Abstract
Pasteurella multocida is a Gram-negative bacterium causing significant livestock diseases, like fowl cholera and hemorrhagic septicemia in cattle, and wound infection in humans. Classified into four subspecies and five capsular serotypes, it possesses multiple virulence factors, including capsular polysaccharides (CPSs), lipopolysaccharides (LPSs), outer membrane proteins (OMPs), iron acquisition proteins, and toxins that serve as vaccine targets. Antimicrobial treatment is challenging, so vaccination is key. Commercial vaccines include killed and live attenuated types, which are commonly used, though they have intrinsic problems. Advanced vaccines like recombinant subunit and DNA vaccines are emerging. Subunit vaccines targeting OMPs (OmpH, OmpA, PlpE, VacJ, and PmSLP) and recombinant Pasteurella multocida toxin (rPMT) show high efficacy in animal models, and their recombinant proteins induce strong immune responses. DNA vaccines have promise but limited use. The challenges in vaccine development are the strain diversity, short-term immunity, and inconsistent cross-protection. There is also a lack of research on recombinant and subunit vaccine development for small ruminants. Future research should focus on multivalent vaccines, optimization, including improving adjuvants and optimizing DNA vaccine delivery.
1. Introduction
Pasteurella species are facultatively anaerobic, Gram-negative bacteria, coccobacillary- to rod-shaped organisms that belong to the family Pasteurellaceae [1]. There are multiple species of Pasteurella, with Pasteurella multocida being the most clinically significant since it infects a wide host range, including domestic and wild mammals, birds, and reptiles [2]. Pasteurella is a disease of economic importance to global animal husbandry that causes fowl cholera, hemorrhagic septicemia (HS), and respiratory disease in ruminants, progressive atrophic rhinitis (PAR) and pneumonic pasteurellosis in swine, snuffles in rabbits, lower respiratory infection in ungulates, and opportunistic infections due to bites from pets or scratch wounds in humans [3,4,5].
P. multocida can be classified into four subspecies, namely, P. multocida multocida, P. multocida gallicida, P. multocida septica [1,6], and P. multocida tigris [7]. Additionally, P. mutolocida was further categorized into five capsular serotypes (A, B, D, E, and F) and sixteen somatic serotypes (1–16) [8]. Among these serotypes, B2 and E2 are known to cause hemorrhagic septicemia, and they may lead to potential cases of pneumonia, enteritis, or septicemia caused by various capsular serogroups and somatic serotypes [9].
Various somatic serotypes (A, B, D, and E) cause hemorrhagic septicemia in buffalo, cattle, pigs, elephants, bison, goats, and mithun, whereas D, B, A, and A/D in pigs, chickens, buffalo, and turkeys cause respiratory diseases, such as bronchopneumonia, pneumonia, and atrophic rhinitis. Serotypes A and B in poultry and bovine cause fowl cholera and hemorrhagic septicemia, respectively. Serotype F affects sheep and cattle [10,11]. P. multocida type A plays a significant role in the respiratory infection of sheep [12].
Most capsular serogroups are associated with specific diseases. For instance, strains of hemorrhagic septicemia are related to capsular serotypes B and E, fowl cholera strains are typically classified as capsular serotype A, and atrophic rhinitis strains usually fall under capsular serotype D [13]. Types B and E are the main causes of bovine hemorrhagic septicemia, which causes substantial annual damage to livestock globally, especially in low- and middle-income countries [14].
These serotypes are the main determinant of hosts and diseases: for example, types B2 and E2 cause bovine hemorrhagic septicemia, serogroup A (specifically A, A3, and A4) is the main cause of fowl cholera, and subgroups D and A are linked to F porcine atrophic rhinitis. Among these, P. multocida is a leading human pathogen, especially in infected wounds from bites of pet animals from serogroup D [15].
Typically, manifested disease outbreaks are associated with a respiratory disease complex, and their impact is observed at the individual-animal level. Globally, P. multocida has been isolated from multiple host species of origin [9,10]. P. multocida is a strict opportunistic bacterium that resides in the nasopharyngeal and oral mucous membranes. Pasteurellosis can be caused by P. multocida, occurring as more or less acute septicemia and causing signs associated with the primary infection organ. Local and systemic defense mechanisms can be impaired by factors such as prolonged transportation, overcrowding, climate change, or respiratory viral infections [16,17].
Due to the widespread use of antimicrobials for prophylaxis and curative treatment, the emergence of antimicrobial resistance genes in Pasteurellosis in food-producing animals requires the development of rapid diagnostic tests and effective vaccines to mitigate the microbial burden and reduce antimicrobial dependence [18,19,20,21,22,23]. Even though biosecurity measures have contributed to reducing the spread of P. multocida, immunization methods are the most potent preventive measure [5,24].
Despite the availability of multiple P. multocida vaccines, their efficacy remains inadequately studied due to a lack of well-structured studies and significant variability in reported outcomes, making it challenging to derive a reliable pooled protection rate estimate [25]. According to Mostaan et al. [26], multiple formulations of vaccines were developed to protect against P. multocida bacteria, but no single vaccine could fully control all the serotypes. Strategies for vaccine design that result in improved cross-protection vaccines represent the most effective means of control. There has been a growing effort to develop vaccines that are more potent and cause less damage. Existing vaccines, including inactivated and live vaccines, have intrinsic problems related to their safety, efficacy, and immunity duration. So far, the new generation of vaccines have also been considered to bring good progress in disease control, and may soon be an option to cope with the drawbacks of killed and live vaccines. Therefore, a review was undertaken to explore the available P. multocida vaccine types, development strategies, and components in various hosts, along with their safety and efficacy.
2. Immunogenic Components of Pasteurella multocida
The pathogenesis of P. multocida is shaped by numerous virulence factors. These include genes associated with capsule formation, lipopolysaccharides (LPSs), fimbriae and adhesins, toxins, proteins involved in iron-controlled mechanisms and iron acquisition, components related to sialic acid metabolism, hyaluronidase, and outer membrane proteins (OMPs) [27,28]. Several hemin- and hemoglobin-binding proteins also contribute to the virulence. None of them was protected in mouse trials under P. multocida experimental infection when inoculated as a single formulation. These hemin- and hemoglobin-binding proteins require other combination strategies to produce vaccines against pathogens that present several alternative iron-uptake proteins [29]. The functions of virulence genes and major virulence genes are shown in Table 1.
Components within the bacterial outer membrane, especially transmembrane proteins and lipoproteins, have a crucial role in the pathogen’s interaction with the host environment and in pathogenesis and immunity. OMPs include several functional groups, such as transporter proteins, structural proteins, binding proteins, adhesins, putative adhesins, membrane-associated enzymes, and protein assembly machinery. OMPs comprising porins are potentially immunogenic, where they are conserved within bacterial phylogeny, rendering them attractive as vaccine candidates [30].
In general, P. multocida type A expresses capsules consisting of hyaluronic acid that are involved in adherence to immune cells [31], whereas type B contains a polysaccharide capsule consisting of galactose, arabinose, and mannose sugar residue, as well as a type F chondroitin or type D heparin/heparin surface [8,32].

Table 1.
Pasteurella multocida virulence factors and associated genes.
Table 1.
Pasteurella multocida virulence factors and associated genes.
| Virulence Factors | Corresponding Virulence Genes | References |
|---|---|---|
| Adehesins and fimbriae | PtfA, pfhA, fimA, tadD, hsf-1, hsf2 | [2,16,20,22,28,33,34,35] |
| Toxins/dermonecrotic | toxA | |
| Iron acquisition | ExbB, exbD, tonB, hgbA, tbpA, hgbB, fur | |
| Sialidases/neuraminidase | nanH, nanB | |
| Hyaluronidase | pmHAS | |
| Protections/outer membrane proteins | ompH, oma87, ompA, plpB, plpB, psl | |
| Superoxide dismutase | sodA, sodC |
Capsular Serotype and Virulence Genes
P. multocida infects a wide range of hosts. Expressions of different capsular serotypes and virulence genes may show differences across different animals. Accordingly, capsular type A strains had a high level of adaptation to bovine and poultry species. In swine, there were capA and capD strains. A high number of capD-positive strains were found in small ruminants. Additionally, capF was found on wild-type strains isolated from diseased cattle. From these reports, none of the isolates incorporated capE, while all strains of capB originated solely from buffaloes. Even though there is a significant difference in proportion, most combinations of genes encoding outer membrane proteins, colonization factors, iron acquisition factors, and superoxide dismutase have no basic differences regarding host specificity. In general, virulence genes do not exhibit host specificity [2,11,23,35,36,37]. The frequency of isolation capsular serotype versus host relationship is shown in Figure 1.
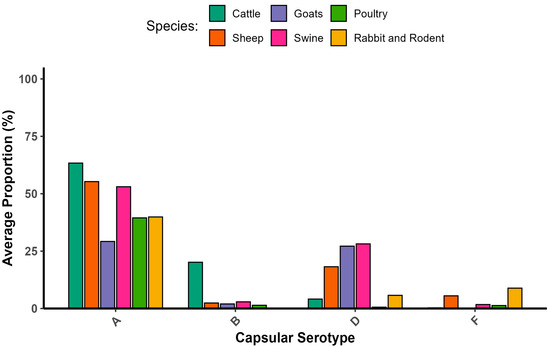
Figure 1.
Frequency of isolation capsular serotype versus host. [2,12,20,21,22,23,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50].
Capsular types A, B, and D commonly occur in domestic ruminants, and a high occurrence of the toxA gene and capsular D isolate was noticed in small ruminants [36]. Studies investigated the proportions of capsular types and their virulence-associated genes; specifically, there were 22 important genes in P. multocida (pmHAS, tbpA, hgbA, hgbB, sodA, sodC, oma87, ompH, plpB, ptfA, fimA, hsf1, hsf2, pfhA, tadD, nanB, exBD-tonB, nanH, exbB, fur, toxA, and ompA), almost all of which were reliably present in domestic ruminants [8,34,36].
Significant relationships were observed between virulence genes and their relevant disease epidemiology, such as gene markers, e.g., the toxA, tbpA, hgbB, and pfhA genes. Swine disease was found to be in association with the toxA gene, whereas pfhA was associated with bovine diseases [2]. tbpA and nanH were found in 100% of nasal swabs and lung tissue in cattle [51]. tbpA and toxA genes played a considerable role in the disease epidemiology of sheep respiratory disorders [12,52]. The isolation proportions of toxA and tbpA genes from goats showed similarity to sheep isolates. This comparison of virulence gene profiles shows the probability of P. multocida transmission between sheep and goats [39,53]. Regarding the bovine respiratory disease complex, prevalent isolates were those containing pfhA, tbpA, and capA. On the other hand, in sheep, the dominant isolates were those with tbpA, toxA, and capD [23]. In sheep and goat, P. multocida capsular type A was the most frequently isolated, followed by type D. Additionally, the high isolation frequency of tbpA, followed by the pfhA, toxA, and hgbB genes, showed probable significance in the pathogenesis in sheep and goats [43,48,49,54]. The toxA gene was detected in sheep with pneumonia and was found to be toxigenic [55].
Several reports involving the capsular serotypes versus host relationships were described. Two capsular serogroups, types A and D, are common capsular types, and the toxA gene is an important marker gene for describing the pathogenic potential of P. multocida strains in swine [34]. In poultry, type A was predominant [35,36,37,44,56,57]. Similarly, in cattle, even though serotypes D, B, and F were reported, serotype A was also predominant [21,36,38]. The presence of the virulence gene ptfA demonstrated a positive association with the disease outcome of cattle, and thus, is an important epidemiological marker gene for characterizing P. multocida isolates [40]. In the rabbit, serogroups A, D, and F were isolated. Type A was the most frequently identified as a major cause of rhinitis and pneumonia. However, type D was detected in metritis, while type F was detected in mastitis, otitis, subcutaneous abscesses, and septicemia. The sodC gene was present in a single strain that was tested. Among the strains that caused respiratory lesions, the pfhA gene was commonly detected in type A strains compared with type D strains. Moreover, pfhA was identified in all F strains. Regarding the strains associated with rhinitis, the fur gene was more frequently isolated from type D strains. The hgbB genes were predominantly detected in the strains that were responsible for metritis [50]. The proportions of virulence genes and their associated hosts reported by various scholars are described in Figure 2.
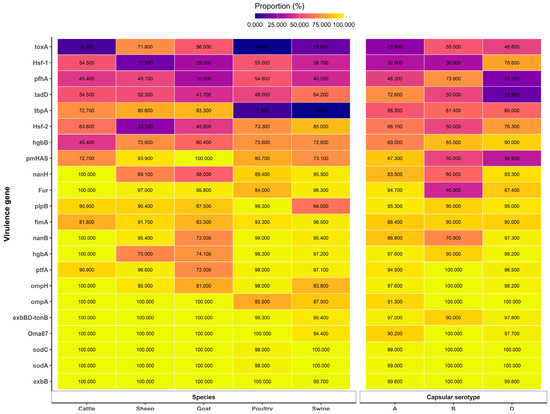
Figure 2.
Faceted heatmap of virulence—gene proportions across host species and capsular serotypes. Each tile displays the mean percentage of isolates positive for a given virulence gene within a species group (Cattle, Sheep, Goat, Poultry, and Swine) or within a capsular serotype (A, B, and D). Values were computed by expanding multi-valued cells, parsing numeric content, excluding missing entries, averaging within each gene–category cell, and constraining results to the valid range of 0.000–100.000. Virulence genes are ordered by their overall mean prevalence across both facets to aid comparability. Sources for Figure 2: [12,22,34,36,46,47,48,49,58,59]. Notes: (ref. [12]) reported only for sheep; (refs. [47,59]) reported only for swine; (ref. [22]) reported only for swine and poultry of serogroups A and D; (ref. [49]) reported only for sheep and goats; (ref. [48]) reported only for goats; (ref. [58]) reported only for avian species and sheep.
Molecular epidemiology describes the association between virulence genes and related serotypes of P. multocida. There were significant associations observed between capsular serotypes and virulence genes. Accordingly, there is a relationship between capsular serogroup A and ompH1, ompH3, plpE, and pfhB1; capsular serogroup B and hgbA and ptfA; and capsular serogroup F and ptfA and PlpP [35]. Furthermore, notable links were found between pfhA, tadD, tbpA, and hayluniradase and the capsule type A isolates [21]. In swine and poultry cases, toxA and hsf-1 showed a significant relationship with subgroup D, and pmHAS and pfhA with serogroup A [56]. The proportions of virulence genes and their associated capsular serotypes reported by various scholars are described in Figure 2.
In strains with similar genetic backgrounds, the molecular mass variability of ompA and ompH indicates that these proteins possess diversifying selection within the host. This implies they likely play a significant role in host–pathogen interactions. Comparing the outer membrane protein profile of bovine isolates with avian, ovine, and porcine species strains clearly shows that a large percentage of respiratory diseases of each species are caused by distinct strains of P. multocida. The discovery of closely related strains across multiple species suggests bacteria transfer between host species as part of the dynamics, structure, and interaction within P. multocida bacteria. However, this variability allows the bacterium to evade immune detection, colonize diverse host niches, and maintain virulence [38].
P. multocida encodes proteins such as those for the outer membrane and porin proteins (especially oma87, psl, and ompH), type 4 fimbriae (denoted by ptfA and pfhA), neuraminidase (including nanB and nanH), proteins involved in iron acquisition (exbBD-tonB, tbpA, hgbA, and hgbB), dermonecrotoxin (toxA), and superoxide dismutase (sodA and sodC). Among these, certain antigens have the potential to act as vaccine candidates [2,29]. Additionally, colonization factors (ptfA, fimA, and hsf-2), nanH, and outer membrane proteins are common characteristics of serogroups A and D. The circulation of these virulence gene patterns offers several indications. It suggests that certain factors involved in cross-protection could potentially be developed into vaccine prospects. These candidates may be able to generate homologs of protective immunity against all serotypes of P. multocida [27,28].
3. Immunizations and Types of Vaccine and Vaccine Candidates
3.1. Immunization Types
3.1.1. Passive Immunization
Several passive immunizations were reported regarding hyperimmune serum and monoclonal antibodies. Passively acquired anti-P. multocida OMP antibodies were verified in dairy farm sera within 24 h after; the P. multocida antibody concentrations were found to be good until 156–419 days and then declined after 198 to 490 days of age [60]. Furthermore, serum antibody concentrations were also associated with different farm management factors [16]. Another report indicated the passive transfer of antibodies challenged with strains A: 1 (75%) and B: 2 (50%). Mice protected against a challenge using the phage lysate inactivation method suggested that the antibody alone could provide cross-protection against the serovar of a P. mutocida infection [61].
Six monoclonal antibodies (MAbs) against P. multocida serotype B:2 LPS were tested for passive protection in mice. The MAbs interacted with B:2 and serotype 5 LPSs, but not others. This mix of MAbs gave partial, serotype-specific protection, extending the mouse survival slightly. This implies that non-LPS antigens are involved in the immune response too [62].
Basic trials against swine atrophic rhinitis indicate that pregnant sows that received immunization with recombinant subunit PMT had more maternal antibodies present in their colostrum compared with those who were immunized with a conventional PAR toxoid vaccine [63]. A nontoxic truncated form of recombinant PMT (PMT2.3) vaccination of mice also develops a high level of anti-PMT antibodies with a high neutralizing serum antibody titer. High levels of serum antibody titers, cellular immunity, and good growth performance were observed in offspring from sows vaccinated with PMT2.3 [64]. Protective levels of maternal antibody were transferred effectively to progenies via the colostrum [65].
A report of two foremost outer membrane proteins of P. multocida, incorporating ompH and ompA, was used to continuously deliver specific MAbs to mice with hybridoma tumors, demonstrating that IgG MAbs against ompH are involved in the protection of mice against a lethal infection challenge using opsonization and inhibition of multiplication of P. multocida as a result of the increased PMN influx into the infection site [66]. Furthermore, rabbit antiserum prepared against recombinant Oma87 passively protected mice against a homologous infection [28].
Cross-protection was observed in the oldest work on sheep immunization, indicating that a formalin-inactivated serotype D strain vaccine serum protects mice against four strains of serotype A and three nontypable strains but does not protect against types B and E [67]. Similarly, live P. multocida-serotypes-B:3,4-vaccinated cattle serum cross-protected passively immunized mice against P. multocida serotypes E:2, F:3,4, and A:3,4 [68].
3.1.2. Active Immunization
Vaccination represents the most effective approach for preventing the transmission and spread of disease outbreaks by boosting the immune systems of animals against harmful pathogens. As such, it ensures comprehensive security regarding animal health and has a positive impact on public health. Given the repeated emphasis on the importance of vaccination, research focused on large-scale industrial vaccine production for the control of the disease has become crucial. This is essential to satisfy the ever-increasing demand for such vaccines [25].
Existing vaccines designed to combat P. multocida are of two main types: inactivated whole-cell bacterin vaccines and live attenuated bacterial vaccines. Unfortunately, both of these vaccine varieties have drawbacks. They are known for having inconsistent safety records, being less effective, and having a relatively short-lived protective period. In particular, bacterin vaccines typically safeguard against the disease for less than six months, while live attenuated vaccines offer prevention for around a year [69]. The weak efficacy of existing vaccines might be attributed to several factors. Poor immunogenicity (poor at triggering cellular immunity) could be one reason, as well as reversion to virulence of live attenuated strains. There is also an issue of vaccine mismatch between the vaccinal serotype strain and the circulating wild-type bacteria, which would prevent effective targeting. Additionally, these vaccines often fail to induce long-lasting immunity. Safety is another major concern, especially with bacterins. The high dose of bacterial-derived endotoxin and other substances in the administered doses of bacterins can lead to systematic reactions [26].
However, one must recognize that inactivated vaccines, even though they produce short-lived immunity and offer insufficient cross-protection, are effective and possess an encouraging cost–benefit ratio [70]. Furthermore, compared with other vaccine types, inactivated vaccines face fewer regulatory restrictions. Even with the progress in genetic engineering and biotechnology, in the short term (three to five years), inactivated vaccines and those adjuvanted with aluminum hydroxide will sustain their commercial dominance. The future of bacterial vaccines in animal production looks bright. Thanks to improvements in vaccine formulations and bioengineering, these vaccines hold the potential to enhance the sustainability of the vaccine production industry. However, it is essential to research methods to boost the efficacy of vaccines and increase their accessibility [71].
The commonly used formalin- or heat-killed whole-cell bacteria vaccines are also commonly circulating vaccines that may affect some major epitopes of Pasteurella. Oil-adjuvanted formulation and/or saponin-added vaccine preparations showed variable efficiency in long-term immunity for cattle, chickens, ducks, turkeys, and rabbits, but not sheep. Alum, Freund’s incomplete adjuvant, PMT toxoid, and DNA were all utilized concurrently to augment the impact of bacteria. Typically, the inactivated bacterin was unable to provide cross-protection or long-lasting immunity. Moreover, it induces local inflammation at the site of administration [24].
In summary, most works on vaccine developments related to virus-like particles (VLPs) hold greater possibilities as a vaccine platform. Their distinguishing characteristics enable them to boost the immune response, and they can serve as a vehicle for foreign antigens to combat infectious diseases. Vaccines formulated based on VLPs are part of the new-generation vaccine strategies that have received approval. Most studies on these vaccines were in the advanced stage of evaluation [70]. Most available vaccines and vaccine candidates for their target host, antigenic composition, method of delivery, and drawbacks summarized below in Table 2, Table 3 and Table 4.
3.2. Vaccine Types
3.2.1. Whole-Cell Vaccine
As previously discussed, administering vaccinations using either inactivated bacterin or live attenuated bacteria represents an efficient and cost-effective approach. This method serves to enhance the health status of animals and safeguard them from P. multocida infections [24]. However, bacterins against P. multocida offer restricted protection against heterologous serotypes. Moreover, there is a concern that live/attenuated vaccines might revert to a virulent state, which could then lead to the infection of animals [72].
These currently available biological products, such as modified live vaccines and bactrins, have their potencies tested based on counting bacterial colonies. Regarding the bacterin potency, the approach involves vaccinating and then challenging mice and/or birds. Inactivated vaccines are used for the prevention of atrophic rhinitis. However, there are no standard criteria for potency testing of P. multocida type D toxoid. Somatic antigens, especially lipopolysaccharide (LPS), seem to play a potential role in the immune mechanism [73].
The OIE [9] recommends that the effective vaccines against hemorrhagic septicemia are formalin-killed bacterins or dense bacterins with an adjuvant. These adjuvant-added vaccines not only increase the level of immunity but also extend its duration. The seed culture used in vaccine production must consist of a bacterial capsule. Vaccines are standardized according to their bacterial load, which is determined through turbidity tests and measurement of the dry bacterial weight. Potency tests are typically conducted using rodents.
Currently, bovine vaccines for P. multocida disease are available on the market; however, these vaccines are mainly restricted to aluminum-adjuvanted whole killed bacterins [71] or live attenuated vaccines [69], both of which provide protection specific to certain serogroups. However, they can cause strong reactions and are not well-matched to the currently circulating strains. This situation makes it extremely challenging to standardize the vaccines, carry out large-scale production, and maintain quality control during vaccine manufacturing. It is important to reduce the drawbacks of traditional vaccine development of modified live attenuated vaccines, such as PMZ2 for ducks [74] and PmCQ2Δ4555–4580 for cattle [75]; these vaccines exhibit cross-protection, yet their safety needs further verification. Even though there are several commercially accessible vaccine formulations, such as alum-precipitated, oil-adjuvanted, and multiple-emulsion vaccines, the pursuit of appropriate, highly protective hemorrhagic septicemia vaccines that offer long-lasting immunity is growing in intensity. Simultaneously, efforts are underway to clarify uncertainties regarding the bacteria, including their virulence factor, pathogenesis, immune mechanism, and diversity within P. multocida organisms [72].
Whole-cell bacterins can offer a certain level of protection. However, this protection is restricted to the homologous lipopolysaccharide (LPS) serotypes. There is substantial information indicating that cross-protecting antigens are expressed only in in vivo situations. Live attenuated vaccines developed empirically have the potential to safeguard against heterologous serotypes. Nevertheless, since the attenuation mechanism is not clearly defined, the reversion of these vaccines to virulence is quite common [28].
The OIE [76] recommends several commercial vaccines for atrophic rhinitis control. These include whole-cell bacterins B. bronchiseptica combined with toxigenic P. multocida bacterin (capsular type D) and/or a P.multocida toxoid. Moreover, some toxigenic and non-toxigenic type A strains of live attenuated B. bronchiseptica vaccines exist too. Vaccines prepared with only B. bronchiseptica are unsuitable for PAR, except in non-progressive cases. P. multocida and B. bronchiseptica vaccines reduce bacterial colonization but do not eliminate them or stop infection. Most commercial vaccines have an oil adjuvant or aluminum hydroxide gel.
3.2.2. Killed Vaccines
According to the OIE [9], there are three vaccines for preventing hemorrhagic septicemia (HS): whole-cell bacterins, alum-precipitated vaccine (APV), and oil-adjuvant vaccine (OAV). Bacterins require frequent vaccination for adequate immunity and can cause shock reactions when dense, while the APV has fewer shock reactions, and the OAV has virtually none.
A formalin-killed vaccine strain of type D4 shows significant protection against homologs of six type D strains and heterologous strains of four type A strains [67]. This whole-broth-culture formalin-killed P. multocida type was widely used for sheep and goats in Ethiopia, NVI [77], but some improvements were made to the killed P. multocida type A antigens formulated with bacterial DNA as an adjuvant as a new vaccine against P. multocida in sheep [78]. However, the vaccine applied against P. multocida bio-type A was found to be less effective in developing protective antibodies against sheep disease; this may have been because the circulating P. multocida serotypes may have been present in low amounts with the vaccine applied. Accordingly, the creation of a multivalent vaccine that incorporates the most commonly occurring Pasteurella serotypes sourced from diverse geographical origins is expected to facilitate efficient prevention. This requires further study on the identification of strains of P. multocida in sheep for further multivalent vaccine development [79].
The initial vaccine for HS was developed in the early 1900s. Unfortunately, the bacterin vaccine elicited weak antibody responses. It provided immunity for merely six months and induced a certain degree of shock in animals. To address the protein shock issue associated with killed-broth-culture vaccine organisms, formalized or agar-wash heat-killed vaccines were developed. These improved vaccines offered immunity for up to four months [72]. Some of the main types of killed vaccines include bacterins, alum-precipitated vaccines, aluminum hydroxide gel vaccines, oil-adjuvanted vaccines, and multiple-emulsion vaccines. Bacterin represents the most basic form of an HS vaccine. It is produced from killed P. multocida using either physical methods, such as heat, drying, or ultraviolet radiation, or chemical agents, such as phenol, Lysol, formalin, or sodium azide [69].
In swine, an inactivated whole-cell antigen of multiple serogroups A (L3 and L6) and D (L6) aluminum hydroxide gel adjuvant vaccines produced no heterologous protection of type A (L3 to L6), but some cross-protection was absorbed from serotype D6 against heterologous strains [80]. In chickens, a formalin inactivated with alum adjuvant vaccine of serotype A was commonly used but improvements were made through P. multocida A:1 grown in the presence of low FeCl3 concentrations, inactivated with a high FeCl3 concentration, and adjuvanted with bacterial DNA from P. multocida type B:2 containing immune-stimulatory CPG motifs that protect chickens with a lethal P. multocida A:1 [81,82].
3.2.3. Live Attenuated Vaccine
This vaccine formulation strategy involving attenuated or avirulent vaccines can be implemented through various methods, including subjecting the bacteria to an iron-deficient condition, using chemically mutagenic substances, and deleting virulence genes [26]. A P. multocida strain B:3,4, specifically a fallow deer strain, was used to prepare a live HS vaccine that has now been utilized for disease control in cattle and water buffalo older than six months, administered as an intranasal aerosol application [68]. Serum of vaccinated cattle cross-protected mice against infection with the serotypes E:2; F:3,4 and A:3,4. The OIE [9] reported that the Food and Agriculture Organization (FAO) has recommended the vaccine as safe and effective for use in Asian countries. Nevertheless, there are no reports of utilization in other countries. In countries affected by hemorrhagic septicemia (HS), only killed vaccines are currently in use. Similar reports also discuss a type A mutant strain, PmCQ2Δ4555, 4580, that was a wild-type strain PmCQ2 with six obvious genes missing; it can protect mice challenged by serogroup B and slightly protect against serogroup F [76].
In an older study by Rice et al. [83], which built on the aforementioned work, the vaccination routes for chickens using a live avirulent P. multocida vaccine were evaluated. Across every experiment, the subcutaneous vaccination route offered the most substantial protection. Among broilers vaccinated via the subcutaneous route, the protection levels scored 95% and 97.5%. Notably, there were no occurrences of unwanted lesions or cheesy mass formation beneath the skin on the backs of broilers’ necks. A novel, live attenuated P. multocida vaccine strain for ducks named PMZ2 features a deletion of the gatA gene and the initial four bases of the hptE gene. These genes play crucial roles in the synthesis of the lipopolysaccharide (LPS) outer core. Despite its cross-protection capabilities remaining unstudied, PMZ2 is a promising live attenuated vaccine for ducks, with the potential for delivery via oral and intranasal routes [74].
3.2.4. Recombinant Subunit Vaccine
Subunit vaccines enable straightforward large-scale production. Additionally, they possess the capacity to modify and enhance proteins. Subunit vaccines comprise individual immunogenic bacterial components, such as proteins and polysaccharides, that can confer immunity [24,63]. In this bacterium, most genes were found to encode membrane proteins through a bioinformatics analysis of the P. multicida genome, where an extensive set of OMPs and outer membrane-associated portions were identified. Given their predicted localization as either secreted or surface-exposed proteins, these proteins were examined for their immunogenicity and capacity to safeguard against lethal P. multocida infections. However, only the recombinant protein plpE was found to be more likely to trigger a protective immune response [30].
An in silico analysis revealed that unique B and T cell epitopes determined based on the adopted antigenic LPS outer membrane complex proteins found in fowl, buffalo, and goats can be appropriate targets for vaccine development against fowl cholera (FC) and hemorrhagic septicemia (HS) [84]. Similarly, Mostaan et al. [85] reported on the immunogenicity, antigenicity, various serotype coverage, half-life, and antibody accessibility epitopes of PlpE (regions 1 + 2 + 3), including regions of the P. multocida plpE protein that can be used as an appropriate serotype-independent vaccine candidate against pasteurellosis.
In bovine pasteurellosis, part of the bacteria’s surface lipoprotein (PmSLP-3) formulated with Montanide ISA 61 fully protects against a serogroup B challenge by creating sustained serum IgG titers that stay for 3 years after administration of two doses and can simultaneously protect against a serogroup E challenge [86]. Similarly, vaccines comprising PmSLP (1 and 3) antigens can be applied as an effective solution for preventing HS- and BRD-related P. multocida infections, indicating that PMSLP is an important vaccine component [87]. PlpE has been reported as a crucial cross-reactive outer membrane protein in P. multocida. The plpE genes of P. multocida serotypes A:3, B:2, and D1 were studied, followed by the expression and immunoblotting analysis of plpE from B:2. OMPs are powerful immunogens that offer protection to mice, rabbits, chickens, and calves. P. multocida A:3 (strain P1059) was discovered to be cross-protective, inducing both active and passive cross-protection in chickens and turkeys. It was also found that the plpE gene of hemorrhagic septicemia which causes serotype B:2, expressed in E. coli, and the recombinant plpE was strongly immunostained by antiserum against the whole-cell antigen. This indicates that the protein is expressed in vivo [88]. Similarly, Wu et al. [89] cloned P. multocida lipoprotein E (PlpE) from P. multocida strain X-73 (A:1) and expressed it in Escherichia coli to show that mice and chickens immunized with r-PlpE were protected against challenge infections with serotypes A:1, A:3, and A:4. Therefore, plpE serves as a cross-protective antigen.
According to Okay et al. [90], recombinant OmpH, plpE, and plpE-OmpH fusion protein formulated with oil-based CpG oligodeoxy nucleotide stimulated 100% protection, indicating that the recombinant PlpE is a possible acellular vaccine candidate for cattle. In avian species, new vaccine formulations involving serotype A:1 recombinant VacJ, PlpE, and ompH [91] and A:3 recombinant adhesive protein (rCp39) [92] for duck and chicken, respectively, are also very promising recombinant vaccine candidates.
In regard to swine, P. multocida toxin (PMT2.3) vaccination in mice delivered a high anti-PMT antibody level with a high neutralizing antibody titer and cellular immune response, with high levels of serum antibody titers and growth performance passed down to their offspring. Therefore, PMT2.3 in the truncated and nontoxigenic recombinant PMT form is a good candidate subunit vaccine against PAR-induced infection in pigs [64]. Similarly, recombinant subunit P. multocida toxin (rsPMT) containing either the N or C terminal portion of PMT developed high neutralizing antibody titers [63]. Wang et al. [93] found that a recombinant vaccination of rTorA, rPrx, and/or rPGAM of serogroup D proteins also protected 60~80% of the tested mice against the challenge with P. multocida field isolates of A, B, and F, and thus, was found to be a good vaccine prospect.
3.2.5. DNA Vaccine
DNA vaccines have been put forward as a possible solution. As subunit vaccines, they carry no infective component or reversion to a virulence state. These vaccines have the advantage of enabling simultaneous immunization against numerous pathogens. They are comparatively straightforward to formulate and cost-effective to produce. The administration of DNA vaccines has been demonstrated to trigger immune responses and offer protection against trials in various animal models. However, despite these benefits, they are not currently suitable for mass vaccination. This is because their application requires biological or physical carriers [94]. Further shortcomings, such as the potential to impact related cell growth, the risk of prompting the production of antibodies against DNA, the development of tolerance to the antigen (protein) generated, and the possibility of abnormal processing of bacterial protein, are factors that can be seen as limiting the development of these types of vaccine [85].
A DNA vaccine constructed using two distinct outer membrane proteins, namely, pOMPH and pOMPA, demonstrated its ability to safeguard against avian pasteurellosis. Moreover, the fusion vaccine developed from these two OMPs exhibited the highest level of protection [95]. An ompH gene amplified from P. multocida strains was sequenced, found one restriction site, found two fragments. The OmpH gene DNA expressed in E. coli protects vaccinated rats, which makes it a candidate vaccine for major farm animals [96].
OmpH and ompA are two major immunogenic proteins in avian P. multocida. Studies on the DNA genes of these OMPs found that a divalent combination (pcDNA-OMPH + pcDNA-OMPA, pOMPH + pOMPA) and a combination of the two gene vaccines (pcDNA-OMPH/OMPA, pOMPHA) provide good protection, equivalent to the attenuated live vaccine of fowl cholera, while the monovalent form is not protective unless combined [97]. Again, chitosan showed its potential to trigger the immune response associated with a naked DNA vaccine centered on ptfA of P. multocida. Thus, the ptfA chitosan construct provides robust protection against P. multocida [98].
Conventional vaccines, such as alum-precipitated and oil-adjuvanted broth bacteria, were subcutaneously injected to protect against hemorrhagic septicemia. Unfortunately, this offered only short-duration immunity and demanded frequent administration. Gene fragments from P. multocida serotype B (ABA39) were sub-cloned into DNA expression plasmid pVAX1-ABA39; research work showed the resulting recombinant vaccine has the potential to be a successful future vaccine against HS [99].

Table 2.
Cattle, buffalo, sheep, and goat P. multocida vaccines and vaccine candidates.
Table 2.
Cattle, buffalo, sheep, and goat P. multocida vaccines and vaccine candidates.
| Target Host/Animal Species | Type of Vaccine | Target Serotype and Strain to Protect | Antigenic Composition | Administration Route | Immunological Effect | Drawbacks | Animal Immunized and Status | References |
|---|---|---|---|---|---|---|---|---|
| Bovine | Live attenuated | B:2 | Wild-type strain 85020 contains a deleted aroA gene (JRMT12) | Intramuscular (IM) | Higher IgG and IgM | Dose-dependent | 108 CFU was safe and effective on calves | [100] |
| Bovine | Live attenuated | A | PmCQ2Δ4555–4580 wild-type strain PmCQ2, with six obvious genes missing | IM | 100% protection against A and B, 40% against F, good cross-protection against B, and slightly protects against F | Trial with mice | [75] | |
| Cattle and buffalo HS | Killed | B6 | B6 | Subcutaneous (SC) | Specific but 100% protection | 4 to 6 months immunity | Cattle and buffalo in use | [69] |
| Cattle and buffalo | Live | B2 | B3,4 | SC | 9 to 12 months of protection | Route of administration and serotype mismatch | Cattle and buffalo in use | [69] |
| Cattle | Formalin-killed | B2 | B2 | SC | 6 to 8 months of protection | Anaphylactic shock in some animals | Cattle in use | [77] |
| Best with cattle/buffalo | Live | B2 | B3,4 | Intranasal | High AB titer; E:2, F:3,4 and A:3,4 | - | Cattle and buffalo in use | [68] |
| Cattle | Live | P. multocida A:3 | A:3 | IM | Reduced clinical lesions | Cattle | [101] | |
| Bovine | Recombinant | P. multocida A:3 | Recombinant proteins PlpE and PlpEC-OmpH | Intraperitoneal | 100% protection; increased IgG and serum IFN-gamma | - | Mice trial | [90] |
| Bovine and buffalo | Subunit | B2 | Native OMP | Subcutaneous | 100% protection | - | Mice | [102] |
| Cattle | Subunit | B and E | PmSLP-3 | SC/IM | Highest level of mucosal PmSLP-3 specific IgG; cross-protection with serogroup E | No cross-protection against BRD strains | Cattle and mice | [86] |
| Cattle with BRD/fowl cholera | Recombinant subunit | A (P. multocida P488 challenge) | OMVs (OmpA, OmpH, and P6) | Intranasal | High AB titer and mucosal immune responses, cross-protection with M, and hemolytic | Mice trial | [4] | |
| Cattle HS/BRD | Bivalent subunit | B2 and A3 | PmSLP-1 (BRD-PmSLP) and PmSLP-3 (HS-PmSLP) | SC/IM | High serum IgG and a good vaccine with good cross-protection | Cattle | [87] | |
| Cattle | Subunit | B:2 | B:2 OMPs plus anti-idiotype AB | SC | 100% protection in rabbits, better protection than whole bacterin | - | Rabbit trial | [103] |
| Bovine | Recombinant subunit | A, B, F | OMPs of A, B, and F | SC | Effective against A and B | Still needs verification in cattle | Mice | [104] |
| Cattle HS | Recombinant | B:2 | rOmpH adjuvanted with CpG-ODN | Intranasal | High serum IgG and secretory IgA levels | Calves | [105] | |
| Cattle and buffalo | DNA | B | B2: Clone pVAX1- ABA392 | IM | Increased serum IgG and no organ lesions | Rat trial | [99] | |
| To all but cattle strain as a challenge | DNA | Mainly A | The ompH conserved gene of ten strains | IM | High AB titer with good cross-protection | Mice trial | [96] | |
| Cattle and buffalo | DNA | B:2 | tbpA gene of B:2 | - | Increases humoral and cell-mediated immune response | - | Mice trial | [106] |
| Goat | Inactivated recombinant | B:2 | B:2 fimbrial protein | Intranasal | High IgG and IgA | Goat trial | [107] | |
| Sheep | Inactivated multivalent | A and D4 | D4 and type A (8473 strain) | SC | Good cross-protection against some A strains | The short duration of immunity (6 months) | Sheep | [67] |
| Sheep | Inactivated/killed | A (challenge strain PMSHI-9) | A+ iron inactivation with iron and bDNA adjuvant type A | SC | Higher Ab titer and cellular (IL-4, IFN-γ, and TNF-α); good humoral and cellular immunity and safety | - | Sheep | [78] |
| Sheep and goat | Formalin-killed | A | A | SC | 6 to 8 months of protection | - | Sheep and goats in use | [77] |

Table 3.
Poultry and avian species related to P. multocida vaccines and vaccine candidates.
Table 3.
Poultry and avian species related to P. multocida vaccines and vaccine candidates.
| Target Host/Animal Species | Type of Vaccine | Target Serotype and Strain to Protect | Antigenic Composition | Administration Route | Immunological Effect | Drawbacks | Animal Immunized and Status | References |
|---|---|---|---|---|---|---|---|---|
| Duck | Live attenuated | A LPS1/PMZ2 | PMZ2 gene with the deleted gatA gene and part of the hptE gene | Oral and intranasal | High level of serum IgG with strong bactericidal effect and a significant increase in IgA response | Cross-protection has not evaluated fully | Duck trial with good effect | [74] |
| Chicken | DNA | A1 challenge study | ptfA gene with added chitosan particle | IM | High AB concentration | Only a 68% protection level | Chicken trial | [98] |
| Chicken | DNA | P. multocida CVCC474 strain challenge | ompA and ompH | IM | High AB and equivalent protection against the attenuated live vaccine | The delivery system is not appropriate | Chicken trial | [97] |
| Turkey | Recombinant peptide | P. multocida x73 (A:1) challenge | A:3 FHAB2 peptides (filament) | Intradermal | Reduce mortality and organ pathology with cross-protection | Turkey trial | [108] | |
| Duck | Recombinant subunit | A:1 | A:1 (PMWSG-4) recombinant VacJ, PlpE, and ompH | SC | 100% protection; reduces tissue damage and colonization | - | Duck | [91] |
| Chicken | Inactivated | A1 | Inactivated A1 + B2 DNA adjuvant | SC | Cost reduction with safe and innate stimulation | - | Chicken | [81] |
| Chicken | Recombinant PlpE | A1 | Recombinant PlpE of (A:1) | SC | 80–100% survival rate with cross-protection (A:3,4) | - | Mice trial | [89] |
| Chicken | Live | A and B | A virulent (A1) | SC | 95 to 97.5% protection level | - | Chicken | [83] |
| Chicken | Inactivated with formalin and FeCl3 | A | A:1 adjuvant with bDNA | SC | 100% long-term protection and good humoral and cellular immunity in mice | - | Mice trial | [82] |
| Chicken | Recombinant protein | A1 | A:3 recombinant adhesive protein (rCp39) | SC | Cross-protection and 60 to 100% protection | Chicken | [92] | |
| Chicken | DNA | A | A pOmpH and pOmpA | IM | Higher AB titer than the live attenuated vaccine | The vaccine delivery system is not appropriate | Chicken | [95] |
| Duck | Recombinant | A:1 | rHVT (herpes virus) OmpH | IM | Ensures good safety and protection | Duration may be short | Duck | [109] |
| Chicken | Recombinant | A:1 | A (rOmpH) | IM | 100% protection from fowl cholera | Chicken | [110] |

Table 4.
Swine- and rabbit-related P. multocida vaccines and vaccine candidates.
Table 4.
Swine- and rabbit-related P. multocida vaccines and vaccine candidates.
| Target Host/Animal Species | Type of Vaccine | Target Serotype and Strain to Protect | Antigenic Composition of Vaccine | Administration Route | Immunological Effect | Drawbacks | Animal Immunized and Status | References |
|---|---|---|---|---|---|---|---|---|
| Swine | DNA | Wild-type P. multocida strain 4533 | toxA | IM | Secrete IFN-ᵞ, increased AB titer | Toxin-specific only | Swine | [111] |
| Rabbit | Killed | A and F | pfhA, sodC, soda, exbB, oma87, fur, fim4, nanB, nanH, and fimA | -- | Reduced the severity of the disease | Still does not protect against some strains of A and D | Rabbit | [112] |
| Swine | Killed | A and D | A:L3 D:L6 | SC | High serum IgG | No cross-protection against others | Mice trial | [80] |
| Pig | Recombinant subunit | PMT (toxA) | Recombinant toxA (Tox1, Tox2, and Tox7) | IM | Protective humoral and cellular immunity | - | Pig | [63] |
| Rabbit | Subunit | A3 | A:3 OMP (IROMP) adjuvanted with cholera toxin (CT) | Intranasal | Mucosal and systemic AB increased; reduced bacterial count | Only reduced bacterial count | Rabbit | [113] |
| Swine | Recombinant subunit and whole-cell bacterin | A and D | D (toxA) and D and A | IM | Controlled prevalence and severity of the disease | Piglet | [65] | |
| Swine | Recombinant subunit | D | toxA (PMT2, 3) | SC | Good humoral and cellular immune responses with passive transfer | Swine | [64] | |
| Swine | Recombinant | A and D | Full-length rOmpH | SC | High Ab | Mice | [114] | |
| Swine | Recombinant subunit | A | Serogroup D (rTorA, rPrx, and/or rPGAM) | Intraperitoneal | High antibodies and IFN-γ, IL-4, and IL-10 in mice with good cross-protection | Mice trial | [93] | |
| Swine | Recombinant bivalent subunit | A | PMT NC adjuvanted with rSly or CpG | IM | Enhanced humoraand cellular immune responses | Piglet | [115] |
4. Conclusions and Future Directions
Pasteurella multocida is an economically important livestock disease that causes various diseases in animals and opportunistic infections in humans. It has four subspecies, five capsular serogroups, and 16 somatic serotypes. Virulence factors include capsular polysaccharides, LPSs; LPS, PMT, and iron acquisition genes; and OMP relationships responsible for phagocytosis, inflammation, adhesion, and nutrient acquisition. Currently, some commonly used antibiotics are developing resistance. Vaccines (inactivated and live) have limitations. Live attenuated vaccines can stimulate cross-protection, while killed vaccines confer serotype-specific immunity. Most vaccines are inactivated whole-cell bacterin vaccines and live attenuated bacterial vaccines. These vaccines have many drawbacks, such as having inconsistent safety records, being less effective, a relatively short-lived protective period, reversion to virulence of live attenuated strains, and a mismatch between the vaccinal serotype strain and circulating wild-type bacteria. Adjuvant formulations vary in immunity duration. Recombinant subunit vaccines are composed of immunogenic proteins selected through in silico analysis and bioinformatics; these are mostly tested extensively to trigger a protective immune response and can be more scalable and intended for large-scale production. Therefore, current vaccine development focuses on identifying immunogenic proteins. For example, PmSLP-3, OmpH, PlpE, and VacJ have shown immunogenic and protective properties in different animal models. In avian, swine, and other species, recombinant and subunit vaccines using these proteins show promise but need more research on antigenic formulations, adjuvants, dosages, and vaccination schedules. DNA vaccines have limited adoption but show some protection. Research on recombinant and subunit vaccines for P. multocida in small ruminants should be emphasized as an alternative to killed vaccines.
Author Contributions
All authors contributed to the writing and approval of the current title. A.B.T. designed, wrote, and prepared the draft review paper. L.F. conceptualized the paper, read the content, and edited the internal composition of the paper, and decided and approved the title. Y.C. conceptualized the content, provided comments, and approved the title. G.M.W. wrote the subsections, provided edits, such as pooling, analyzing, and interpreting the data from the collected literature, and developed the figures. Z.T., L.Y., R.H., and J.Z. contributed sub-sections of the paper, edited the details of the paper, and provided comments and suggestions. The authors were responsible for the final approval of the version set for publication. All authors, drawing on their complementary areas of expertise, contributed to this review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development program of China (2022YFD1800904); National Natural Science Foundation of China (NSFC) (32102678, 32373019); Major Science and Technology Project of Gansu Province (22ZD6NA001; Basic Research Innovation Group Project of Gansu Province (25JRRA434), Gansu, China; and Program of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2021-LVRI).
Conflicts of Interest
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this review paper.
References
- Boyce, J.D.; Harper, M.; Wilkie, I.; Adler, B. Pasteurella. In Pathogenesis of Bacterial Infections in Animals, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 325–346. [Google Scholar]
- Ewers, C.; Lübke-Becker, A.; Bethe, A.; Kießling, S.; Filter, M.; Wieler, L.H. Virulence genotype of Pasteurella multocida strains isolated from different hosts with various disease status. Vet. Microbiol. 2006, 114, 304–317. [Google Scholar] [CrossRef]
- Wilkie, I.W.; Harper, M.; Boyce, J.D.; Adler, B. Pasteurella multocida: Diseases and pathogenesis. Curr. Top. Microbiol. Immunol. 2012, 361, 1–22. [Google Scholar] [CrossRef]
- Roier, S.; Fenninger, J.C.; Leitner, D.R.; Rechberger, G.N.; Reidl, J.; Schild, S. Immunogenicity of Pasteurella multocida and Mannheimia haemolytica outer membrane vesicles. Int. J. Med. Microbiol. 2013, 303, 247–256. [Google Scholar] [CrossRef]
- Radostitis, O.M.; Gay, C.C.; Constable, P.D.; Hinchcliff, K.W. A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats, and Horses, 10th ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 2009. [Google Scholar]
- World Organisation for Animal Health (OIE). Pasteurella spp. Aetiology Epidemiology Diagnosis Prevention and Control Potential Impacts of Disease Agent Beyond Clinical Illness References. 2020. Available online: https://www.woah.org/app/uploads/2021/05/pasteurella-spp-infection-with.pdf (accessed on 16 January 2025).
- Capitini, C.M.; Herrero, I.A.; Patel, R.; Ishitani, M.B.; Boyce, T.G. Wound infection with Neisseriaweaveri and a novel subspecies of Pasteurella multocida in a child who sustained a tiger bite. Clin. Infect. Dis. 2002, 34, E74–E76. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.M.; Boyce, J.D.; Chung, J.Y.; Frost, A.J.; Adler, B. Genetic organization of Pasteurella multocida cap loci and development of a multiplex capsular PCR typing system. J. Clin. Microbiol. 2001, 39, 924–929. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OIE). Terrestrial Manual: Haemorrhagic Septicaemia (Pasteurella multocida Serotypes 6:B and 6:E). Chapter 3.4.10. 2021. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.04.10_HAEMORRHAGIC_SEPTICAEMIA.pdf (accessed on 4 February 2025).
- Sarangi, L.N.; Thoma, P.; Gupta, S.K.; Kumar, S.; Viswas, K.N.; Singh, V.P. Molecular epidemiology of Pasteurella multocida circulating in India by multilocus sequence typing. Transbound. Emerg. Dis. 2014, 63, e286–e292. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, X.; Zhou, R.; Chen, H.; Wilson, B.A.; Wu, B. Pasteurella multocida: Genotypes and genomics. Microbiol. Mol. Biol. Rev. 2019, 83, e00014-19. [Google Scholar] [CrossRef]
- Shayegh, J.; Atashpaz, S.; Hejazi, M.S. Virulence genes profile and typing of ovine Pasteurella multocida. Asian J. Anim. Vet. Adv. 2008, 3, 206–213. [Google Scholar] [CrossRef]
- Boyce, J.D.; Chung, J.Y.; Adler, B. Pasteurella multocida capsule: Composition, function and genetics. J. Biotechnol. 2000, 83, 153–160. [Google Scholar] [CrossRef]
- Richardson, N.I.; Ravenscroft, N.; Kuttel, M.M. Conformational comparisons of Pasteurella multocida types B and E and structurally related capsular polysaccharides. Glycobiology 2023, 33, 745–754. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Dabo, S.M.; Taylor, J.D.; Confer, A.W. Pasteurella multocida and bovine respiratory disease. Anim. Health Res. Rev. 2008, 8, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Lowenstine, L.J.; Osborn, K.G. Respiratory system diseases of nonhuman primates. Nonhum. Primates Biomed. Res. 2012, 2, 413–481. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Schulze-Tanzil, G.; Martel, J.L.; Chaslus-Dancla, E.; Schwarz, S. Antimicrobial resistance in Pasteurella and Mannheimia: Epidemiology and genetic basis. Vet. Res. 2001, 32, 323–339. [Google Scholar] [CrossRef]
- Mwanga, I.; Mzula, A.; Mwega, E.; Chota, A.C.; Wambura, P.N. Virulence attributes and antimicrobial profile of Pasteurella multocida isolated from pneumonic goats in Northern Tanzania. Sci. Afr. 2024, 26, e02490. [Google Scholar] [CrossRef]
- Khamesipour, F.; Momtaz, H.; Azhdary Mamoreh, M. Occurrence of virulence factors and antimicrobial resistance in Pasteurella multocida strains isolated from slaughter cattle in Iran. Front. Microbiol. 2014, 5, 536. [Google Scholar] [CrossRef]
- Katsuda, K.; Hoshinoo, K.; Ueno, Y.; Kohmoto, M.; Mikami, O. Virulence genes and antimicrobial susceptibility in Pasteurella multocida isolates from calves. Vet. Microbiol. 2013, 167, 737–774. [Google Scholar] [CrossRef]
- Furian, T.Q.; Borges, K.A.; Laviniki, V.; Rocha, S.L.D.S.; Almeida, C.N.D.; Nascimento, V.P.D.; Salle, C.T.P.; Moraes, H.L.D.S. Virulence genes and antimicrobial resistance of Pasteurella multocida isolated from poultry and swine. Braz. J. Microbiol. 2016, 47, 210–216. [Google Scholar] [CrossRef]
- Cucco, L.; Massacci, F.R.; Sebastiani, C.; Mangili, P.; Bano, L.; Cocchi, M.; Luppi, A.; Ortenzi, R.; Pezzotti, G.; Magistrali, C.F. Molecular characterization and antimicrobial susceptibility of Pasteurella multocida strains isolated from hosts affected by various diseases in Italy. Vet. Ital. 2017, 53, 21–27. [Google Scholar] [CrossRef]
- Ahmad, T.A.; Rammah, S.S.; Sheweita, S.A.; Haroun, M.; El-Sayed, H. Development of immunization trials against Pasteurella multocida. Vaccine 2014, 32, 909–917. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Yusoff, A.H.; Sulaiman, A.Z.; Awang, M.A.; Lazim, A.M.; Lim, S.J.; Oslan, S.N.; Saad, M.Z.; Ariff, A.B. Pasteurellosis vaccine commercialization: Physiochemical factors for optimum production. Processes 2022, 10, 1248. [Google Scholar] [CrossRef]
- Mostaan, S.; Ghasemzadeh, A.; Sardari, S.; Shokrgozar, M.A.; Brujeni, G.N.; Abolhassani, M.; Ehsani, P.; Karam, M.R.A. Pasteurella multocida vaccine candidates: A systematic review. Avicenna J. Med. Biotechnol. 2020, 12, 140. [Google Scholar]
- Harper, M.; Boyce, J.D.; Adler, B. Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol. Lett. 2006, 265, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; Bulach, D.; Chung, J.; Doughty, S.; Hunt, M.; Rajakumar, K.; Serrano, M.; Van Zanden, A.; Zhang, Y.; Ruffolo, C. Candidate vaccine antigens and genes in Pasteurella multocida. J. Biotechnol. 1999, 73, 83–90. [Google Scholar] [CrossRef]
- Bosch, M.; Garrido, M.E.; De Rozas, A.M.P.; Badiola, I.; Barbé, J.; Llagostera, M. Pasteurella multocida contains multiple immunogenic haemin and haemoglobin-binding proteins. Vet. Microbiol. 2004, 99, 103–112. [Google Scholar] [CrossRef]
- Hatfaludi, T.; Al-Hasani, K.; Boyce, J.D.; Adler, B. Outer membrane proteins of Pasteurella multocida. Vet. Microbiol. 2010, 144, 1–17. [Google Scholar] [CrossRef]
- Guan, L.; Zhang, L.; Xue, Y.; Yang, J.; Zhao, Z. Molecular pathogenesis of the hyaluronic acid capsule of Pasteurella multocida. Microb. Pathog. 2020, 149, 104380. [Google Scholar] [CrossRef]
- Harper, M.; Boyce, J.D.; Adler, B. The key surface components of Pasteurella multocida: Capsule and lipopolysaccharide. Curr. Top. Microbiol. Immunol. 2012, 361, 39–51. [Google Scholar] [CrossRef]
- Fernández-Rojas, M.A.; Vaca, S.; Reyes-López, M.; de la Garza, M.; Aguilar-Romero, F.; Zenteno, E.; Soriano-Vargas, E.; Negrete-Abascal, E. Outer membrane vesicles of Pasteurella multocida contain virulence factors. Microbiol. Open 2014, 3, 711–717. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Z.; Hu, J.; Wu, B.; Cai, X.; He, Q.; Chen, H. Isolation, antimicrobial resistance, and virulence genes of Pasteurella multocida strains from swine in China. J. Clin. Microbiol. 2009, 47, 951–958. [Google Scholar] [CrossRef]
- Smith, E.; Miller, E.; Aguayo, J.M.; Figueroa, C.F.; Nezworski, J.; Studniski, M.; Wileman, B.; Johnson, T. Genomic diversity and molecular epidemiology of Pasteurella multocida. PLoS ONE 2021, 16, e0249138. [Google Scholar] [CrossRef]
- Aski, H.S.; Tabatabaei, M. Occurrence of virulence-associated genes in Pasteurella multocida isolates obtained from different hosts. Microb. Pathog. 2016, 96, 52–57. [Google Scholar] [CrossRef]
- Arumugam, N.D.; Ajam, N.; Blackall, P.J.; Asiah, N.M.; Ramlan, M.; Maria, J.; Yuslan, S.; Thong, K.L. Capsular serotyping of Pasteurella multocida from various animal hosts comparison of phenotypic and genotypic methods. Trop. Biomed. 2011, 28, 55–63. [Google Scholar] [PubMed]
- Davies, R.L.; MacCorquodale, R.; Reilly, S. Characterization of bovine strains of Pasteurella multocida and comparison with isolates of avian, ovine, and porcine origin. Vet. Microbiol. 2004, 99, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Shayegh, J.; Sharaf, J.; Mikaili, P.; Namvar, H. Pheno- and genotyping of Pasteurella multocida isolated from goat in Iran. Afr. J. Biotechnol. 2009, 8, 3707–3710. [Google Scholar]
- Verma, S.; Sharma, M.; Katoch, S.; Verma, L.; Kumar, S.; Dogra, V.; Chahota, R.; Dhar, P.; Singh, G. Profiling of virulence associated genes of Pasteurella multocida isolated from cattle. Vet. Res. Commun. 2013, 37, 83–89. [Google Scholar] [CrossRef]
- Liu HuiSheng, L.H.; Zhao ZhanQin, Z.Z.; Xi XiaoJian, X.X.; Xue Qiao, X.Q.; Long Ta, L.T.; Xue Yun, X.Y. Occurrence of Pasteurella multocida among pigs with respiratory disease in China between 2011 and 2015. Ir. Vet. J. 2017, 70, 2. [Google Scholar] [CrossRef]
- Devi, L.B.; Bora, D.P.; Das, S.K.; Sharma, R.K.; Mukherjee, S.; Hazarika, R.A. Virulence gene profiling of porcine Pasteurella multocida isolates of Assam. Vet. World 2018, 11, 348. [Google Scholar] [CrossRef]
- Fernandez, S.; Galapero, J.; Gomez, L.; Perez, C.J.; Ramos, A.; Cid, D.; Garcia, A.; Rey, J. Identification, capsular typing and virulence factors of Pasteurella multocida isolates from Merino lambs in Extremadura (Southwestern Spain). Vet. Med. 2018, 63, 117–124. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, F.; Lan, S.; Guo, J.; Liu, W.; Li, X.; Luo, Z.; Zhang, M.; Wu, J.; Shi, Y. Investigation of genetic diversity and epidemiological characteristics of Pasteurella multocida isolates from poultry in southwest China by population structure, multi-locus sequence typing and virulence-associated gene profile analysis. J. Vet. Med. Sci. 2018, 80, 921–929. [Google Scholar] [CrossRef]
- Wang, J.; Sang, L.; Sun, S.; Chen, Y.; Chen, D.; Xie, X. Characterization of Pasteurella multocida isolated from dead rabbits with respiratory disease in Fujian, China. BMC Vet. Res. 2019, 15, 438. [Google Scholar] [CrossRef]
- Prajapati, A.; Chanda, M.M.; Yogisharadhya, R.; Parveen, A.; Ummer, J.; Dhayalan, A.; Mohanty, N.N.; Shivachandra, S.B. Comparative genetic diversity analysis based on virulence and repetitive genes profiling of circulating Pasteurella multocida isolates from animal hosts. Infect. Genet. Evol. 2020, 85, 104564. [Google Scholar] [CrossRef] [PubMed]
- Vu-Khac, H.; Trinh, T.H.; Nguyen, T.G.; Nguyen, X.T.; Nguyen, T.T. Prevalence of virulence factor, antibiotic resistance, and serotype genes of Pasteurella multocida strains isolated from pigs in Vietnam. Vet. World 2020, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Mombeni, E.G.; Gharibi, D.; Ghorbanpoor, M.; Jabbari, A.R.; Cid, D. Toxigenic and non-toxigenic Pasteurella multocida genotypes, based on capsular, LPS, and virulence profile typing, associated with pneumonic pasteurellosis in Iran. Vet. Microbiol. 2021, 257, 109077. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Abdolahi, F. Molecular evaluation of sheep and goats isolates of Pasteurella multocida and their antibiotic resistance. Vet. Res. Forum 2023, 14, 481–487. [Google Scholar] [CrossRef]
- D’Amico, F.; Messina, D.; Casalino, G.; Schiavitto, M.; Bove, A.; Romito, D.; D’Onghia, F.P.; Camarda, A.; Circella, E. Characterization of Pasteurella multocida Strains from Different Lesions in Rabbits. Animals 2024, 14, 1569. [Google Scholar] [CrossRef]
- Bostan, T.I.; Torky, H.A.; Ahmed, A.M.; Hassan, O.F. Phenotypic and genotyping characterization of Pasteurella multocida in farm animals. Kafrelsheikh Vet. Med. J. 2016, 14, 99–113. [Google Scholar] [CrossRef]
- Atashpaz, S.; Shayegh, J.; Hejazi, M.S. Rapid virulence typing of Pasteurella multocida by multiplex PCR. Res. Vet. Sci. 2009, 87, 355–357. [Google Scholar] [CrossRef]
- Shayegh, J.; Parvizi, M.; Hejazi, M.S. Diversity of caprine and ovine Pasteurella multocida isolates based on 16S rRNA gene sequencing. Iran. J. Vet. Res. 2010, 11, 373–378. [Google Scholar]
- Cao, X.; Gu, L.; Gao, Z.; Fan, W.; Zhang, Q.; Sheng, J.; Zhang, Y.; Sun, Y. Pathogenicity and Genomic Characteristics Analysis of Pasteurella multocida Serotype A Isolated from Argali Hybrid Sheep. Microorganisms 2024, 12, 1072. [Google Scholar] [CrossRef]
- Cid, D.; García-Alvarez, A.; Domínguez, L.; Fernández-Garayzábal, J.F.; Vela, A.I. Pasteurella multocida isolates associated with ovine pneumonia are toxigenic. Vet. Microbiol. 2019, 232, 70–73. [Google Scholar] [CrossRef]
- Furian, T.Q.; Borges, K.A.; Pilatti, R.M.; Almeida, C.; do Nascimento, V.P.; Salle, C.T.P.; Moraes, H.D.S. Identification of the capsule type of Pasteurella multocida isolates from cases of fowl cholera by multiplex PCR and comparison with phenotypic methods. Braz. J. Poult. Sci. 2014, 16, 31–36. [Google Scholar] [CrossRef]
- El-Demerdash, A.S.; Mowafy, R.E.; Fahmy, H.A.; Matter, A.A.; Samir, M. Pathognomonic features of Pasteurella multocida isolates among various avian species in Sharkia Governorate, Egypt. World J. Microbiol. Biotechnol. 2023, 39, 335. [Google Scholar] [CrossRef]
- Farahani, M.F.; Esmaelizad, M.; Jabbari, A.R. Investigation of iron uptake and virulence gene factors (fur, tonB, exbD, exbB, hgbA, hgbB1, hgbB2 and tbpA) among isolates of Pasteurella multocida from Iran. Iran. J. Microbiol. 2019, 11, 191. [Google Scholar] [PubMed] [PubMed Central]
- García, N.; Fernández-Garayzábal, J.F.; Goyache, J.; Domínguez, L.; Vela, A.I. Associations between biovar and virulence factor genes in Pasteurella multocida isolates from pigs in Spain. Vet. Rec. 2011, 169, 362. [Google Scholar] [CrossRef] [PubMed]
- Step, D.L.; Confer, A.W.; Kirkpatrick, J.G.; Richards, J.B.; Fulton, R.W.; Payton, M.E. Respiratory tract infections in dairy calves from birth to breeding age: Detection by laboratory isolation and antibody responses. Bov. Pract. 2005, 39, 44–53. [Google Scholar] [CrossRef]
- Durairajan, R.; Verma, H.; Prajapati, A.; Abbas, M.; Rawat, M.; Verma, R. Active immunization with Pasteurella multocida lysate elicits antibody that protects rabbits against virulent Pasteurella multocida and protects mice by passive immunization. Indian J. Anim. Res. 2023, 57, 231–235. [Google Scholar] [CrossRef]
- Adler, B.; Chancellor, R.; Homchampa, P.; Hunt, M.; Ruffolo, C.; Strugnell, R.; Wapling, D. Immunity and vaccine development in Pasteurella multocida infections. J. Biotechnol. 1996, 44, 139–144. [Google Scholar] [CrossRef]
- Liao, C.M.; Huang, C.; Hsuan, S.L.; Chen, Z.W.; Lee, W.C.; Liu, C.I.; Winton, J.R.; Chien, M.S. Immunogenicity and efficacy of three recombinant subunit Pasteurella multocida toxin vaccines against progressive atrophic rhinitis in pigs. Vaccine 2006, 24, 27–35. [Google Scholar] [CrossRef]
- Lee, J.; Kang, H.E.; Woo, H.J. Protective immunity conferred by the C-terminal fragment of recombinant Pasteurella multocida toxin. Clin. Vaccine Immunol. 2012, 19, 1526–1531. [Google Scholar] [CrossRef]
- Hsuan, S.L.; Liao, C.M.; Huang, C.; Winton, J.R.; Chen, Z.W.; Lee, W.C.; Liao, J.W.; Chen, T.H.; Chiou, C.J.; Yeh, K.S.; et al. Efficacy of a novel Pasteurella multocida vaccine against progressive atrophic rhinitis of swine. Vaccine 2009, 27, 2923–2929. [Google Scholar] [CrossRef]
- Vasfi Marandi, M.; Mittal, K.R. Role of outer membrane protein H (OmpH)- and OmpA-specific monoclonal antibodies from hybridoma tumors in the protection of mice against Pasteurella multocida. Infect. Immun. 1997, 65, 4502–4508. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.M.; Bester, F.J. Formulation of an effective Pasteurella multocida vaccine for sheep. Onderstepoort J. Vet. Res. 1984, 51, 189–191. [Google Scholar] [PubMed]
- Myint, A.; Nyunt, H.H.; Jones, T.O. Safety, efficacy and cross-protectivity of a live intranasal aerosol haemorrhagic septicaemia vaccine. Vet. Rec. 2005, 156, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Jaiswal, T.N. Haemorrhagic septicaemia vaccines. Vaccine 1998, 16, 1184–1192. [Google Scholar] [CrossRef]
- Almoheer, R.; Abd Wahid, M.E.; Zakaria, H.A.; Jonet, M.A.B.; Al-Shaibani, M.M.; Al-Gheethi, A.; Addis, S.N.K. Spatial, temporal, and demographic patterns in the prevalence of hemorrhagic septicemia in 41 countries in 2005–2019: A systematic analysis with Special Focus on the potential development of a New-Generation Vaccine. Vaccines 2022, 10, 315. [Google Scholar] [CrossRef]
- Domínguez-Odio, A.; Delgado, D.L.C. Global commercialization and research of veterinary vaccines against Pasteurella multocida: 2015–2022 technological surveillance. Vet. World 2023, 16, 946. [Google Scholar] [CrossRef]
- Shivachandra, S.B.; Viswas, K.N.; Kumar, A. A review of hemorrhagic septicemia in cattle and buffalo. Anim. Health Res. Rev. 2011, 12, 67–82. [Google Scholar] [CrossRef]
- Confer, A.W. Immunogens of pasteurella. Vet. Microbiol. 1993, 37, 353–368. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Shen, H.; Liao, Y.; Zhu, D.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Yang, Q.; et al. Immunogenicity and protection of a Pasteurella multocida strain with a truncated lipopolysaccharide outer core in ducks. Vet. Res. 2022, 53, 17. [Google Scholar] [CrossRef]
- He, F.; Xiong, P.; Zhang, H.; Yang, L.; Qiu, Y.; Li, P.; Zhao, G.; Li, N.; Peng, Y. Attenuated vaccine PmCQ2Δ4555–4580 effectively protects mice against Pasteurella multocida infection. BMC Vet. Res. 2024, 20, 94. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Terrestrial Manual: Atrophic Rhinitis of Swine. Chapter 3.9.2. 2018. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.09.02_ATROPHIC_RHINITIS.pdf (accessed on 4 February 2025).
- National Veterinary Institute Ethiopia (NVI). Vaccine Product Catalog. 2016. Available online: https://www.nvi.com.et/wp-content/uploads/2017/11/NVI-Product-catalog-4-1.pdf (accessed on 4 February 2025).
- Abbasi, K.; Tahamtan, Y.; Moazamian, E.; Hosseini, M.H. Formalin and ferric chloride inactivated Pasteurella multocida type adjuvanted with bacterial DNA and alum as a new vaccine candidate in sheep pasteurellosis. Microb. Pathog. 2023, 183, 106282. [Google Scholar] [CrossRef]
- Qasim, M.T.; Hachim, S.K.; Jabr, S.; Mohammed, K.A.; Hamood, S.A. Ovine Pasteurellosis Vaccine: Assessment of the Protective Antibody Titer and Recognition of the Prevailing Serotypes. Arch. Razi Inst. 2022, 77, 1207. [Google Scholar] [CrossRef]
- Guan, L.J.; Yang, J.Q.; Xu, Q.Y.; Feng, Y.F.; Zhang, X.C.; Tang, B.; Zhao, Z.Q. Immunogenicity and efficacy of serogroup A and D bacterins against Pasteurella multocida in mice. Front. Vet. Sci. 2023, 10, 1132536. [Google Scholar] [CrossRef]
- Herath, C.; Kumar, P.; Singh, M.; Kumar, D.; Ramakrishnan, S.; Goswami, T.K.; Singh, A.; Ram, G.C. Experimental iron-inactivated Pasteurella multocida A: 1 vaccine adjuvanted with bacterial DNA is safe and protects chickens from fowl cholera. Vaccine 2010, 28, 2284–2289. [Google Scholar] [CrossRef]
- Homayoon, M.; Tahamtan, Y.; Kargar, M.; Hosseini, S.M.H.; Akhavan Sepahy, A. Pasteurella multocida inactivated with ferric chloride and adjuvanted with bacterial DNA is a potent and efficacious vaccine in Balb/c mice. J. Med. Microbiol. 2018, 67, 1383–1390. [Google Scholar] [CrossRef]
- Rice, J.T.; Dick, J.W.; Bierer, B.W. Subcutaneous vaccination of chickens with a live, avirulent Pasteurella multocida, Vaccine. Poult. Sci. 1978, 57, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Tariq, A. In-silico analysis of Pasteurella multocida to identify common epitopes between fowl, goat, and buffalo. Gene 2016, 580, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Mostaan, S.; Ghasemzadeh, A.; Ehsani, P.; Sardari, S.; Shokrgozar, M.A.; Abolhassani, M.; Brujeni, G.N. In silico analysis of Pasteurella multocida PlpE protein epitopes as novel subunit vaccine candidates. Iran. Biomed. J. 2020, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Fegan, J.E.; Waeckerlin, R.C.; Tesfaw, L.; Islam, E.A.; Deresse, G.; Dufera, D.; Assefa, E.; Woldemedhin, W.; Legesse, A.; Akalu, M.; et al. Developing a PmSLP3-based vaccine formulation that provides robust long-lasting protection against hemorrhagic septicemia–causing serogroup B and E strains of Pasteurella multocida in cattle. Front. Immunol. 2024, 15, 1392681. [Google Scholar] [CrossRef]
- Islam, E.A.; Fegan, J.E.; Tefera, T.A.; Curran, D.M.; Waeckerlin, R.C.; Ng, D.; Ahn, S.K.; Lai, C.H.R.; Nguyen, Q.H.; Shah, M.; et al. Reverse vaccinology-based identification of a novel surface lipoprotein that is an effective vaccine antigen against bovine infections caused by Pasteurella multocida. PLoS Pathog. 2023, 19, e1011249. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, S.; Ranjan, R.; Gupta, S.K.; Singh, V.P.; Sharma, B. Molecular heterogeneity of plpE gene in Indian isolates of Pasteurella multocida and expression of recombinant PlpE in vaccine strain of P. multocida serotype B: 2. J. Vet. Sci. 2010, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.R.; Shien, J.H.; Shieh, H.K.; Chen, C.F.; Chang, P.C. Protective immunity conferred by recombinant Pasteurella multocida lipoprotein E (PlpE). Vaccine 2007, 25, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Okay, S.; Özcengiz, E.; Gürsel, I.; Özcengiz, G. Immunogenicity and protective efficacy of the recombinant Pasteurella lipoprotein E and outer membrane protein H from Pasteurella multocida A:3 in mice. Res. Vet. Sci. 2012, 93, 1261–1265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Xiao, J.; Chang, Y.-F.; Zhang, H.; Teng, Y.; Lin, W.; Li, H.; Chen, W.; Zhang, X.; Xie, Q. Immunogenicity and protective efficacy of the recombinant Pasteurella multocida lipoproteins VacJ and PlpE, and outer membrane protein H from P. multocida A: 1 in ducks. Front. Immunol. 2022, 13, 985993. [Google Scholar] [CrossRef]
- Sthitmatee, N.; Numee, S.; Kawamoto, E.; Sasaki, H.; Yamashita, K.; Takahashi, N.; Kataoka, Y.; Sawada, T. Protection of chickens from fowl cholera by vaccination with recombinant adhesive protein of Pasteurella multocida. Vaccine 2008, 26, 2398–2407. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Ai, W.; Zeng, D.; Liang, W.; Hua, L.; Liu, H.; Wang, X.; Tian, Y.; Chen, H.; et al. Three novel immunogenic proteins determined through 2-Dimensional electrophoresis and mass spectrometry with immune serum confer protection against challenge with porcine Pasteurella multocida in mouse models. Res. Vet. Sci. 2021, 136, 303–309. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Poh, C.L. Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Vet. Res. 2019, 50, 78. [Google Scholar] [CrossRef]
- Qiang, G.; Ming, C.; Ming-fu, N.I.U. Out membrane protein DNA vaccines for protective immunity against virulent avian Pasteurella multocida in chickens. Procedia Environ. Sci. 2011, 8, 723–729. [Google Scholar] [CrossRef][Green Version]
- Yassein, A.A.; Teleb, A.A.; Hassan, G.M.; El Fiky, Z.A. The immune response and protective efficacy of a potential DNA vaccine against virulent Pasteurella multocida. J. Genet. Eng. Biotechnol. 2021, 19, 81. [Google Scholar] [CrossRef]
- Gong, Q.; Qu, N.; Niu, M.; Qin, C.; Cheng, M.; Sun, X.; Zhang, A. Immune responses and protective efficacy of a novel DNA vaccine encoding outer membrane protein of avian Pasteurella multocida. Vet. Immunol. Immunopathol. 2013, 152, 317–324. [Google Scholar] [CrossRef]
- Gong, Q.; Kong, L.Y.; Niu, M.F.; Qin, C.L.; Yang, Y.; Li, X.; Ruan, M.D.; Tian, Y.; Li, Z.L. Construction of a ptfA chitosan nanoparticle DNA vaccine against Pasteurella multocida and the immune response in chickens. Vet. J. 2018, 231, 1–7. [Google Scholar] [CrossRef]
- Chelliah, S.; Velappan, R.D.; Lim, K.T.; Swee, C.W.K.; Nor Rashid, N.; Rothan, H.A.; Kabir, N.; Ismail, S. Potential DNA vaccine for hemorrhagic septicemia disease. Mol. Biotechnol. 2020, 62, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Dagleish, M.P.; Hodgson, J.C.; Ataei, S.; Finucane, A.; Finlayson, J.; Sales, J.; Parton, R.; Coote, J.G. Safety and protective efficacy of intramuscular vaccination with a live aroA derivative of Pasteurella multocida B: 2 against experimental hemorrhagic septicemia in calves. Infect. Immun. 2007, 75, 5837–5844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chengappa, M.M.; McLaughlin, B.G.; Kadel, W.L.; Maddux, R.L.; Greer, S.C. Efficacy of a live Pasteurella multocida vaccine for the prevention of experimentally induced bovine pneumonic pasteurellosis. Vet. Microbiol. 1989, 21, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Tewari, K.; Singh, R. Comparative immunogenicity and protective efficacy of different preparations of outer membrane proteins of Pasteurella multocida (B:2) in a mouse model. Veterinarski Arhiv 2013, 83, 665–676. [Google Scholar]
- Arif, J.; Rahman, S.U.; Arshad, M.; Akhtar, P. Immunopotentiation of outer membrane protein through anti-idiotype Pasteurella multocida vaccine in rabbits. Biologicals 2013, 41, 339–344. [Google Scholar] [CrossRef]
- Du, H.; Wu, C.; Li, C.; Fang, R.; Ma, J.; Ji, J.; Li, Z.; Li, N.; Peng, Y.; Zhou, Z. Two novel cross-protective antigens for bovine Pasteurella multocida. Mol. Med. Rep. 2017, 16, 4627–4633. [Google Scholar] [CrossRef]
- Muangthai, K.; Tankaew, P.; Varinrak, T.; Uthi, R.; Rojanasthien, S.; Sawada, T.; Sthitmatee, N. Intranasal immunization with a recombinant outer membrane protein H based Haemorrhagic septicemia vaccine in dairy calves. J. Vet. Med. Sci. 2018, 80, 68–76. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.P.; Cheema, P.S.; Sandey, M.; Ranjan, R.; Gupta, S.K.; Sharma, B. Immune response to DNA vaccine expressing transferrin binding protein a gene of Pasteurella multocida. Braz. J. Microbiol. 2011, 42, 750–760. [Google Scholar] [CrossRef]
- Mohd Yasin, I.S.; Mohd Yusoff, S.; Mohd, Z.S.; Abd Wahid Mohd, E. Efficacy of an inactivated recombinant vaccine encoding a fimbrial protein of Pasteurella multocida B: 2 against hemorrhagic septicemia in goats. Trop. Anim. Health Prod. 2011, 43, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tatum, F.M.; Tabatabai, L.B.; Briggs, R.E. Cross-protection against fowl cholera disease with the use of recombinant Pasteurella multocida FHAB2 peptides vaccine. Avian Dis. 2012, 56, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Apinda, N.; Yao, Y.; Zhang, Y.; Muenthaisong, A.; Sangkaka, K.; Nambooppha, B.; Rittipornlertrak, A.; Koonyosying, P.; Nair, V.; Sthitmatee, N. Efficiency of NHEJ-CRISPR/Cas9 and Cre-LoxP Engineered Recombinant Turkey Herpesvirus Expressing Pasteurella multocida OmpH Protein for Fowl Cholera Prevention in Ducks. Vaccines 2023, 11, 1498. [Google Scholar] [CrossRef] [PubMed]
- Thanasarasakulpong, A.; Poolperm, P.; Tankaew, P.; Sawada, T.; Sthitmatee, N. Protectivity conferred by immunization with intranasal recombinant outer membrane protein H from Pasteurella multocida serovar A: 1 in chickens. J. Vet. Med. Sci. 2015, 77, 321–326. [Google Scholar] [CrossRef][Green Version]
- Register, K.B.; Sacco, R.E.; Brockmeier, S.L. Immune response in mice and swine to DNA vaccines derived from the Pasteurella multocida toxin gene. Vaccine 2007, 25, 6118–6128. [Google Scholar] [CrossRef]
- Casalino, G.; D’Amico, F.; Bozzo, G.; Dinardo, F.R.; Schiavitto, M.; Galante, D.; Aceti, A.; Ceci, E.; Romito, D.; D’Onghia, F.P.; et al. In-field evaluation of the impact on clinical signs of an inactivated autogenous vaccine against Pasteurella multocida in rabbits. Int. J. Vet. Sci. Med. 2024, 12, 39–47. [Google Scholar] [CrossRef]
- Confer, A.W.; Suckow, M.A.; Montelongo, M.; Dabo, S.M.; Miloscio, L.J.; Gillespie, A.J.; Meredith, G.L. Intranasal vaccination of rabbits with Pasteurella multocida A: 3 outer membranes that express iron-regulated proteins. Am. J. Vet. Res. 2001, 62, 697–703. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, Y.B.; Kwon, M.S. Outer membrane protein H for protective immunity against Pasteurella multocida. J. Microbiol. 2007, 45, 179–184. [Google Scholar] [PubMed]
- Wu, M.C.; Wu, H.; Lee, J.W.; Chang, W.C.; Chu, C.Y. A protein-based subunit vaccine with biological adjuvants provides effective protection against Pasteurella multocida in pigs. Vet. Res. 2023, 54, 17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).