 245 | 87.19 |
| Yes (12.81%) | 1 | 26 | 9.25 | |
| 2 | 7 | 2.49 | ||
| 3 | 3 | 1.07 | ||
| Fever | No | 0 | 278 | 98.93 |
| Yes (1.07%) | 1 | 2 | 0.71 | |
| 2 | 1 | 0.36 | ||
| Loss of appetite | No | 0 | 275 | 97.86 |
| Yes (2.14%) | 1 | 4 | 1.42 | |
| 2 | 1 | 0.36 | ||
| 3 | 1 | 0.36 | ||
| Nausea | No | 0 | 281 | 100.00 |
| Rash | No | 0 | 281 | 100.00 |
| Dizziness | No | 0 | 278 | 98.93 |
| Yes (1.07%) | 1 | 1 | 0.36 | |
| 2 | 1 | 0.36 | ||
| 3 | 1 | 0.36 | ||
| Headache | No | 0 | 270 | 96.09 |
| Yes (3.91%) | 1 | 6 | 2.14 | |
| 2 | 3 | 1.07 | ||
| 3 | 2 | 0.71 |
| Type of AEs | Response | Severity | Tdap (%) | TT (%) | p-Value |
|---|---|---|---|---|---|
| Local AEs | |||||
| Pain | No | 0 | 158 (32.6%) | 130 (26.8%) | 0.008 ** |
| Yes | 1 | 66 (13.6%) | 54 (11.1%) | ||
| 2 | 50 (10.3%) | 20 (4.1%) | |||
| 3 | 7 (1.4%) | 0 (0%) | |||
| Erythema | No | 0 | 270 (55.7%) | 196 (40.4%) | 0.443 |
| Yes | 1 | 9 (1.9%) | 8 (1.6%) | ||
| 2 | 2 (0.4%) | 0 (0%) | |||
| Swelling | No | 0 | 270 (55.7%) | 192 (39.6%) | 0.598 |
| Yes | 1 | 8 (1.6%) | 17 (1.9%) | ||
| 2 | 3 (0.6%) | 3 (0.6%) | |||
| Systemic AEs | |||||
| Fatigue | No | 0 | 245 (50.5%) | 186 (38.4%) | 0.551 |
| Yes | 1 | 26 (5.4%) | 14 (2.9%) | ||
| 2 | 7 (1.4%) | 3 (0.6%) | |||
| 3 | 3 (0.6%) | 1 (0.2%) | |||
| Fever | No | 0 | 278 (57.3%) | 203 (41.9%) | 0.661 |
| Yes | 1 | 2 (0.4%) | 1 (0.2%) | ||
| 2 | 1 (0.2%) | 0 (0%) | |||
| Loss of appetite | No | 0 | 275 (56.7%) | 201 (41.4%) | 0.692 |
| Yes | 1 | 4 (0.8%) | 3 (0.6%) | ||
| 2 | 1 (0.2%) | 0 (0%) | |||
| 3 | 1 (0.2%) | 0 (0%) | |||
| Nausea | No | 0 | 281 (57.9%) | 203 (41.9%) | 0.240 |
| Yes | 1 | 0 (0%) | 1 (0.2%) | ||
| Rash | No | 0 | 281 (57.9%) | 204 (42.1%) | – |
| Yes | 1 | 0 (0%) | 0 (0%) | ||
| Dizziness | No | 0 | 278 (57.3%) | 202 (41.6%) | 0.532 |
| Yes | 1 | 1 (0.2%) | 2 (0.4%) | ||
| 2 | 1 (0.2%) | 0 (0%) | |||
| 3 | 1 (0.2%) | 0 (0%) | |||
| Headache | No | 0 | 270 (55.7%) | 201 (41.4%) | 0.148 |
| Yes | 1 | 2 (0.4%) | 2 (0.4%) | ||
| 2 | 7 (1.4%) | 0 (0%) | |||
| 3 | 2 (0.4%) | 1 (0.2%) | |||
| AE type | Reaction | Tdap (n = 281) | TT (n = 204) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|
| Total grade 3 AEs | No | 267 (95.0%) | 202 (99.0%) | 0.018 | 5.30 (1.19–23.56) p-value calculated using Fisher’s exact test |
| Yes | 14 (5.0%) | 2 (1.0%) |
| Adverse Event | Tdap (n = 204) | Tdap + IIV4 (n = 77) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| Local AEs | ||||
| Pain | 87 (42.6%) | 37 (48.1%) | 0.416 | 1.244 (0.735–2.105) |
| Erythema | 6 (2.9%) | 5 (6.5%) | 0.171 | 2.292 (0.679–7.740) |
| Swelling | 6 (2.9%) | 5 (6.5%) | 0.171 | 2.292 (0.679–7.740) |
| Systemic AEs | ||||
| Fatigue | 26 (12.7%) | 10 (13.0%) | 0.957 | 1.022 (0.468–2.233) |
| Fever | 2 (1.0%) | 1 (1.3%) | 0.817 | 1.329 (0.119–14.869) |
| Loss of appetite | 5 (2.5%) | 1 (1.3%) | 0.551 | 0.524 (0.060–4.556) |
| Dizziness | 2 (1.0%) | 1 (1.3%) | 0.817 | 1.329 (0.119–14.869) |
| Headache | 6 (2.9%) | 5 (6.5%) | 0.171 | 2.292 (0.679–7.740) |
| Nausea | 0 (0%) | 0 (0%) | — | — |
| Rash | 0 (0%) | 0 (0%) | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.M.; Tran, N.T.; Pham, Q.H.; Cao, H.N. Safety Evaluation of Tetanus, Diphtheria, and Acellular Pertussis Vaccine (Tdap) During Pregnancy Among Vietnamese Women. Vaccines 2025, 13, 1036. https://doi.org/10.3390/vaccines13101036
Nguyen HM, Tran NT, Pham QH, Cao HN. Safety Evaluation of Tetanus, Diphtheria, and Acellular Pertussis Vaccine (Tdap) During Pregnancy Among Vietnamese Women. Vaccines. 2025; 13(10):1036. https://doi.org/10.3390/vaccines13101036
Chicago/Turabian StyleNguyen, Hien Minh, Nhat Thang Tran, Quoc Huy Pham, and Huu Nghia Cao. 2025. "Safety Evaluation of Tetanus, Diphtheria, and Acellular Pertussis Vaccine (Tdap) During Pregnancy Among Vietnamese Women" Vaccines 13, no. 10: 1036. https://doi.org/10.3390/vaccines13101036
APA StyleNguyen, H. M., Tran, N. T., Pham, Q. H., & Cao, H. N. (2025). Safety Evaluation of Tetanus, Diphtheria, and Acellular Pertussis Vaccine (Tdap) During Pregnancy Among Vietnamese Women. Vaccines, 13(10), 1036. https://doi.org/10.3390/vaccines13101036







