Immunity to Varicella Zoster Virus in Healthcare Workers: A Systematic Review and Meta-Analysis (2024)
Abstract
1. Introduction
- Provide an estimate of the seroprevalence of the seronegative status for VZV in HCWs;
- As the VZV vaccine can be delivered either as a monovalent formulate or associated with measles, parotitis, and rubella (MPR) vaccine, estimate whether seroprevalence rates for VZV can be compared to other exanthema such as measles and rubella;
- As occupational physicians often are requested to perform medical surveillance without serological data, ascertain whether medical history could be a reliable hint for seroprevalence status.
2. Materials and Methods
2.1. Research Concept
2.2. Research Strategy
2.2.1. PubMed
2.2.2. EMBASE
2.2.3. Scopus
2.3. Inclusion and Exclusion Criteria
- (1)
- Reporting on HCWs directly involved in the management of patients, of any age group, in any healthcare setting (e.g., hospitals, nursing homes, etc.);
- (2)
- Providing the total number of sampled HCWs;
- (3)
- Providing the VZV seroprevalence either as crude prevalence or percent values.
- (1)
- Including workers from healthcare settings not directly involved in the management of patients (e.g., laboratory workers; occupational cleaners, and hospital waste handlers, etc.);
- (2)
- Reporting on medical students from pre-clinical years;
- (3)
- Studies not including original data (i.e., systematic reviews, meta-analysis, editorial comments); case reports and/or case series; original studies still not peer-reviewed (i.e., in preprint status);
- (4)
- Not providing the total number of sampled HCWs;
- (5)
- Not providing the timeframe and/or geographical settings of the study;
- (6)
- The full text was unavailable through online repositories or by inter-library loan;
- (7)
- The main text of the relevant study was written in a language not understood by any of authors (i.e., English, Italian, German, French, Spanish, Portuguese, Turkish);
- (8)
- Not providing details on the laboratory diagnosis of VZV seroprevalence.
2.4. Selection Criteria
2.5. Data Extraction
- (a)
- Main characteristics of the study: first author’s name, year of publication, timeframe of the study; geographical settings;
- (b)
- Characteristics of the study group: sample size, baseline data of sampled HCWs (gender; age groups: proportion of individuals aged <30 y.o. vs. ≥30 y.o.), occupational groups (nurses, physicians, other professionals);
- (c)
- Outcome data: seroprevalence of VZV;
- (d)
- Supplementary data: seroprevalence of rubella and measles; self-reported data on the previous infection by VZV.
2.6. Quality Assessment (Risk of Bias)
2.7. Data Analysis
2.7.1. Descriptive Analysis
2.7.2. Diagnostic Accuracy of Medical History
2.7.3. Meta-Analysis
3. Results
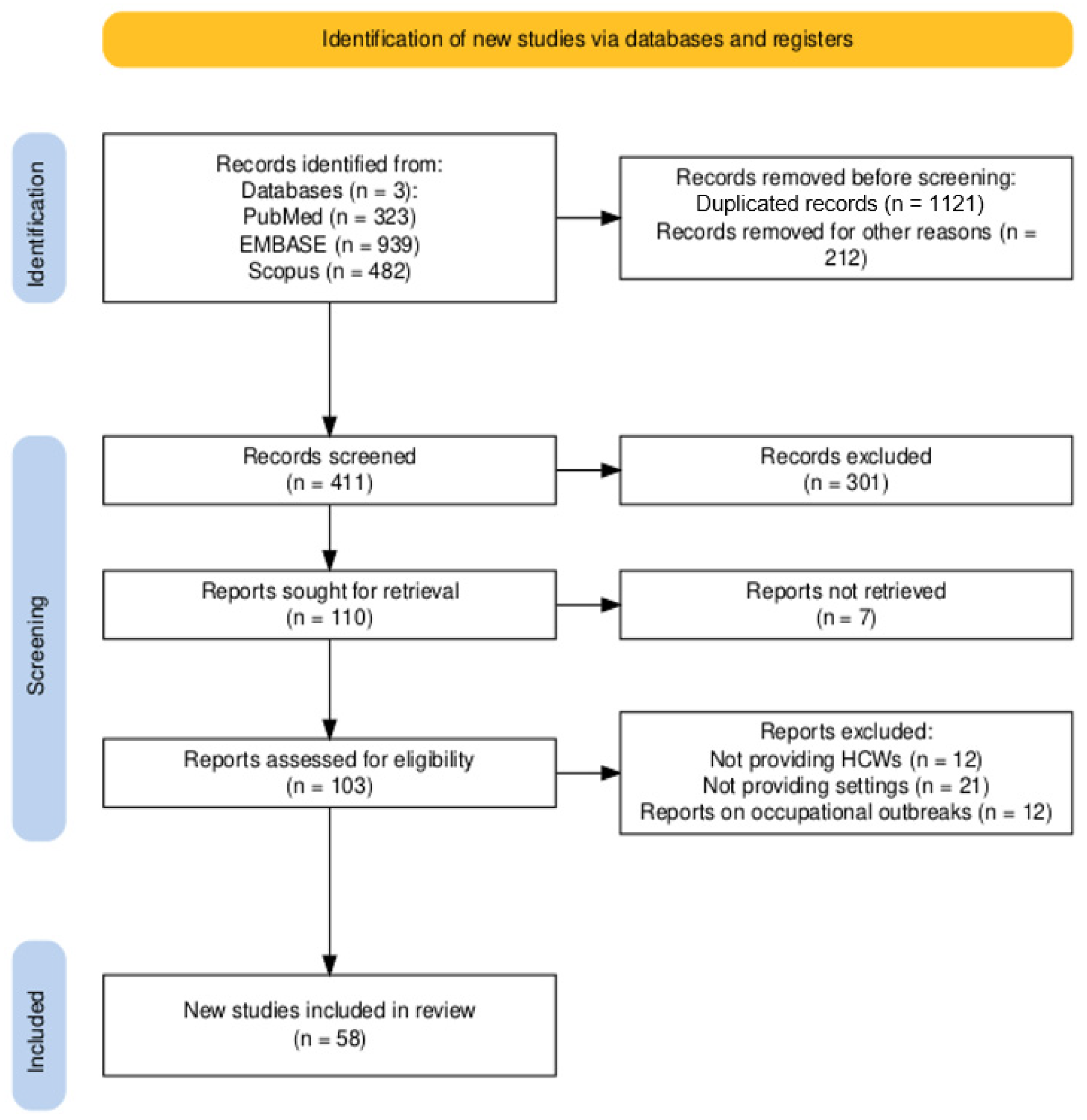
3.1. Summary of Retrieved Studies
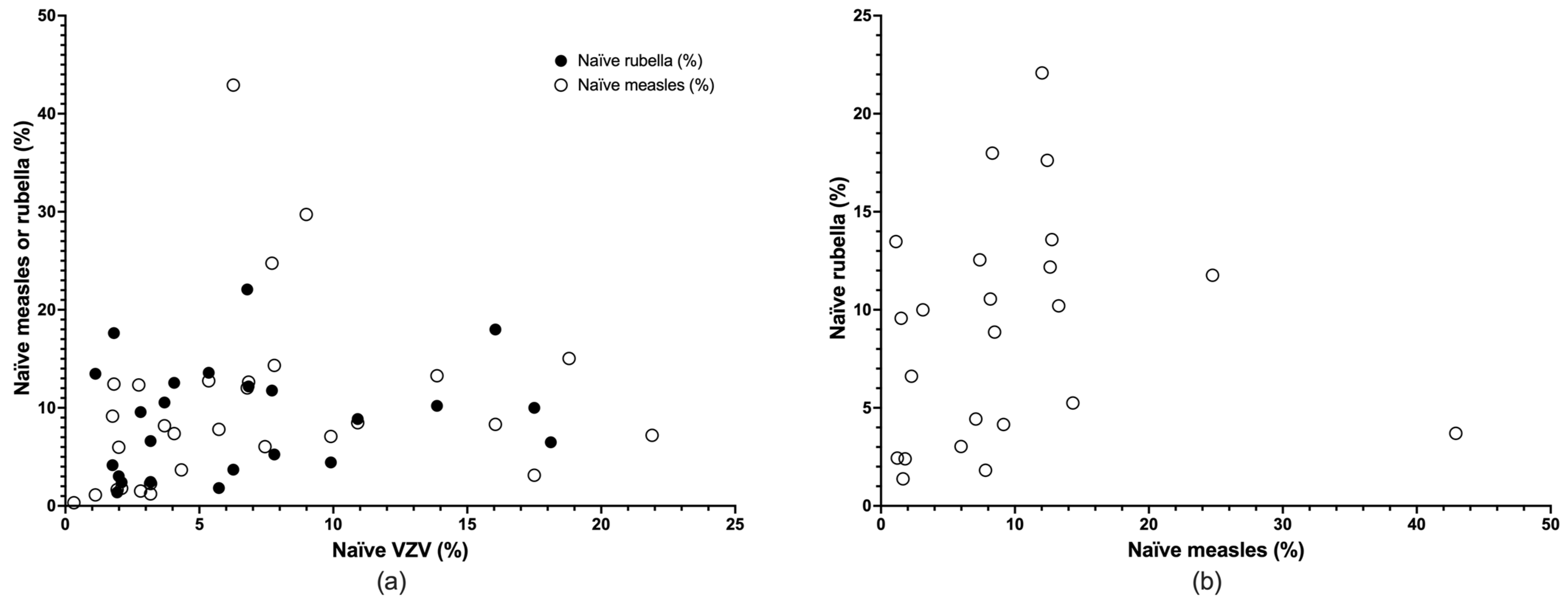
3.2. Descriptive Results
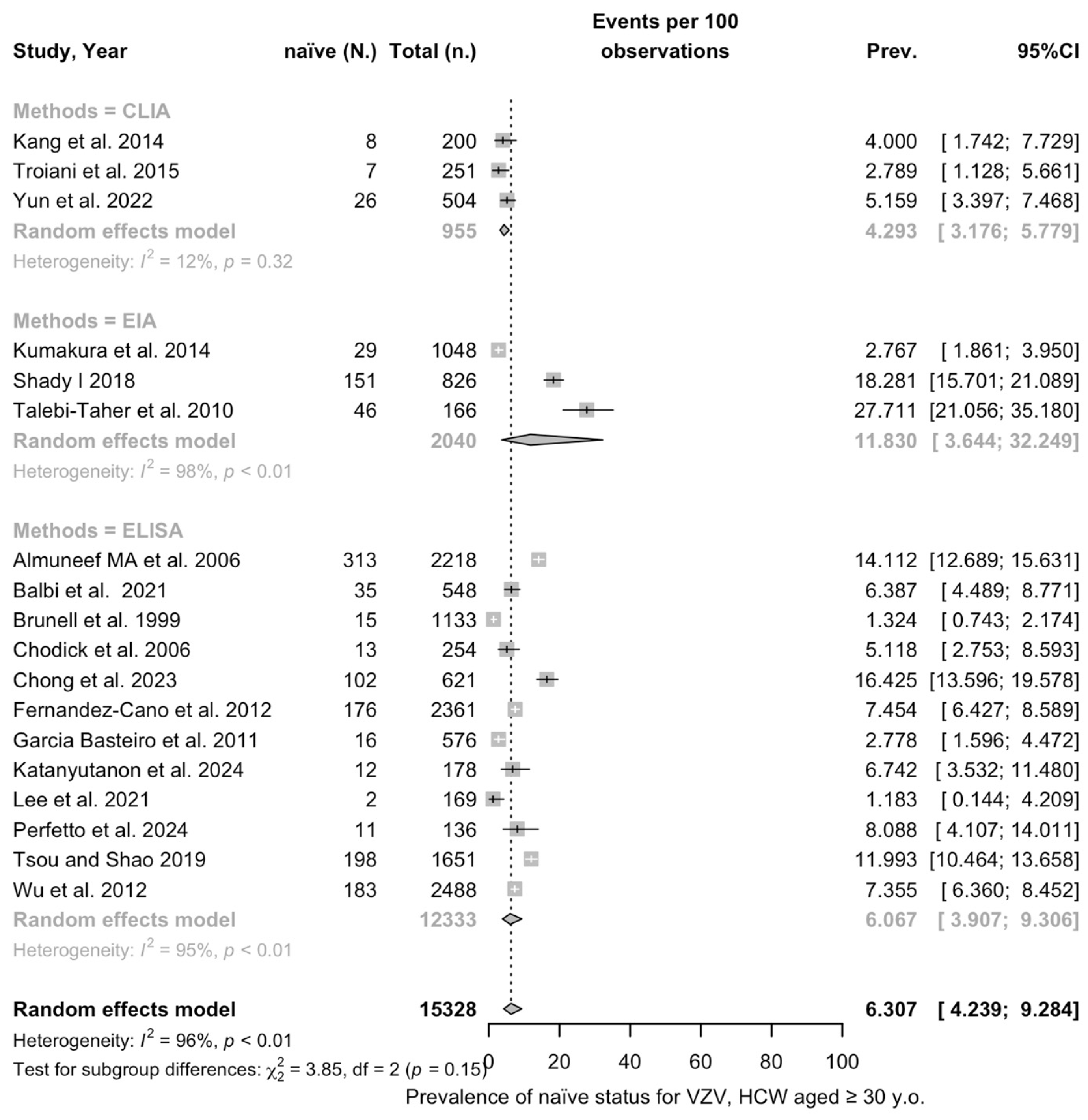
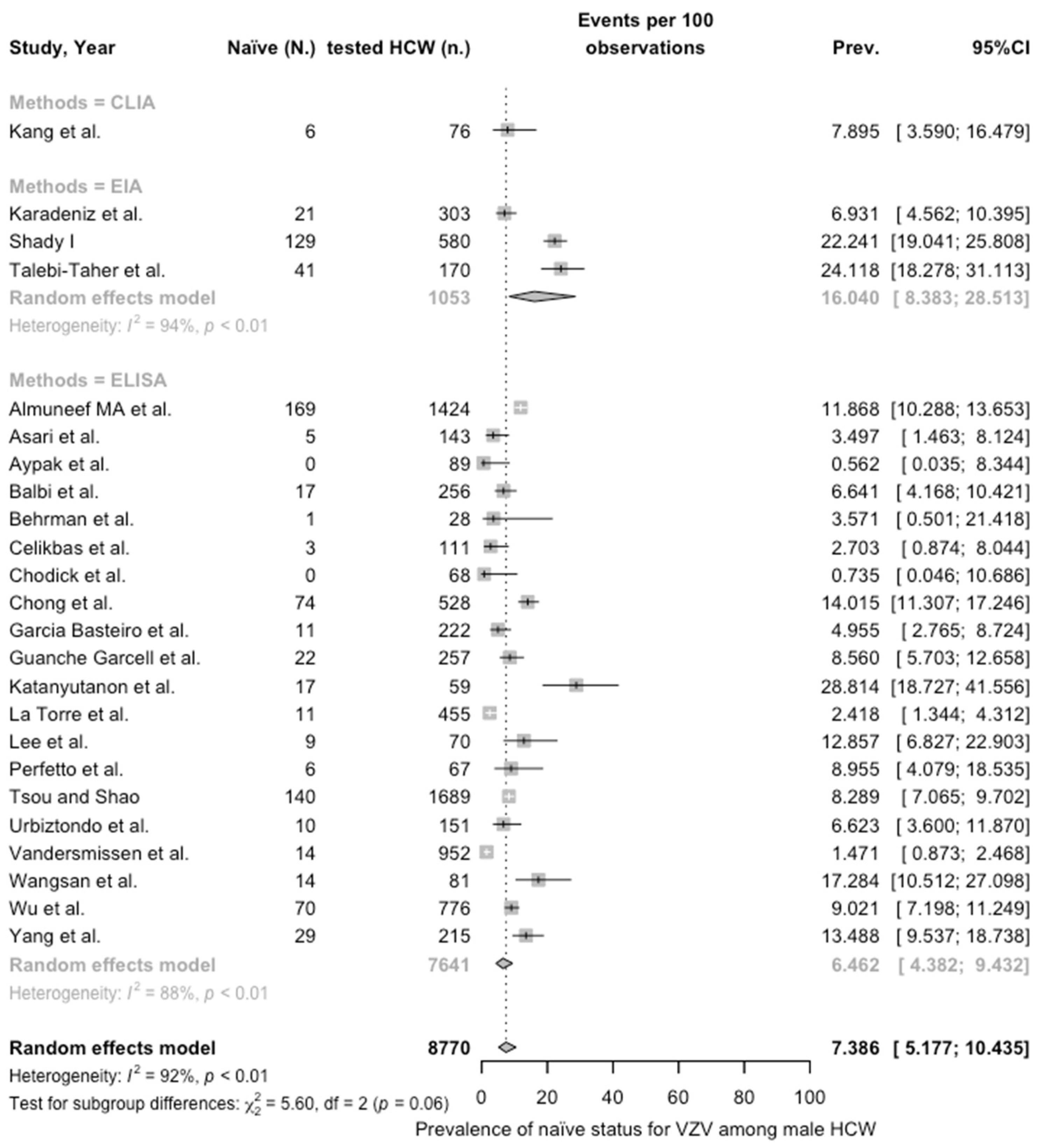
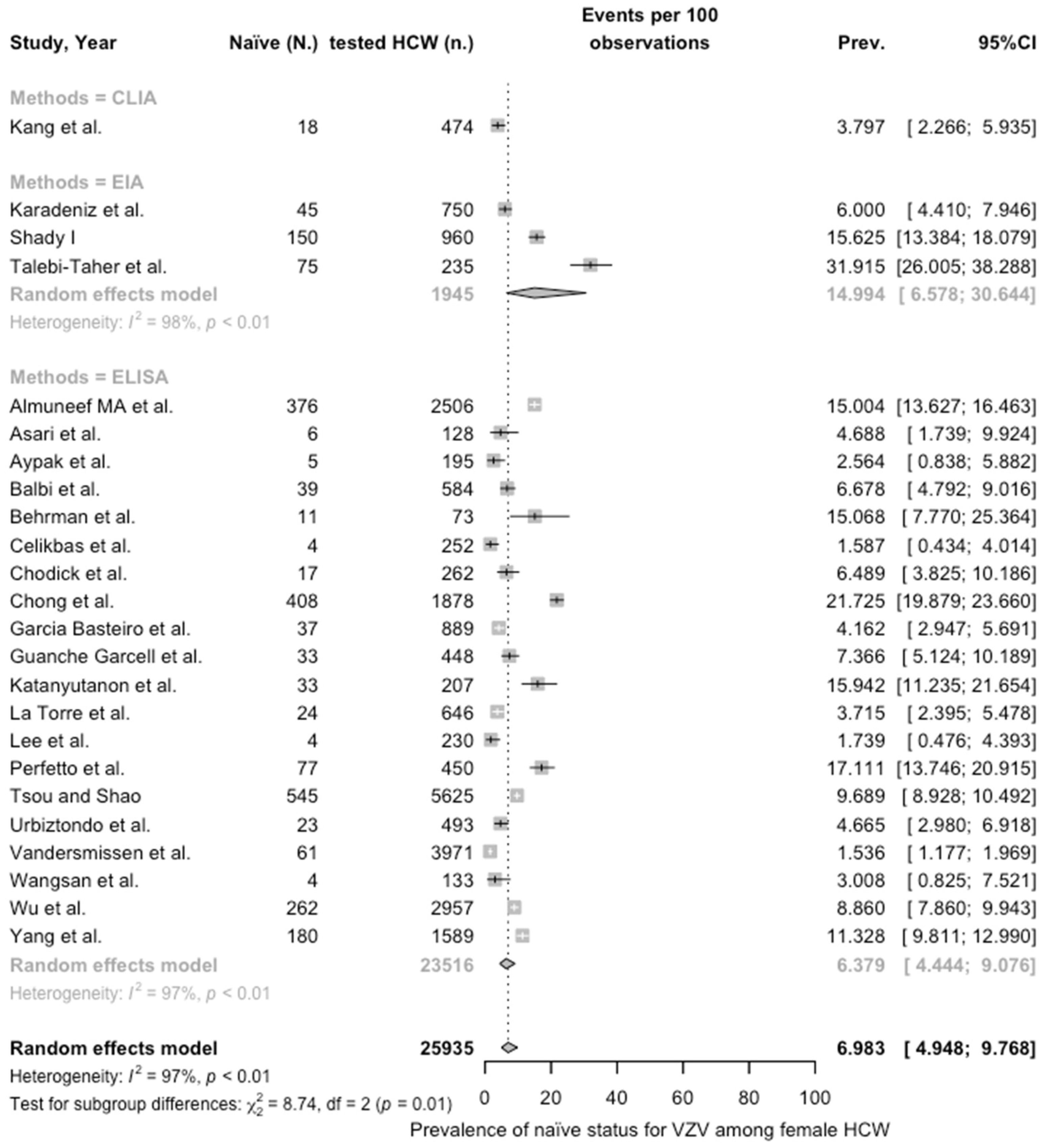
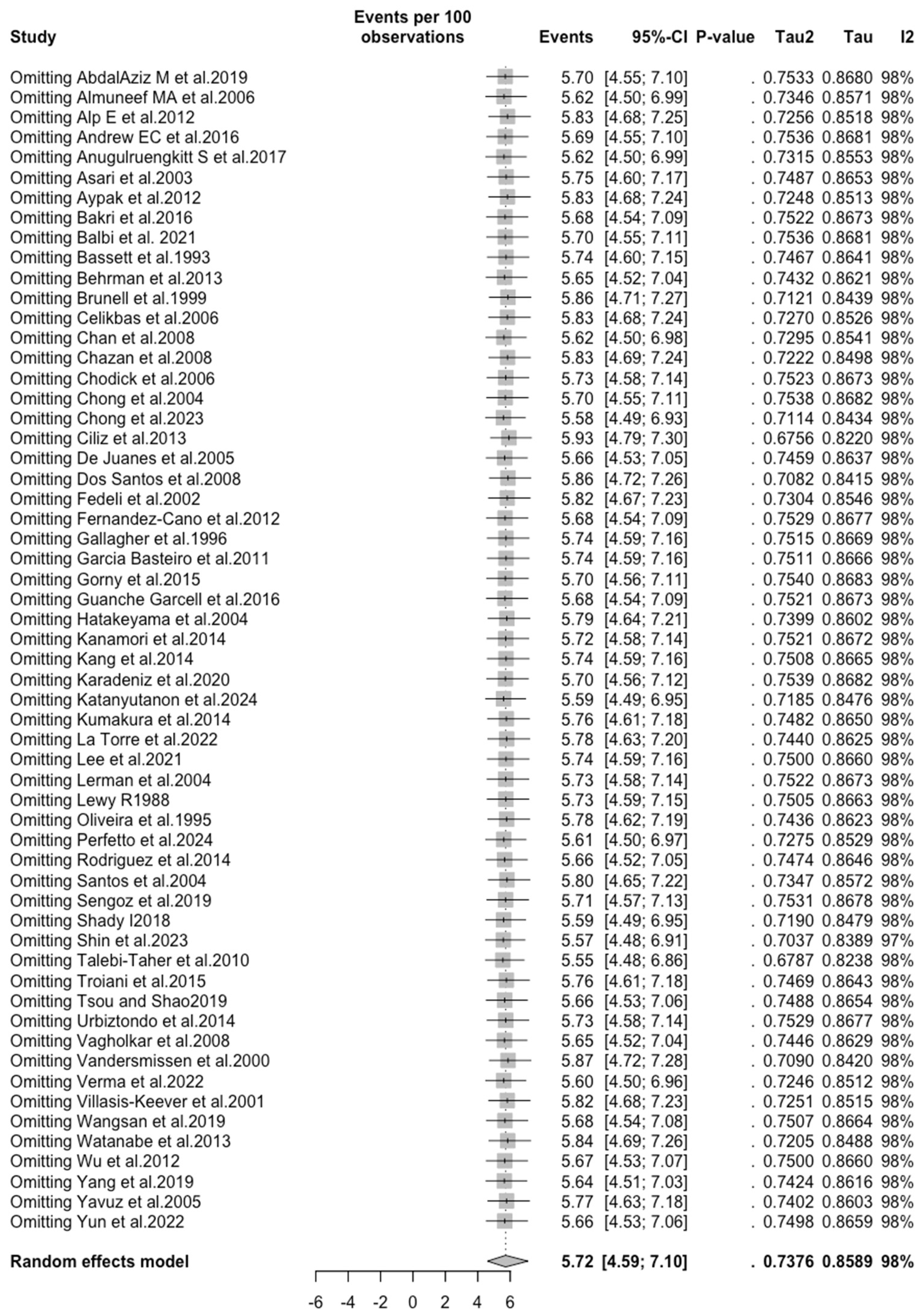
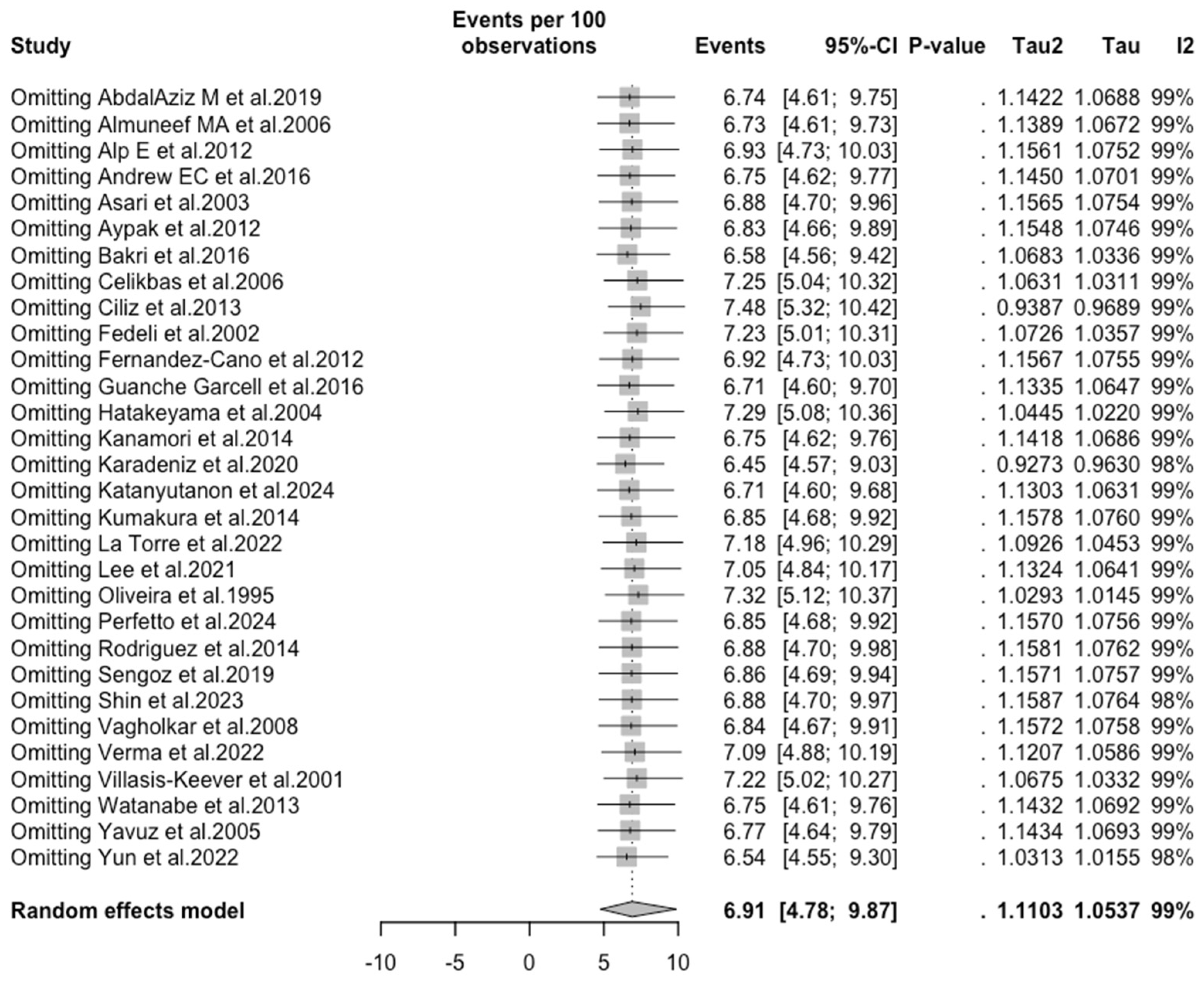
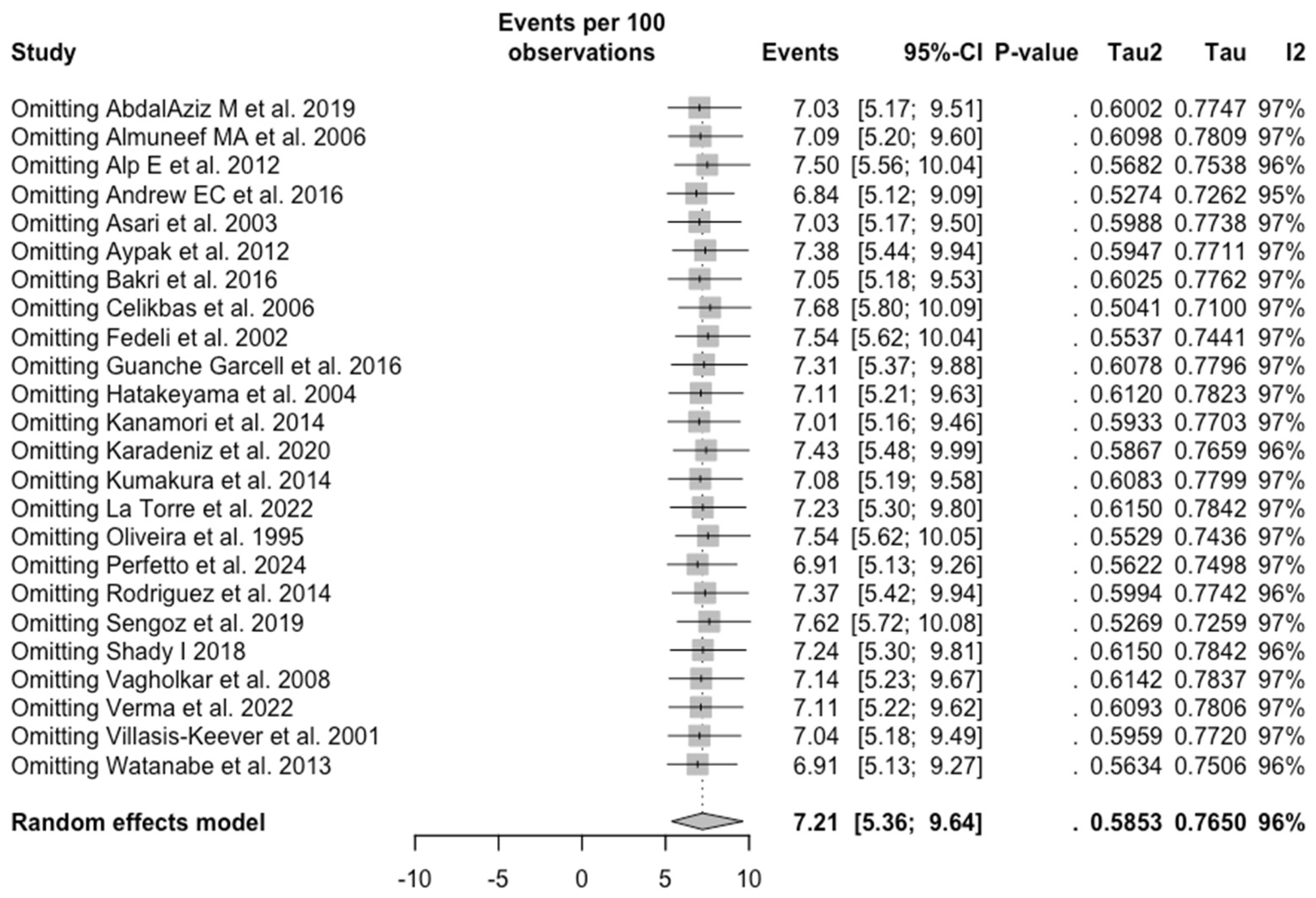
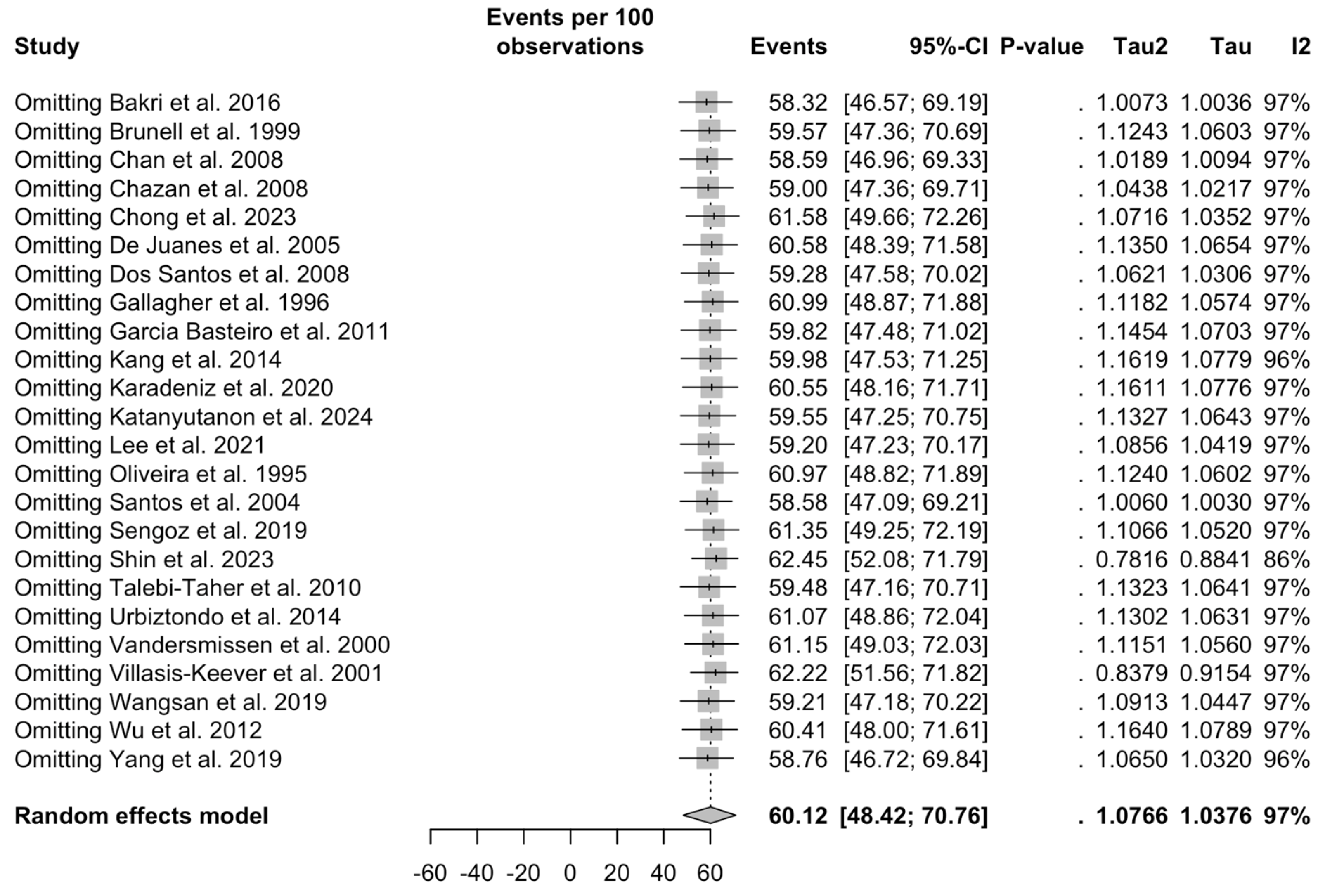
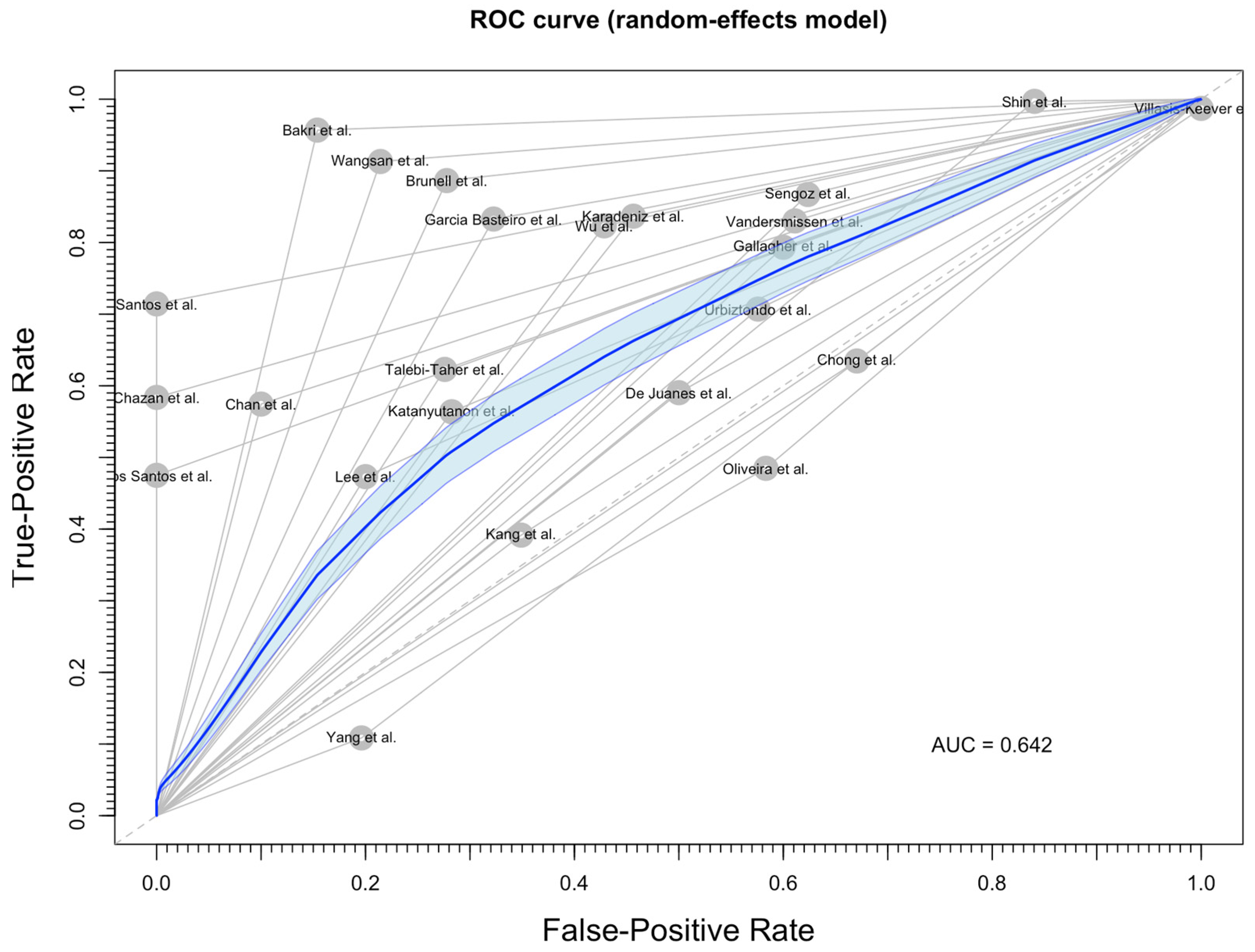
3.3. Meta-Analysis
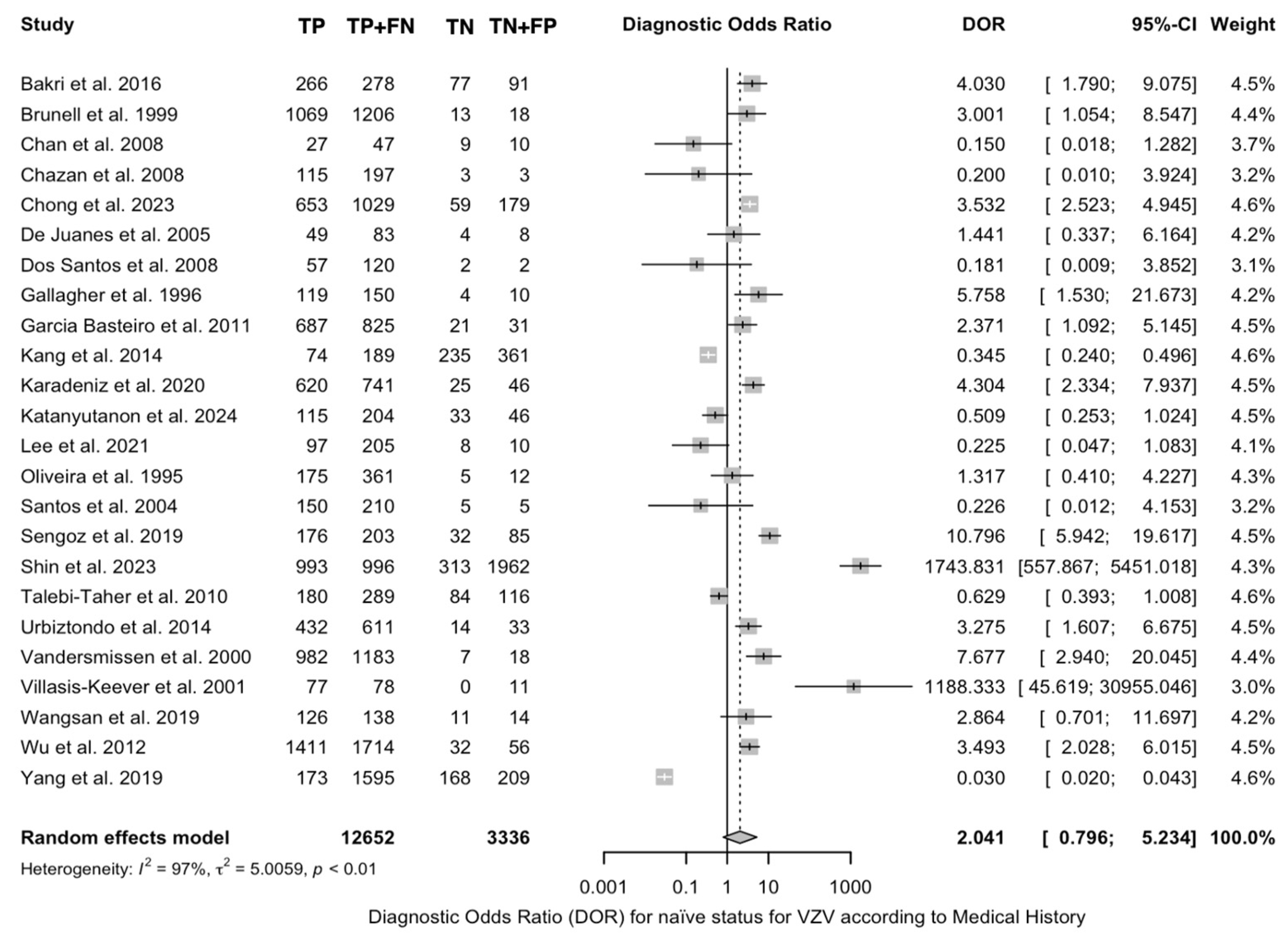
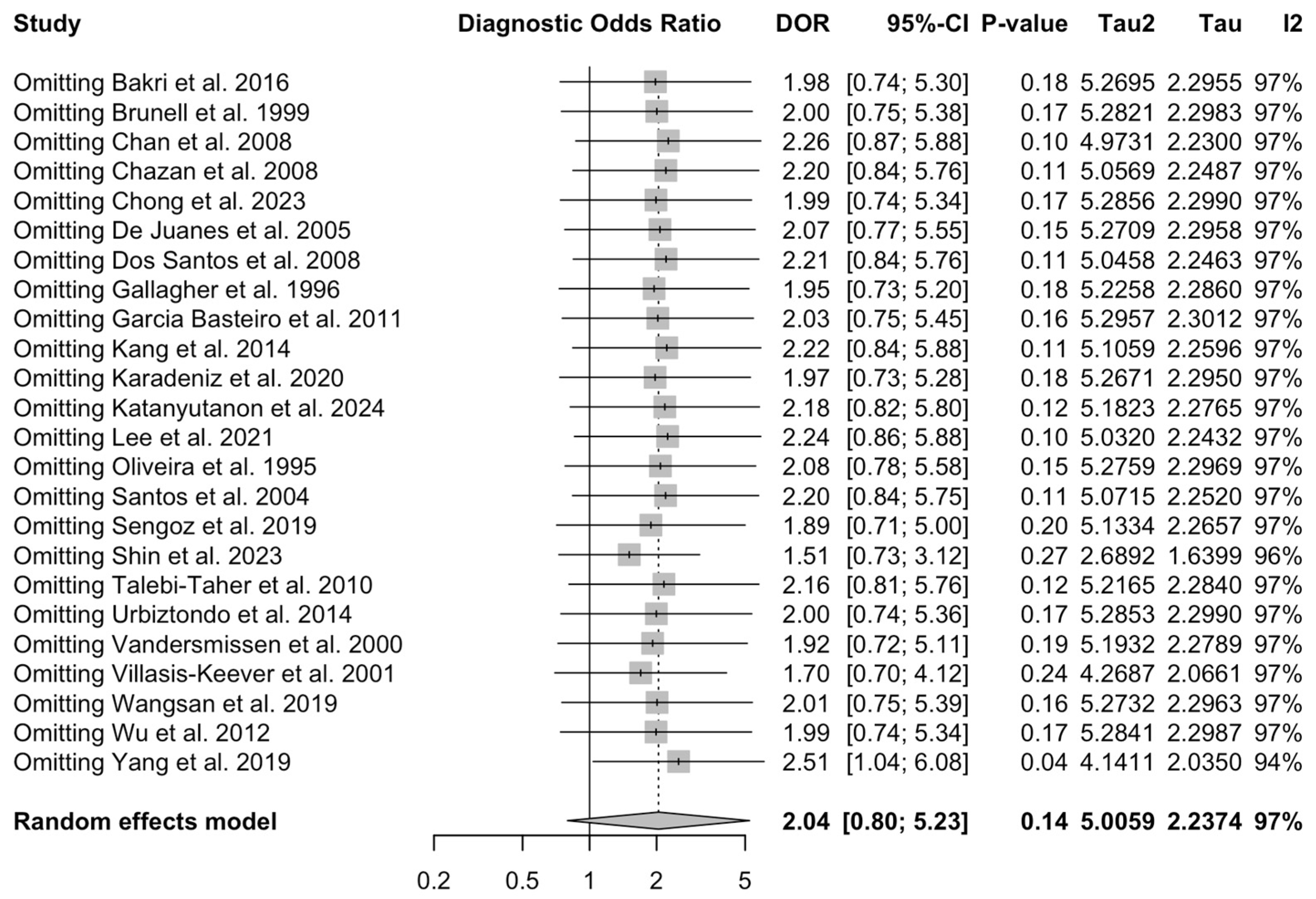
3.4. Diagnostic Performance of Medical History
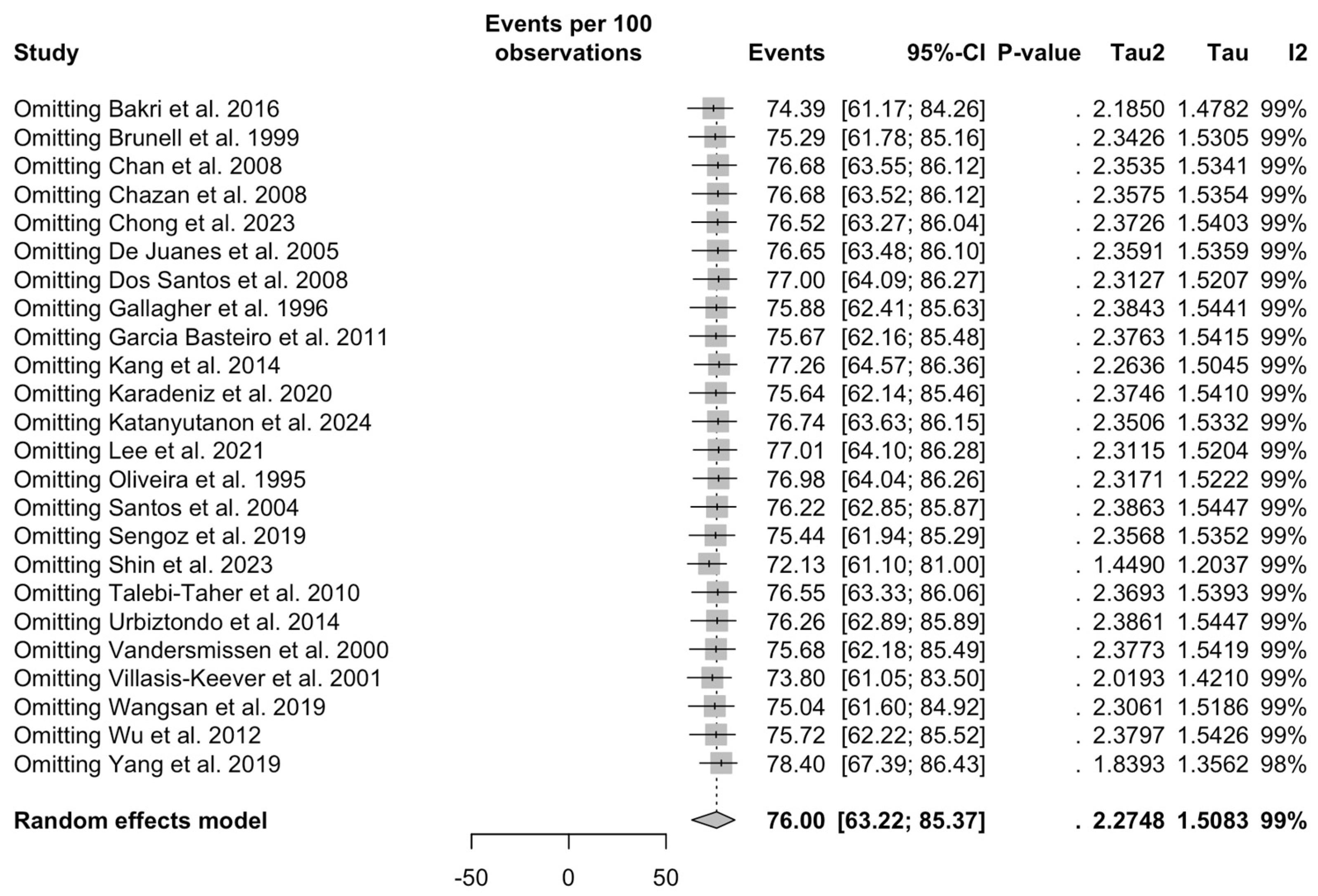
3.5. Sensitivity Analysis
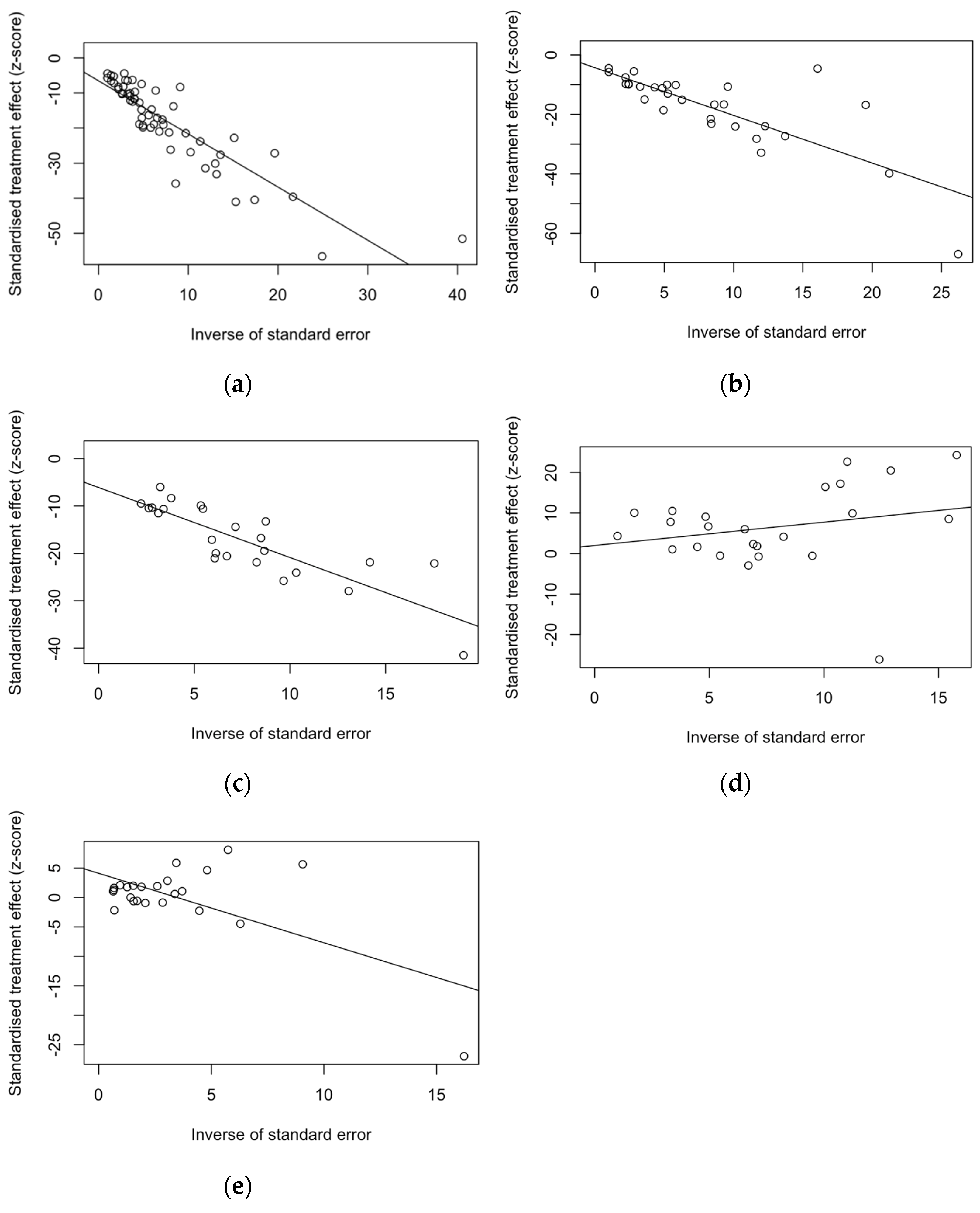
3.6. Analysis of Publication Bias
4. Discussion
4.1. Summary of Main Findings
4.2. Generalizability and Implication for Daily Practice
4.3. Implications for Practice and Policy
4.4. Limitation of Evidence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Search Strategy | N. of Entries |
|---|---|---|
| PubMed | (“Herpesvirus 3, Human”[Mesh] OR “Varicella Zoster Virus Infection”[Mesh] OR “Chickenpox”[Mesh] OR “Herpes Zoster”[Mesh]) AND (“Health Personnel”[Mesh] OR “Allied Health Personnel”[Mesh] OR “healthcare worker*” OR “health care worker*”) | 323 |
| EMBASE | ((“chickenpox”/exp OR chickenpox) OR “varicella zoster virus” OR “herpes zoster”) AND “health care personnel” | 939 |
| Scopus | (“Varicella Zoster Virus Infection” OR “Chickenpox” OR “Herpes Zoster”) AND (“Health Personnel” OR “Allied Health Personnel” OR “healthcare worker*” OR “health care worker*”) | 482 |
| Domain | Explanation |
|---|---|
| D1 | Did selection of study participants result in appropriate comparison groups? |
| D2 | Did the study design or analysis account for important and modifying variables? |
| D3 | Were outcome data complete without attrition or exclusion from analysis? |
| D4 | Can we be confident in the exposure characterization? |
| D5 | Can we be confident in the outcome assessment? |
| D6 | Were all measured outcomes reported? |
| D7 | Were there no other potential threats to internal validity (e.g., statistical methods were appropriate, and researchers adhered to the study protocol)? |
 : definitively high;
: definitively high;  : probably high;
: probably high;  : probably low;
: probably low;  : definitively low. Detailed description of domains is provided in Appendix A Table A2.
: definitively low. Detailed description of domains is provided in Appendix A Table A2.
 : definitively high;
: definitively high;  : probably high;
: probably high;  : probably low;
: probably low;  : definitively low. Detailed description of domains is provided in Appendix A Table A2.
: definitively low. Detailed description of domains is provided in Appendix A Table A2.| Study | D1 | D2 | D3 | D4 | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| AbdalAziz et al., 2019 [79] |  |  |  |  |  |  |  |
| Almuneef et al., 2006 [80] |  |  |  |  |  |  |  |
| Alp et al., 2012 [81] |  |  |  |  |  |  |  |
| Andrew et al., 2016 [46] |  |  |  |  |  |  |  |
| Anugulruengkitt et al., 2017 [82] |  |  |  |  |  |  |  |
| Asari et al., 2003 [83] |  |  |  |  |  |  |  |
| Aypak et al., 2012 [84] |  |  |  |  |  |  |  |
| Bakri et al., 2016 [85] |  |  |  |  |  |  |  |
| Balbi et al., 2021 [86] |  |  |  |  |  |  |  |
| Bassett et al., 1993 [87] |  |  |  |  |  |  |  |
| Behrman et al., 2013 [62] |  |  |  |  |  |  |  |
| Brunell et al., 1999 [57] |  |  |  |  |  |  |  |
| Celikbas et al., 2006 [88] |  |  |  |  |  |  |  |
| Chan et al., 2008 [89] |  |  |  |  |  |  |  |
| Chazan et al., 2008 [90] |  |  |  |  |  |  |  |
| Chodick et al., 2006 [91] |  |  |  |  |  |  |  |
| Chong et al., 2004 [92] |  |  |  |  |  |  |  |
| Chong et al., 2023 [93] |  |  |  |  |  |  |  |
| Ciliz et al., 2013 [94] |  |  |  |  |  |  |  |
| De Juanes et al., 2005 [95] |  |  |  |  |  |  |  |
| Dos Santos et al., 2008 [96] |  |  |  |  |  |  |  |
| Fedeli et al., 2002 [97] |  |  |  |  |  |  |  |
| Fernandez-Cano et al., 2012 [98] |  |  |  |  |  |  |  |
| Gallagher et al., 1996 [99] |  |  |  |  |  |  |  |
| Garcia Basteiro et al., 2011 [100] |  |  |  |  |  |  |  |
| Gorny et al., 2015 [101] |  |  |  |  |  |  |  |
| Guanche Garcell et al., 2016 [102] |  |  |  |  |  |  |  |
| Hatakayama et al., 2004 [103] |  |  |  |  |  |  |  |
| Kanamori et al., 2014 [104] |  |  |  |  |  |  |  |
| Kang et al., 2014 [105] |  |  |  |  |  |  |  |
| Karadeniz et al., 2020 [106] |  |  |  |  |  |  |  |
| Katanyutanon et al., 2024 [107] |  |  |  |  |  |  |  |
| Kumakura et al., 2014 [108] |  |  |  |  |  |  |  |
| La Torre et al., 2022 [109] |  |  |  |  |  |  |  |
| Lee et al., 2021 [110] |  |  |  |  |  |  |  |
| Lerman et al., 2004 [111] |  |  |  |  |  |  |  |
| Lewy et al., 1988 [112] |  |  |  |  |  |  |  |
| Oliveira et al., 1995 [113] |  |  |  |  |  |  |  |
| Perfetto et al., 2024 [114] |  |  |  |  |  |  |  |
| Rodriguez et al., 2014 [115] |  |  |  |  |  |  |  |
| Santos et al., 2004 [116] |  |  |  |  |  |  |  |
| Sengoz et al., 2019 [117] |  |  |  |  |  |  |  |
| Shady I 2018 [118] |  |  |  |  |  |  |  |
| Shin et al., 2023 [119] |  |  |  |  |  |  |  |
| Talebi-Taher et al., 2010 [120] |  |  |  |  |  |  |  |
| Troiani et al., 2015 [58] |  |  |  |  |  |  |  |
| Tsou and Shao 2019 [61] |  |  |  |  |  |  |  |
| Urbiztondo et al., 2014 [121] |  |  |  |  |  |  |  |
| Vagholkar et al., 2008 [63] |  |  |  |  |  |  |  |
| Vandermissen et al., 2000 [122] |  |  |  |  |  |  |  |
| Verma et al., 2022 [123] |  |  |  |  |  |  |  |
| Villasis-Keever et al., 2001 [124] |  |  |  |  |  |  |  |
| Wangsan et al., 2019 [125] |  |  |  |  |  |  |  |
| Watanabe et al., 2013 [126] |  |  |  |  |  |  |  |
| Wu et al., 2012 [127] |  |  |  |  |  |  |  |
| Yang et al., 2019 [55] |  |  |  |  |  |  |  |
| Yavuz et al., 2005 [128] |  |  |  |  |  |  |  |
| Yun et al., 2022 [129] |  |  |  |  |  |  |  |



References
- Rahaus, M.; Schünemann, S.; Wolff, M.H. General Aspects Morphological and Biological Characteristics of Varicella-Zoster Virus. In Pathogenesis, and Clinical Aspects. Contrib Microbiol; Wolff, M., Schünemann, S., Schmidt, A., Eds.; Karger: Basel, Switzerland, 1999; Volume 3, pp. 1–9. [Google Scholar]
- Arvin, A.M. Varicella-Zoster Virus. Clin. Microbiol. Rev. 1996, 9, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella Zoster Virus Infection. Nat. Rev. Dis. Primers 2015, 1, 15016. [Google Scholar] [CrossRef]
- Tommasi, C.; Breuer, J. The Biology of Varicella-Zoster Virus Replication in the Skin. Viruses 2022, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Heininger, U.; Seward, J.F. Varicella. Lancet 2006, 368, 1365–1376. [Google Scholar] [CrossRef]
- García, J.M.M.; Guillén, S.M.; Rodríguez-Artalejo, F.; Ruiz-Galiana, J.; Cantón, R.; Ramos, P.D.L.; García-Botella, A.; García-Lledó, A.; Hernández-Sampelayo, T.; Gómez-Pavón, J.; et al. Status of Herpes Zoster and Herpes Zoster Vaccines in 2023: A Position Paper. Rev. Esp. Quimioter. 2023, 36, 223–235. [Google Scholar] [CrossRef]
- Patil, A.; Goldust, M.; Wollina, U. Herpes Zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Wang, M.; Jiang, J.; Zhu, Y.; Kumar, R.; Lin, S. The Global Disease Burden of Varicella-Zoster Virus Infection from 1990 to 2019. J. Med. Virol. 2022, 94, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, R. Do Varicella Vaccination Programs Change the Epidemiology of Herpes Zoster? A Comprehensive Review, with Focus on the United States. Expert Rev. Vaccines 2019, 18, 793–811. [Google Scholar] [CrossRef]
- Otani, N.; Shima, M.; Yamamoto, T.; Okuno, T. Effect of Routine Varicella Immunization on the Epidemiology and Immunogenicity of Varicella and Shingles. Viruses 2022, 14, 588. [Google Scholar] [CrossRef]
- Giannelos, N.; Curran, D.; Nguyen, C.; Kagia, C.; Vroom, N.; Vroling, H. The Incidence of Herpes Zoster Complications: A Systematic Literature Review. Infect. Dis. Ther. 2024, 13, 1461–1486. [Google Scholar] [CrossRef]
- Marchetto, S.; De Benedictis, F.M.; De Martino, M.; Versace, A.; Chiappini, E.; Bertaine, C.; Osimani, P.; Cordiali, R.; Gabiano, C.; Galli, L. Epidemiology of Hospital Admissions for Chickenpox in Children: An Italian Multicentre Study in the Pre-Vaccine Era. Acta Paediatr. Int. J. Paediatr. 2007, 96, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Díez-Domingo, J.; Aristegui, J.; Calbo, F.; Gonzalez-Hachero, J.; Moraga, F.; Peña Guitian, J.; Ruiz Contreras, J.; Torrellas, A. Epidemiology and Economic Impact of Varicella in Immunocompetent Children in Spain. A Nation-Wide Study. Vaccine 2003, 21, 3236–3239. [Google Scholar] [CrossRef] [PubMed]
- Bonsignori, F.; Chiappini, E.; Frenos, S.; Peraldo, M.; Galli, L.; De Martino, M. Hospitalization Rates for Complicated and Uncomplicated Chickenpox in a Poorly Vaccined Pediatric Population. Infection 2007, 35, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Arama, V.; Rafila, A.; Streinu-Cercel, A.; Pistol, A.; Bacruban, R.; Sandu, R.; Pitigoi, D.; Negoita, A. Varicella in Romania: Epidemiological Trends, 1986-2004. Eurosurveillance 2005, 10, 2775. [Google Scholar] [CrossRef]
- Dubos, F.; Grandbastien, B.; Hue, V.; Martinot, A. Epidemiology of Hospital Admissions for Paediatric Varicella Infections: A One-Year Prospective Survey in the Pre-Vaccine Era. Epidemiol. Infect. 2007, 135, 131–138. [Google Scholar] [CrossRef]
- Liese, J.G.; Grote, V.; Rosenfeld, E.; Fischer, R.; Belohradsky, B.H.; Kries, R.V. The Burden of Varicella Complications before the Introduction of Routine Varicella Vaccination in Germany. Pediatr. Infect. Dis. J. 2008, 27, 119–124. [Google Scholar] [CrossRef]
- Freer, G.; Pistello, M. Varicella-Zoster Virus Infection: Natural History, Clinical Manifestations, Immunity and Current and Future Vaccination Strategies. New Microbiol. 2018, 41, 95–105. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). Varicella Vaccination in the European Union; European Centre for Disease Prevention and Control (ECDC): Stokholm, Sweden, 2015. [Google Scholar]
- Grimprel, E.; Levy, C.; de la Rocque, F.; Cohen, R.; Soubeyrand, B.; Caulin, E.; Derrough, T.; Lecuyer, A.; D’Athis, P.; Gaudelus, J. Paediatric Varicella Hospitalisations in France: A Nationwide Survey. Clin. Microbiol. Infect. 2007, 13, 546–549. [Google Scholar] [CrossRef]
- Theodoridou, M.; Laina, I.; Hadjichristodoulou, C.; Syriopoulou, V. Varicella-Related Complications and Hospitalisations in a Tertiary Pediatric Medical Center before Vaccine Introduction. Eur. J. Pediatr. 2006, 165, 273–274. [Google Scholar] [CrossRef]
- Daulagala, S.W.P.L.; Noordeen, F. Epidemiology and Factors Influencing Varicella Infections in Tropical Countries Including Sri Lanka. Virusdisease 2018, 29, 277–284. [Google Scholar] [CrossRef]
- Barrero Guevara, L.A.; Goult, E.; Rodriguez, D.; Hernandez, L.J.; Kaufer, B.; Kurth, T.; Domenech De Cellès, M. Delineating the Seasonality of Varicella and Its Association With Climate in the Tropical Country of Colombia. J. Infect. Dis. 2023, 228, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Vos, R.A.; Mollema, L.; van Boven, M.; van Lier, A.; Smits, G.; Janga-Jansen, A.V.A.; Baboe-Kalpoe, S.; Hulshof, K.; Stienstra, Y.; van der Klis, F.R.M.; et al. High Varicella Zoster Virus Susceptibility in Caribbean Island Populations: Implications for Vaccination. Int. J. Infect. Dis. 2020, 94, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Fujita, D.M.; da Silva Nali, L.H.; da Costa, R.R.; de Andrade Júnior, H.F.; de Albuquerque Luna, E.J. Low Vaccine Coverage and Varicella Outbreaks in Brazil—2019–2022. Vaccine 2024, 42, 3384–3388. [Google Scholar] [CrossRef]
- Dubos, F.; Hue, V.; Grandbastien, B.; Catteau, B.; Martinot, A. Bacterial Skin Infections in Children Hospitalized with Varicella: A Possible Negative Impact of Non-Steroidal Anti-Inflammatory Drugs? Acta Derm. Venereol. 2008, 88, 26–30. [Google Scholar] [CrossRef]
- Cameron, J.C.; Allan, G.; Johnston, F.; Finn, A.; Heath, P.T.; Booy, R. Severe Complications of Chickenpox in Hospitalised Children in the UK and Ireland. Arch. Dis. Child. 2007, 92, 1062–1066. [Google Scholar] [CrossRef]
- Gil, A.; San-Martín, M.; Carrasco, P.; González, A. Epidemiology of Severe Varicella-Zoster Virus Infection in Spain. Vaccine 2004, 22, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Tozzi, A.E.; Bozzola, M.; Krzysztofiak, A.; Valentini, D.; Grandin, A.; Villani, A. Neurological Complications of Varicella in Childhood: Case Series and a Systematic Review of the Literature. Vaccine 2012, 30, 5785–5790. [Google Scholar] [CrossRef]
- Meyer, P.A.; Seward, J.F.; Jumaan, A.O.; Wharton, M. Varicella Mortality: Trends before Vaccine Licensure in the United States, 1970–1994. J. Infect. Dis. 2000, 182, 383–390. [Google Scholar] [CrossRef]
- Leung, J.; Marin, M. Update on Trends in Varicella Mortality during the Varicella Vaccine Era—United States, 1990–2016. Hum. Vaccin Immunother. 2018, 14, 2460–2463. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Jumaan, A.O.; Seward, J.F. Decline in Mortality Due to Varicella after Implementation of Varicella Vaccination in the United STates. N. Engl. J. Med. 2005, 352, 450–458. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Varicella Vaccine in the European Union; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2014. [Google Scholar]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic Review of incidence and Complications of Herpes Zoster: Towards a Global Perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Spence, O.; Giannelos, N.; Kaan, I. Herpes Zoster Recurrence: A Narrative Review of the Literature. Dermatol. Ther. 2024, 14, 569–592. [Google Scholar] [CrossRef] [PubMed]
- Scampoli, P.; Di Martino, G.; Cedrone, F.; Odio, C.; Di Giovanni, P.; Romano, F.; Staniscia, T. The Burden of Herpes Zoster on Hospital Admissions: A Retrospective Analysis in the Years of 2015–2021 from the Abruzzo Region, Italy. Vaccines 2024, 12, 462. [Google Scholar] [CrossRef]
- Parikh, R.; Singer, D.; Chmielewski-Yee, E.; Dessart, C. Effectiveness and Safety of Recombinant Zoster Vaccine: A Review of Real-World Evidence. Hum. Vaccin. Immunother. 2023, 19, 2263979. [Google Scholar] [CrossRef] [PubMed]
- Wutzler, P.; Bonanni, P.; Burgess, M.; Gershon, A.; Sáfadi, M.A.; Casabona, G. Varicella Vaccination—The Global Experience. Expert Rev. Vaccines 2017, 16, 833–843. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choe, Y.J.; Lee, J.; Kim, E.; Lee, J.Y.; Hong, K.; Yoon, Y.; Kim, Y.K. Global Varicella Vaccination Programs. Clin. Exp. Pediatr. 2022, 65, 555–562. [Google Scholar] [CrossRef]
- Hirose, M.; Gilio, A.E.; Ferronato, A.E.; Ragazzi, S.L.B. The Impact of Varicella Vaccination on Varicella-Related Hospitalization Rates: Global Data Review. Rev. Paul. Pediatr. Engl. Ed. 2016, 34, 359–366. [Google Scholar] [CrossRef]
- Bibi, Z.; Nawaz, A.D.; Al Kurbi, M.; Fakhroo, S.; Ferih, K.; Al-Jaber, N.; Alex, M.; Elawad, K.H.; Chivese, T.; Zughaier, S.M. Real-World Effectiveness of the Varicella Vaccine among Children and Adolescents in Qatar: A Case–Control Study. Vaccines 2023, 11, 1567. [Google Scholar] [CrossRef]
- Lang, J.C.; Samant, S.; Cook, J.R.; Ranjan, S.; Senese, F.; Starnino, S.; Giuffrida, S.; Azzari, C.; Baldo, V.; Pawaskar, M. The Clinical and Economic Costs Associated with Regional Disparities in Varicella Vaccine Coverage in Italy over 50 Years (2020–2070). Sci. Rep. 2024, 14, 11929. [Google Scholar] [CrossRef]
- Advisory Committee on Immunization Practices (ACIP). Prevention of Varicella Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007, 56, 1–48. [Google Scholar]
- Advisory Committee on Immunization Practices (ACIP). Immunization of Health-Care Personnel Recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbidity and Mortality Weekly Report. MMWR 2011, 60, 1–48. [Google Scholar]
- Preda, M.; Manolescu, L.S.C.; Chivu, R.D. Advances in Alpha Herpes Viruses Vaccines for Human. Vaccines 2023, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Andrew, E.C.; Gibney, K.B.; Denholm, J.; Leder, K. Seroprotection to Vaccine-Preventable Diseases among Workers at a Victorian Tertiary Hospital. Aust. N. Z. J. Public Health 2016, 40, 284–289. [Google Scholar] [CrossRef][Green Version]
- Losa, L.; Antonazzo, I.C.; Di Martino, G.; Mazzaglia, G.; Tafuri, S.; Mantovani, L.G.; Ferrara, P. Immunogenicity of Recombinant Zoster Vaccine: A Systematic Review, Meta-Analysis, and Meta-Regression. Vaccines 2024, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Tseroni, M.; Drositis, I.; Gamaletsou, M.N.; Koukou, D.M.; Bolikas, E.; Peskelidou, E.; Daflos, C.; Panagiotaki, E.; Ledda, C.; et al. Vaccination Coverage Rates and Attitudes towards Mandatory Vaccinations among Healthcare Personnel in Tertiary-Care Hospitals in Greece. Expert Rev. Vaccines 2022, 21, 853–859. [Google Scholar] [CrossRef]
- Fortunato, F.; Tafuri, S.; Cozza, V.; Martinelli, D.; Prato, R. Low Vaccination Coverage among Italian Healthcare Workers in 2013: Contributing to the Voluntary vs. Mandatory Vaccination Debate. Hum. Vaccines Immunother. 2015, 11, 133–139. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Tafuri, S.; Larocca, A.M.V.; Germinario, C.A.; Stefanizzi, P. Long -Term Persistence of Antibodies against Varicella in Fully Immunized Healthcare Workers: An Italian Retrospective Cohort Study. BMC Infect. Dis. 2021, 21, 475. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Marchesi, F. Vaccinating Front-Line Healthcare Workers: Results of a Pre-Pandemic Cross-Sectional Study from North-Eastern Italy on First Responders. Vaccines 2022, 10, 1492. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Bragazzi, N.L.; Balzarini, F. Pertussis Immunization in Healthcare Workers Working in Pediatric Settings: Knowledge, Attitudes and Practices (KAP) of Occupational Physicians. Preliminary Results from a Web-Based Survey (2017). J. Prev. Med. Hyg. 2020, 61, E66. [Google Scholar]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Vaccinations of Health Care Workers: A Cross Sectional Pilot Study in North-Eastern Italy. Int. J. Occup. Med. Environ. Health 2017, 30, 775–790. [Google Scholar] [CrossRef]
- Odemis, I.; Sukran, K.; Akbulut, I.; Albayrak, H. Seroprevalence of Measles, Mumps, Rubella, and Varicella Zoster Virus Antibodies among Healthcare Students: Analysis of Vaccine Efficacy and Cost-Effectiveness. Rev. Esp. Qimioter 2019, 32, 525–531. [Google Scholar]
- Yang, J.; Liu, J.; Xing, F.; Ye, H.; Dai, G.; Liu, M.; Lo, S.K.F.; Lau, R.W.T.; Chiu, K.H.Y.; Chan, J.F.W.; et al. Nosocomial Transmission of Chickenpox and Varicella Zoster Virus Seroprevalence Rate amongst Healthcare Workers in a Teaching Hospital in China. BMC Infect. Dis. 2019, 19, 582. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, A.; Nicolli, A.; De Nuzzo, D.; Lago, L.; Artuso, E.; Maso, S. Varicella Seroepidemiology and Immunization in a Cohort of Future Healthcare Workers in the Pre-Vaccination Era. Int. J. Infect. Dis. 2020, 96, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Brunell, P.A.; Wood, D. Varicella Serological Status of Healthcare Workers as a Guide to Whom to Test or Immunize. Infect. Control Hosp. Epidemiol. 1999, 20, 355–357. [Google Scholar] [CrossRef]
- Troiani, L.; Hill, J.J.; Consoli, S.; Weber, D.J. Varicella-Zoster Immunity in Us Healthcare Personnel with Self-Reported History of Disease. Infect. Control Hosp. Epidemiol. 2015, 36, 1467–1468. [Google Scholar] [CrossRef]
- Genovese, C.; Picerno, I.A.M.; Trimarchi, G.; Cannavò, G.; Egitto, G.; Cosenza, B.; Merlina, V.; Icardi, G.; Panatto, D.; Amicizia, D.; et al. Vaccination Coverage in Healthcare Workers: A Multicenter Cross-Sectional Study in Italy. J. Prev. Med. Hyg. 2019, 60, E12–E17. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Balzarini, F.; Ranzieri, S. Mandate or Not Mandate: Knowledge, Attitudes, and Practices of Italian Occupational Physicians towards SARS-CoV-2 Immunization at the Beginning of Vaccination Campaign. Vaccines 2021, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.T.; Shao, H.H. Varicella Seroprevalence in Healthcareworkers at a Medical Center Following Changes in National and Local Hospital Vaccination Policies. Int. J. Environ. Res. Public Health 2019, 16, 889. [Google Scholar] [CrossRef]
- Behrman, A.; Lopez, A.S.; Chaves, S.S.; Watson, B.M.; Schmid, D.S. Varicella Immunity in Vaccinated Healthcare Workers. J. Clin. Virol. 2013, 57, 109–114. [Google Scholar] [CrossRef]
- Vagholkar, S.; Ng, J.; Chan, R.C.; Bunker, J.M.; Zwar, N.A. Healthcare Workers and Immunity to Infectious Diseases. Aust. N. Z. J. Public Health 2008, 32, 367–371. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Mintzker, Y.; Blum, D.; Adler, L. Replacing PICO in Non-Interventional Studies. BMJ Evid. Based Med. 2022, 28, 284. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Von Hippel, P.T. The Heterogeneity Statistic I2 Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Krumpal, I. Determinants of Social Desirability Bias in Sensitive Surveys: A Literature Review. Qual. Quant. 2013, 47, 2025–2047. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). In OHAT Risk of Bias Rating Tool for Human and Animal Studies; National Toxicology Program: Research Triangle Park, NC, USA, 2015.
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing Risk of Bias in Human Environmental Epidemiology Studies Using Three Tools: Different Conclusions from Different Tools. Syst. Rev. 2020, 9, 249. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A Re-Evaluation of Random-Effects Meta-Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Jones, C.M.; Athanasiou, T. Summary Receiver Operating Characteristic Curve Analysis Techniques in the Evaluation of Diagnostic Tests. Ann. Thorac. Surg. 2005, 79, 16–20. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- R Development Core Team. R a Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3900051070. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- AbdalAziz, M.; Farahat, F.; Ossenkopp, J.; AlThaqafy, M.; Hassan, O.; AlSaedi, A. 31 Immune Status to Measles, Rubella, and Varicella-Zoster among Newly Recruited Healthcare Workers at King Abdulaziz Medical City, Jeddah, Saudi Arabia. BMJ Open Qual. 2019, 8, A1–A33. [Google Scholar] [CrossRef]
- Almuneef, M.A.; Memish, Z.A.; Balkhy, H.H.; Otaibi, B.; Helmi, M. Seroprevalence Survey of Varicella, Measles, Rubella, and Hepatitis A and B Viruses in a Multinational Healthcare Workforce in Saudi Arabia. Infect. Control Hosp. Epidemiol. 2006, 27, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Alp, E.; Cevahir, F.; Gökahmetoglu, S.; Demiraslan, H.; Doganay, M. Prevaccination Screening of Health-Care Workers for Immunity to Measles, Rubella, Mumps, and Varicella in a Developing Country: What Do We Save? J. Infect. Public Health 2012, 5, 127–132. [Google Scholar] [CrossRef]
- Anugulruengkitt, S.; Puthanakit, T.; Siengboon, S.; Paitoonpong, L.; Kowitdamrong, E.; Pancharoen, C.; Pichitchok, Y. Prevalence and Characteristics of Pediatric Healthcare Workers without Immunity to Varicella Zoster Virus. Jpn. J. Infect. Dis. 2017, 70, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Asari, S.; Deguchi, M.; Tahara, K.; Taniike, M.; Toyokawa, M.; Nishi, I.; Watanabe, M.; Iwatani, Y.; Makimoto, K. Seroprevalence Survey of Measles, Rubella, Varicella, and Mumps Antibodies in Health Care Workers and Evaluation of a Vaccination Program in a Tertiary Care Hospital in Japan. Am. J. Infect. Control 2003, 31, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Aypak, C.; Bayram, Y.; Eren, H.; Altunsoy, A.; Berktas, M. Susceptibility to Measles, Rubella, Mumps, and Varicella-Zoster Viruses among Healthcare Workers. J. Nippon. Med. Sch. 2012, 79, 453–458. [Google Scholar] [CrossRef]
- Bakri, F.G.; Abdelrahim, Z.M.; Alkalbani, A.S.; Khrais, G.M.; Shamroukh, D.S.; Hayajneh, F.A.; Mahafza, A. Seroprevalence of Measles, Mumps, Rubella, and Varicella among Physicians and Nurses in Jordan. Turk. J. Med. Sci. 2016, 46, 614–619. [Google Scholar] [CrossRef]
- Balbi, O.; Baldi, S.; Rizza, S.; Pietroiusti, A.; Perrone, S.; Coppeta, L. Seroprevalence Survey for Varicella among Healthcare Workers and Medical Students in Italy. Hum. Vaccin. Immunother. 2020, 17, 372–376. [Google Scholar] [CrossRef]
- Bassett, D.C.J.; Ho, A.K.C.; Cheng, A.F.B. Susceptibility of Hospital Staff to Varicella Zoster Virus Infection in Hong Kong. J. Hosp. Infect. 1993, 23, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Celikbas, A.; Ergonul, O.; Aksaray, S.; Tuygun, N.; Esener, H.; Tanir, G.; Eren, S.; Baykam, N.; Guvener, E.; Dokuzoguz, B. Measles, Rubella, Mumps, and Varicella Seroprevalence among Health Care Workers in Turkey: Is Prevaccination Screening Cost-Effective? Am. J. Infect. Control 2006, 34, 583–587. [Google Scholar] [CrossRef]
- Sam, I.C.; Tariman, H.; Chan, Y.-F.; Kahar Bador, M.; Mohd Yusof, M.Y.; Hassan, H. Varicella-Zoster Virus Seroprevalence in Healthcare Workers in Kuala Lumpur, Malaysia. Med. J. Malaysia 2008, 63, 429–430. [Google Scholar]
- Chazan, B.; Colodner, R.; Teitler, N.; Chen, Y.; Raz, R. Varicella Zoster Virus in Health Care Workers in Northern Israel: Seroprevalence and Predictive Value of History of Varicella Infection. Am. J. Infect. Control 2008, 36, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Chodick, G.; Ashkenazi, S.; Livni, G.; Lerman, Y. Journal of Occupational Health Increased Susceptibility to Varicella-Zoster Virus among Israeli Physicians and Nurses Born in the Middle-East Region. J. Occup. Health 2006, 48, 246–252. [Google Scholar] [CrossRef]
- Chong, C.Y.; Lim, S.H.; Ng, W.Y.M.; Tee, N.; Lin, R.V.T.P. Varicella Screening and Vaccination for Healthcare Workers at KK Women’s and Children’s Hospital. Ann. Acad. Med. Singap. 2004, 33, 243–247. [Google Scholar] [CrossRef]
- Chong, C.H.; Liu, C.E.; Leong, Y.Y.; Liao, S.Y.; Lai, H.W.; Lee, Y.L. Seroprevalence of Varicella-Zoster Virus Antibody and Immunogenicity of Live Attenuated Varicella Vaccine in Healthcare Workers in Taiwan. J. Microbiol. Immunol. Infect. 2023, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ciliz, N.; Gazi, H.; Ecemiş, T.; Şenol, Ş.; Akçali, S.; Kurutepe, S. Seroprevalance of Measles, Rubella, Mumps, Varicella, Diphtheria, Tetanus and Hepatitis B in Healthcare Workers. KLIMIK Derg. 2013, 26, 26–30. [Google Scholar] [CrossRef]
- De Juanes, J.R.; Gil, A.; San-Martín, M.; González, A.; Esteban, J.; García De Codes, A. Seroprevalence of Varicella Antibodies in Healthcare Workers and Health Sciences Students: Reliability of Self-Reported History of Varicella. Vaccine 2005, 23, 1434–1436. [Google Scholar] [CrossRef]
- dos Santos, A.M.N.; Ono, E.; Lobato, R.T.; do Prado, S.I.; Kopelman, B.I.; Cavalcanti, C.M.; Monomi, M.K.I.; Weckx, L.Y.; de Moraes-Pinto, M.I. Diphtheria, Tetanus, and Varicella Immunity in Health Care Workers in Neonatal Units. Am. J. Infect. Control 2008, 36, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, U.; Zanetti, C.; Saia, B. Susceptibility of Healthcare Workers to Measles, Mumps Rubella and Varicella. J. Hosp. Infect. 2002, 51, 133–135. [Google Scholar] [CrossRef]
- Fernández-Cano, M.I.; Armadans, L.; Sulleiro, E.; Espuga, M.; Ferrer, E.; Martínez-Gómez, X.; Vaqué, J.; Campins, M. Susceptibilidad Frente a Sarampión y Varicela En El Personal Sanitario de Un Hospital de Tercer Nivel En Cataluña. Enferm. Infecc. Microbiol. Clin. 2012, 30, 184–188. [Google Scholar] [CrossRef]
- Gallagher, J.; Quaid, B.; Cryan, B. Susceptibility to Varicella Zoster Virus Infection in Health Care Workers. Occup. Med. 1996, 46, 289–292. [Google Scholar] [CrossRef]
- García-Basteiro, A.L.; Bayas, J.M.; Campins, M.; Torres, M.; Serra, C.; Varela, P.; Barbé, E.; Vidal, J. Susceptibility to Varicella among Health Care Workers. Acceptability and Response to Vaccination. Med. Clin. 2011, 137, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Gorny, A.W.; Mittal, C.; Saw, S.; Venkatachalam, I.; Fisher, D.A.; Tambyah, P.A. Varicella Seroprevalence in Healthcare Workers in a Tertiary Hospital: An Audit of Cross-Sectional Data Infectious Diseases. BMC Res. Notes 2015, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Garcell, H.G.; Guanche Garcell, H.; Arias, V.; García, G.; Serrano, A. Seroprotection against Vaccine-Preventable Diseases amongst Health Care Workers in a Community Hospital, Qatar. Int. J. Occup. Environ. Med. 2016, 7, 234–240. [Google Scholar] [CrossRef][Green Version]
- Hatakeyama, S.; Moriya, K.; Itoyama, S.; Nukui, Y.; Uchida, M.; Shintani, Y.; Morisawa, Y.; Kimura, S. Prevalence of Measles, Rubella, Mumps, and Varicella Antibodies Among Healthcare Workers in Japan. Infect. Control Hosp. Epidemiol. 2004, 25, 591–594. [Google Scholar] [CrossRef]
- Kanamori, H.; Tokuda, K.; Ikeda, S.; Endo, S.; Ishizawa, C.; Hirai, Y.; Takahashi, M.; Aoyagi, T.; Hatta, M.; Gu, Y.; et al. Prevaccination Antibody Screening and Immunization Program for Healthcare Personnel against Measles, Mumps, Rubella, And Varicella in a Japanese Tertiary Care Hospital. Tohoku J. Exp. Med. 2014, 234, 111–116. [Google Scholar] [CrossRef][Green Version]
- Kang, J.H.; Park, Y.S.; Park, S.Y.; Kim, S.B.; Ko, K.P.; Seo, Y.H. Varicella Seroprevalence among Health Care Workers in Korea: Validity of Self-Reported History and Cost-Effectiveness of Prevaccination Screening. Am. J. Infect. Control 2014, 42, 885–887. [Google Scholar] [CrossRef]
- Karadeniz, A.; Akduman Alaşehir, E. Seroepidemiology of Hepatitis Viruses, Measles, Mumps, Rubella and Varicella among Healthcare Workers and Students: Should We Screen before Vaccination? J. Infect. Public Health 2020, 13, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Katanyutanon, A.; Thanasopon, W.; Sonthichai, C.; Chansaenroj, J.; Nakabut, R.; Naritpavalun, S.; Prasitsomsakul, Y.; Surakhot, P.; Pompim, P.; Wanlapakorn, N.; et al. Seroprevalence of Measles and Varicella in Healthcare Workers in Chonburi Province, Thailand between October 2022 and January 2023. medRxiv 2024, 2, 24303008. [Google Scholar] [CrossRef]
- Kumakura, S.; Shibata, H.; Onoda, K.; Nishimura, N.; Matsuda, C.; Hirose, M. Seroprevalence Survey on Measles, Mumps, Rubella and Varicella Antibodies in Healthcare Workers in Japan: Sex, Age, Occupational-Related Differences and Vaccine Efficacy. Epidemiol. Infect. 2014, 142, 12–19. [Google Scholar] [CrossRef] [PubMed]
- La Torre, G.; Marte, M.; Imeshtari, V.; Colaprico, C.; Ricci, E.; Shaholli, D.; Barletta, V.I.; Serruto, P.; Gaeta, A.; Antonelli, G. Susceptibility towards Chickenpox, Measles and Rubella among Healthcare Workers at a Teaching Hospital in Rome. Vaccines 2022, 10, 1573. [Google Scholar] [CrossRef]
- Lee, J.S.; Jeong, O.; Yang, H. Screening and Vaccination Against Measles and Varicella Among Health Care Workers: A Cost-Effectiveness Analysis. Asia Pac. J. Public Health 2021, 33, 508–515. [Google Scholar] [CrossRef]
- Lerman, Y.; Chodick, G.; Tepper, S.; Livni, G.; Ashkenazi, S. Seroepidemiology of Varicella-Zoster Virus Antibodies among Health-Care Workers and Day-Care-Centre Workers. Epidemiol. Infect. 2004, 132, 1135–1138. [Google Scholar] [CrossRef]
- Lewy, R. Immunization Status of Entering Housestaff Physicians. J. Occup. Med. 1988, 30, 822–823. [Google Scholar] [PubMed]
- Oliveira, J.; Da Cunha, S.; Côrte-Real, R.; Sampaio, L.; Dias, N.; Melico-Silvestre, A. Prevalência Dos Anticorpos Anti-Sarampo, Rubéola, Papeira e Varicela Numa Populaçâo de Trabalhadores Da Saúde. Acta Med. Port. 1996, 8, 207–216. [Google Scholar]
- Perfetto, B.; Paduano, G.; Grimaldi, E.; Sansone, V.; Donnarumma, G.; Di Giuseppe, G. Seroprevalence for Measles, Varicella, Mumps and Rubella in the Trainee Obstetric Population: A Survey in Southern Italy. Vaccines 2024, 12, 3355. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.L.; Martinez, D.; Santos-Sancho, J.M.; Borda, J.R.; Orero, A. Seroprevalence of Measles, Rubella, Mumps and Varicella in Health Workers in the Community of Madrid. Rev. Esp. Quimioter. 2014, 27, 98–101. [Google Scholar]
- Santos, A.M.N.; Ono, E.; Weckx, L.Y.; Coutinho, A.P.; de Moraes-Pinto, M.I. Varicella Zoster Antibodies in Healthcare Workers from Two Neonatal Units in Säo Paulo, Brazil—Assessment of a Staff Varicella Policy. J. Hosp. Infect. 2004, 56, 228–231. [Google Scholar] [CrossRef]
- Şengöz, M.; Pişkin, N.; Aydemir, H.; Köktürk, F.; Tekin, İ.Ö.; Çelebi, G.; Atakent, D. Seroprevalence of Measles, Rubella, Mumps and Varicella in Health Care Workers. KLIMIK Derg. 2019, 32, 46–51. [Google Scholar] [CrossRef]
- Shady, I. Seroprevalence of Antibodies against Varicella Zoster Virus and Rubella Virus among Newly Recruited Expatriate Healthcare Workers: A Cross-Sectional Study. BMJ Open 2018, 8, e019339. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.; Choi, J.R.; Huh, K.; Chung, D.R.; Cho, S.Y.; Jeong, J.; Ko, J.H.; Kang, C.I.; Peck, K.R. Trend of Immunity against Measles and Varicella Zoster Virus in Healthcare Workers in Korea. Vaccine 2023, 41, 4679–4684. [Google Scholar] [CrossRef] [PubMed]
- Talebi-Taher, M.; Noori, M.; Shamshiri, A.R.; Barati, M. Varicella Zoster Antibodies among Health Care Workers in a University Hospital, Teheran, Iran. Int. J. Occup. Med. Environ. Health 2010, 23, 27–32. [Google Scholar] [CrossRef][Green Version]
- Urbiztondo, L.; Borrás, E.; Bayas, J.M.; Bayas, J.M.; Borrás, E.; Domínguez, A.; Broner, S.; Borrás, E.; Domínguez, A.; Costa, J.; et al. Varicella-Zoster Virus Immunity among Health Care Workers in Catalonia. Vaccine 2014, 32, 5945–5948. [Google Scholar] [CrossRef]
- Vandersmissen, G.; Moens, G.; Vranckx, R.; De Schryver, A.; Jacques, P.; Vandersmissen, G.; Moens, G.; De Schryver, A.; Jacques, P. Occupational Risk of Infection by Varicella Zoster Virus in Belgian Healthcare Workers: A Seroprevalence Study. Occup. Environ. Med. 2000, 57, 621–626. [Google Scholar] [CrossRef]
- Verma, S.; Ravinder, K.S.; Mahajan, V.; Rawat, A.; Nehra, U.; Subramani, V.; Jassal, M.; Bharti, B. Immune Status of Indian Pediatric Health Care Workers against Various Preventable Diseases. Open Forum Infect. Dis. 2022, 9, S103. [Google Scholar] [CrossRef]
- Villasís-Keever, M.A.; Peña, L.A.; Miranda-Novales, G.; Alvarez y Muñoz, T.; Damasio-Santana, L.; López-Fuentes, G.; Girón-Carrillo, J.L. Prevalence of Serological Markers against Measles, Rubella, Varicella, Hepatitis B, Hepatitis C, and Human Immunodeficiency Virus among Medical Residents in Mexico. Prev. Med. 2001, 32, 424–428. [Google Scholar] [CrossRef]
- Wangsan, K.; Chaiear, N.; Krisorn, P.; Soonthornvinit, W. Varicella Zoster Immunity amongst New Health Personnel in a University Hospital in Northeastern Thailand Article. J. Med. Assoc. Thail. 2019, 102, S7–S11. [Google Scholar]
- Watanabe, T.; Tsuchiya, M.; Suzuki, T.; Niwa, T.; Ohta, H.; Murakami, N. P280: Seroprevalence of Measles, Rubella, Mumps, and Varicella among Health Care Workers in Japan. Antimicrob. Resist. Infect. Control 2013, 2, P280. [Google Scholar] [CrossRef]
- Wu, M.F.; Yang, Y.W.; Lin, W.Y.; Chang, C.Y.; Soon, M.S.; Liu, C.E. Varicella Zoster Virus Infection among Healthcare Workers in Taiwan: Seroprevalence and Predictive Value of History of Varicella Infection. J. Hosp. Infect. 2012, 80, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, T.; Ozdemir, I.; Sencan, I.; Arbak, P.; Behçet, M.; Sert, E. Seroprevalence of Varicella, Measles and Hepatitis B among Female Health Care Workers of Childbearing Age. Jpn. J. Infect. Dis. 2005, 58, 383–386. [Google Scholar] [CrossRef]
- Yun, J.H.; Lee, E.; Choi, J.H.; Ki, H.K.; Park, J. Seroprevalence of Varicella-Zoster Virus and Measles among Healthcare Workers in a Tertiary Medical Center in Korea. Vaccines 2022, 10, 1956. [Google Scholar] [CrossRef]
- National Institute of Environmental Health Sciences. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2019.
- Shapiro, E.D.; Marin, M. The Effectiveness of Varicella Vaccine: 25 Years of Postlicensure Experience in the United States. J. Infect. Dis. 2022, 226, S425–S430. [Google Scholar] [CrossRef]
- Goldman, G.S. Universal Varicella Vaccination: Efficacy Trends and Effect on Herpes Zoster. Int. J. Toxicol. 2005, 24, 205–213. [Google Scholar] [CrossRef]
- Riera-Montes, M.; Bollaerts, K.; Heininger, U.; Hens, N.; Gabutti, G.; Gil, A.; Nozad, B.; Mirinaviciute, G.; Flem, E.; Souverain, A.; et al. Estimation of the Burden of Varicella in Europe before the Introduction of Universal Childhood Immunization. BMC Infect. Dis. 2017, 17, 353. [Google Scholar] [CrossRef]
- Papaloukas, O.; Giannouli, G.; Papaevangelou, V. Successes and Challenges in Varicella Vaccine. Ther. Adv. Vaccines 2014, 2, 39–55. [Google Scholar] [CrossRef]
- Branda, F.; Giovanetti, M.; Romano, C.; Benvenuto, D.; Ciccozzi, A.; Sanna, D.; Ciccozzi, M.; Scarpa, F. Global Measles Surveillance: Trends, Challenges, and Implications for Public Health Interventions. Infect. Dis. Rep. 2024, 16, 367–379. [Google Scholar] [CrossRef]
- Memish, Z.A.; Bamgboye, E.A.; Mohammed, M.; AlHakeem, R.; Al-Tawfiq, J.A.; Assiri, A. Secular Trend and Epidemiology of Measles in the Kingdom of Saudi Arabia: 2009-2012. Travel. Med. Infect. Dis. 2015, 13, 74–79. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al Mutair, A.; Alhumaid, S.; Garout, M.; Alsubki, R.A.; Alshahrani, F.S.; Alfouzan, W.A.; Alestad, J.H.; Alsaleh, A.E.; Al-Mozaini, M.A.; et al. Updates on Measles Incidence and Eradication: Emphasis on the Immunological Aspects of Measles Infection. Medicina 2022, 58, 680. [Google Scholar] [CrossRef]
- Spoulou, V.; Alain, S.; Gabutti, G.; Giaquinto, C.; Liese, J.; Martinon-Torres, F.; Vesikari, T. Implementing Universal Varicella Vaccination in Europe: The Path Forward. Pediatr. Infect. Dis. J. 2019, 38, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Azzari, C.; Baldo, V.; Giuffrida, S.; Gani, R.; O’brien, E.; Alimenti, C.; Daniels, V.J.; Wolfson, L.J. The Cost-Effectiveness of Universal Varicella Vaccination in Italy: A Model-Based Assessment of Vaccination Strategies. Clin. Econ. Outcomes Res. 2020, 12, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Moro, P.L.; Leung, J.; Marquez, P.; Kim, Y.; Wei, S.; Su, J.R.; Marin, M. Safety Surveillance of Varicella Vaccines in the Vaccine Adverse Event Reporting System, United States, 2006–2020. J. Infect. Dis. 2022, 226, S431–S440. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Al Olama, F.M.A.S.; Al Qaseer, M.; Al Ubaidani, I.; Dinleyici, E.C.; Hayajneh, W.A.; Bizri, A.R.; Loulou, M.; Ndao, T.; Wolfson, L.J. The Clinical and Economic Burden of Varicella in the Middle East: A Systematic Literature Review. Hum. Vaccin. Immunother. 2020, 16, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rieck, T.; Feig, M.; an der Heiden, M.; Siedler, A.; Wichmann, O. Assessing Varicella Vaccine Effectiveness and Its Influencing Factors Using Health Insurance Claims Data, Germany, 2006 to 2015. Eurosurveillance 2017, 22, 21–32. [Google Scholar] [CrossRef]
- Betsch, C.; Wicker, S. Personal Attitudes and Misconceptions, Not Official Recommendations Guide Occupational Physicians’ Vaccination Decisions. Vaccine 2014, 32, 4478–4484. [Google Scholar] [CrossRef]
- Falato, R.; Ricciardi, S.; Franco, G. Influenza Risk Perception and Vaccination Attitude in Medical and Nursing Students during the Vaccination Campaigns of 2007/2008 (Seasonal Influenza) and 2009/2010 (H1N1 Influenza). Med. Lav. 2011, 102, 208–215. [Google Scholar]
- La Torre, G.; Scalingi, S.; Garruto, V.; Siclari, M.; Chiarini, M.; Mannocci, A. Knowledge, Attitude and Behaviours towards Recommended Vaccinations among Healthcare Workers. Healthcare 2017, 5, 13. [Google Scholar] [CrossRef]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef]
- Loulergue, P.; Moulin, F.; Vidal-Trecan, G.; Absi, Z.; Demontpion, C.; Menager, C.; Gorodetsky, M.; Gendrel, D.; Guillevin, L.; Launay, O. Knowledge, Attitudes and Vaccination Coverage of Healthcare Workers Regarding Occupational Vaccinations. Vaccine 2009, 27, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, V.; Bracci, M.; Nunnari, G.; Ferrante, M.; Ledda, C. Tetanus Immunity in Construction Workers in Italy. Occup. Med. 2014, 64, 217–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riccò, M.; Vezzosi, L.; Cella, C.; Pecoraro, M.; Novembre, G.; Moreo, A.; Ognibeni, E.M.; Schellenberg, G.; Maranelli, G. Tetanus Vaccination Status in Construction Workers: Results from an Institutional Surveillance Campaign. Acta Biomed. 2019, 90, 269–278. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Veronesi, L.; Colucci, M.E. Knowledge, Attitudes, Beliefs and Practices of Construction Workers towards Tetanus Vaccine in Northern Italy. Ind. Health 2016, 54, 554–563. [Google Scholar] [CrossRef]
- Auffret, Y.; Rousseaux, J.Y.; Gatineau, F.; Hamoniaux, F.; Gouillou, M.; Abalea Le Dreff, L.; Pina Silas, S.; Rakatobe, F.; Alavi, Z. Should We Believe Emergency Department Patients Self-Reported Tetanus Vaccine Status? Am. J. Emerg. Med. 2019, 37, 983–990. [Google Scholar] [CrossRef]
- Tafuri, S.; Martinelli, D.; Caputi, G.; Arbore, A.; Lopalco, P.L.; Germinario, C.; Prato, R. An Audit of Vaccination Coverage among Vaccination Service Workers in Puglia, Italy. Am. J. Infect. Control 2009, 37, 414–416. [Google Scholar] [CrossRef]
- Baum, S.G. Notes from the Field: Expanded Laboratory Testing for Varicella-Minnesota 2016-2023. MMWR 2024, 73, 245–246. [Google Scholar]
- Marin, M.; Leung, J.; Anderson, T.C.; Lopez, A.S. Monitoring Varicella Vaccine Impact on Varicella Incidence in the United States: Surveillance Challenges and Changing Epidemiology, 1995–2019. J. Infect. Dis. 2022, 226, S392–S399. [Google Scholar] [CrossRef] [PubMed]
- Prymula, R.; Bergsaker, M.R.; Esposito, S.; Gothefors, L.; Man, S.; Snegova, N.; Štefkovičova, M.; Usonis, V.; Wysocki, J.; Douha, M.; et al. Protection against Varicella with Two Doses of Combined Measles-Mumps-Rubella-Varicella Vaccine versus One Dose of Monovalent Varicella Vaccine: A Multicentre, Observer-Blind, Randomised, Controlled Trial. Lancet 2014, 383, 1313–1324. [Google Scholar] [CrossRef]
- Vafai, N.; Self, K.; Sheffield, B.; Hojvat, S.; Kusi-Appiah, A.; Vaughan, P.; Cowan, E.; Vafai, A. Rapid, Sensitive, and Specific Lateral-Flow Immunochromatographic Point-of-Care Device for Detection of Varicella-Zoster Virus Immunoglobulin G Antibodies in Fingerstick Blood. J. Immunol. Methods 2023, 514, 113429. [Google Scholar] [CrossRef]
- Bagnasco, A.; Zanini, M.; Catania, G.; Watson, R.; Hayter, M.; Dasso, N.; Dini, G.; Agodi, A.; Pasquarella, C.; Zotti, C.M.; et al. Predicting needlestick and sharps injuries in nursing students: Development of the SNNIP scale. Nurs. Open 2020, 7, 1578–1587. [Google Scholar] [CrossRef]
- Campagna, M.; Bacis, M.; Belotti, L.; Biggi, N.; Carrer, P.; Cologni, L.; Gattinis, V.; Lodi, V.; Magnavita, N.; Micheloni, G.; et al. Exanthemic Diseases (Measles, Chickenpox, Rubella and Parotitis). Focus on Screening and Health Surveillance of Health Workers_ Results and Perspectives of a Multicenter Working Group. G. Ital. Med. Lav. Ergon. 2010, 32, 298–303. [Google Scholar] [PubMed]
- Johnson, C.; Rome, L.P.; Stancin, T.; Kumar, M.L. Humoral Immunity and Clinical Reinfections Following Varicella Vaccine in Healthy Children. Pediatrics 1989, 84, 418–421. [Google Scholar] [CrossRef]
- Pan, D.; Wang, W.; Cheng, T. Current Methods for the Detection of Antibodies of Varicella-Zoster Virus: A Review. Microorganisms 2023, 11, 519. [Google Scholar] [CrossRef]
- Breuer, J.; Schmid, D.S.; Gershon, A.A. Use and Limitations of Varicella-Zoster Virus-Specific Serological Testing to Evaluate Breakthrough Disease in Vaccinees and to Screen for Susceptibility to Varicella. J. Infect. Dis. 2008, 197, S147–S151. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Poland, G.A. Vaccination Policies for Healthcare Workers in Europe. Vaccine 2014, 32, 4876–4880. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Theodoridou, K.; Ledda, C.; Rapisarda, V.; Theodoridou, M. Vaccination of Healthcare Workers: Is Mandatory Vaccination Needed? Expert Rev. Vaccines 2019, 18, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Botelho-Nevers, E.; Brantsæter, A.B.; Carlsson, R.M.; Heininger, U.; Hübschen, J.M.; Josefsdottir, K.S.; Kassianos, G.; Kyncl, J.; Ledda, C.; et al. Vaccination of Healthcare Personnel in Europe: Update to Current Policies. Vaccine 2019, 37, 7576–7584. [Google Scholar] [CrossRef] [PubMed]
- Childre, F.; Nord, D.D. Measles, Mumps, Rubella, and Varicella Immunization Status of First Responders—A Policy Proposal. AAOHN J. 2009, 57, 187–189. [Google Scholar] [CrossRef]
- Kang, C.K.; Chang, E.; Jung, J.; Lee, E.; Song, K.H.; Choe, P.G.; Bang, J.H.; Kim, E.S.; Park, S.W.; Kim, H.B.; et al. Different Levels of Humoral and Cellular Immunity to Varicella-Zoster Virus in Seropositive Healthcare Workers. J. Infect. Public Health 2022, 15, 734–738. [Google Scholar] [CrossRef]
- Li, S.; Chan, I.S.F.; Matthews, H.; Heyse, J.F.; Chan, C.Y.; Kuter, B.J.; Kaplan, K.M.; Rupert Vessey, S.J.; Sadoff, J.C. Inverse Relationship between Six. Week Postvaccination Varicella Antibody Response to Vaccine and Likelihood of Long. Term. Breakthrough Infection. Pediatr. Infect. Dis. J. 2002, 21, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Boniol, M.; Kunjumen, T.; Nair, T.S.; Siyam, A.; Campbell, J.; Diallo, K. The Global Health Workforce Stock and Distribution in 2020 and 2030: A Threat to Equity and â € Universal’ Health Coverage? BMJ Glob. Health 2022, 7, e009316. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV Disease in Infants and Young Children: Can We See a Brighter Future? Hum. Vaccines Immunother. 2022, 18, 2079322. [Google Scholar] [CrossRef] [PubMed]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. Fixed-Effect vs Random-Effects Models for Meta-Analysis: 3 Points to Consider. Global Spine J. 2022, 12, 1624–1626. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Fairbrother, M.; Jones, K. Fixed and Random Effects Models: Making an Informed Choice. Qual. Quant. 2019, 53, 1051–1074. [Google Scholar] [CrossRef]
- Esterhuizen, T.M.; Thabane, L. Con: Meta-Analysis: Some Key Limitations and Potential Solutions. Nephrol. Dial. Transplant. 2016, 31, 882–885. [Google Scholar] [CrossRef]



| Study | Timeframe | Country | Laboratory Study | Total Sample (N) | Tested VZV (n/N, %) | Tested Measles (n/N, %) | Tested Rubella (n/N, %) | Tested Nurses (n/N, %) | Tested Physicians (n/N, %) | Age (Years) | Medical History on VZV (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AbdalAziz et al., 2019 [79] | N.A. | Saudi Arabia | ELISA | 673 | 673, 100% | 673, 100% | 673, 100% | N.A. | N.A. | 26.5 ± 5.5 (A) | No |
| Almuneef et al., 2006 [80] | September 2001 to March 2005 | Saudi Arabia | ELISA | 4006 | 3930, 98.10 | 3818, 97.80% | 3972, 99.15% | 913, 22.79% | 267, 6.67% | N.A. | No |
| Alp et al., 2012 [81] | December 2010 to April 2011 | Turkey | ELISA | 1255 | 1255, 100% | 1255, 100% | 1255, 100% | 611, 48.69% | N.A. | 30 (19–60) (B) | No |
| Andrew et al., 2016 [46] | January 2012 to December 2013 | Australia | ELISA | 1901 | 1664, 85.53% | 1779, 93.58% | 1789, 94.11% | N.A. | N.A. | 30 (25–38) (B) | No |
| Anugulruengkitt et al., 2017 [82] | May 2015 to January 2015 | Thailand | ELISA | 760 | 107, 14.08% | - | - | 347, 45.66% | 146, 19.21% | 35.8 (28.5–47.2) (B) | No |
| Asari et al., 2003 [83] | April 2000 | Japan | ELISA | 271 | 271, 100% | 271, 100% | 271, 100% | 72, 26.57% | 199, 73.43% | N.A. | No |
| Aypak et al., 2012 [84] | N.A. | Turkey | ELISA | 284 | 284, 100% | 284, 100% | 284, 100% | 111, 39.08% | 87, 30.63% | 33.5 ± 11 (A) | No |
| Bakri et al., 2016 [85] | March 2011 to March 2012 | Jordan | ELISA | 493 | 493, 100% | 493, 100% | 493, 100% | 241, 48.88% | 252, 51.12% | 28.8 ± 6.3 (A) | Yes |
| Balbi et al., 2021 [86] | January 2018 to August 2018 | Italy | ELISA | 840 | 840, 100% | - | - | 297, 35.36% | 463, 55.12% | 36.6 (18–70) (B) | No |
| Bassett et al., 1993 [87] | N.A. | Hong Kong | EIA | 97 | 70, 72.16% | - | - | 97, 100% | - | N.A. | No |
| Behrman et al., 2013 [62] | November 2005 to May 2007 | USA | ELISA | 101 | 101, 100% | - | - | N.A. | N.A. | 30 (18–70) (B) | No |
| Brunell et al., 1999 [57] | N.A. | USA | ELISA | 1359 | 1359, 100% | - | - | N.A. | N.A. | N.A. | Yes |
| Celikbas et al., 2006 [88] | March 2005 to May 2005 | Turkey | ELISA | 363 | 363, 100% | 363, 100% | 363, 100% | 118, 32.51% | 186, 51.24% | 29.1 (C) | No |
| Sam et al., 2008 [89] | September 2006 to April 2008 | Malaysia | ELISA | 88 | 57, 64.77% | - | - | N.A. | N.A. | 26.2 (C) | Yes |
| Chazan et al., 2008 [90] | N.A. | Israel | ELISA | 200 | 200, 100% | - | - | 101, 50.50% | 42, 21.00% | N.A. | Yes |
| Chodick et al., 2006 [91] | N.A. | Israel | ELISA | 335 | 330, 98.51% | - | - | 188, 56.12% | 147, 43.88% | 41.96 ± 12.0 (A) | No |
| Chong et al., 2004 [92] | September 1997 to February 1998 | Singapore | EIA | 2284 | 2284, 100% | - | - | 1325, 58.01% | 241, 10.55% | N.A. | No |
| Chong et al., 2023 [93] | August 2011 to July 2017 | Taiwan | ELISA | 2406 | 2406, 100% | - | - | 959, 39.86% | 366, 15.21% | N.A. | Yes |
| Ciliz et al., 2013 [94] | November 2011 to July 2012 | Turkey | ELISA | 309 | 309, 100% | 309, 100% | - | 66, 21.36% | 151, 48.87% | 33.8 ± 7.6 (A) | No |
| De Juanes et al., 2005 [95] | March 2003 to August 2003 | Spain | ELISA | 93 | 93, 100% | - | - | N.A. | N.A. | 30.6 ± 4.0 (A) | Yes |
| Dos Santos et al., 2008 [96] | September 2002 to November 2002 | Brazil | ELISA | 215 | 215, 100% | - | - | 134, 62.33% | 55, 25.58% | 35.3 (20.7–64.0) (B) | Yes |
| Fedeli et al., 2002 [97] | September 1998 to February 2002 | Italy | ELISA | 333 | 333, 100% | 333, 100% | 333, 100% | 203, 60.96% | 25, 7.51% | 38 (23–60) (B) | No |
| Fernandez-Cano et al., 2012 [98] | January 2006 to December 2008 | Spain | ELISA | 2752 | 2511, 91.24% | 2528, 91.86% | - | 1014, 36.85% | 632, 22.97% | 42.9 ± 11.8 (A) | No |
| Gallagher et al., 1996 [99] | January 1990 to Dec. 1994 | UK | LAA | 894 | 894, 100% | - | - | N.A. | N.A. | N.A. | Yes |
| Garcia Basteiro et al., 2011 [100] | November 2000 to September 2001 | Spain | ELISA | 1111 | 1111, 100% | - | - | 412, 37.08% | 270, 24.30% | 32.2 ± 9.2 (A) | Yes |
| Gorny et al., 2015 [101] | 2009 to 2014 | Singapore | ELISA | 6701 | 3906, 58.29% | - | - | 2221, 33.14% | 124, 1.85% | N.A. | No |
| Guanche Garcell et al., 2016 [102] | August 2012 to December 2015 | Qatar | ELISA | 705 | 705, 100% | 705, 100% | 705, 100% | 400, 56.74% | 177, 25.11% | N.A. | No |
| Hatakayama et al., 2004 [103] | September 2002 to October 2002 | Japan | EIA | 877 | 854, 97.38% | 860, 98.06% | 867, 98.86% | 426, 48.57% | 212, 24.17% | 34.4 ± 10.3 (A) | No |
| Kanamori et al., 2014 [104] | April 2012 to Mar 2013 | Japan | EIA | 243 | 243, 100% | 243, 100% | 243, 100% | 72, 29.63% | 75, 30.86% | N.A. | No |
| Kang et al., 2014 [105] | March 2008 to March 2010 | South Korea | CLIA | 550 | 550, 100% | - | - | 393, 71.45% | 103, 18.73% | 27 (21–56) (B) | Yes |
| Karadeniz et al., 2020 [106] | September 2016 to September 2017 | Turkey | EIA | 1053 | 1053, 100% | 1053, 100% | 1053, 100% | 481, 45.68% | 395, 37.51% | 22.3 ± 5.3 (A) | Yes |
| Katanyutanon et al., 2024 [107] | October 2022 to January 2023 | Thailand | ELISA | 266 | 266, 100% | 266, 100% | - | N.A. | N.A. | 38.3 ± 11.5 (A) | Yes |
| Kumakura et al., 2014 [108] | 2005 to 2009 | Japan | EIA | 18,111 | 1811, 100% | 1811, 100% | 1811, 100% | 622, 34.35% | 662, 36.55% | 34.3 ± 10.2 (A) | No |
| La Torre et al., 2022 [109] | February 2017 to Jan 2020 | Italy | ELISA | 1106 | 1101, 99.55% | 1097, 99.19% | 1105, 99.91% | 462, 41.77% | 336, 30.38% | 54.1 ± 8.8 (A) | No |
| Lee et al., 2021 [110] | June 2019 to September 2019 | South Korea | ELISA | 300 | 300, 100% | 300, 100% | - | 203, 67.67% | 34, 11.33% | 33.3 ± 8.3 (A) | Yes |
| Lerman et al., 2004 [111] | N.A. | Israel | ELISA | 335 | 335, 100% | - | - | - | - | N.A. | No |
| Lewy et al., 1988 [112] | 1987 | USA | ELISA | 164 | 164, 100% | - | - | - | - | 25 to 36 (D) | No |
| Oliveira et al., 1995 [113] | N.A. | Portugal | ELISA | 409 | 409, 100% | 409, 100% | 409, 100% | - | - | 40.9 ± 9.7 (A) | Yes |
| Perfetto et al., 2024 [114] | 2017 to 2022 | Italy | ELISA | 517 | 517, 100% | 517, 100% | 517, 100% | - | - | 26.3 ± 5.7 (A) | No |
| Rodriguez et al., 2014 [115] | January 2009 to June 2010 | Spain | ELISA | 1060 | 1060, 100% | 1060, 100% | 1060, 100% | 616, 58.11% | 261, 24.62% | 40.2 ± 12.6 (A) | No |
| Santos et al., 2004 [116] | September 2002 to November 2002 | Brazil | ELISA | 215 | 215, 100% | - | - | - | - | 33 (20–64) (B) | Yes |
| Sengoz et al., 2019 [117] | March 2014 to January 2015 | Turkey | ELISA | 384 | 384, 100% | 384, 100% | 384, 100% | 202, 52.60% | 65, 16.93% | 32.4 ± 6.4 (A) | Yes |
| Shady I 2018 [118] | April 2015 to January 2016 | Kuwait | EIA | 1540 | 1540, 100% | - | 1540, 100% | 792, 51.43% | 174, 11.30% | N.A. | No |
| Shin et al., 2023 [119] | 2017 to 2022 | South Korea | ELISA | 10,576 | 9607, 90.84% | 10278, 97.18% | - | 5356, 51.43% | 1862, 17.61% | N.A. | Yes |
| Talebi-Taher et al., 2010 [120] | February 2009 to March 2009 | Iran | EIA | 405 | 405, 100% | - | - | 217, 53.58% | 125, 30.86% | N.A. | Yes |
| Troiani et al., 2015 [58] | January 2014 to December 2014 | USA | CLIA | 413 | 413, 100% | - | - | N.A. | N.A. | N.A. | No |
| Tsou and Shao 2019 [61] | January 2008 to September 2018 | Taiwan | ELISA | 7314 | 7314, 100% | - | - | 2826, 38.64% | 1394, 19.06% | 26.8 ± 8.0 (A) | No |
| Urbiztondo et al., 2014 [121] | June 2008 to December 2010 | Spain | ELISA | 644 | 644, 100% | - | - | 249, 38.66% | 191, 29.66% | N.A. | Yes |
| Vagholkar et al., 2008 [63] | September 2003 to July 2005 | Australia | ELISA | 1900 | 1320, 69.47% | 1320, 69.47% | 1320, 69.47% | N.A. | N.A. | N.A. | No |
| Vandermissen et al., 2000 [122] | February 1996 to June 1996 | Belgium | ELISA | 4923 | 4923, 100% | - | - | N.A. | N.A. | N.A. | Yes |
| Verma et al., 2022 [123] | July 2018 to December 2018 | India | ELISA | 160 | 160, 100% | 160, 100% | 160, 100% | 31, 19.38% | 106, 66.25% | 30.6 ± 7.8 (A) | No |
| Villasis-Keever et al., 2001 [124] | March 1998 to May 1998 | Mexico | ELISA | 89 | 89, 100% | 89, 100% | 89, 100% | - | 89, 100% | 28 (23–41) (B) | Yes |
| Wangsan et al., 2019 [125] | January 2017 to September 2017 | Thailand | ELISA | 214 | 214, 100% | - | - | 40, 18.69% | 137, 64.02% | 24 (24–27) (B) | Yes |
| Watanabe et al., 2013 [126] | 2007 to 2012 | Japan | ELISA | 1385 | 1385, 100% | 1385, 100% | 1385, 100% | N.A. | N.A. | N.A. | No |
| Wu et al., 2012 [127] | N.A. | Taiwan | ELISA | 3733 | 3733, 100% | - | - | 1580, 42.33% | 537, 14.39% | 34.6 (18–68) (B) | Yes |
| Yang et al., 2019 [55] | January 2014 to December 2017 | Mainland China | ELISA | 1804 | 1804, 100% | - | - | 1238, 68.63% | 153, 8.48% | N.A. | Yes |
| Yavuz et al., 2005 [128] | January 2005 to March 2005 | Turkey | ELISA | 73 | 73, 100% | 73, 100% | - | N.A. | N.A. | 32.7 ± 5.4 (A) | No |
| Yun et al., 2022 [129] | October 2015 to October 2021 | South Korea | CLIA | 2070 | 2070, 100% | 1827, 88.26% | - | 8, 54.01% | 473, 22.85% | N.A. | No |
| Variable | No./Total | % |
|---|---|---|
| Sampled HCWs | 77,362 | 100% |
| Age | ||
| <30 years | 23,202 | 29.99% |
| ≥30 years | 22,125 | 28.60% |
| Not reported | 32,025 | 41.41% |
| Gender | ||
| Male | 15,344 | 19.83% |
| Female | 45,407 | 58.70% |
| Not reported | 16,611 | 21.47% |
| Job title | ||
| Nurses | 29,749 | 38.45% |
| Physicians | 11,214 | 14.50% |
| Other | 25,524 | 32.99% |
| Not provided | 10,875 | 14.06% |
| Tested HCWs | ||
| Varicella zoster virus | 71,720 | 92.71% |
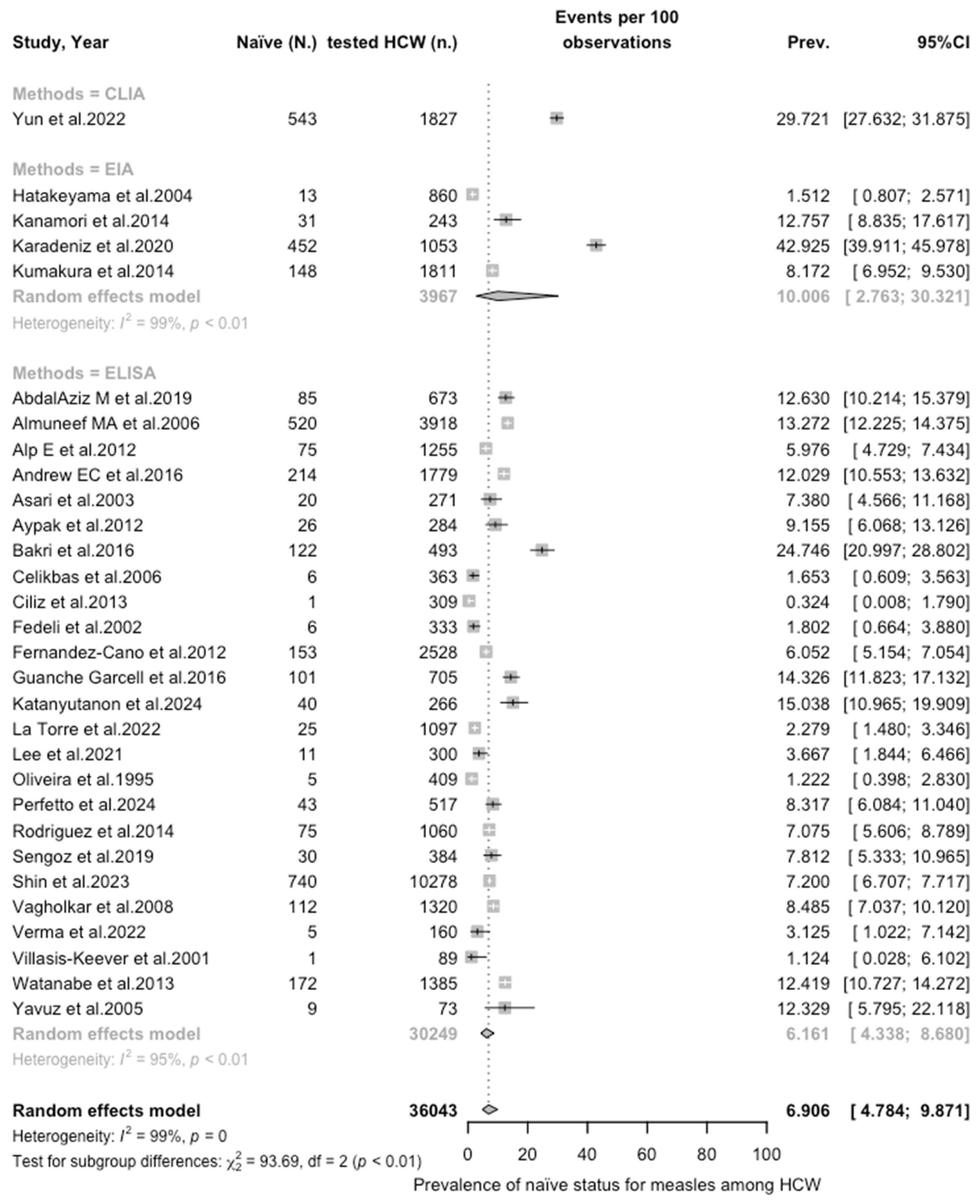
| Measles | 36,043 | 46.59% |
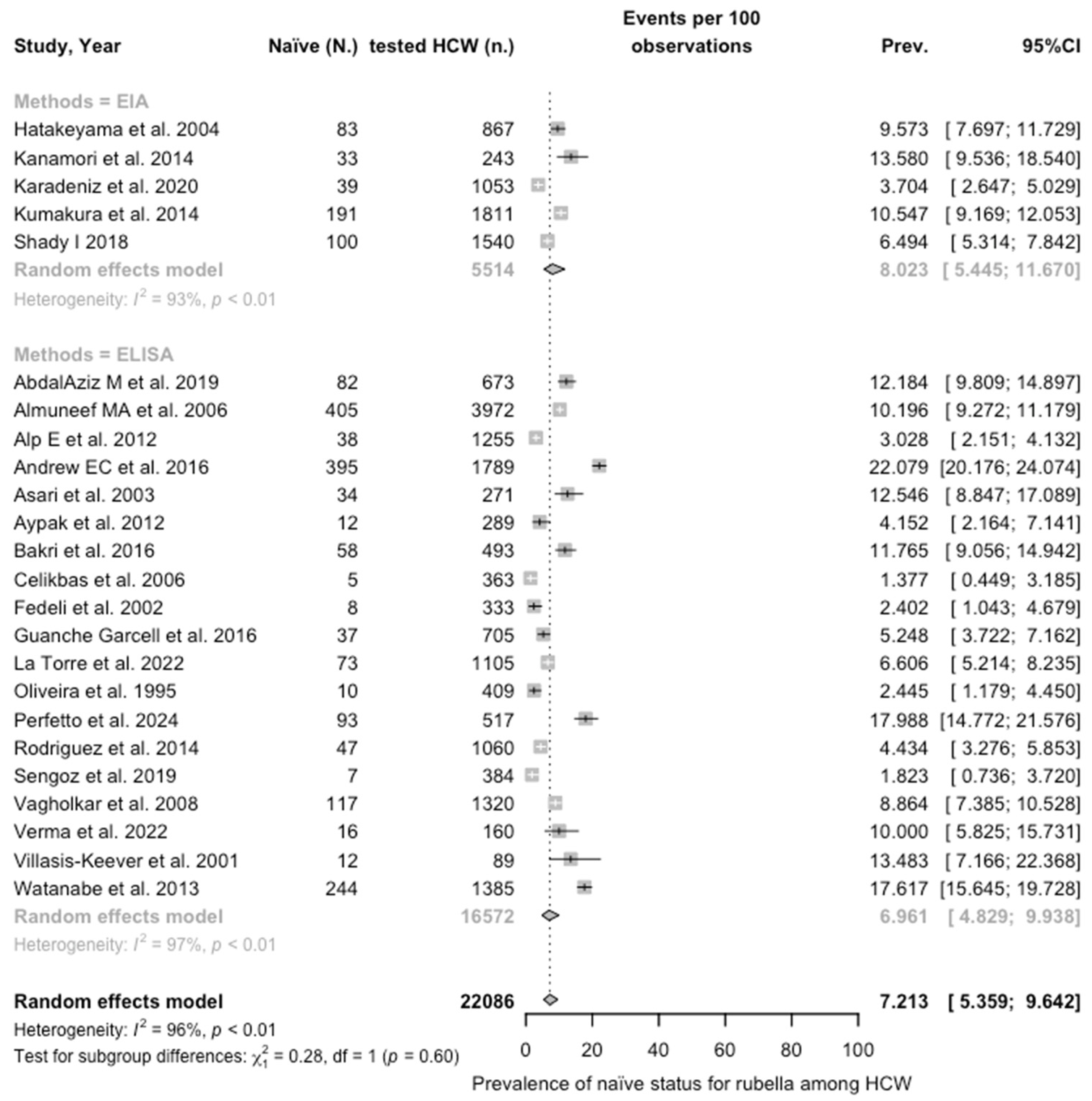
| Rubella | 22,086 | 28.55% |
| Pathogen | N. | % | Naïve (n/N, %) | Risk Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|
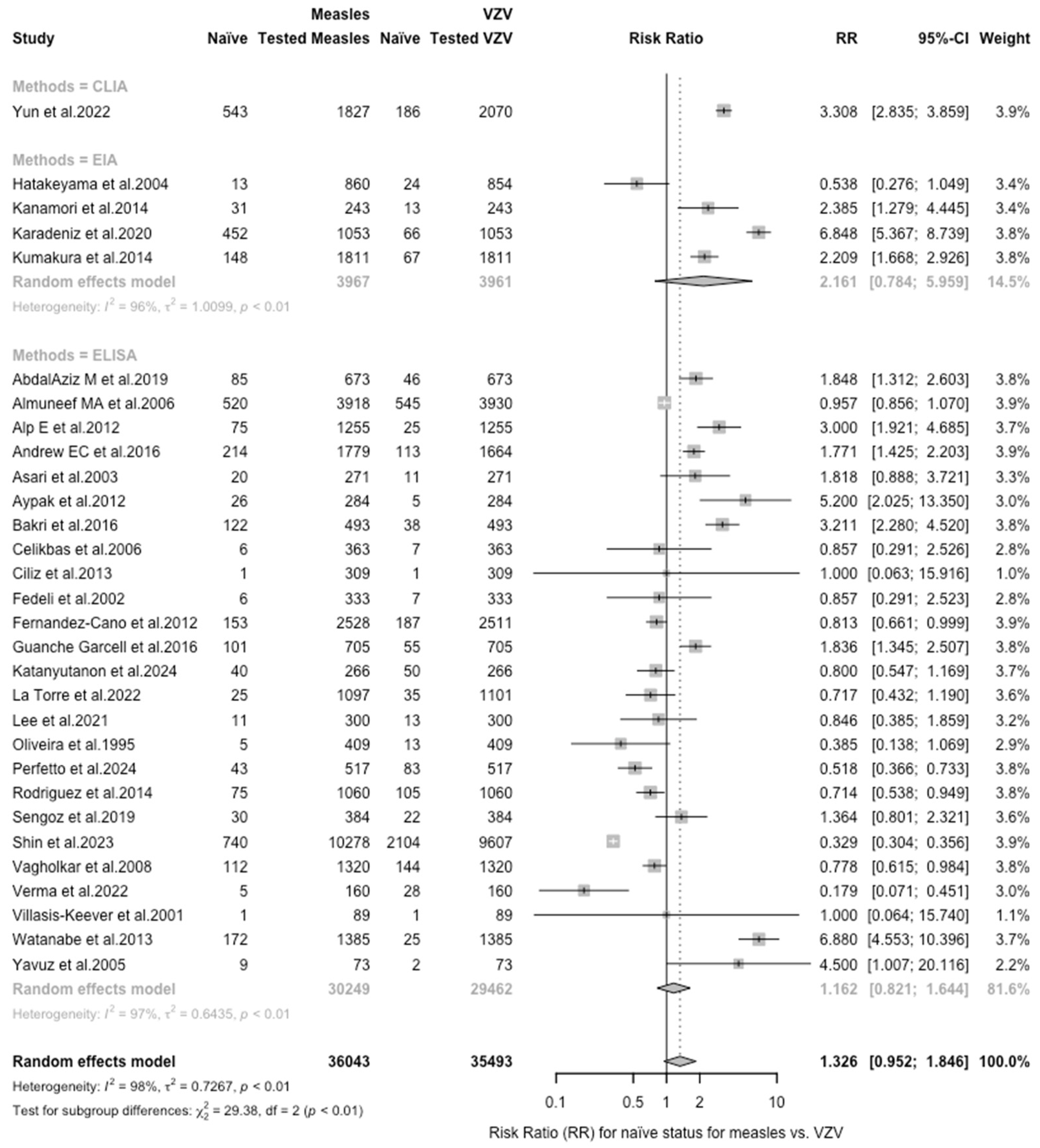
| Varicella Zoster | 71,720 | 92.71% | 6960, 9.70% | REFERENCE | |
| Measles | 36,043 | 46.59% | 3784, 10.50% | 1.081 | 1.042; 1.123 |
| Rubella | 22,086 | 28.55% | 1830, 8.29% | 0.854 | 0.813; 0.897 |
| Variable | % | Naïve (n/N, %) | Risk Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Gender | N./35,663 | ||||
| Male | 8867 | 24.86% | 801, 9.04% | REFERENCE | |
| Female | 26,796 | 75.14% | 2522, 9.41% | 1.042 | 0.966; 1.124 |
| Age | N./27,891 | ||||
| <30 years | 14,451 | 51.81% | 1793, 12.41% | 1.288 | 1.204; 1.378 |
| ≥30 years | 13,440 | 48.29% | 1295, 9.64% | REFERENCE | |
| Timeframe of the study | N./71,720 | ||||
| Before 2000 | 2896 | 4.04% | 85, 2.94% | 0.173 | 0.140; 0.214 |
| 2000–2009 | 15,885 | 22.15% | 1032, 6.50% | 0.384 | 0.359; 0.410 |
| 2010–2019 | 34,619 | 48.27% | 2740, 7.91% | 0.467 | 0.445; 0.490 |
| 2020 onwards | 18,320 | 25.54% | 3103, 16.94% | REFERENCE | |
| Settings of the study | N./71,447 | ||||
| EUR | 18,949 | 26.52% | 784, 4.14% | REFERENCE | |
| EMR | 7746 | 10.84% | 1079, 13.93% | 3.367 | 3.083; 3.677 |
| SEAR | 747 | 1.05% | 113, 15.13% | 3.656 | 3.044; 4.391 |
| WPR | 41,649 | 58.29% | 4847, 11.64% | 2.813 | 2.614; 3.027 |
| AMR | 2356 | 3.30% | 91, 3.86% | 0.934 | 0.755; 1.155 |
| Pathogen | Grouping Variable | Pooled Prevalence (N Per 100 Samples, 95% CI) | τ2 | (I2; 95% CI) | Q | p Value |
|---|---|---|---|---|---|---|
| VZV | All | 5.719 (4.590; 7.104) | 0.738 | 98.1% (97.8 to 98.3) | 2939.67 (df = 57) | <0.001 |
| Age | ||||||
| <30 y.o. | 9.775 (6.907; 13.660) | 0.615 | 95.6% (94.2 to 96.7) | 389.16 (df = 17) | <0.001 | |
| ≥30 y.o. | 6.307 (4.239; 9.284) | 0.760 | 96.0% (94.8 to 97.0) | 428.04 (df = 17) | <0.001 | |
| Gender | ||||||
| Male | 7.386 (5.177; 10.435) | 0.757 | 91.7% (88.9 to 93.8) | 277.85 (df = 23) | <0.001 | |
| Female | 6.983 (4.948; 9.768) | 0.776 | 97.4% (96.8 to 97.8) | 868.98 (df = 23) | <0.001 | |
| Measles | All | 6.906 (4.785; 9.871) | 1.110 | 98.6% (98.3 to 98.8) | 2001.61 (df = 29) | <0.001 |
| Rubella | All | 7.213 (5.359; 9.643) | 0.585 | 96.5% (95.6 to 97.2) | 656.83 (df = 23) | <0.001 |
| Grouping Variable | RR (95% CI) | τ2 | (I2; 95% CI) | Q | p Value |
|---|---|---|---|---|---|
| Age < 30 vs. ≥30 y.o. | 1.434 (1.172; 1.755) | 0.122 | 79.9% (68.9 to 86.9) | 84.41 (df = 17) | <0.001 |
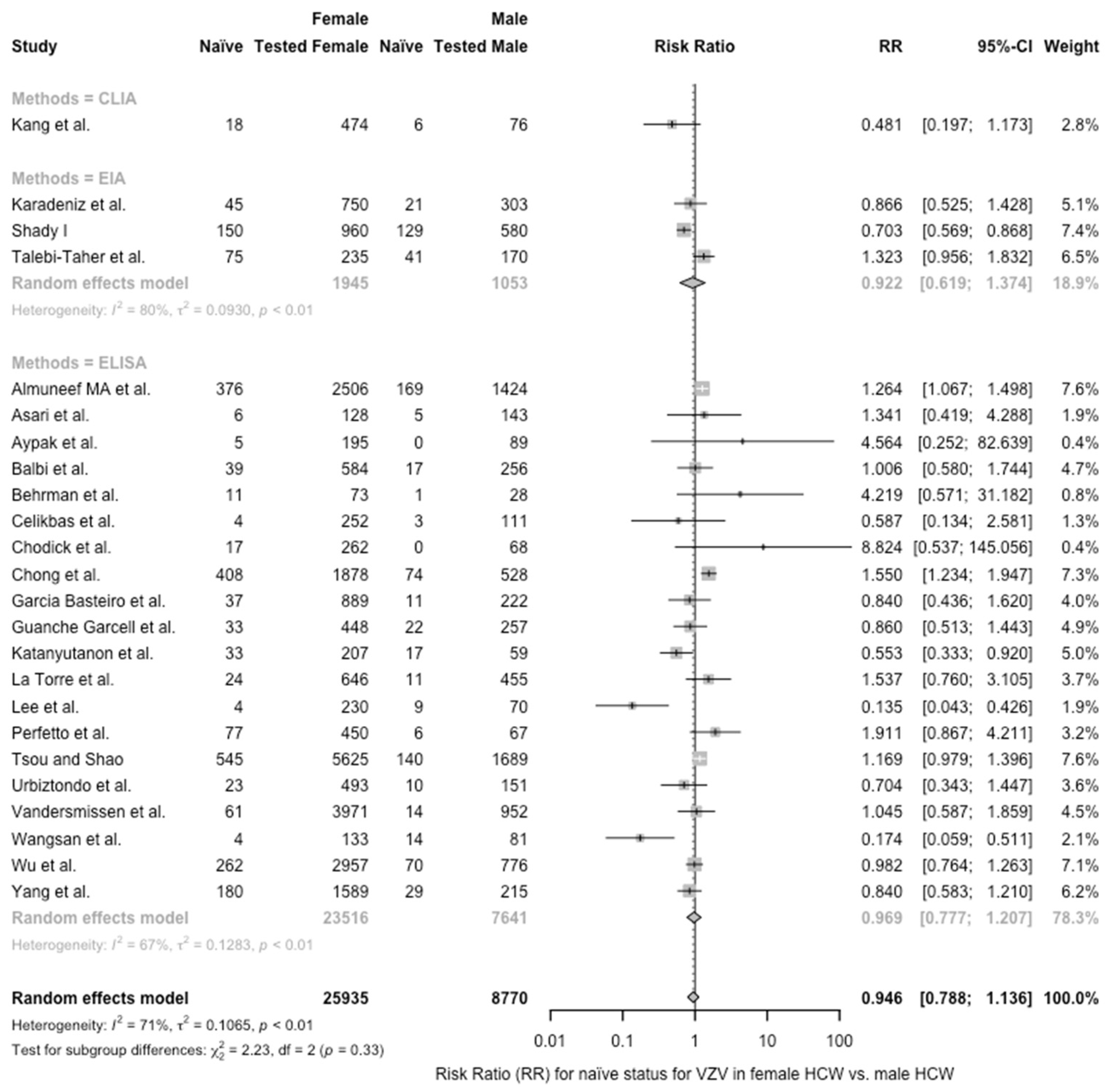
| Female vs. Male | 0.946 (0.788; 1.136) | 0.107 | 70.8% (55.8 to 80.7) | 78.68 (df = 23) | <0.001 |
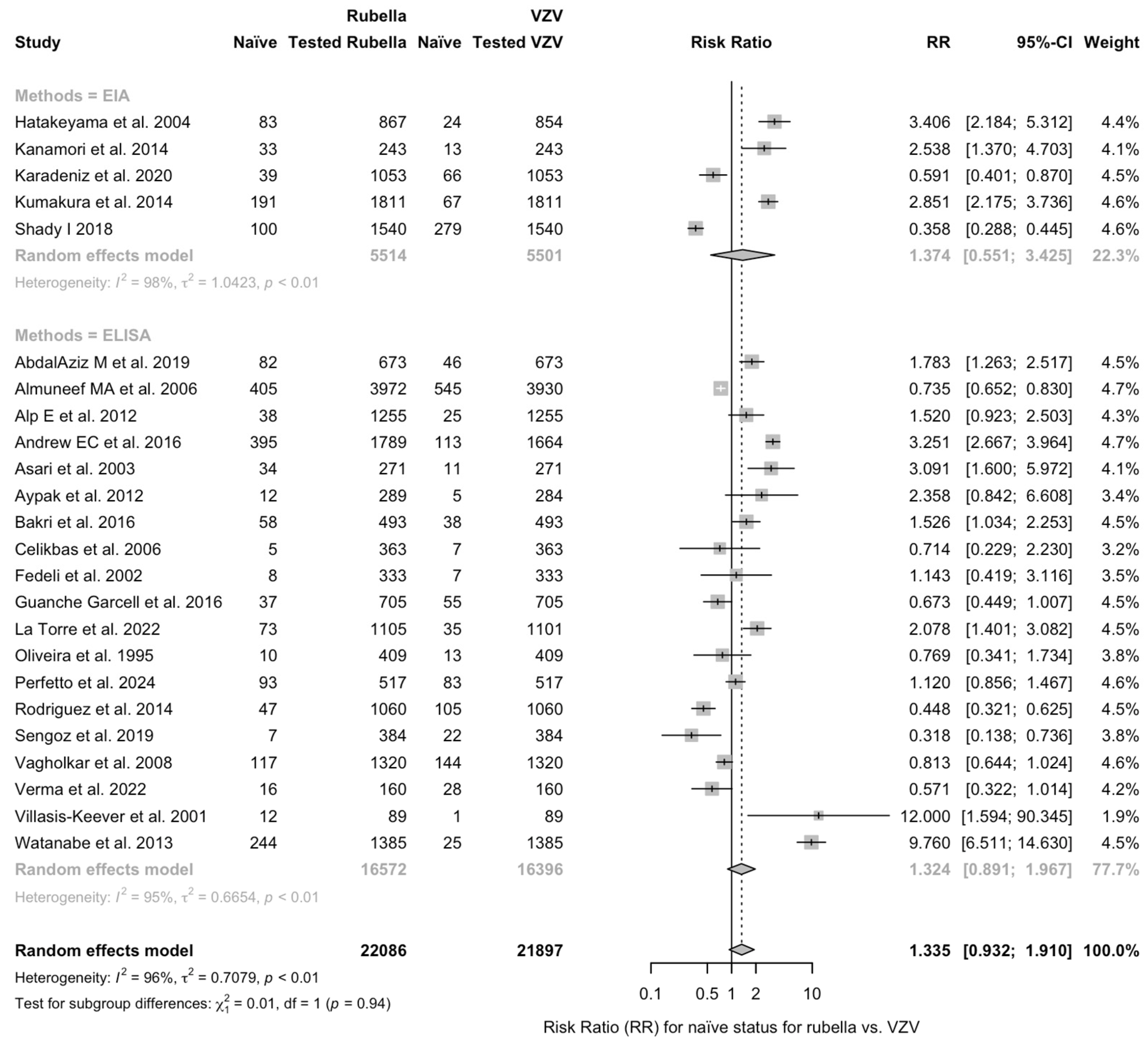
| Measles vs. VZV | 1.326 (0.953; 1.846) | 0.727 | 98.1% (97.8 to 98.4) | 1518.00 (df = 29) | <0.001 |
| Rubella vs. VZV | 1.335 (0.932; 1.910) | 0.708 | 95.9% (94.8 to 96.7) | 554.91 (df = 23) | <0.001 |
| Estimate | t | df | Bias (SE) | p Value |
|---|---|---|---|---|
| Proportion of naïve HCW, VZV | −5.90 | 56 | −6.529 (1.106) | <0.001 |
| Proportion of naïve HCW, measles | −1.76 | 28 | −4.262 (2.426) | 0.090 |
| Proportion of naïve HCW, rubella | −3.46 | 22 | −6.088 (1.759) | 0.002 |
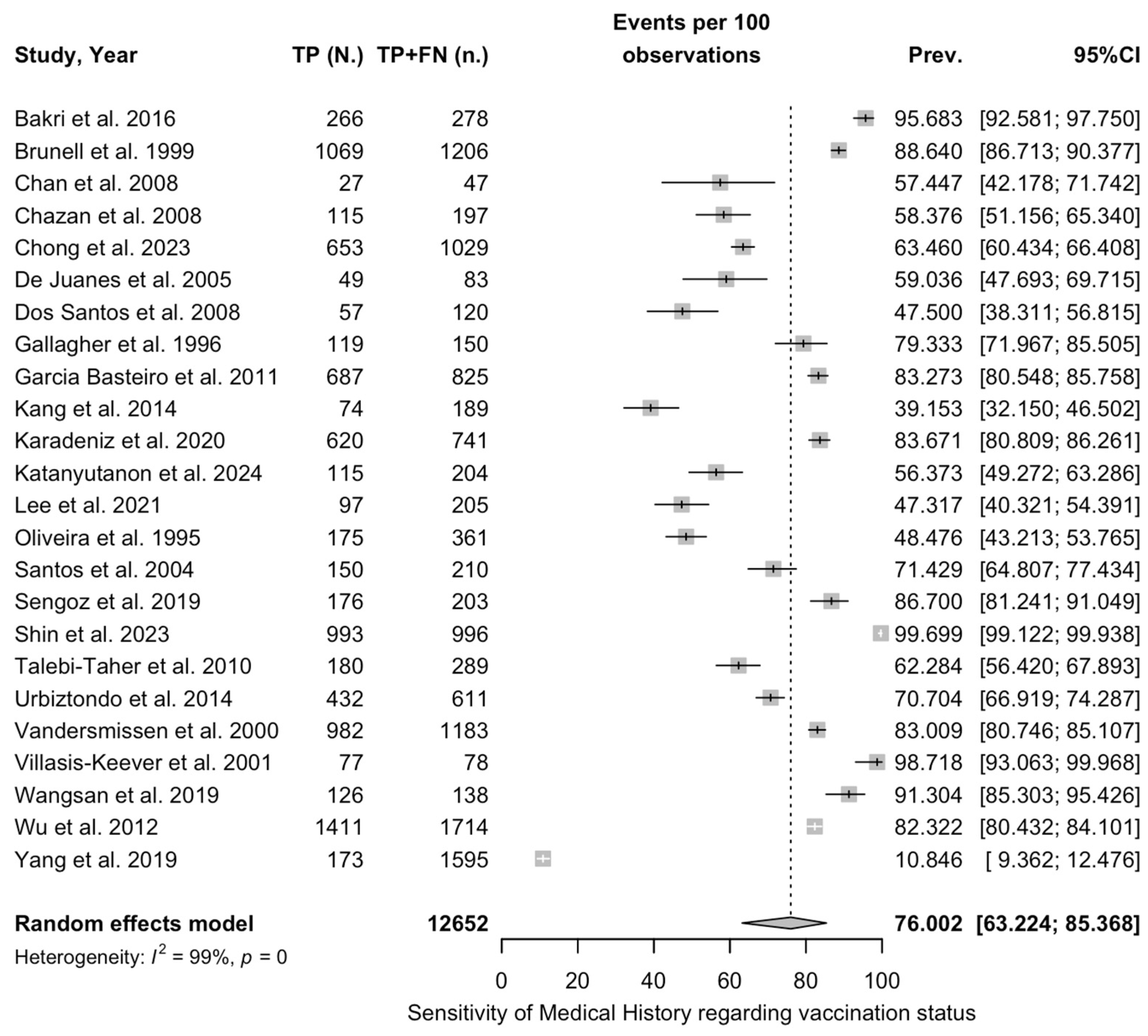
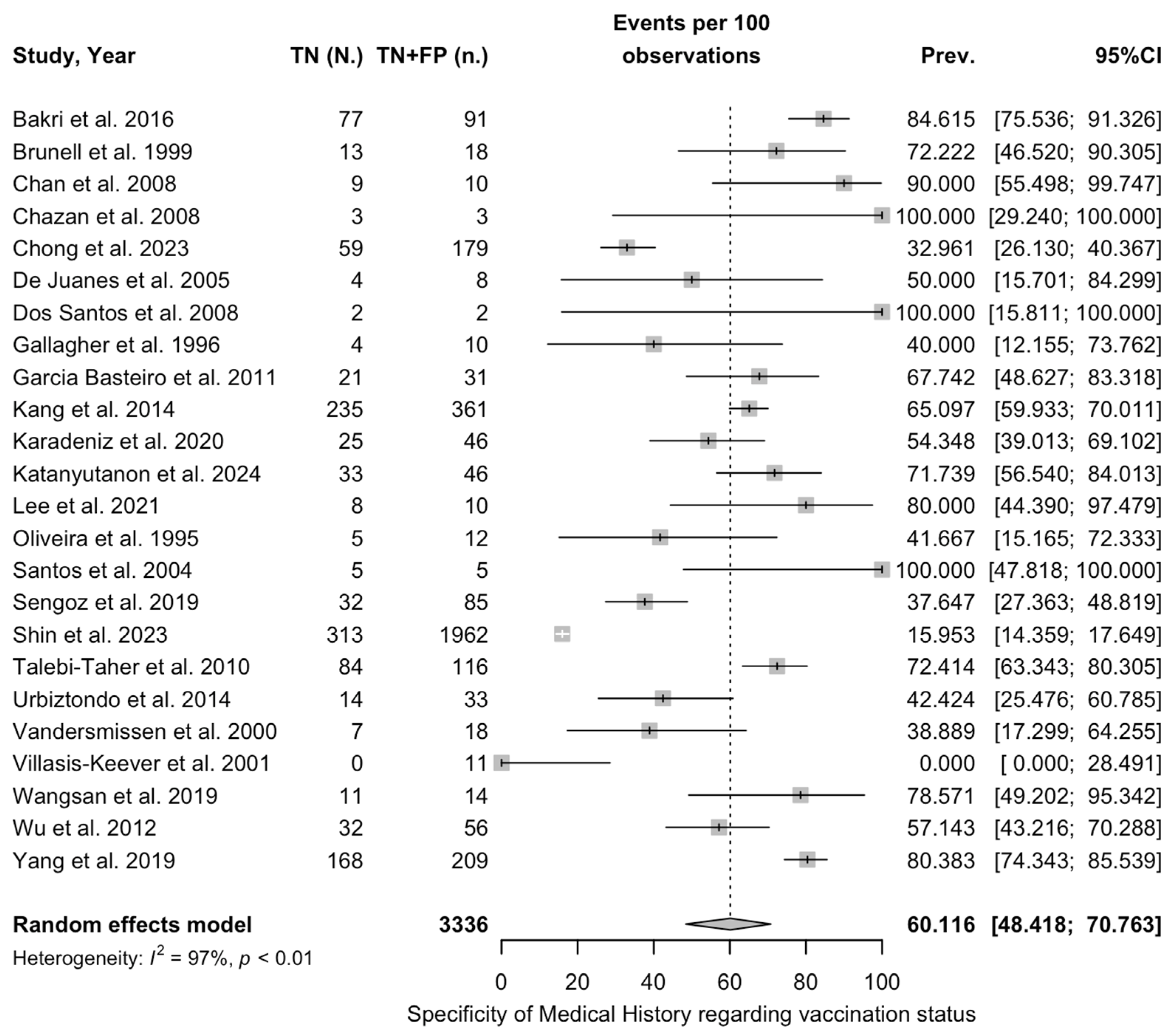
| Sensitivity of medical history, VZV | 0.44 | 22 | 1.981 (4.519) | 0.665 |
| Specificity of medical history, VZV | 2.81 | 22 | 4.109 (1.462) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Ferraro, P.; Zaffina, S.; Camisa, V.; Marchesi, F.; Franzoso, F.F.; Ligori, C.; Fiacchini, D.; Magnavita, N.; Tafuri, S. Immunity to Varicella Zoster Virus in Healthcare Workers: A Systematic Review and Meta-Analysis (2024). Vaccines 2024, 12, 1021. https://doi.org/10.3390/vaccines12091021
Riccò M, Ferraro P, Zaffina S, Camisa V, Marchesi F, Franzoso FF, Ligori C, Fiacchini D, Magnavita N, Tafuri S. Immunity to Varicella Zoster Virus in Healthcare Workers: A Systematic Review and Meta-Analysis (2024). Vaccines. 2024; 12(9):1021. https://doi.org/10.3390/vaccines12091021
Chicago/Turabian StyleRiccò, Matteo, Pietro Ferraro, Salvatore Zaffina, Vincenzo Camisa, Federico Marchesi, Francesca Fortin Franzoso, Cosimo Ligori, Daniel Fiacchini, Nicola Magnavita, and Silvio Tafuri. 2024. "Immunity to Varicella Zoster Virus in Healthcare Workers: A Systematic Review and Meta-Analysis (2024)" Vaccines 12, no. 9: 1021. https://doi.org/10.3390/vaccines12091021
APA StyleRiccò, M., Ferraro, P., Zaffina, S., Camisa, V., Marchesi, F., Franzoso, F. F., Ligori, C., Fiacchini, D., Magnavita, N., & Tafuri, S. (2024). Immunity to Varicella Zoster Virus in Healthcare Workers: A Systematic Review and Meta-Analysis (2024). Vaccines, 12(9), 1021. https://doi.org/10.3390/vaccines12091021










