Development of a Cationic Polymeric Micellar Structure with Endosomal Escape Capability Enables Enhanced Intramuscular Transfection of mRNA-LNPs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
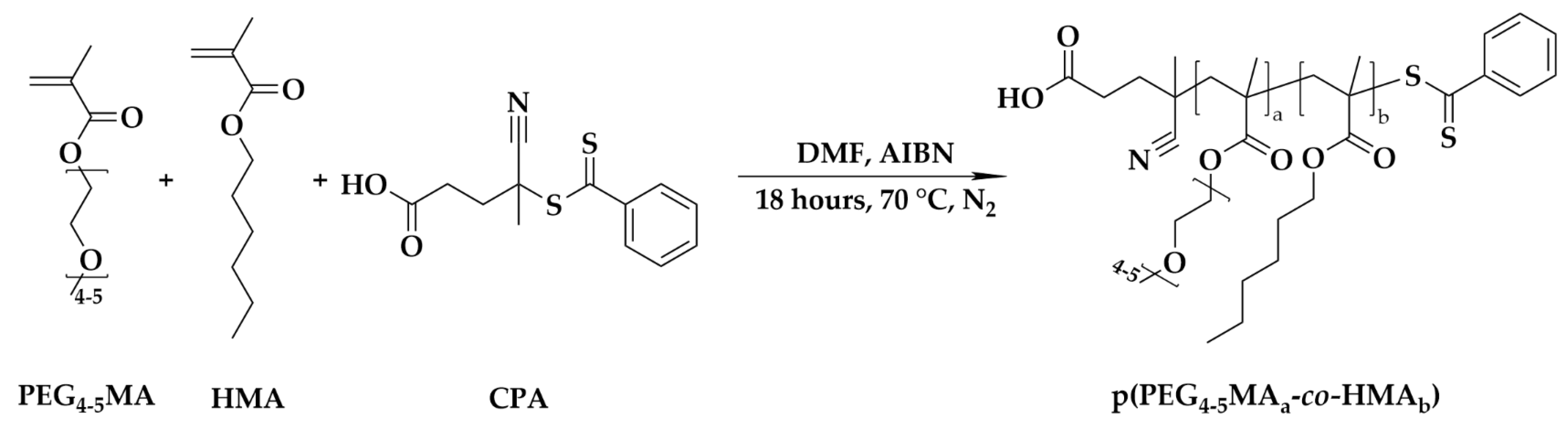
2.2. Synthesis of Macrochain Transfer Agent (Macro-CTA): Poly(poly(ethylene glycol)4-5 methacrylatea-co-hexyl methacrylate)b (p(PEG4-5MAa-co-HMAb))
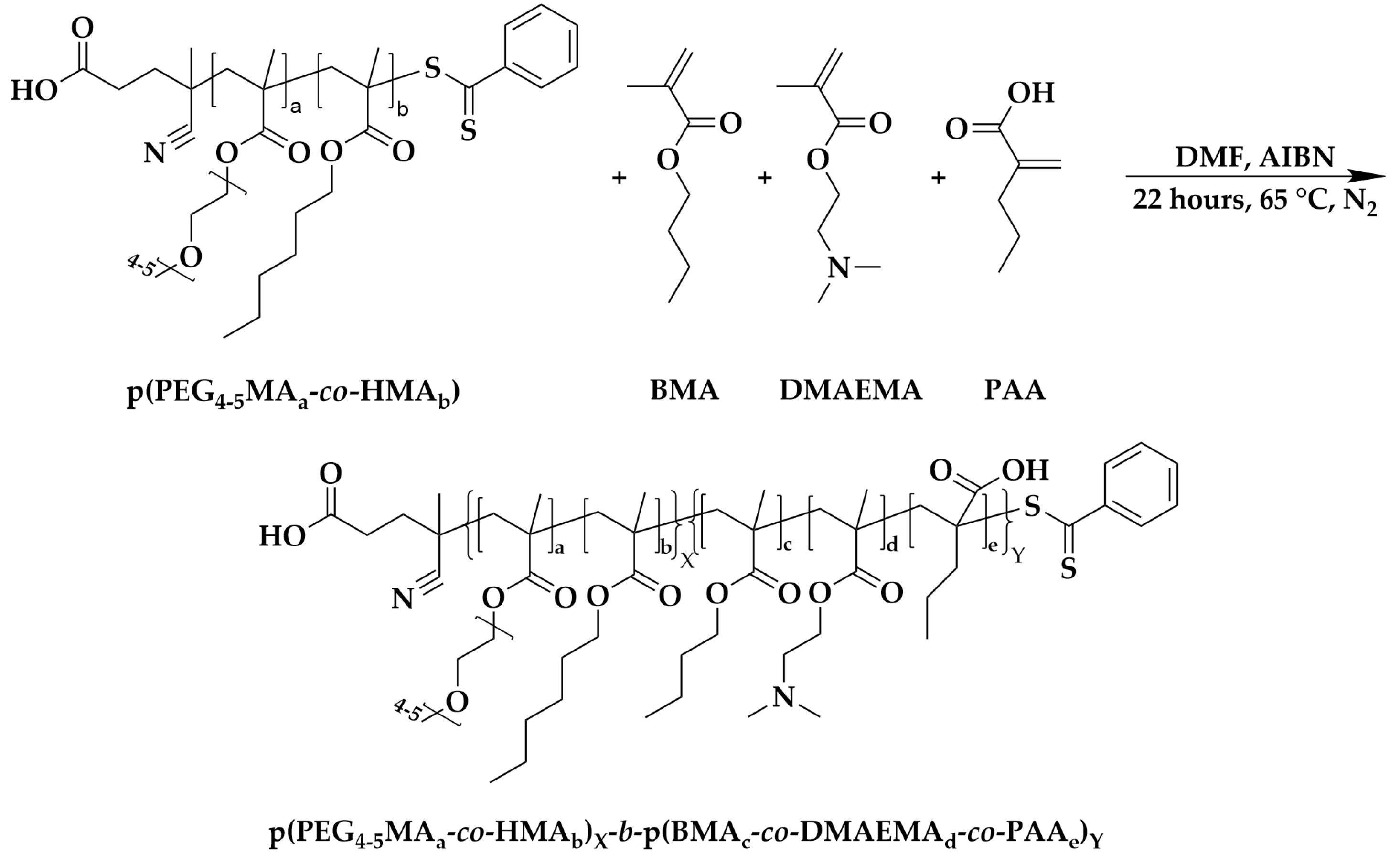
2.3. Synthesis of Cationic Copolymer: Poly (poly (ethylene glycol)4-5 methacrylatea-co-hexyl methacrylateb)X-b-poly(butyl methacrylatec-co-dimethylaminoethyl methacrylated-co-propyl acrylatee)Y (p(PEG4-5MAa-co-HMAb)X-b-p(BMAc-co-DMAEMAd-co-PAAe)Y)
2.4. Proton Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
2.5. Gel Permeation Chromatography (GPC)
2.6. Cationic Polymeric Micelle (cPM) Formulation
2.7. Plasma Isolation and Protein Corona-Coated cPM (PC-cPM) Formulation
2.8. LNP Formulation
2.9. Dynamic Light Scattering (DLS)
2.10. Cryo-Transmission Electron Microscopy (CryoTEM)
2.11. RiboGreen Assay
2.12. Membrane-Destabilizing Activity of cPMs and PC-cPMs
2.12.1. Hemolytic Analysis
2.12.2. The Impact of cPMs and PC-cPM on the Stability of LNPs
2.13. In Vitro Cell Cytotoxicity Assay
2.14. In Vivo Animal Imaging Using the IVIS Spectrum System
2.15. Statistical Analysis
3. Results
3.1. Characterization of Synthesized Macro-Initiator and Cationic Ampholytic Di-Block Copolymer
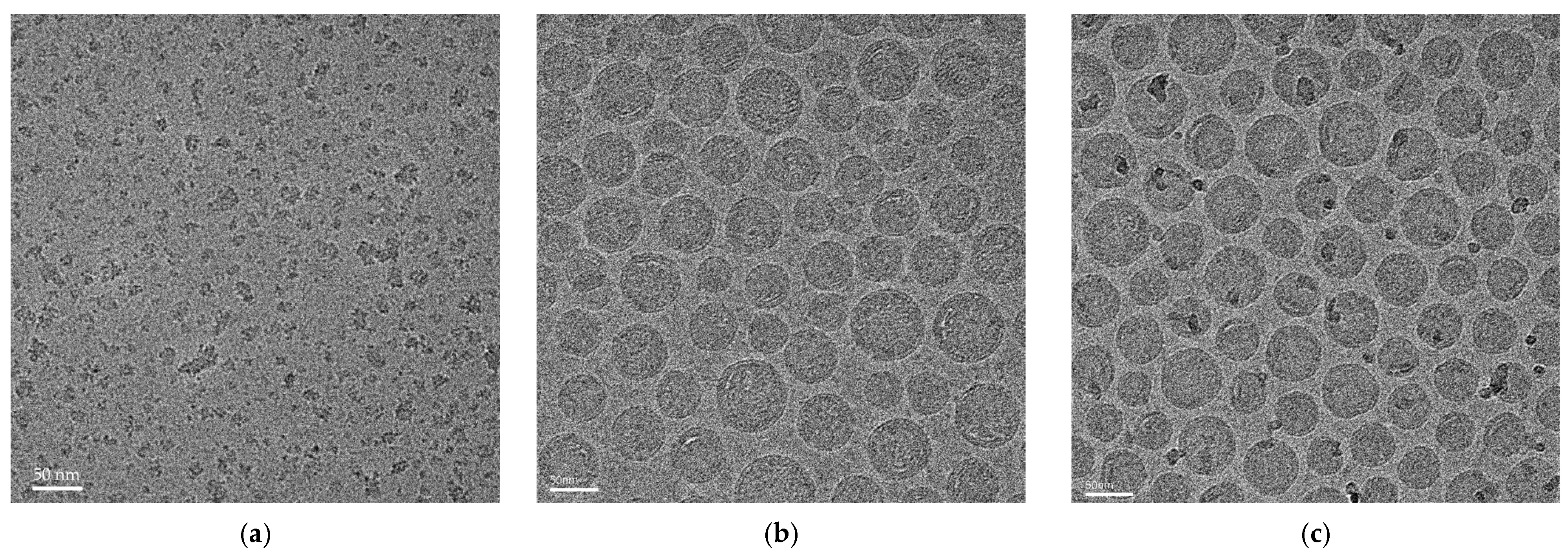
3.2. Characterization of LNPs and cPMs
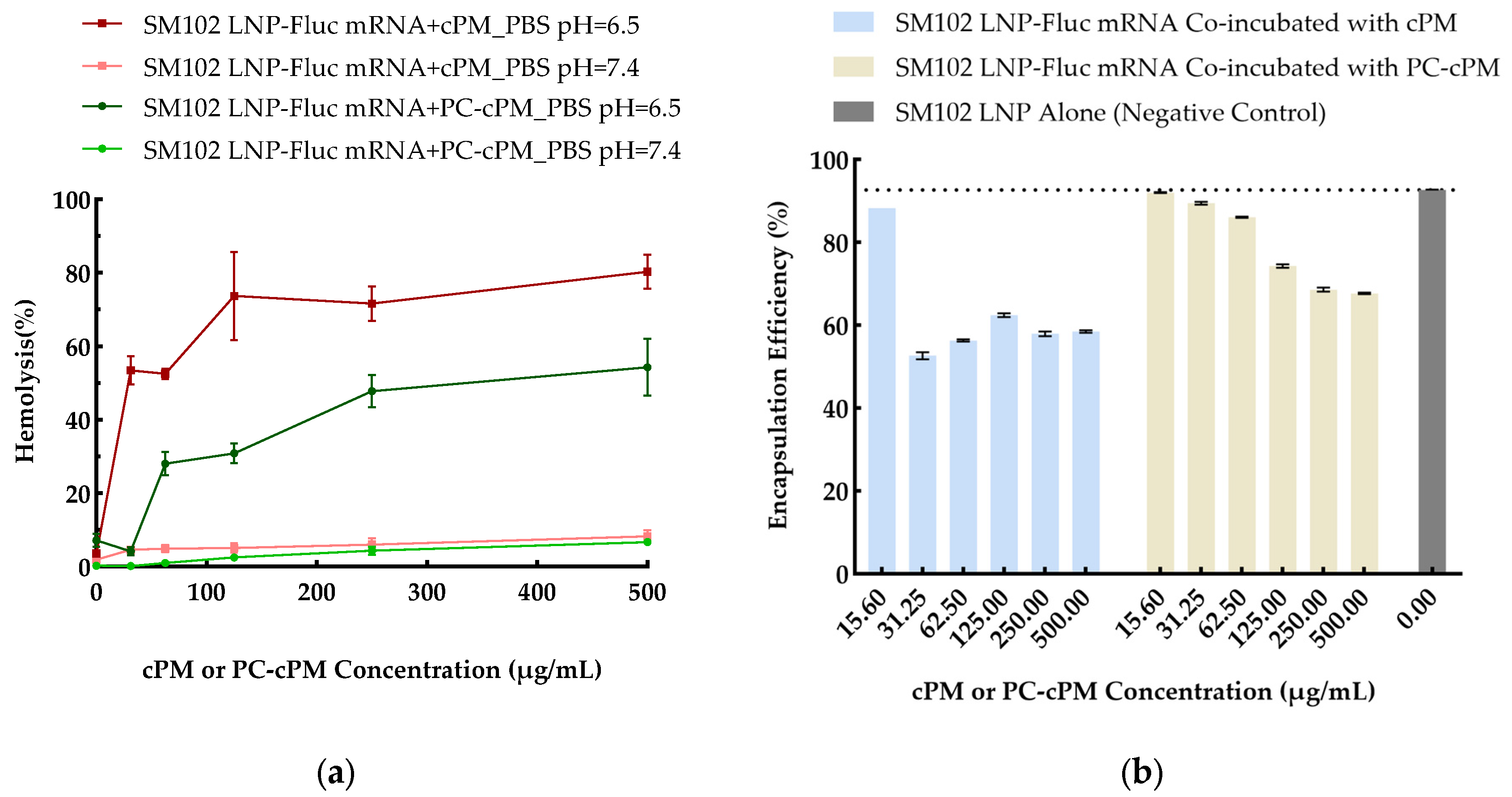
3.3. The Impact of cPMs and PC-cPMs on the Structural Stability and Endosomal Escape Capabilities of LNPs
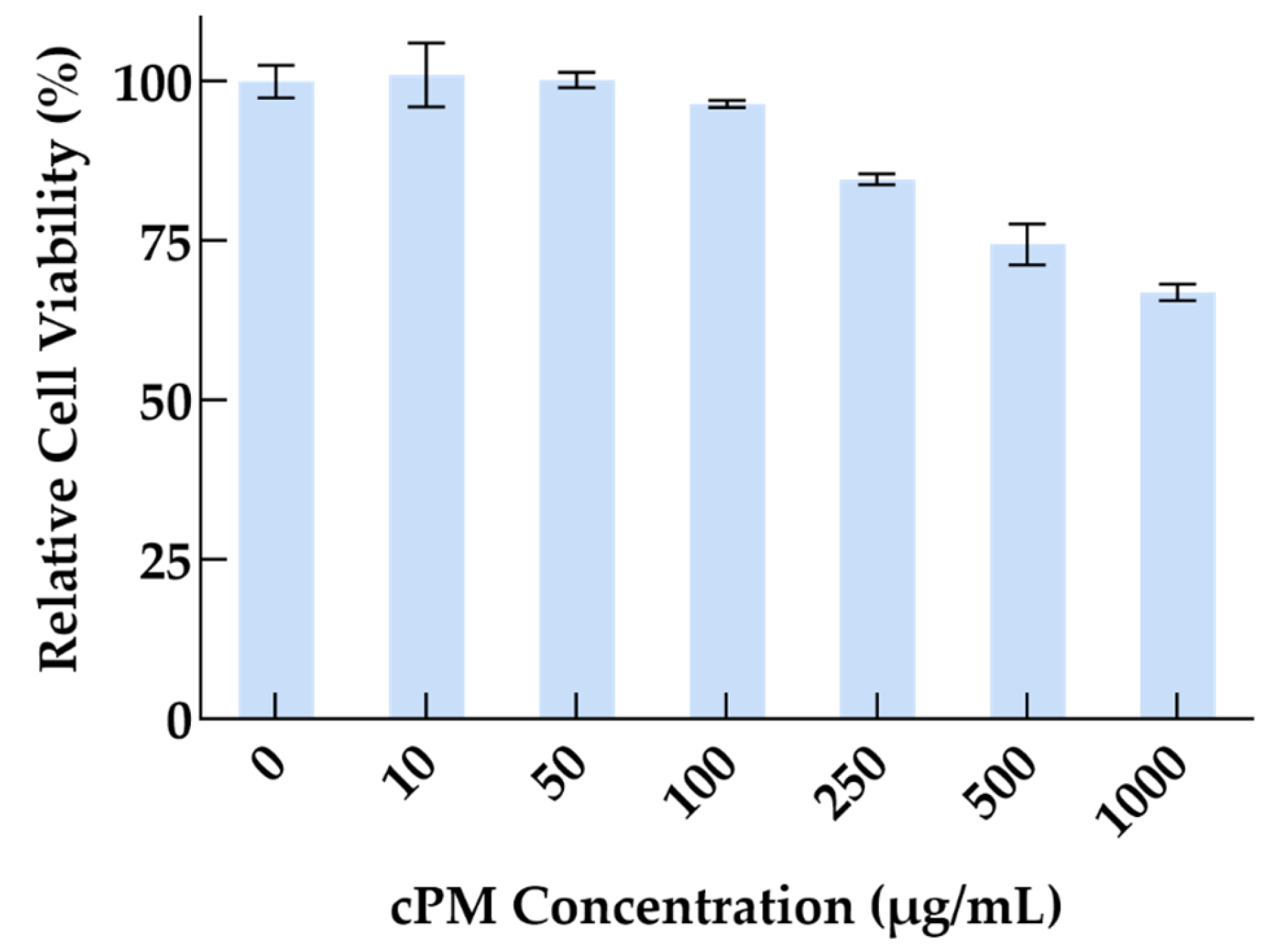
3.4. Cytocompatibility of cPMs
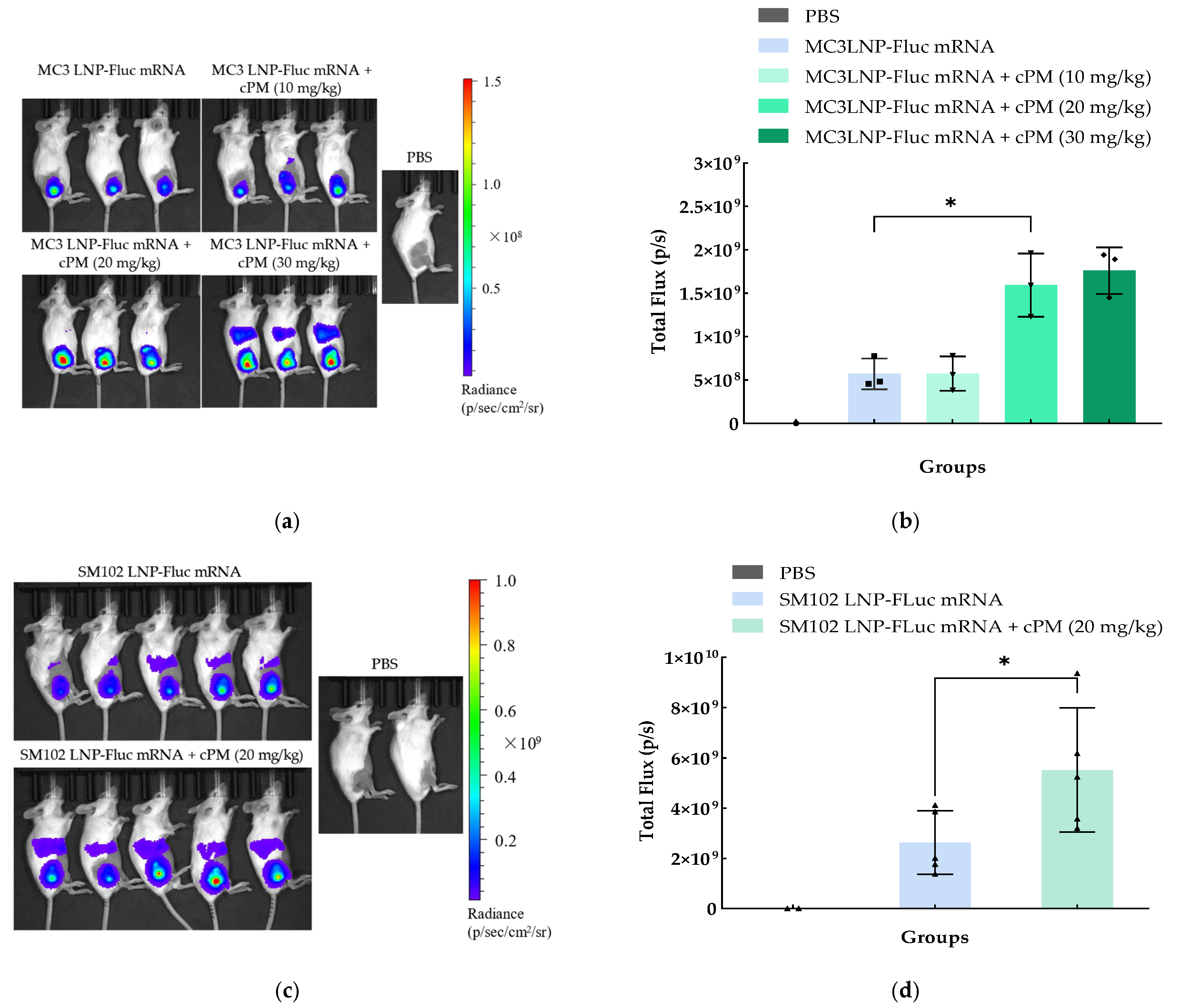
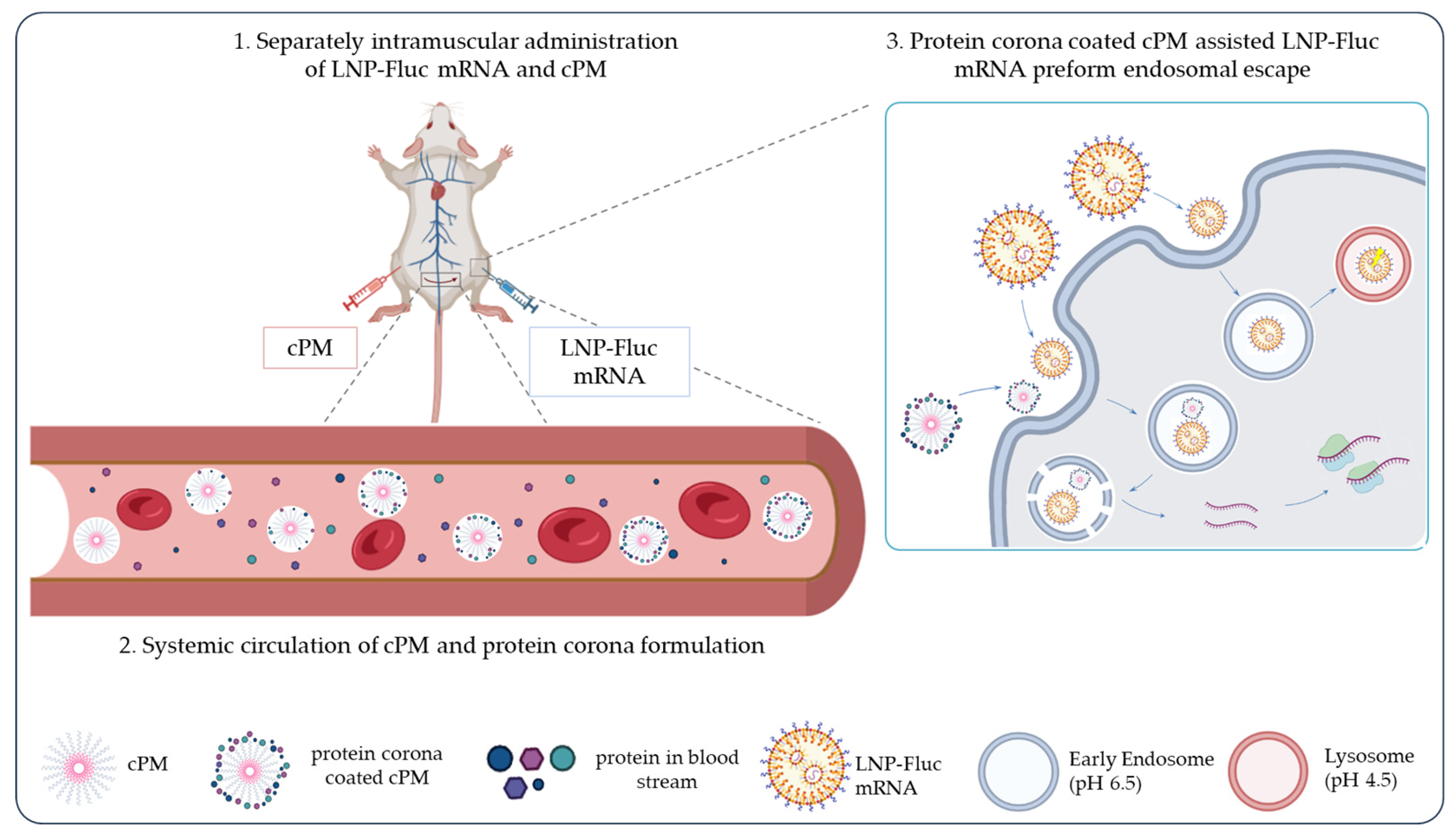
3.5. Bioluminescence Imaging of LNP-Fluc mRNA and cPMs Following Intramuscular Administration
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines; Springer International Publishing: Cham, Switzerland, 2020; Volume 440, pp. 71–110. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal Escape: A Bottleneck for LNP-Mediated Therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Lorenz, A.; Dahlman, J.; Sahay, G. Challenges in Carrier-mediated Intracellular Delivery: Moving beyond Endosomal Barriers. WIREs Nanomed. Nanobiotechnol. 2016, 8, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm. Res. 2021, 38, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Huotari, J.; Helenius, A. Endosome Maturation: Endosome Maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Whitehead, K.A. Tools for Translation: Non-Viral Materials for Therapeutic mRNA Delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic Delivery of Nucleic Acids: The Case of Ionizable Lipid Nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, M.Z.; Payne, T.; Porter, C.J.H.; Pouton, C.W.; Johnston, A.P.R. Beyond the Endosomal Bottleneck: Understanding the Efficiency of mRNA/LNP Delivery. Adv. Funct. Mater. 2024, 34, 2404510. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-Human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based Analysis of Lipid Nanoparticle–Mediated siRNA Delivery, Intracellular Trafficking and Endosomal Escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Wittrup, A.; Ai, A.; Liu, X.; Hamar, P.; Trifonova, R.; Charisse, K.; Manoharan, M.; Kirchhausen, T.; Lieberman, J. Visualizing Lipid-Formulated siRNA Release from Endosomes and Target Gene Knockdown. Nat. Biotechnol. 2015, 33, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Rybak, S.L.; Murphy, R.F. Primary Cell Cultures from Murine Kidney and Heart Differ in Endosomal pH. J. Cell. Physiol. 1998, 176, 216–222. [Google Scholar] [CrossRef]
- Klipp, A.; Burger, M.; Leroux, J.-C. Get out or Die Trying: Peptide- and Protein-Based Endosomal Escape of RNA Therapeutics. Adv. Drug Deliv. Rev. 2023, 200, 115047. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery In Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef] [PubMed]
- Delehedde, C.; Even, L.; Midoux, P.; Pichon, C.; Perche, F. Intracellular Routing and Recognition of Lipid-Based mRNA Nanoparticles. Pharmaceutics 2021, 13, 945. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-L.; Majzoub, R.N.; Shirazi, R.S.; Ewert, K.K.; Chen, Y.-J.; Liang, K.S.; Safinya, C.R. Endosomal Escape and Transfection Efficiency of PEGylated Cationic Liposome–DNA Complexes Prepared with an Acid-Labile PEG-Lipid. Biomaterials 2012, 33, 4928–4935. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, W.; Liu, D.; Wu, J.; Guo, Z.; Ji, X.; Bharwani, Z.; Zhao, L.; Zhao, X.; Farokhzad, O.C.; et al. Surface De-PEGylation Controls Nanoparticle-Mediated siRNA Delivery in Vitro and in Vivo. Theranostics 2017, 7, 1990–2002. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating Endosomal Escape of Polymorphic Lipid Nanoparticles That Boost mRNA Delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Bandara, S.R.; Tan, Z.; Leal, C. Lipid Nanoparticle Topology Regulates Endosomal Escape and Delivery of RNA to the Cytoplasm. Proc. Natl. Acad. Sci. USA 2023, 120, e2301067120. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Paramasivam, P.; Franke, C.; Stöter, M.; Höijer, A.; Bartesaghi, S.; Sabirsh, A.; Lindfors, L.; Arteta, M.Y.; Dahlén, A.; Bak, A.; et al. Endosomal Escape of Delivered mRNA from Endosomal Recycling Tubules Visualized at the Nanoscale. J. Cell Biol. 2022, 221, e202110137. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of Lipid Nanoparticles and Its Impact on the Efficacy of mRNA Vaccines and Therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, A.I.S.; Yun, C.-O.; Schiffelers, R.M.; Hennink, W.E. Polymeric Delivery Systems for Nucleic Acid Therapeutics: Approaching the Clinic. J. Control. Release 2021, 331, 121–141. [Google Scholar] [CrossRef]
- Ulkoski, D.; Bak, A.; Wilson, J.T.; Krishnamurthy, V.R. Recent Advances in Polymeric Materials for the Delivery of RNA Therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1149–1167. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kim, H.J.; Lim, S.B.; Naito, M.; Miyata, K. Recent Progress in the Endosomal Escape Mechanism and Chemical Structures of Polycations for Nucleic Acid Delivery. Macromol. Biosci. 2024, 24, 2300366. [Google Scholar] [CrossRef] [PubMed]
- Degors, I.M.S.; Wang, C.; Rehman, Z.U.; Zuhorn, I.S. Carriers Break Barriers in Drug Delivery: Endocytosis and Endosomal Escape of Gene Delivery Vectors. Acc. Chem. Res. 2019, 52, 1750–1760. [Google Scholar] [CrossRef]
- Winkeljann, B.; Keul, D.C.; Merkel, O.M. Engineering Poly- and Micelleplexes for Nucleic Acid Delivery—A Reflection on Their Endosomal Escape. J. Control. Release 2023, 353, 518–534. [Google Scholar] [CrossRef]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Karimi Zade, A.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-Based Nanocarriers in Co-Delivery of Drug and Gene: A Developing Horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef]
- Kim, M.; Oh, J.; Lee, Y.; Lee, E.-H.; Ko, S.H.; Jeong, J.H.; Park, C.H.; Lee, M. Delivery of Self-Replicating Messenger RNA into the Brain for the Treatment of Ischemic Stroke. J. Control. Release 2022, 350, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Wightman, L.; Kircheis, R.; Rössler, V.; Carotta, S.; Ruzicka, R.; Kursa, M.; Wagner, E. Different Behavior of Branched and Linear Polyethylenimine for Gene Deliveryin Vitro Andin Vivo. J. Gene Med. 2001, 3, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Suh, H.-W.; Suberi, A.; Whang, C.-H.; Ene, M.; Grundler, J.; Grun, M.K.; Saltzman, W.M. Branching in Poly(Amine-Co-Ester) Polyplexes Impacts mRNA Transfection. Biomaterials 2024, 311, 122692. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, N.D.; Szoka, F.C.; Verkman, A.S. Chloride Accumulation and Swelling in Endosomes Enhances DNA Transfer by Polyamine-DNA Polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Zhao, Z. Poly (β Amino Esters) Copolymers: Novel Potential Vectors for Delivery of Genes and Related Therapeutics. Int. J. Pharm. 2022, 611, 121289. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Itaka, K.; Nomoto, T.; Ishii, T.; Suma, T.; Ikegami, M.; Miyata, K.; Oba, M.; Nishiyama, N.; Kataoka, K. Modulated Protonation of Side Chain Aminoethylene Repeats in N-Substituted Polyaspartamides Promotes mRNA Transfection. J. Am. Chem. Soc. 2014, 136, 12396–12405. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.; Kim, B.S.; Ogura, S.; Kamegawa, R.; Naito, M.; Yamasaki, Y.; Kim, H.J.; Miyata, K. Fine-Tuning of Polyaspartamide Derivatives with Alicyclic Moieties for Systemic mRNA Delivery. J. Control. Release 2022, 342, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ulkoski, D.; Munson, M.J.; Jacobson, M.E.; Palmer, C.R.; Carson, C.S.; Sabirsh, A.; Wilson, J.T.; Krishnamurthy, V.R. High-Throughput Automation of Endosomolytic Polymers for mRNA Delivery. ACS Appl. Bio. Mater. 2021, 4, 1640–1654. [Google Scholar] [CrossRef] [PubMed]
- Betker, J.L.; Tilden, S.G.; Anchordoquy, T.J. Escaping to Silence Using an Endosome-Disrupting Polymer. Mol. Ther. 2021, 29, 2893–2894. [Google Scholar] [CrossRef] [PubMed]
- Helmschrodt, C.; Höbel, S.; Schöniger, S.; Bauer, A.; Bonicelli, J.; Gringmuth, M.; Fietz, S.A.; Aigner, A.; Richter, A.; Richter, F. Polyethylenimine Nanoparticle-Mediated siRNA Delivery to Reduce α-Synuclein Expression in a Model of Parkinson’s Disease. Mol. Ther. Nucleic Acids 2017, 9, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mondal, S.K.; Tzeng, S.Y.; Rui, Y.; Al-kharboosh, R.; Kozielski, K.K.; Bhargav, A.G.; Garcia, C.A.; Quiñones-Hinojosa, A.; Green, J.J. Poly(Ethylene Glycol)–Poly(Beta-Amino Ester)-Based Nanoparticles for Suicide Gene Therapy Enhance Brain Penetration and Extend Survival in a Preclinical Human Glioblastoma Orthotopic Xenograft Model. ACS Biomater. Sci. Eng. 2020, 6, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.E.; Kintzing, J.R.; Hanna, A.; Shannon, J.M.; Gupta, M.K.; Duvall, C.L. Balancing Cationic and Hydrophobic Content of PEGylated siRNA Polyplexes Enhances Endosome Escape, Stability, Blood Circulation Time, and Bioactivity in Vivo. ACS Nano 2013, 7, 8870–8880. [Google Scholar] [CrossRef]
- Kim, H.J.; Ogura, S.; Otabe, T.; Kamegawa, R.; Sato, M.; Kataoka, K.; Miyata, K. Fine-Tuning of Hydrophobicity in Amphiphilic Polyaspartamide Derivatives for Rapid and Transient Expression of Messenger RNA Directed toward Genome Engineering in Brain. ACS Cent. Sci. 2019, 5, 1866–1875. [Google Scholar] [CrossRef]
- Meenakshi Sundaram, D.N.; Plianwong, S.; Kc, R.; Ostergaard, H.; Uludağ, H. In Vitro Cytotoxicity and Cytokine Production by Lipid-Substituted Low Molecular Weight Branched PEIs Used for Gene Delivery. Acta Biomater. 2022, 148, 279–297. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.P.; Sarisozen, C.; Luther, E.; Pan, J.; Torchilin, V.P. Surface-Engineered Polyethyleneimine-Modified Liposomes as Novel Carrier of siRNA and Chemotherapeutics for Combination Treatment of Drug-Resistant Cancers. Drug Deliv. 2019, 26, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Miura, Y.; Kobayashi, M.; Chintrakulchai, W.; Toyoda, M.; Ogi, K.; Michinishi, J.; Ohtake, T.; Honda, Y.; Nomoto, T.; et al. Fine Tuning of the Net Charge Alternation of Polyzwitterion Surfaced Lipid Nanoparticles to Enhance Cellular Uptake and Membrane Fusion Potential. Sci. Technol. Adv. Mater. 2024, 25, 2338785. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Q.; Jiang, Q.; Huang, Y.; Liu, H.; Zhao, Y.; Cao, W.; Ma, G.; Dai, F.; Liang, X.; et al. Enhanced Endosomal/Lysosomal Escape by Distearoyl Phosphoethanolamine-Polycarboxybetaine Lipid for Systemic Delivery of siRNA. J. Control. Release 2014, 176, 104–114. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Kauffman, K.J.; Fenton, O.S.; Sadtler, K.; Patel, A.K.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Optimization of a Degradable Polymer–Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett. 2018, 18, 6449–6454. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Patel, A.K.; Rhym, L.H.; Palmiero, U.C.; Bhat, B.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Systemic Delivery of mRNA and DNA to the Lung Using Polymer-Lipid Nanoparticles. Biomaterials 2021, 275, 120966. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Vaccine Induces Neutralizing Antibodies and Poly-Specific T Cells in Humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Evans, W.H.; Hardison, W.G. Phospholipid, Cholesterol, Polypeptide and Glycoprotein Composition of Hepatic Endosome Subfractions. Biochem. J. 1985, 232, 33–36. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex Vivo Red Blood Cell Hemolysis Assay for the Evaluation of pH-Responsive Endosomolytic Agents for Cytosolic Delivery of Biomacromolecular Drugs. JoVE 2013, 73, 50166. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wang, Y.; Zhou, J.; Jiao, X.; Han, M.; Zhang, X.; Hu, H.; Su, R.; Zhang, Y.; et al. Overcoming Endosomal Escape Barriers in Gene Drug Delivery Using De Novo Designed pH-Responsive Peptides. ACS Nano 2024, 18, 10324–10340. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.L.; Reuter, K.G.; Kai, M.P.; Herlihy, K.P.; Jones, S.W.; Luft, J.C.; Napier, M.; Bear, J.E.; DeSimone, J.M. PEGylated PRINT Nanoparticles: The Impact of PEG Density on Protein Binding, Macrophage Association, Biodistribution, and Pharmacokinetics. Nano Lett. 2012, 12, 5304–5310. [Google Scholar] [CrossRef]
- Jones, R.A.; Poniris, M.H.; Wilson, M.R. pDMAEMA Is Internalised by Endocytosis but Does Not Physically Disrupt Endosomes. J. Control. Release 2004, 96, 379–391. [Google Scholar] [CrossRef]
- Ma, D. Enhancing Endosomal Escape for Nanoparticle Mediated siRNA Delivery. Nanoscale 2014, 6, 6415. [Google Scholar] [CrossRef]
- Felber, A.E.; Dufresne, M.-H.; Leroux, J.-C. pH-Sensitive Vesicles, Polymeric Micelles, and Nanospheres Prepared with Polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Convertine, A.J.; Benoit, D.S.W.; Duvall, C.L.; Hoffman, A.S.; Stayton, P.S. Development of a Novel Endosomolytic Diblock Copolymer for siRNA Delivery. J. Control. Release 2009, 133, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Cheung, C.Y.; Black, F.E.; Zia, J.K.; Stayton, P.S.; Hoffman, A.S.; Wilson, M.R. Poly(2-Alkylacrylic Acid) Polymers Deliver Molecules to the Cytosol by pH-Sensitive Disruption of Endosomal Vesicles. Biochem. J. 2003, 372, 65–75. [Google Scholar] [CrossRef]
- Convertine, A.J.; Diab, C.; Prieve, M.; Paschal, A.; Hoffman, A.S.; Johnson, P.H.; Stayton, P.S. pH-Responsive Polymeric Micelle Carriers for siRNA Drugs. Biomacromolecules 2010, 11, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Prieve, M.G.; Harvie, P.; Monahan, S.D.; Roy, D.; Li, A.G.; Blevins, T.L.; Paschal, A.E.; Waldheim, M.; Bell, E.C.; Galperin, A.; et al. Targeted mRNA Therapy for Ornithine Transcarbamylase Deficiency. Mol. Ther. 2018, 26, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Lundy, B.B.; Convertine, A.; Miteva, M.; Stayton, P.S. Neutral Polymeric Micelles for RNA Delivery. Bioconjugate Chem. 2013, 24, 398–407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, C.; Convertine, A.J.; Stayton, P.S.; Bryers, J.D. Multifunctional Triblock Copolymers for Intracellular Messenger RNA Delivery. Biomaterials 2012, 33, 6868–6876. [Google Scholar] [CrossRef]
- Duvall, C.L.; Convertine, A.J.; Benoit, D.S.W.; Hoffman, A.S.; Stayton, P.S. Intracellular Delivery of a Proapoptotic Peptide via Conjugation to a RAFT Synthesized Endosomolytic Polymer. Mol. Pharm. 2010, 7, 468–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Bassi, E.; Passarelli, C.; Braghetta, P.; Ferlini, A. Biodistribution Studies of Polymeric Nanoparticles for Drug Delivery in Mice. Hum. Gene Ther. 2014, 25, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Åslund, A.K.O.; Vandebriel, R.J.; Caputo, F.; De Jong, W.H.; Delmaar, C.; Hyldbakk, A.; Rustique, E.; Schmid, R.; Snipstad, S.; Texier, I.; et al. A Comparative Biodistribution Study of Polymeric and Lipid-Based Nanoparticles. Drug Deliv. Transl. Res. 2022, 12, 2114–2131. [Google Scholar] [CrossRef]
- Obst, K.; Yealland, G.; Balzus, B.; Miceli, E.; Dimde, M.; Weise, C.; Eravci, M.; Bodmeier, R.; Haag, R.; Calderón, M.; et al. Protein Corona Formation on Colloidal Polymeric Nanoparticles and Polymeric Nanogels: Impact on Cellular Uptake, Toxicity, Immunogenicity, and Drug Release Properties. Biomacromolecules 2017, 18, 1762–1771. [Google Scholar] [CrossRef]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic Understanding of in Vivo Protein Corona Formation on Polymeric Nanoparticles and Impact on Pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the Nanoparticle–Protein Corona Using Methods to Quantify Exchange Rates and Affinities of Proteins for Nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein Adsorption and Cellular Uptake of Cerium Oxide Nanoparticles as a Function of Zeta Potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Blundell, E.L.C.J.; Healey, M.J.; Holton, E.; Sivakumaran, M.; Manstana, S.; Platt, M. Characterisation of the Protein Corona Using Tunable Resistive Pulse Sensing: Determining the Change and Distribution of a Particle’s Surface Charge. Anal Bioanal. Chem. 2016, 408, 5757–5768. [Google Scholar] [CrossRef] [PubMed]
- Alberg, I.; Kramer, S.; Schinnerer, M.; Hu, Q.; Seidl, C.; Leps, C.; Drude, N.; Möckel, D.; Rijcken, C.; Lammers, T.; et al. Polymeric Nanoparticles with Neglectable Protein Corona. Small 2020, 16, 1907574. [Google Scholar] [CrossRef] [PubMed]
- Smolková, B.; MacCulloch, T.; Rockwood, T.F.; Liu, M.; Henry, S.J.W.; Frtús, A.; Uzhytchak, M.; Lunova, M.; Hof, M.; Jurkiewicz, P.; et al. Protein Corona Inhibits Endosomal Escape of Functionalized DNA Nanostructures in Living Cells. ACS Appl. Mater. Interfaces 2021, 13, 46375–46390. [Google Scholar] [CrossRef]
- Xiao, W.; Gao, H. The Impact of Protein Corona on the Behavior and Targeting Capability of Nanoparticle-Based Delivery System. Int. J. Pharm. 2018, 552, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; He, B.; Tao, J.; He, Y.; Deng, H.; Wang, X.; Zheng, Y. Application of Förster Resonance Energy Transfer (FRET) Technique to Elucidate Intracellular and In Vivo Biofate of Nanomedicines. Adv. Drug Deliv. Rev. 2019, 143, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The Shape Effect of Mesoporous Silica Nanoparticles on Biodistribution, Clearance, and Biocompatibility In Vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef]
| Polymer | Mn 1 (kDa) | Mw 1 (kDa) | Ð 1 | 1st Block | 2nd Block | |||
|---|---|---|---|---|---|---|---|---|
| % PEG4-5MA 2 | % HMA 2 | % BMA 2 | % DMAEMA 2 | % PAA 2 | ||||
| p(PEG4-5MAa-co-HMAb) | 7.3 | 8.1 | 1.1 | 85.7 | 14.3 | / | / | / |
| p(PEG4-5MAa-co-HMAb)X-b-p(BMAc-co-DMAEMAd-co-PAAe)Y | 39.8 | 64.3 | 1.6 | 85.7 | 14.3 | 50.0 | 36.2 | 13.8 |
| Nanoparticle | Size (nm) | PDI | Zeta (mV) | EE (%) | |||
|---|---|---|---|---|---|---|---|
| Ave. | Sta.Dev. | Ave. | Sta.Dev. | Ave. | Sta.Dev. | ||
| SM102 LNP | 90.11 | 1.3 | 0.14 | 0.09 | 10.15 | 0.28 | 91 |
| MC3 LNP | 83.63 | 0.19 | 0.15 | 0.03 | 10.77 | 0.80 | 93 |
| cPM | 61.31 | 0.68 | 0.21 | 0.01 | 37.76 | 2.18 | / |
| PC-cPM | 265.70 | 15.31 | 0.25 | 0.05 | −13.67 | 1.46 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Shao, H.; Shang, H.; Pang, L.; Chen, X.; Cao, J.; Wang, Y.; Zhao, Z. Development of a Cationic Polymeric Micellar Structure with Endosomal Escape Capability Enables Enhanced Intramuscular Transfection of mRNA-LNPs. Vaccines 2025, 13, 25. https://doi.org/10.3390/vaccines13010025
Deng S, Shao H, Shang H, Pang L, Chen X, Cao J, Wang Y, Zhao Z. Development of a Cationic Polymeric Micellar Structure with Endosomal Escape Capability Enables Enhanced Intramuscular Transfection of mRNA-LNPs. Vaccines. 2025; 13(1):25. https://doi.org/10.3390/vaccines13010025
Chicago/Turabian StyleDeng, Siyuan, Han Shao, Hongtao Shang, Lingjin Pang, Xiaomeng Chen, Jingyi Cao, Yi Wang, and Zhao Zhao. 2025. "Development of a Cationic Polymeric Micellar Structure with Endosomal Escape Capability Enables Enhanced Intramuscular Transfection of mRNA-LNPs" Vaccines 13, no. 1: 25. https://doi.org/10.3390/vaccines13010025
APA StyleDeng, S., Shao, H., Shang, H., Pang, L., Chen, X., Cao, J., Wang, Y., & Zhao, Z. (2025). Development of a Cationic Polymeric Micellar Structure with Endosomal Escape Capability Enables Enhanced Intramuscular Transfection of mRNA-LNPs. Vaccines, 13(1), 25. https://doi.org/10.3390/vaccines13010025






