Leveraging Immunofocusing and Virus-like Particle Display to Enhance Antibody Responses to the Malaria Blood-Stage Invasion Complex Antigen PfCyRPA
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning
2.2. Protein Expression and Purification
2.3. Phage Display
2.4. Capsid Virus-like Particle Production
2.5. Mice Immunizations
2.6. Antibody Purification
2.7. General ELISA Protocol
2.8. Serum ELISA
2.9. Quantification of Serum IgG Concentration
2.10. Quantification of PfCyRPA Specific IgG by ELISA
2.11. Polyclonal IgG-Binding Kinetics Determined Using Attana © Biosensor
2.12. Growth Inhibition Activity Assay
2.13. Data Analysis and Visualization
3. Results
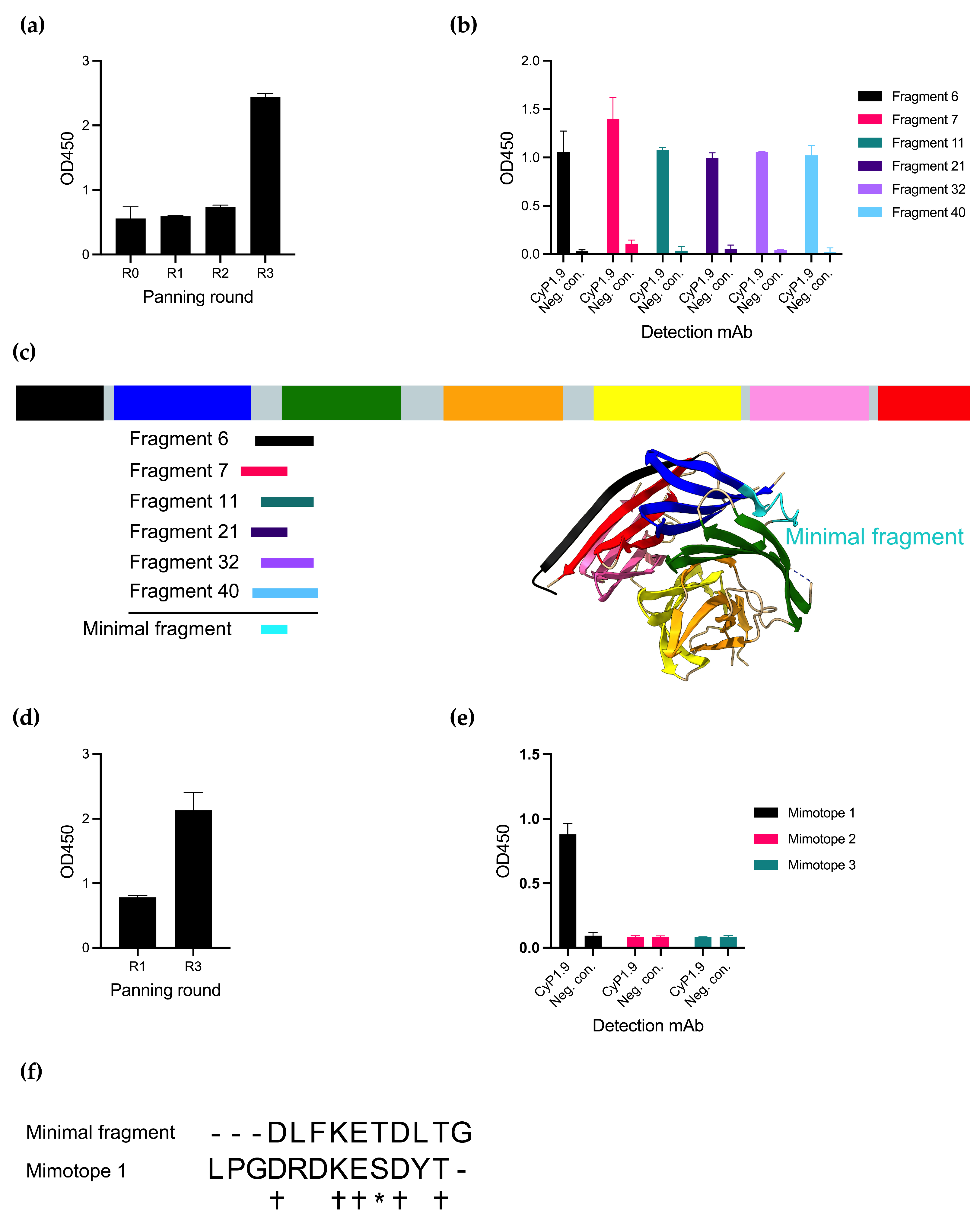
3.1. The Neutralizing mAb CyP1.9 Recognizes a Peptide Fragment of PfCyRPA and a 12-mer Mimotope
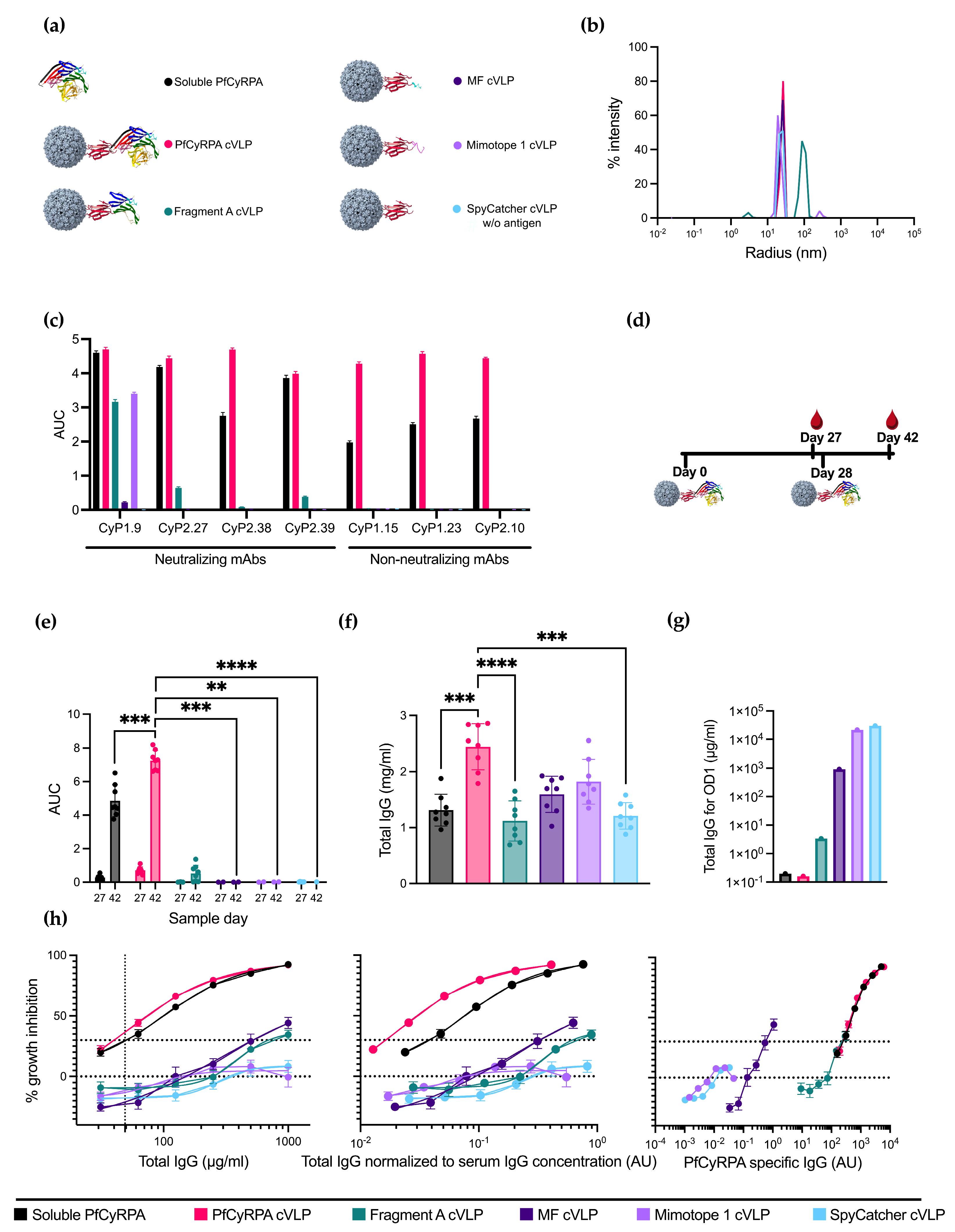
3.2. PfCyRPA-Derived Antigens Were Conjugated to cVLPs and Were Recognized by Neutralizing mAbs
3.3. Displaying PfCyRPA on cVLP Increases Antigen-Specific IgG Production
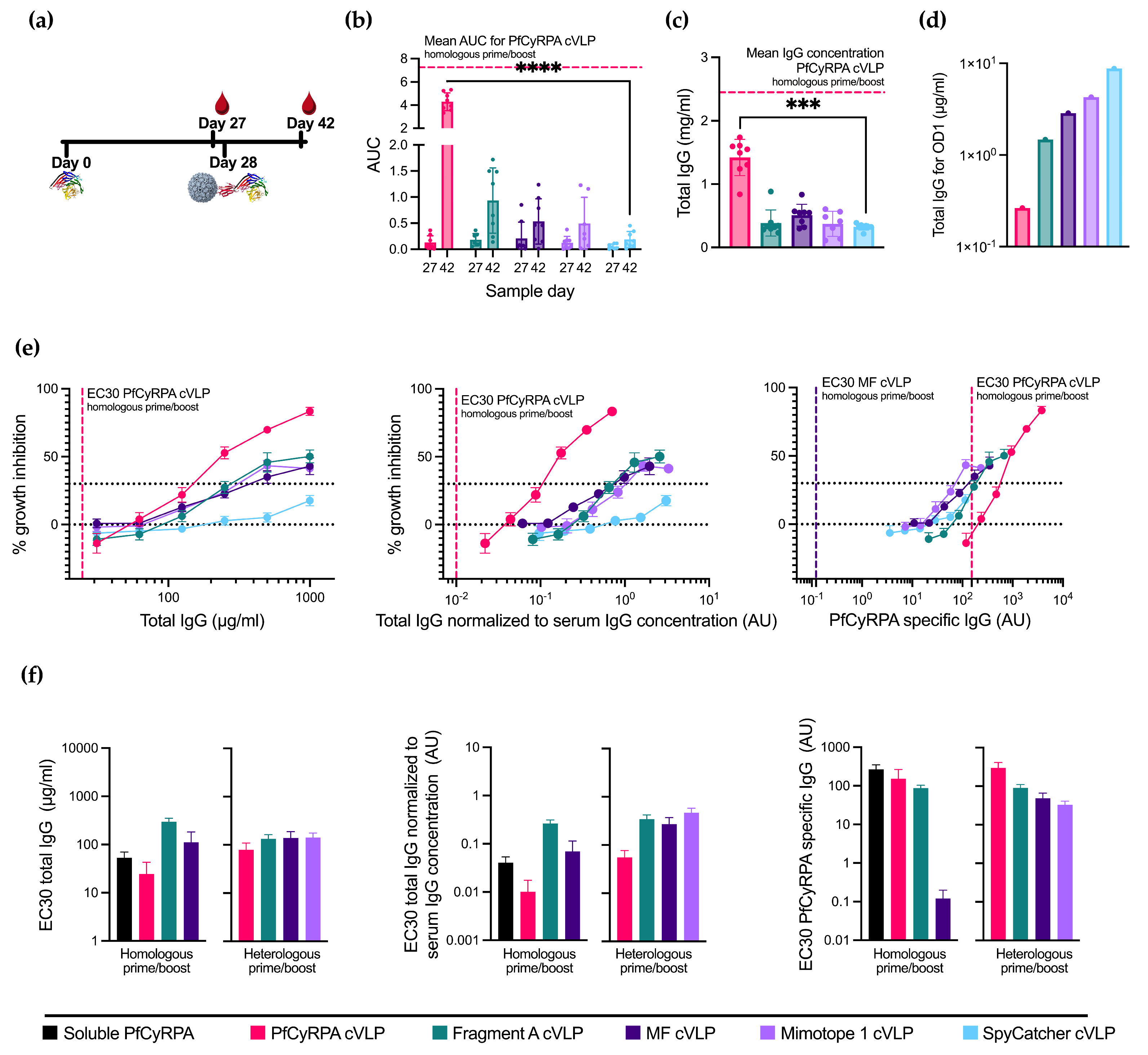
3.4. Boosting with Linear Epitopes Selectively Increases the Induction of Neutralizing Antibodies
3.5. Immunization with PfCyRPA cVLP Induces the Lowest EC30 When Normalized to Serum IgG Concentration
3.6. Immunization with PfCyRPA cVLP Induces Higher IgG2a, IgG2b, and IgG3 Responses Compared to Soluble PfCyRPA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scally, S.W.; Triglia, T.; Evelyn, C.; Seager, B.A.; Pasternak, M.; Lim, P.S.; Healer, J.; Geoghegan, N.D.; Adair, A.; Tham, W.H.; et al. PCRCR Complex Is Essential for Invasion of Human Erythrocytes by Plasmodium falciparum. Nat. Microbiol. 2022, 7, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lopaticki, S.; Riglar, D.T.; Dekiwadia, C.; Uboldi, A.D.; Tham, W.H.; O’Neill, M.T.; Richard, D.; Baum, J.; Ralph, S.A.; et al. An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011, 7, e1002199. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Chen, L.; Healer, J.; Lopaticki, S.; Boyle, M.; Triglia, T.; Ehlgen, F.; Ralph, S.A.; Beeson, J.G.; Cowman, A.F. Reticulocyte-Binding Protein Homologue 5—An Essential Adhesin Involved in Invasion of Human Erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 2009, 39, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.M.; Matile, H.; Papastogiannidis, P.; Kamber, J.; Favuzza, P.; Voss, T.S.; Wittlin, S.; Pluschke, G. Passive Immunoprotection of Plasmodium falciparum-Infected Mice Designates the CyRPA as Candidate Malaria Vaccine Antigen. J. Immunol. 2012, 188, 6225–6237. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.; Amlabu, E.; Pandey, A.K.; Mitra, P.; Chauhan, V.S.; Gaur, D. Multiprotein Complex between the GPI-Anchored CyRPA with PfRH5 and PfRipr Is Crucial for Plasmodium falciparum Erythrocyte Invasion. Proc. Natl. Acad. Sci. USA 2015, 112, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Volz, J.C.; Yap, A.; Sisquella, X.; Thompson, J.K.; Lim, N.T.Y.; Whitehead, L.W.; Chen, L.; Lampe, M.; Tham, W.H.; Wilson, D.; et al. Essential Role of the PfRh5/PfRipr/CyRPA Complex during Plasmodium falciparum Invasion of Erythrocytes. Cell Host Microbe 2016, 20, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Huang, R.; Menant, S.; Hong, C.; Sandow, J.J.; Birkinshaw, R.W.; Healer, J.; Hodder, A.N.; Kanjee, U.; Tonkin, C.J.; et al. Structure of Plasmodium falciparum Rh5–CyRPA–Ripr Invasion Complex. Nature 2019, 565, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin Is a Receptor Essential for Erythrocyte Invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Waweru, H.; Kanoi, B.N.; Kuja, J.O.; Maranga, M.; Kongere, J.; Maina, M.; Kinyua, J.; Gitaka, J. Limited Genetic Variations of the Rh5-CyRPA-Ripr Invasion Complex in Plasmodium falciparum Parasite Population in Selected Malaria-Endemic Regions, Kenya. Front. Trop. Dis. 2023, 4, 1102265. [Google Scholar] [CrossRef]
- MalariaGEN; Ahouidi, A.; Ali, M.; Almagro-Garcia, J.; Amambua-Ngwa, A.; Amaratunga, C.; Amato, R.; Amenga-Etego, L.; Andagalu, B.; Anderson, T.J.C.; et al. An Open Dataset of Plasmodium falciparum Genome Variation in 7000 Worldwide Samples [version 2; peer review: 2 approved]. Wellcome Open Res. 2021, 6, 42. [Google Scholar] [CrossRef]
- Ragotte, R.J.; Higgins, M.K.; Draper, S.J. The RH5-CyRPA-Ripr Complex as a Malaria Vaccine Target. Trends Parasitol. 2020, 36, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, L.Y.; Bartholdson, S.J.; Crosnier, C.; Campos, M.G.; Wanaguru, M.; Nguon, C.; Kwiatkowski, D.P.; Wright, G.J.; Rayner, J.C. A Full-Length Recombinant Plasmodium falciparum PfRH5 Protein Induces Inhibitory Antibodies That Are Effective across Common PfRH5 Genetic Variants. Vaccine 2013, 31, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Sony Reddy, K.; Pandey, A.K.; Singh, H.; Sahar, T.; Emmanuel, A.; Chitnis, C.E.; Chauhan, V.S.; Gaur, D. Bacterially Expressed Full-Length Recombinant Plasmodium falciparum RH5 Protein Binds Erythrocytes and Elicits Potent Strain-Transcending Parasite-Neutralizing Antibodies. Infect. Immun. 2014, 82, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Wong, W.; Thompson, J.K.; He, W.; Birkinshaw, R.W.; Miura, K.; Long, C.A.; Soroka, V.; Søgaard, T.M.M.; Jørgensen, T.; et al. Neutralising Antibodies Block the Function of Rh5/Ripr/CyRPA Complex during Invasion of Plasmodium falciparum into Human Erythrocytes. Cell Microbiol. 2019, 21, e13030. [Google Scholar] [CrossRef] [PubMed]
- Ntege, E.H.; Arisue, N.; Ito, D.; Hasegawa, T.; Palacpac, N.M.Q.; Egwang, T.G.; Horii, T.; Takashima, E.; Tsuboi, T. Identification of Plasmodium falciparum Reticulocyte Binding Protein Homologue 5-Interacting Protein, PfRipr, as a Highly Conserved Blood-Stage Malaria Vaccine Candidate. Vaccine 2016, 34, 5612–5622. [Google Scholar] [CrossRef]
- Knudsen, A.S.; Björnsson, K.H.; Bassi, M.R.; Walker, M.R.; Kok, A.; Cristinoi, B.; Jensen, A.R.; Barfod, L. Strain-Dependent Inhibition of Erythrocyte Invasion by Monoclonal Antibodies Against Plasmodium falciparum CyRPA. Front. Immunol. 2021, 12, 716305. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.S.; Walker, M.R.; Agullet, J.P.; Björnsson, K.H.; Bassi, M.R.; Barfod, L. Enhancing Neutralization of Plasmodium falciparum Using a Novel Monoclonal Antibody against the Rhoptry-Associated Membrane Antigen. Sci. Rep. 2022, 12, 3040. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Wong, W.; Thompson, J.K.; Healer, J.; Goddard-Borger, E.D.; Lawrence, M.C.; Cowman, A.F. Structural Basis for Inhibition of Erythrocyte Invasion by Antibodies to Plasmodium falciparum Protein CyRPA. Elife 2017, 6, e21347. [Google Scholar] [CrossRef] [PubMed]
- Favuzza, P.; Guffart, E.; Tamborrini, M.; Scherer, B.; Dreyer, A.M.; Rufer, A.C.; Erny, J.; Hoernschemeyer, J.; Thoma, R.; Schmid, G.; et al. Structure of the Malaria Vaccine Candidate Antigen CyRPA and Its Complex with a Parasite Invasion Inhibitory Antibody. Elife 2017, 6, e20383. [Google Scholar] [CrossRef]
- Douglas, A.D.; Williams, A.R.; Knuepfer, E.; Illingworth, J.J.; Furze, J.M.; Crosnier, C.; Choudhary, P.; Bustamante, L.Y.; Zakutansky, S.E.; Awuah, D.K.; et al. Neutralization of Plasmodium falciparum Merozoites by Antibodies against PfRH5. J. Immunol. 2014, 192, 245–258. [Google Scholar] [CrossRef]
- Douglas, A.D.; Williams, A.R.; Illingworth, J.J.; Kamuyu, G.; Biswas, S.; Goodman, A.L.; Wyllie, D.H.; Crosnier, C.; Miura, K.; Wright, G.J.; et al. The Blood-Stage Malaria Antigen PfRH5 Is Susceptible to Vaccine-Inducible Cross-Strain Neutralizing Antibody. Nat. Commun. 2011, 2, 601. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.O.; Silk, S.E.; Elias, S.C.; Miura, K.; Diouf, A.; Galaway, F.; De Graaf, H.; Brendish, N.J.; Poulton, I.D.; Griffiths, O.J.; et al. Human Vaccination against RH5 Induces Neutralizing Antimalarial Antibodies That Inhibit RH5 Invasion Complex Interactions. JCI Insight 2017, 2, e96381. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Thompson, J.K.; Mackwell, K.L.; Browne, C.D.; Seager, B.A.; Ngo, A.; Lowes, K.N.; Silk, S.E.; Pulido, D.; King, L.D.W.; et al. RH5.1-CyRPA-Ripr Antigen Combination Vaccine Shows Little Improvement over RH5.1 in a Preclinical Setting. Front. Cell Infect. Microbiol. 2022, 12, 1049065. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.D.; Baldeviano, G.C.; Lucas, C.M.; Lugo-Roman, L.A.; Crosnier, C.; Bartholdson, S.J.; Diouf, A.; Miura, K.; Lambert, L.E.; Ventocilla, J.A.; et al. A PfRH5-Based Vaccine Is Efficacious against Heterologous Strain Blood-Stage Plasmodium falciparum Infection in Aotus Monkeys. Cell Host Microbe 2015, 17, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.D.; Baldeviano, G.C.; Jin, J.; Miura, K.; Diouf, A.; Zenonos, Z.A.; Ventocilla, J.A.; Silk, S.E.; Marshall, J.M.; Alanine, D.G.W.; et al. A Defined Mechanistic Correlate of Protection against Plasmodium falciparum Malaria in Non-Human Primates. Nat. Commun. 2019, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Minassian, A.M.; Silk, S.E.; Barrett, J.R.; Nielsen, C.M.; Miura, K.; Diouf, A.; Loos, C.; Fallon, J.K.; Michell, A.R.; White, M.T.; et al. Reduced Blood-Stage Malaria Growth and Immune Correlates in Humans Following RH5 Vaccination. Med 2021, 2, 701–719.e19. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Sousa, M.; Castro, R.; Schäfer, A.; Hauser, J.; Schulze, K.; Amacker, M.; Tamborrini, M.; Pluschke, G.; Alves, P.M.; et al. Scalable Process for High-Yield Production of PfCyRPA Using Insect Cells for Inclusion in a Malaria Virosome-Based Vaccine Candidate. Front. Bioeng. Biotechnol. 2022, 10, 879078. [Google Scholar] [CrossRef]
- Ragotte, R.J.; Pulido, D.; Lias, A.M.; Quinkert, D.; Alanine, D.G.W.; Jamwal, A.; Davies, H.; Nacer, A.; Lowe, E.D.; Grime, G.W.; et al. Heterotypic Interactions Drive Antibody Synergy against a Malaria Vaccine Candidate. Nat. Commun. 2022, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Malkin, E.M.; Diemert, D.J.; McArthur, J.H.; Perreault, J.R.; Miles, A.P.; Giersing, B.K.; Mullen, G.E.; Orcutt, A.; Muratova, O.; Awkal, M.; et al. Phase 1 Clinical Trial of Apical Membrane Antigen 1: An Asexual Blood-Stage Vaccine for Plasmodium falciparum Malaria. Infect. Immun. 2005, 73, 3677–3685. [Google Scholar] [CrossRef]
- Azasi, Y.; Gallagher, S.K.; Diouf, A.; Dabbs, R.A.; Jin, J.; Mian, S.Y.; Narum, D.L.; Long, C.A.; Gaur, D.; Draper, S.J.; et al. Bliss’ and Loewe’s Additive and Synergistic Effects in Plasmodium falciparum Growth Inhibition by AMA1-RON2L, RH5, RIPR and CyRPA Antibody Combinations. Sci. Rep. 2020, 10, 11802. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Jegerlehner, A.; Storni, T.; Lipowsky, G.; Schmid, M.; Pumpens, P.; Bachmann, M.F. Regulation of IgG Antibody Responses by Epitope Density and CD21-Mediated Costimulation. Eur. J. Immunol. 2002, 32, 3305–3314. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Rohrer, U.H.; Kündig, T.M.; Bürki, K.; Hengartner, H.; Zinkernagel, R.M. The Influence of Antigen Organization on B Cell Responsiveness. Science 1993, 262, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Lowy, D. Explanations for the High Potency of HPV Prophylactic Vaccines. Vaccine 2018, 36, 4768–4773. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide Tag Forming a Rapid Covalent Bond to a Protein, through Engineering a Bacterial Adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Thrane, S.; Janitzek, C.M.; Matondo, S.; Resende, M.; Gustavsson, T.; Jongh, W.A.; Clemmensen, S.; Roeffen, W.; Vegte-Bolmer, M.; Gemert, G.J.; et al. Bacterial Superglue Enables Easy Development of Efficient Virus-like Particle Based Vaccines. J. Nanobiotechnol. 2016, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Fougeroux, C.; Goksøyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Søgaard, M.; Aves, K.L.; Horsted, E.W.; et al. Capsid-like Particles Decorated with the SARS-CoV-2 Receptor-Binding Domain Elicit Strong Virus Neutralization Activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Bruun, T.U.J.; Andersson, A.M.C.; Draper, S.J.; Howarth, M. Engineering a Rugged Nanoscaffold to Enhance Plug-and-Display Vaccination. ACS Nano 2018, 12, 8855–8866. [Google Scholar] [CrossRef]
- Martinez, F.G.; Zielke, R.A.; Fougeroux, C.E.; Li, L.; Sander, A.F.; Sikora, A.E. Development of a Tag/Catcher-Mediated Capsid Virus-like Particle Vaccine Presenting the Conserved Neisseria Gonorrhoeae SliC Antigen That Blocks Human Lysozyme. Infect. Immun. 2023, 91, e00245-23. [Google Scholar] [CrossRef] [PubMed]
- Goksøyr, L.; Skrzypczak, M.; Sampson, M.; Nielsen, M.A.; Salanti, A.; Theander, T.G.; Remaley, A.T.; De Jongh, W.A.; Sander, A.F. A CVLP-Based Vaccine Displaying Full-Length PCSK9 Elicits a Higher Reduction in Plasma PCSK9 Than Similar Peptide-Based CVLP Vaccines. Vaccines 2023, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.J.; Sander, A.F.; Ariaans, M.B.P.A.; Fougeroux, C.; Heinzel, C.; Fendel, R.; Esen, M.; Kremsner, P.G.; ter Heine, R.; Wertheim, H.F.; et al. First-in-Human Use of a Modular Capsid Virus-like Vaccine Platform: An Open-Label, Non-Randomised, Phase 1 Clinical Trial of the SARS-CoV-2 Vaccine ABNCoV2. Lancet Microbe 2023, 4, e140–e148. [Google Scholar] [CrossRef]
- Aves, K.L.; Janitzek, C.M.; Fougeroux, C.E.; Theander, T.G.; Sander, A.F. Freeze-Drying of a Capsid Virus-like Particle-Based Platform Allows Stable Storage of Vaccines at Ambient Temperature. Pharmaceutics 2022, 14, 1301. [Google Scholar] [CrossRef]
- Rappuoli, R.; Bottomley, M.J.; D’Oro, U.; Finco, O.; De Gregorio, E. Reverse Vaccinology 2.0: Human Immunology Instructs Vaccine Antigen Design. J. Exp. Med. 2016, 213, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Pabst, M.J. Removal of Endotoxin from Protein Solutions by Phase Separation Using Triton X-114. J. Oflmmunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef]
- Iglewicz, B.; Hoaglin, D.C. How to Detect and Handle Outliers; ASQC Quality Press: Milwaukee, WI, USA, 1993. [Google Scholar]
- Pickett, G.G.; Peabody, D.S. Encapsidation of Heterologous RNAs by Bacteriophage MS2 Coat Protein. Nucleic Acids Res. 1993, 21, 4621–4626. [Google Scholar] [CrossRef] [PubMed]
- Shishovs, M.; Rumnieks, J.; Diebolder, C.; Jaudzems, K.; Andreas, L.B.; Stanek, J.; Kazaks, A.; Kotelovica, S.; Akopjana, I.; Pintacuda, G.; et al. Structure of AP205 Coat Protein Reveals Circular Permutation in SsRNA Bacteriophages. J. Mol. Biol. 2016, 428, 4267–4279. [Google Scholar] [CrossRef]
- Chang, X.; Krenger, P.; Krueger, C.C.; Zha, L.; Han, J.; Yermanos, A.; Roongta, S.; Mohsen, M.O.; Oxenius, A.; Vogel, M.; et al. TLR7 Signaling Shapes and Maintains Antibody Diversity Upon Virus-Like Particle Immunization. Front. Immunol. 2022, 12, 827256. [Google Scholar] [CrossRef]
- Thrane, S.; Aves, K.L.; Uddbäck, I.E.M.; Janitzek, C.M.; Han, J.; Yang, Y.R.; Ward, A.B.; Theander, T.G.; Nielsen, M.A.; Salanti, A.; et al. A Vaccine Displaying a Trimeric Influenza-A HA Stem Protein on Capsid-Like Particles Elicits Potent and Long-Lasting Protection in Mice. Vaccines 2020, 8, 389. [Google Scholar] [CrossRef]
- Harrison, T.E.; Alam, N.; Farrell, B.; Quinkert, D.; Lias, A.M.; King, L.D.W.; Draper, S.J.; Campeotto, I.; Higgins, M.K. Rational Structure-Guided Design of a Blood Stage Malaria Vaccine Immunogen Presenting a Single Epitope from PfRH5. bioRxiv 2024. [Google Scholar] [CrossRef]
- Williams, B.G.; King, L.D.W.; Pulido, D.; Quinkert, D.; Lias, A.M.; Silk, S.E.; Ragotte, R.J.; Davies, H.; Barrett, J.R.; McHugh, K.; et al. Development of an Improved Blood-Stage Malaria Vaccine Targeting the Essential RH5-CyRPA-RIPR Invasion Complex. Nat. Commun. 2024, 15, 4857. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Björnsson, K.H.; Bassi, M.R.; Knudsen, A.S.; Aves, K.-L.; Morella Roig, È.; Sander, A.F.; Barfod, L. Leveraging Immunofocusing and Virus-like Particle Display to Enhance Antibody Responses to the Malaria Blood-Stage Invasion Complex Antigen PfCyRPA. Vaccines 2024, 12, 859. https://doi.org/10.3390/vaccines12080859
Björnsson KH, Bassi MR, Knudsen AS, Aves K-L, Morella Roig È, Sander AF, Barfod L. Leveraging Immunofocusing and Virus-like Particle Display to Enhance Antibody Responses to the Malaria Blood-Stage Invasion Complex Antigen PfCyRPA. Vaccines. 2024; 12(8):859. https://doi.org/10.3390/vaccines12080859
Chicago/Turabian StyleBjörnsson, Kasper H., Maria R. Bassi, Anne S. Knudsen, Kara-Lee Aves, Èlia Morella Roig, Adam F. Sander, and Lea Barfod. 2024. "Leveraging Immunofocusing and Virus-like Particle Display to Enhance Antibody Responses to the Malaria Blood-Stage Invasion Complex Antigen PfCyRPA" Vaccines 12, no. 8: 859. https://doi.org/10.3390/vaccines12080859
APA StyleBjörnsson, K. H., Bassi, M. R., Knudsen, A. S., Aves, K.-L., Morella Roig, È., Sander, A. F., & Barfod, L. (2024). Leveraging Immunofocusing and Virus-like Particle Display to Enhance Antibody Responses to the Malaria Blood-Stage Invasion Complex Antigen PfCyRPA. Vaccines, 12(8), 859. https://doi.org/10.3390/vaccines12080859






