Viral Vector-Based Chlamydia trachomatis Vaccines Encoding CTH522 Induce Distinct Immune Responses in C57BL/6J and HLA Transgenic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cells
2.3. Plasmid Construction and Recombinant Virus Generation
2.4. Viruses
2.5. PCR Analysis of Recombinant MVAs
2.6. SDS-PAGE and Western Blot
2.7. Immunofluorescence Microscopy and Flow Cytometry
2.8. Immunization and Tissue Collection
2.9. Peptides
2.10. Cellular-Specific Immune Response Analysis
2.11. Intracellular Staining
2.12. Enzyme-Linked Immunosorbent Assay
2.13. Statistical Analysis
3. Results
3.1. Generation and In Vitro Characterization of MVA-CTH522
3.2. Generation and In Vitro Characterization of MVA-spCTH522 and MVA-CTH522:B7
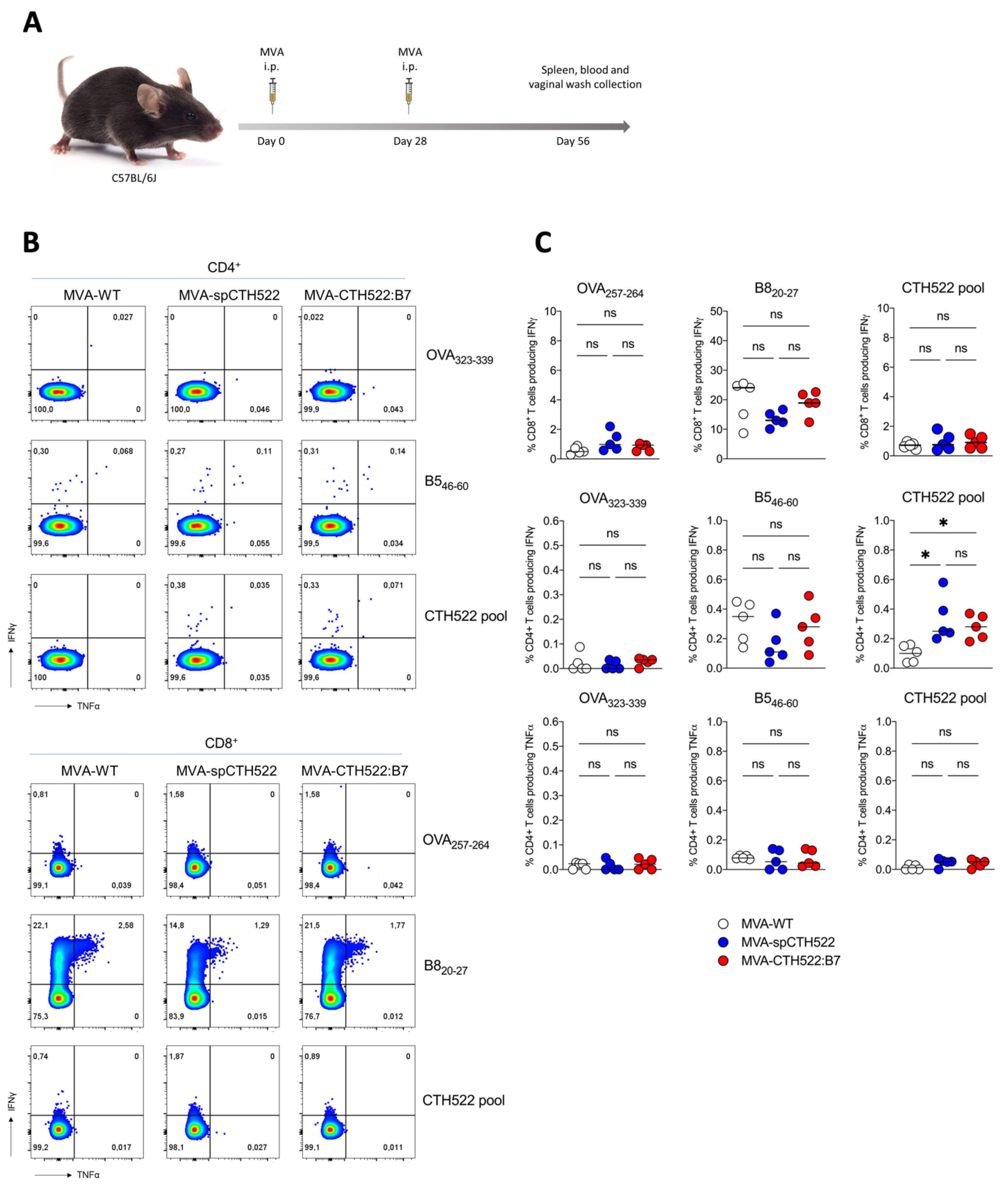
3.3. MVA-spCTH522 and MVA-CTH522:B7 Induced Systemic CD4+ But Not CD8+ T-Cell Responses against CTH522 in C57BL/6J Mice
3.4. MVA-CTH522:B7 But Not MVA-spCTH522 Induced Humoral Responses in C57BL/6J Mice
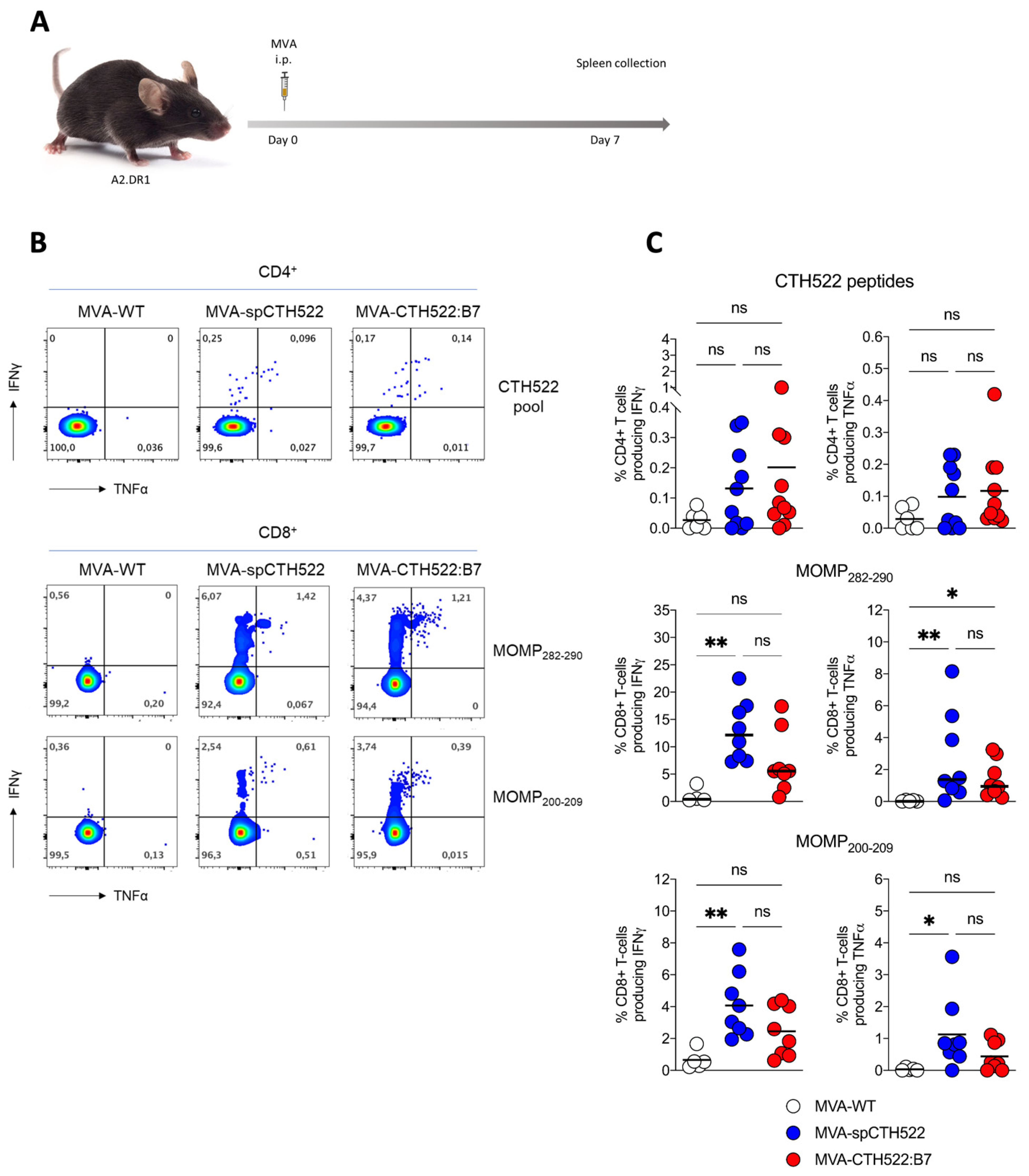
3.5. MVA-CTH522:B7 Induced T-Cell and Antibody Responses in HLA Transgenic Mice
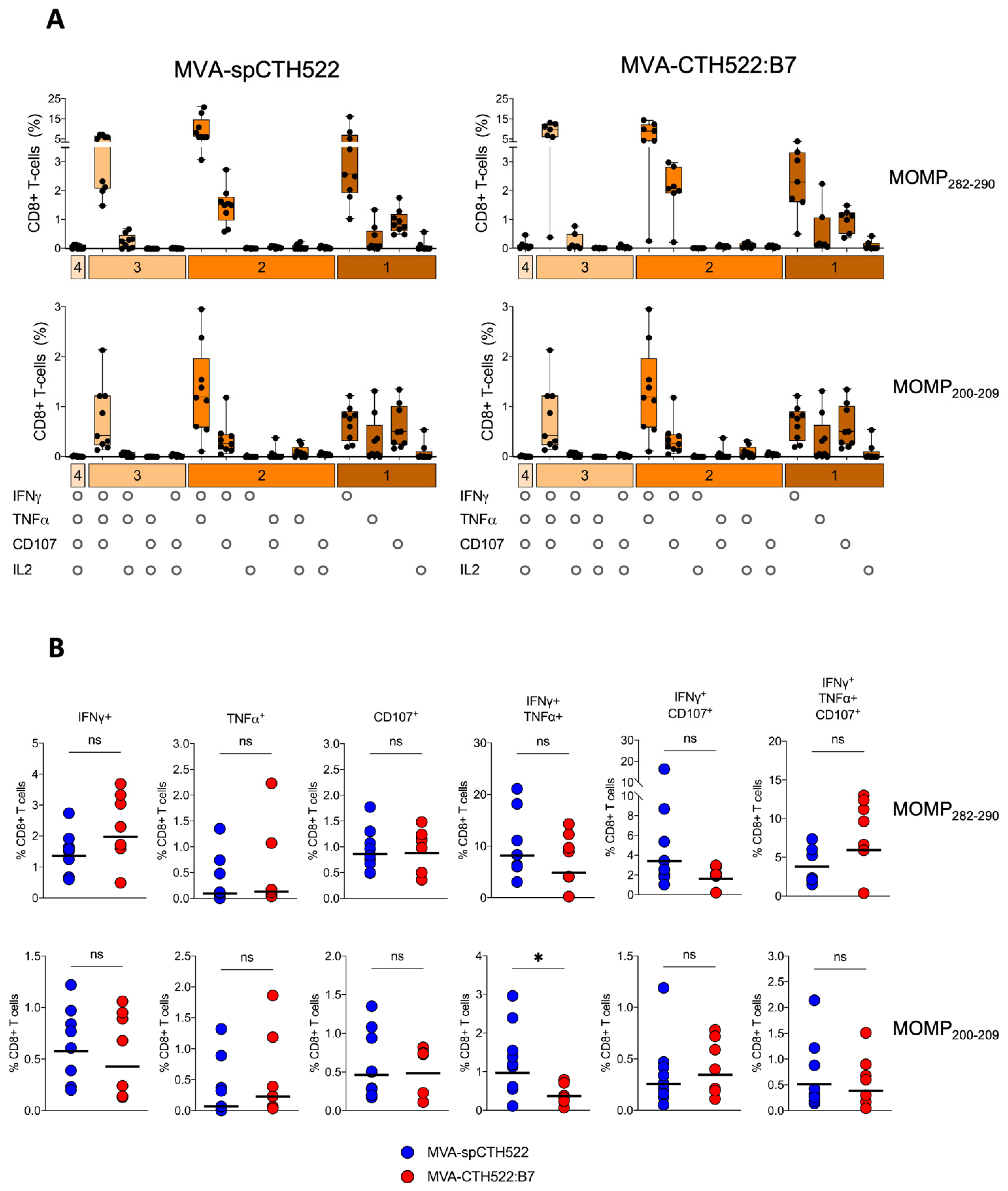
3.6. MVA-spCTH522 and MVA-CTH522:B7 Induced Multifunctional CD8+ T Cell in HLA Transgenic Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021.
- Murray, S.M.; McKay, P.F. Chlamydia trachomatis: Cell biology, immunology and vaccination. Vaccine 2021, 39, 2965–2975. [Google Scholar] [CrossRef]
- Bébéar, C.; de Barbeyrac, B. Genital Chlamydia trachomatis infections. Clin. Microbiol. Infect. 2009, 15, 4–10. [Google Scholar] [CrossRef]
- Peterman, T.A.; Newman, D.R.; Maddox, L.; Schmitt, K.; Shiver, S. Risk for HIV following a diagnosis of syphilis, gonorrhoea or chlamydia: 328,456 women in Florida, 2000–2011. Int. J. STD AIDS 2015, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- de Vrieze, N.H.N.; de Vries, H.J.C. Lymphogranuloma venereum among men who have sex with men. An epidemiological and clinical review. Expert Rev. Anti-Infect. Ther. 2014, 12, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Batteiger, B.E.; Tu, W.; Ofner, S.; Stothard, D.R.; Orr, D.P.; Katz, B.P.; Fortenberry, J.D. Repeated Chlamydia trachomatis Genital Infections in Adolescent Women. J. Infect. Dis. 2010, 201, 42–51. [Google Scholar] [CrossRef]
- Gupta, K.; Bakshi, R.K.; Van Der Pol, B.; Daniel, G.; Brown, L.; Press, C.G.; Gorwitz, R.; Papp, J.; Lee, J.Y.; Geisler, W.M. Repeated Chlamydia trachomatis infections are associated with lower bacterial loads. Epidemiol. Infect. 2019, 147, e18. [Google Scholar] [CrossRef]
- Walker, J.; Tabrizi, S.N.; Fairley, C.K.; Chen, M.Y.; Bradshaw, C.S.; Twin, J.; Taylor, N.; Donovan, B.; Kaldor, J.M.; McNamee, K.; et al. Chlamydia trachomatis Incidence and Re-Infection among Young Women—Behavioural and Microbiological Characteristics. PLoS ONE 2012, 7, e37778. [Google Scholar] [CrossRef]
- Detels, R.; Green, A.M.; Klausner, J.D.; Katzenstein, D.; Gaydos, C.D.; Handsfield, H.H.; Pequegnat, W.; Mayer, K.; Hartwell, T.D.; Quinn, T.C. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex. Transm. Dis. 2011, 38, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Rappuoli, R. Chlamydia trachomatis Control Requires a Vaccine. Vaccine 2013, 31, 1892. [Google Scholar] [CrossRef]
- Brunham, R.C.; Pourbohloul, B.; Mak, S.; White, R.; Rekart, M.L. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J. Infect. Dis. 2005, 192, 1836–1844. [Google Scholar] [CrossRef]
- Evans, J.R.; Solomon, A.W.; Kumar, R.; Perez, Á.; Singh, B.P.; Srivastava, R.M.; Harding-Esch, E. Antibiotics for trachoma. Cochrane Database Syst. Rev. 2019, 2019, CD001860. [Google Scholar] [CrossRef]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Salhan, S.; Das, B.C.; Mittal, A. Predominance of Chlamydia trachomatis Serovars Associated with Urogenital Infections in Females in New Delhi, India. J. Clin. Microbiol. 2003, 41, 2700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- BYang, B.; Zheng, H.-P.; Feng, Z.-Q.; Xue, Y.-H.; Wu, X.-Z.; Huang, J.-M.; Xue, X.-J.; Jiang, H.-N. The Prevalence and Distribution of Chlamydia trachomatis Genotypes among Sexually Transmitted Disease Clinic Patients in Guangzhou, China, 2005–2008. Jpn. J. Infect. Dis. 2010, 63, 342–345. [Google Scholar]
- Lesiak-Markowicz, I.; Schötta, A.-M.; Stockinger, H.; Stanek, G.; Markowicz, M. Chlamydia trachomatis serovars in urogenital and ocular samples collected 2014–2017 from Austrian patients. Sci. Rep. 2019, 9, 18327. [Google Scholar] [CrossRef]
- Olsen, A.W.; Follmann, F.; Erneholm, K.; Rosenkrands, I.; Andersen, P. Protection Against Chlamydia trachomatis Infection and Upper Genital Tract Pathological Changes by Vaccine-Promoted Neutralizing Antibodies Directed to the VD4 of the Major Outer Membrane Protein. J. Infect. Dis. 2015, 212, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.N.T.; Guleed, S.; Olsen, A.W.; Follmann, F.; Christensen, J.P.; Dietrich, J. Th1/Th17 T cell Tissue-Resident Immunity Increases Protection, But Is Not Required in a Vaccine Strategy Against Genital Infection with Chlamydia trachomatis. Front. Immunol. 2021, 12, 790463. [Google Scholar] [CrossRef]
- Olsen, A.W.; Rosenkrands, I.; Jacobsen, C.S.; Cheeseman, H.M.; Kristiansen, M.P.; Dietrich, J.; Shattock, R.J.; Follmann, F. Immune signature of Chlamydia vaccine CTH522/CAF®01 translates from mouse-to-human and induces durable protection in mice. Nat. Commun. 2024, 15, 1665. [Google Scholar] [CrossRef]
- Wizel, B.; Nyström-Asklin, J.; Cortes, C.; Tvinnereim, A. Role of CD8+ T cells in the host response to Chlamydia. Microbes Infect. 2008, 10, 1420–1430. [Google Scholar] [CrossRef]
- Helble, J.D.; Starnbach, M.N. T cell responses to Chlamydia. Pathog. Dis. 2021, 79, ftab014. [Google Scholar] [CrossRef]
- BReddy, S.; Rastogi, S.; Das, B.; Salhan, S.; Verma, S.; Mittal, A. Cytokine expression pattern in the genital tract of Chlamydia trachomatis positive infertile women—Implication for T-cell responses. Clin. Exp. Immunol. 2004, 137, 552–558. [Google Scholar] [CrossRef]
- Russell, A.N.; Zheng, X.; O’Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Taylor, B.D.; Picard, M.D.; Flechtner, J.B.; Zhong, W.; Frazer, L.C.; et al. Identification of Chlamydia trachomatis Antigens Recognized by T Cells From Highly Exposed Women Who Limit or Resist Genital Tract Infection. J. Infect. Dis. 2016, 214, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Kari, L.; Whitmire, W.M.; Olivares-Zavaleta, N.; Goheen, M.M.; Taylor, L.D.; Carlson, J.H.; Sturdevant, G.L.; Lu, C.; Bakios, L.E.; Randall, L.B.; et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J. Exp. Med. 2011, 208, 2217–2223. [Google Scholar] [CrossRef]
- Olivares-Zavaleta, N.; Whitmire, W.M.; Kari, L.; Sturdevant, G.L.; Caldwell, H.D. CD8+ T Cells Define an Unexpected Role in Live-Attenuated Vaccine Protective Immunity against Chlamydia trachomatis Infection in Macaques. J. Immunol. 2014, 192, 4648–4654. [Google Scholar] [CrossRef] [PubMed]
- Loomis, W.P.; Starnbach, M.N. Chlamydia trachomatis Infection Alters the Development of Memory CD8+ T Cells. J. Immunol. 2006, 177, 4021–4027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Starnbach, M.N. An Excess of the Proinflammatory Cytokines IFN-γ and IL-12 Impairs the Development of the Memory CD8+ T Cell Response to Chlamydia trachomatis. J. Immunol. 2015, 195, 1665–1675. [Google Scholar] [CrossRef]
- Fankhauser, S.C.; Starnbach, M.N. PD-L1 Limits the Mucosal CD8+ T Cell Response to Chlamydia trachomatis. J. Immunol. 2014, 192, 1079–1090. [Google Scholar] [CrossRef]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, Q.; Wang, L.; Sun, X.; Zhang, N.; Xue, M.; Xu, D.; Zhong, G. Characterization of Pathogenic CD8+ T cells in Chlamydia-Infected OT1 Mice. Infect. Immun. 2022, 90, e0045321. [Google Scholar] [CrossRef]
- Hogquist, K.A.; Jameson, S.C.; Heath, W.R.; Howard, J.L.; Bevan, M.J.; Carbone, F.R. T cell receptor antagonist peptides induce positive selection. Cell 1994, 76, 17–27. [Google Scholar] [CrossRef]
- Manam, S.; Nicholson, B.J.; Murthy, A.K. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog. Dis. 2013, 67, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Lijek, R.S.; Helble, J.D.; Olive, A.J.; Seiger, K.W.; Starnbach, M.N. Pathology after Chlamydia trachomatis infection is driven by nonprotective immune cells that are distinct from protective populations. Proc. Natl. Acad. Sci. USA 2018, 115, 2216–2221. [Google Scholar] [CrossRef]
- Olive, A.J.; Gondek, D.C.; Starnbach, M.N. CXCR3 and CCR5 are both required for T cell-mediated protection against C. trachomatis infection in the murine genital mucosa. Mucosal Immunol. 2011, 4, 208–216. [Google Scholar] [CrossRef]
- Kaynarcalidan, O.; Mascaraque, S.M.; Drexler, I. Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design. Biomedicines 2021, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, A.F.; Kreijtz, J.H.C.M.; De Vries, R.D.; Song, F.; Fux, R.; Rimmelzwaan, G.F.; Sutter, G.; Volz, A. Modified Vaccinia Virus Ankara (MVA) as Production Platform for Vaccines against Influenza and Other Viral Respiratory Diseases. Viruses 2014, 6, 2735–2761. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.A.; McCaffery, J.N.; Caceres, J.; Kashentseva, E.; Singh, B.; Dmitriev, I.P.; Curiel, D.T.; Moreno, A. Inclusion of the murine IgGκ signal peptide increases the cellular immunogenicity of a simian adenoviral vectored Plasmodium vivax multistage vaccine. Vaccine 2018, 36, 2799–2808. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, B.-M.; Lu, W.-C.; Su, C.-I.; Prijovich, Z.M.; Chung, W.-C.; Wu, P.-Y.; Chen, K.-C.; Lee, I.-C.; Juan, T.-Y.; et al. The B7-1 Cytoplasmic Tail Enhances Intracellular Transport and Mammalian Cell Surface Display of Chimeric Proteins in the Absence of a Linear ER Export Motif. PLoS ONE 2013, 8, e75084. [Google Scholar] [CrossRef]
- Pajot, A.; Michel, M.; Fazilleau, N.; Pancré, V.; Auriault, C.; Ojcius, D.M.; Lemonnier, F.A.; Lone, Y. A mouse model of human adaptive immune functions: HLA-A2.1-/HLA-DR1-transgenic H-2 class I-/class II-knockout mice. Eur. J. Immunol. 2004, 34, 3060–3069. [Google Scholar] [CrossRef]
- Becker, P.; Nörder, M.; Weissmann, S.; Ljapoci, R.; Erfle, V.; Drexler, I.; Guzmán, C.A. Gene Expression Driven by a Strong Viral Promoter in MVA Increases Vaccination Efficiency by Enhancing Antibody Responses and Unmasking CD8+ T Cell Epitopes. Vaccines 2014, 2, 581–600. [Google Scholar] [CrossRef]
- Kugler, F.; Drexler, I.; Protzer, U.; Hoffmann, D.; Moeini, H. Generation of recombinant MVA-norovirus: A comparison study of bacterial artificial chromosome- and marker-based systems. Virol. J. 2019, 16, 100. [Google Scholar] [CrossRef]
- Barnowski, C.; Ciupka, G.; Tao, R.; Jin, L.; Busch, D.H.; Tao, S.; Drexler, I. Efficient Induction of Cytotoxic T Cells by Viral Vector Vaccination Requires STING-Dependent DC Functions. Front. Immunol. 2020, 11, 1458. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Flesch, I.E.A.; Hollett, N.A.; Wong, Y.C.; Quinan, B.R.; Howard, D.; da Fonseca, F.G.; Tscharke, D.C. Extent of Systemic Spread Determines CD8+ T Cell Immunodominance for Laboratory Strains, Smallpox Vaccines, and Zoonotic Isolates of Vaccinia Virus. J. Immunol. 2015, 195, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Angevine, M.; Demick, K.; Ortiz, L.; Rudersdorf, R.; Watkins, D.; DeMars, R. Induction of HLA class I-restricted CD8+ CTLs specific for the major outer membrane protein of Chlamydia trachomatis in human genital tract infections. J. Immunol. 1999, 162, 6855–6866. [Google Scholar] [CrossRef] [PubMed]
- Orlova, O.V.; Glazkova, D.V.; Bogoslovskaya, E.V.; Shipulin, G.A.; Yudin, S.M. Development of Modified Vaccinia Virus Ankara-Based Vaccines: Advantages and Applications. Vaccines 2022, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- de la Maza, L.M.; Darville, T.L.; Pal, S. Chlamydia trachomatis vaccines for genital infections: Where are we and how far is there to go? Expert Rev. Vaccines 2021, 20, 421–435. [Google Scholar] [CrossRef]
- Phillips, S.; Quigley, B.L.; Timms, P. Seventy Years of Chlamydia Vaccine Research—Limitations of the Past and Directions for the Future. Front. Microbiol. 2019, 10, 433459. [Google Scholar] [CrossRef]
- Ura, T.; Takeuchi, M.; Kawagoe, T.; Mizuki, N.; Okuda, K.; Shimada, M. Current Vaccine Platforms in Enhancing T-Cell Response. Vaccines 2022, 10, 1367. [Google Scholar] [CrossRef]
- Grotenbreg, G.M.; Roan, N.R.; Guillen, E.; Meijers, R.; Wang, J.-H.; Bell, G.W.; Starnbach, M.N.; Ploegh, H.L. Discovery of CD8+ T cell epitopes in Chlamydia trachomatis infection through use of caged class I MHC tetramers. Proc. Natl. Acad. Sci. USA 2008, 105, 3831–3836. [Google Scholar] [CrossRef]
- Starnbach, M.N.; Loomis, W.P.; Ovendale, P.; Regan, D.; Hess, B.; Alderson, M.R.; Fling, S.P. An Inclusion Membrane Protein from Chlamydia trachomatis Enters the MHC Class I Pathway and Stimulates a CD8+ T Cell Response. J. Immunol. 2003, 171, 4742–4749. [Google Scholar] [CrossRef]
- Drexler, I.; Staib, C.; Kastenmüller, W.; Stevanović, S.; Schmidt, B.; Lemonnier, F.A.; Rammensee, H.-G.; Busch, D.H.; Bernhard, H.; Erfle, V.; et al. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc. Natl. Acad. Sci. USA 2003, 100, 217–222. [Google Scholar] [CrossRef]
- Schumacher, T.; Bunse, L.; Pusch, S.; Sahm, F.; Wiestler, B.; Quandt, J.; Menn, O.; Osswald, M.; Oezen, I.; Ott, M.; et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014, 512, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, W.; Shu, J.; Kou, Z.; Jin, X. A novel polyepitope vaccine elicited HIV peptide specific CD4+ T cell responses in HLA-A2/DRB1 transgenic mice. PLoS ONE 2017, 12, e0184207. [Google Scholar] [CrossRef] [PubMed]
- Kruse, S.; Büchler, M.; Uhl, P.; Sauter, M.; Scherer, P.; Lan, T.C.; Zottnick, S.; Klevenz, A.; Yang, R.; Rösl, F.; et al. Therapeutic vaccination using minimal HPV16 epitopes in a novel MHC-humanized murine HPV tumor model. Oncoimmunology 2019, 8, e1524694. [Google Scholar] [CrossRef] [PubMed]
- Le Vu, P.; Vadakekolathu, J.; Idri, S.; Nicholls, H.; Cavaignac, M.; Reeder, S.; Khan, M.A.; Christensen, D.; Pockley, A.G.; McArdle, S.E. A Mutated Prostatic Acid Phosphatase (PAP) Peptide-Based Vaccine Induces PAP-Specific CD8+ T Cells with Ex Vivo Cytotoxic Capacities in HHDII/DR1 Transgenic Mice. Cancers 2022, 14, 1970. [Google Scholar] [CrossRef]
- Peng, S.; Xing, D.; Ferrall, L.; Tsai, Y.-C.; Hung, C.-F.; Wu, T.-C. Identification of human MHC-I HPV18 E6/E7-specific CD8+ T cell epitopes and generation of an HPV18 E6/E7-expressing adenosquamous carcinoma in HLA-A2 transgenic mice. J. Biomed. Sci. 2022, 29, 80. [Google Scholar] [CrossRef]
- Conforti, A.; Peruzzi, D.; Giannetti, P.; Biondo, A.; Ciliberto, G.; La Monica, N.; Aurisicchio, L. A Novel Mouse Model for Evaluation and Prediction of HLA-A2-restricted CEA Cancer Vaccine Responses. J. Immunother. 2009, 32, 744–754. [Google Scholar] [CrossRef]
- Morrison, S.G.; Morrison, R.P. A Predominant Role for Antibody in Acquired Immunity to Chlamydial Genital Tract Reinfection. J. Immunol. 2005, 175, 7536–7542. [Google Scholar] [CrossRef]
- Pal, S.; Peterson, E.M.; de la Maza, L.M. Vaccination with the Chlamydia trachomatis Major Outer Membrane Protein Can Elicit an Immune Response as Protective as That Resulting from Inoculation with Live Bacteria. Infect. Immun. 2005, 73, 8153–8160. [Google Scholar] [CrossRef]
- Ardizzone, C.M.; Albritton, H.L.; Lillis, R.A.; Bagnetto, C.E.L.; Shen, L.; Cavacini, L.A.; Kozlowski, P.A.; Quayle, A.J. Human genital antibody-mediated inhibition of Chlamydia trachomatis infection and evidence for ompA genotype-specific neutralization. PLoS ONE 2021, 16, e0258759. [Google Scholar] [CrossRef]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015, 348, aaa8205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.N.T.; Olsen, A.W.; Lorenzen, E.; Andersen, P.; Hvid, M.; Follmann, F.; Dietrich, J. Parenteral vaccination protects against transcervical infection with Chlamydia trachomatis and generate tissue-resident T cells post-challenge. NPJ Vaccines 2020, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Rotrosen, E.; Kupper, T.S. Assessing the generation of tissue resident memory T cells by vaccines. Nat. Rev. Immunol. 2023, 23, 655–665. [Google Scholar] [CrossRef]
- Muschaweckh, A.; Buchholz, V.R.; Fellenzer, A.; Hessel, C.; König, P.-A.; Tao, S.; Tao, R.; Heikenwälder, M.; Busch, D.H.; Korn, T.; et al. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J. Exp. Med. 2016, 213, 3075–3086. [Google Scholar] [CrossRef]
- Johnson, R.M.; Brunham, R.C. Tissue-Resident T Cells as the Central Paradigm of Chlamydia Immunity. Infect. Immun. 2016, 84, 868–873. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.W.; Rosenkrands, I.; Holland, M.J.; Andersen, P.; Follmann, F. A Chlamydia trachomatis VD1-MOMP vaccine elicits cross-neutralizing and protective antibodies against C/C-related complex serovars. NPJ Vaccines 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.C.W.; Hu, V.; Bailey, R.L.; Burton, M.J.; Holland, M.J. Towards a safe and effective chlamydial vaccine: Lessons from the eye. Vaccine 2014, 32, 1572–1578. [Google Scholar] [CrossRef]
- Pollock, K.M.; Borges, H.; Cheeseman, H.M.; Rosenkrands, I.; Schmidt, K.L.; E Søndergaard, R.; Day, S.; Evans, A.; McFarlane, L.R.; Joypooranachandran, J.; et al. An investigation of trachoma vaccine regimens by the chlamydia vaccine CTH522 administered with cationic liposomes in healthy adults (CHLM-02): A phase 1, double-blind trial. Lancet Infect. Dis. 2024, 24, 829–844. [Google Scholar] [CrossRef]
- Burgener, A.-V.; Seth-Smith, H.M.B.; Kern-Baumann, S.; Durovic, A.; Blaich, A.; Menter, T.; Bruder, E.; Roloff, T.; Martinez, A.; Borel, N.; et al. A Case Study of Zoonotic Chlamydia abortus Infection: Diagnostic Challenges From Clinical and Microbiological Perspectives. Open Forum Infect. Dis. 2022, 9, ofac524. [Google Scholar] [CrossRef]
- Pichon, N.; Guindre, L.; Laroucau, K.; Cantaloube, M.; Nallatamby, A.; Parreau, S. Chlamydia abortus in Pregnant Woman with Acute Respiratory Distress Syndrome. Emerg. Infect. Dis. 2020, 26, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.H.; DeWolfe, J.L.; Salyer, R.D. Disease Outcome Subsequent to Primary and Secondary Urogenital Infection with Murine or Human Biovars of Chlamydia trachomatis. Infect. Immun. 2000, 68, 7186–7189. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.R.; Tifrea, D.; Pal, S.; de la Maza, L.M. Differences in infectivity and induction of infertility: A comparative study of Chlamydia trachomatis strains in the murine model. Microbes Infect. 2013, 15, 219–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Clercq, E.; Kalmar, I.; Vanrompay, D. Animal Models for Studying Female Genital Tract Infection with Chlamydia trachomatis. Infect. Immun. 2013, 81, 3060–3067. [Google Scholar] [CrossRef]
- Bell, J.D.; Bergin, I.L.; Schmidt, K.; Zochowski, M.K.; Aronoff, D.M.; Patton, D.L. Nonhuman Primate Models Used to Study Pelvic Inflammatory Disease Caused by Chlamydia trachomatis. Infect. Dis. Obstet. Gynecol. 2011, 2011, 675360. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreacchio, G.; Longo, Y.; Moreno Mascaraque, S.; Anandasothy, K.; Tofan, S.; Özün, E.; Wilschrey, L.; Ptok, J.; Huynh, D.T.; Luirink, J.; et al. Viral Vector-Based Chlamydia trachomatis Vaccines Encoding CTH522 Induce Distinct Immune Responses in C57BL/6J and HLA Transgenic Mice. Vaccines 2024, 12, 944. https://doi.org/10.3390/vaccines12080944
Andreacchio G, Longo Y, Moreno Mascaraque S, Anandasothy K, Tofan S, Özün E, Wilschrey L, Ptok J, Huynh DT, Luirink J, et al. Viral Vector-Based Chlamydia trachomatis Vaccines Encoding CTH522 Induce Distinct Immune Responses in C57BL/6J and HLA Transgenic Mice. Vaccines. 2024; 12(8):944. https://doi.org/10.3390/vaccines12080944
Chicago/Turabian StyleAndreacchio, Giuseppe, Ylenia Longo, Sara Moreno Mascaraque, Kartikan Anandasothy, Sarah Tofan, Esma Özün, Lena Wilschrey, Johannes Ptok, Dung T. Huynh, Joen Luirink, and et al. 2024. "Viral Vector-Based Chlamydia trachomatis Vaccines Encoding CTH522 Induce Distinct Immune Responses in C57BL/6J and HLA Transgenic Mice" Vaccines 12, no. 8: 944. https://doi.org/10.3390/vaccines12080944
APA StyleAndreacchio, G., Longo, Y., Moreno Mascaraque, S., Anandasothy, K., Tofan, S., Özün, E., Wilschrey, L., Ptok, J., Huynh, D. T., Luirink, J., & Drexler, I. (2024). Viral Vector-Based Chlamydia trachomatis Vaccines Encoding CTH522 Induce Distinct Immune Responses in C57BL/6J and HLA Transgenic Mice. Vaccines, 12(8), 944. https://doi.org/10.3390/vaccines12080944





