Abstract
Enhancing our comprehension of mRNA vaccines may facilitate the future design of novel vaccines aimed at augmenting immune protection while minimising reactogenic responses. Before this design is carried out, it is important to determine whether adaptive immunity correlates with the reactogenicity profile of vaccines. We studied a large cohort that was vaccinated with mRNA vaccines to answer this question. This was an observational study with real-world data. Reactogenicity data were obtained from the VigilVacCOVID study. Immunogenicity (humoral and cellular) data were retrieved from health records. One main population (n = 215) and two subpopulations were defined (subpopulation 1, n = 3563; subpopulation 2, n = 597). Sensitivity analyses were performed with subpopulations 1 and 2 to explore the consistency of results. We analysed the association of the intensity and types of adverse reactions with the development and quantity of elicited antibody titres. As an exploratory analysis in subpopulation 1, we assessed the association between reactogenicity and cellular immunogenicity. A higher incidence of fever, malaise, and myalgia including severe cases was significantly associated with the development and quantity of positive antibody titres. No significant findings were observed with cellular immunity. We observed a positive association between immunogenicity and reactogenicity. These findings can be relevant for the future development of our understanding of how mRNA vaccines function.
1. Introduction
The short-term safety and efficacy of SARS-CoV-2 mRNA vaccines have been widely characterised in pivotal clinical trials [1,2]. After carrying out pivotal clinical trials, several large scale “real-world” studies were conducted, reporting that SARS-CoV-2 vaccines were safe [3,4,5]. In these studies, the most frequently reported adverse reactions (ARs) were local (e.g., pain at the injection site) or systemic ARs (e.g., fever, myalgia, or malaise) [1,2]. These were usually self-limited and manageable ARs, which further reinforces the positive benefit–risk balance of the currently available SARS-CoV-2 vaccines.
However, given the emergence of several variants of concern, it is highly likely that new vaccine platforms that target new age strata, new antigens, and different booster doses will continue to be developed.
Undoubtedly, the ideal vaccine construct should effectively mobilize both the innate and adaptive immune responses without provoking systemic inflammation, which could potentially lead to serious ARs. One remaining question is whether vaccine-associated ARs can be uncoupled from the elicited immune response in an ideal vaccine construct [6,7]. Various authors have described binding and neutralizing antibodies as potential immune correlates of protection for SARS-CoV-2 vaccines [8,9]. The question that follows from their research is whether this adaptive immunity is, to some extent, correlated with the reactogenicity profile of vaccines in current vaccine constructs.
Recent studies have suggested that a higher reported frequency of ARs after vaccination could be associated, to some extent, with an increase in antibody titres [10,11]. Krammer et al. reported that individuals with pre-existing SARS-CoV-2 immunity showed a significant spike antibody immunogenicity and experienced more severe reactogenicity after the first dose compared to individuals without pre-existing antibodies [11]. Additionally, Debes et al. reported that spike IgG antibodies were higher in vaccinees with prior SARS-CoV-2 infection and those that reported clinically significant reactions after vaccination [12]. However, to date, the potential link between immunogenicity and reactogenicity has yielded contradictory results [13,14,15]. Therefore, the association between reactogenicity and immunogenicity after the first and second doses remains to be confirmed in larger vaccinated cohorts, especially in a setting that can be extrapolatable to clinical practice (i.e., in an heterogenous and more representative population beyond health-care professionals (HCPs)).
Overall, a deeper understanding of the immunological correlates of reactogenicity can assist in the future development of newer vaccine constructs through which immune protection can be boosted without hindering a proportional increase in reactogenicity. This becomes increasingly important given the expected increase in mRNA technology development in several clinical indications beyond SARS-CoV-2 vaccines [16].
In our prior VigilVacCOVID study, we observed a correlation between reactogenicity and immunogenicity [17]. This was evidenced by the consistent association of a higher incidence of AR with younger age and prior exposure to SARS-CoV-2, which is also in line with other published reports [18]. Conversely, reduced vaccine reactogenicity was linked to a compromised immune status, leading to improved tolerability. Following this rationale, we took advantage of the reactogenicity data collected within the framework of the study to analyse the immunogenicity data and substantiate the hypothesis of a potential link between reactogenicity and immunogenicity in a large, vaccinated cohort under the conditions of clinical practice.
The VigilvacCOVID study was an observational, single-centre post-authorisation study aimed at characterising short-term reactogenicity after vaccination with mRNA-1273 and BNT162b2 vaccines in two different populations: HCPs and solid organ transplant recipients (SOTRs). The study was designed in December 2020, and reactogenicity data were obtained during the vaccination campaign conducted at Hospital Clínic of Barcelona (HCB) from 7 January, when a vaccination campaign began in the HCB, until 30 April 2021. The first vaccinee group were HCPs. The type of vaccine was determined by national guidance and availability depending on local supply. Accordingly, at the beginning of the vaccination campaign, BNT162b2 was the only vaccine available for HCPs. The vaccination plan was amended on January 22nd with the inclusion of mRNA-1273 for HCPs, and from March 2nd onwards, mRNA-1273 was also administered to SOTRs. Reactogenicity was collected through a structured questionnaire that was delivered to each vaccinee through a telephone interview after receiving each vaccine dose.
2. Materials and Methods
2.1. Study Design and Collected Data
This was an observational, unicentric study with real-world data (RWD) that was implemented as a post hoc subanalysis of the VigilVacCOVID study [17]. The population of the VigilVacCOVID study included HCPs vaccinated with BNT162b2 and mRNA-1273 vaccines and SOTRs vaccinated with mRNA-1273. The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies [19]. The current post hoc subanalysis was approved by the Ethics Committee of HCB as a major amendment of the VigilVacCOVID study (references: HCB/2021/0684-amendment 1 and HCB/2021/0685-amendment 1). An informed consent waiver was approved by the Ethics Committee given the retrospective nature of the data collection and review, with no interventions performed on patients.
Reactogenicity and tolerability results had been previously obtained during the vaccination campaign in the context of the VigilVacCOVID study. The characteristics of the implemented surveillance program, the methodology of the safety data collection, and the overall reactogenicity profile assessed in the main VigilVacCOVID study are described elsewhere [17].
For the current study, we obtained the immunogenicity results of the above-mentioned participants from the electronic health records of HCB, with the collaboration of the Microbiology and Immunology Departments. The immunogenicity data included humoral (i.e., antibody titres) and cellular immunological results. Humoral immunogenicity had been assessed via the analysis of neutralising and anti-spike antibodies (IgG + IgM), based on the semiquantitative measurement of antibody titres on a scale ranging from 0 to >10. Results of >1.0 were considered positive antibody titres [20]. IgG titres were measured on samples that tested positive in the Cobas platform (Cobas; Roche Molecular Systems, Branchburg, NJ, USA) in a subset of patients, as per local clinical practice protocols [21]. IgG was measured only in a subset of the total vaccinated population (N = 322 vaccinees). Cellular immunogenicity was measured as the development of T cell-virus-specific responses as assessed via the interferon-gamma ELISPOT assay applied to isolated peripheral blood mononuclear cells (PBMCs) [22]. Given that cellular immunogenicity assessment was not routine clinical practice at the time of the vaccination campaign, data on cellular immunity were only available for a subset of vaccinees (N = 59 vaccinees). The complete method description is provided in the Supplementary Materials.
2.2. Statistical Analysis
2.2.1. Sample Size Calculation and Planned Analyses
The VigilVacCOVID cohort comprised HCPs vaccinated with BNT162b2 and mRNA-1273 vaccines and SOTRs vaccinated with mRNA-1273. It included a total of 6865 subjects, of whom 5088 responded to the questionnaire and thereby provided reactogenicity data for the current study. Of these, immunogenicity data were available for 3563 participants.
Given that a prospective analysis of immunogenicity had not been planned during our previous VigilVacCOVID study and considering the retrospective and real-world nature of data collection, there were some constraints that we had to take into consideration for the analysis of results. On one hand, the method previously used to measure humoral immunogenicity in clinical practice was semiquantitative and did not distinguish between infection-related (nucleocapsid protein (NCP) IgG antibodies) and vaccine-related (neutralising IgG antibodies to the receptor-binding protein of the S1 subunit of the spike protein) neutralising antibodies. SARS-CoV-2 tests were not routinely performed before and after vaccination. Likewise, in this initial population with available immunogenicity data (N = 3563), some immunity assessments had been performed either within a 4-month period after vaccination or prior to vaccination. We considered that the positive antibody titres of these distant measurements were not representative of ARs occurring immediately after receiving vaccine doses, nor were they necessarily related to vaccination. For instance, the possibility exists that some of the included participants developed immunogenicity before or after vaccination because of SARS-CoV-2 infection and not specifically due to vaccination.
To adjust these potential biases, we decided to perform two sensitivity analyses (see flowchart in Figure 1). We first refined the initial population by restricting the immunogenicity assessment timeframe to 4 weeks, and we defined subpopulation 2 for sensitivity analysis 2 (N = 597). Furthermore, we excluded vaccinees who had immunogenicity data prior to vaccination (N = 215). This was considered the most sensitive and least-biased population, and for this reason, it was considered the main population for analysis. We subsequently redefined the initial population (N = 3563) as subpopulation 1, and we considered the analysis of this population as sensitivity analysis 1. The main aim of this sensitivity analysis was to assess the consistency of results across analyses with a higher sample size. Baseline characteristics of subpopulations 1 and 2 are displayed in the Supplementary Materials (Tables S7 and S17), and the baseline characteristics of the main population are depicted in Table 1.
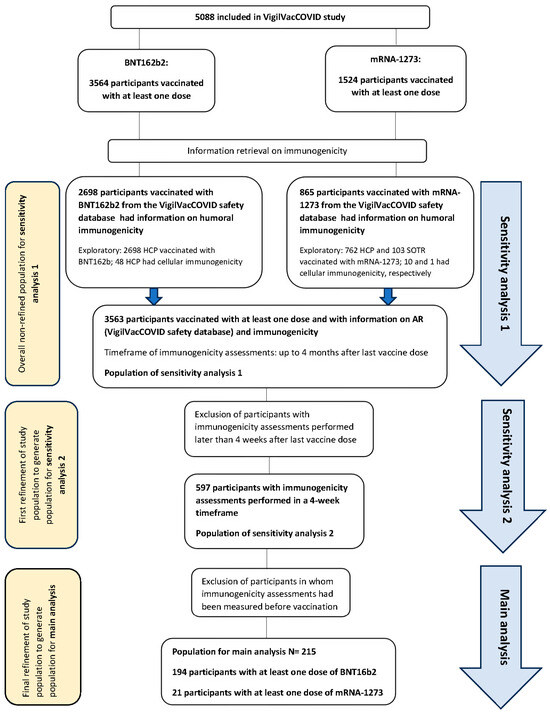
Figure 1.
Flowchart of the study displaying the populations for the main analysis and sensitivity analyses.

Table 1.
Characteristics of the participants (main analysis).
2.2.2. Study Endpoints
The primary endpoint was to assess the association between reactogenicity after vaccination and the development of humoral immunogenicity in the main population. Reactogenicity was defined in terms of the proportion of vaccinees with mild, moderate, and severe ARs for each immunogenicity category and in terms of AR intensity.
The key secondary endpoint was to assess the association between specific solicited and unsolicited AR and the development of immunogenicity.
As exploratory endpoints, we assessed the same reactogenicity and humoral immunogenicity endpoints as in sensitivity analyses 1 and 2. In addition, cellular immunogenicity was assessed as an exploratory endpoint in subpopulation 1. Cellular immunogenicity was not assessed in the main population, nor in subpopulation 2, given the lack of sample size and available data on cellular immunogenicity.
Finally, reactogenicity and humoral immunogenicity were assessed, in a descriptive and exploratory manner, in different subgroups of subpopulation 1, to assess potential trends that could suggest a difference with regard to the type of administered vaccine (mRNA-1273 vs. BTN162b2) or the vaccinated population (HCP vs. SOTR). Due to the sample size limitations and its exploratory nature, this descriptive analysis was only performed on subpopulation 1 and only considered the humoral immunogenicity results.
2.2.3. Statistical Analysis
Categorical variables were analysed as frequencies and percentages, whereas continuous variables were described as the mean ± standard deviation or median (25–75% interquartile range), as deemed suitable. Categorical data underwent comparison via the chi-square test or the Fisher’s exact test, as applicable. Continuous variables were assessed using Mann–Whitney testing.
Reactogenicity data were expressed in terms of the solicited and unsolicited AR rate (n, %), types of solicited and unsolicited ARs, intensity distribution of solicited ARs according to three grades of maximum intensity (mild, moderate, severe), and the mean and median Likert score (LS) values of the solicited ARs. Intensity was assessed as the proportion of vaccinees with mild, moderate, and severe ARs for each immunogenicity category (positive or negative). In addition, the mean (SD) Likert score (LS) (with values ranging from 0 to 10) of the intensity of the developed ARs was assessed for each immunogenicity category. Finally, the distribution of AR intensity in each immunogenicity category was analysed with the maximum intensity grade of AR experienced after vaccination and the distribution (%) of vaccinees in each maximum grade according to whether they tested positive or negative in the immunogenicity tests. These analyses were performed after the first, second, or any vaccine dose. Severe ARs were defined by an LS score of 7–10. Intensity was measured for solicited ARs, which were the primary events for study. Due to the subjectivity of some solicited ARs and the questionnaire-based methodology of the study, intensity was not measured for insomnia, nausea, diarrhoea, and vomiting, or for unsolicited ARs that had been collected through a free text option.
Given that specific IgG titres were only measured in 322 vaccinees, and on a different platform than the one used for total antibody titres, we decided to use only the total semiquantitative antibody titres for the immunogenicity analysis and not IgG titres. For the analysis of immunogenicity, we defined antibody positiveness considering a cut-off of >1.0 (positive humoral immunogenicity above a cut-off of >1.0; for the cut-off and detection method, see the Materials and Methods and Supplementary Materials). Given that antibody titres were measured with a semiquantitative method on a scale ranging from 0 to >10, we calculated the median value of the titres’ level (second quartile, Q2), and then analysed the distribution of vaccinees above and below the median to carry out a semiquantitative assessment that considered the extent of elicited humoral immunogenicity. Results were expressed as absolute and relative numbers of vaccinees with the reported AR and negative antibody titres or titres below the median value vs. vaccinees with the reported AR and positive antibody titres or titres above the median value, with p-values. Cellular immunogenicity was not assessed in the main population, nor in subpopulation 2, given the lack of sample size and available data on cellular immunogenicity (N = 59) and because this was considered as an exploratory analysis.
We applied a two-sided significance level of 5%. All calculations were performed using SAS v9.4 software (Cary, NC, USA). In addition, we conducted exploratory analyses using a less conservative significance level of 10% to explore, in a descriptive manner, any potential relationships that could be of clinical interest despite not meeting the standard 5% threshold.
3. Results
3.1. Immunogenicity and Reactogenicity Results
The overall results are depicted in Table 2, Table 3 and Table 4. The individual absolute and relative numbers of each assessed variable can be found in the tables in the Supplementary Materials. Figure 2, Figure 3 and Figure 4 depict the association between immunogenicity and reactogenicity (as a percentage of solicited ARs). For the purposes of clarity and to highlight the main results of the analyses, only those ARs that showed a significant association are displayed. The complete graphic results of Table 2, Table 3 and Table 4 are shown in the Supplementary Materials, Figures S1–S3.

Table 2.
Results of the main analysis.

Table 3.
Results of sensitivity analysis 1.

Table 4.
Results of sensitivity analysis 2.
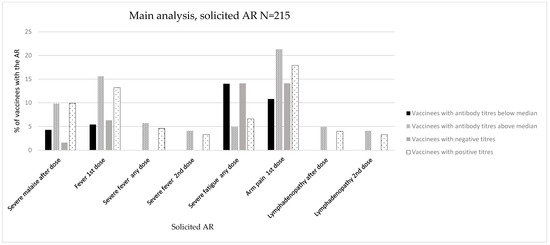
Figure 2.
Immunogenicity and reactogenicity results of the main population. Bar diagram of the results in Table 2. Immunogenicity is expressed as antibody positiveness (positive/negative titres) and antibody titres (antibody titres below/above median) data (%). Reactogenicity results are represented as a percentage of vaccinees developing each solicited AR.
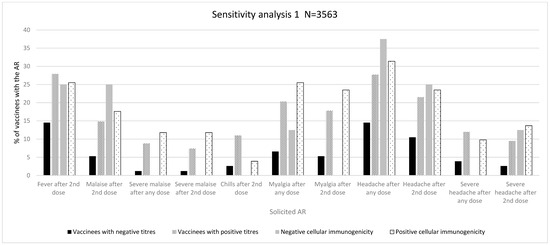
Figure 3.
Immunogenicity and reactogenicity results of sensitivity analysis 1. Bar diagram of the results in Table 3. Immunogenicity is expressed as antibody positiveness (positive/negative titres) and cellular immunogenicity (positive/negative) data (%). Reactogenicity results are represented as a percentage of vaccinees developing each solicited AR.
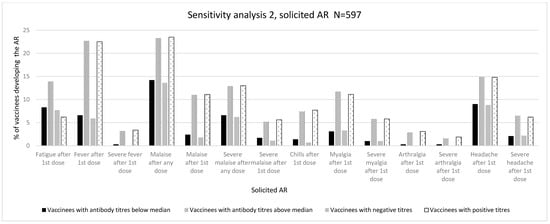
Figure 4.
Immunogenicity and reactogenicity results of sensitivity analysis 2. Bar diagram of the results in Table 4. Immunogenicity is expressed as antibody positiveness (positive/negative titres) and antibody titres (antibody titres below/above median) data (%). Reactogenicity results are represented as a percentage of vaccinees developing each solicited AR.
3.1.1. Main Analysis (Main Population, N = 215)
Immunogenicity Assessed as Positive/Negative Antibody Titres
We did not observe any significant association with the maximum intensity grades or LS (mean, SD). Regarding specific ARs, we observed a significant association between the development of severe malaise after any dose and the development of positive antibody titres against SARS-CoV-2 (vaccinees with malaise: 1 (1.6%) with negative antibody titres, 15 (9.9%) with positive antibody titres, p = 0.033) (Figure 2, Table 2, and Tables S1–S3 in the Supplementary Materials).
Immunogenicity Assessed as Antibody Titres above/below Median
No significant associations were observed in the intensity analysis (Table 2 and Table S4 in the Supplementary Materials).
Regarding specific ARs, we observed a significant association between the development of fever (after first dose: 5 (5.4%) vs. 19 (15.6%), p = 0.019; after any dose: 0 (0.0%) vs. 7 (5.7%), p = 0.019; after second dose: 0 (0.0%) vs. 5 (4.1%), p = 0.048), arm pain (after first dose: 10 (10.8%) vs. 26 (21.3%), p = 0.019), lymphadenopathy (after any dose: 0 (0.0%) vs. 6 (4.9%), p = 0.030; after second dose: 0 (0.0%) vs. 5 (4.1%), p = 0.048), and the measurement of semiquantitative antibody titres above the median. These ARs developed after any vaccine dose and did not demonstrate a specific predominance in frequency over the first or second doses (Figure 2, Table 2, and Table S5 in the Supplementary Materials).
In terms of severe ARs, we observed a significant association with the development of severe fever (after any dose: 0 (0.0%) vs. 7 (5.7%), p = 0.019; after second dose: 0 (0.0%) vs. 5 (4.1%), p = 0.048) (Table 2 and Table S6 in the Supplementary Materials).
3.1.2. Sensitivity Analysis 1 (N = 3563)
Immunogenicity Assessed as Positive/Negative Antibody Titres
A significant association was found in the distribution of ARs of grades 1–3 after any vaccine dose (grade 1: 15 (23.8% vs. 350 (12.2%); grade 2: 23 (36.5%) vs. 1132 (39.3%); grade 3: 25 (39.7%) vs. 1397 (48.5%), p = 0.020) and after a second vaccine dose (grade 1: 12 (27.3%) vs. 261 (11.6%); grade 2: 19 (43.2%) vs. 949 (42.3%); grade 3: 13 (29.5) vs. 1036 (46.1%, p = 0.003)). We also found a significant association with intensity measured by LS (mean, SD) after any vaccine dose (5.48 (2.47%) vs. 6.15 (2.36%), p = 0.031) and after a second vaccine dose (4.95 (2.37%) vs. 6.10 (2.06%), p = 0.001). Likewise, there was a significantly higher proportion of severe ARs after a second dose (13 (17.1%) vs. 1036 (29.7%), p = 0.017) (Table 3, and Table S8 in the Supplementary Materials).
Regarding specific ARs, we observed a significantly higher proportion of fever after a second dose (11 (14.5%) vs. 974 (27.9%), p = 0.010), malaise after any dose (7 (9.2%) vs. 622 (17.8%), p = 0.050) and after a second dose (4 (5.3%) vs. 517 (14.8%), p = 0.020), chills after a second dose (2 (2.6%) vs. 385 (11.0%), p = 0.020), myalgia after any dose (5 (6.6%) vs. 709 (20.3%), p = 0.003) and after a second dose (4 (5.3%) vs. 621 (17.8%), p = 0.004), and headache after any dose (11 (14.5%) vs. 967 (27.7%), p = 0.010) and after a second dose (8 (10.5%) vs. 750 (21.5%), p = 0.020), (Table S9 in the Supplementary Materials). Regarding severe specific ARs, a higher proportion of these were observed for severe malaise after any dose (1 (1.2%) vs. 305 (8.8%), p = 0.010), severe malaise after a second dose (1 (1.2%) vs. 258 (7.4%), p = 0.030), severe headache after any dose (3 (3.9%) vs. 419 (12.0%), p = 0.030), and severe headache after a second dose (2 (2.6%) vs. 331 (9.5%), p = 0.040) (Figure 3, Table 3, and Table S10 in the Supplementary Materials).
Immunogenicity Assessed as Antibody Titres above/below Median
We observed a significant association for ARs of grades 1–3, LS (mean, SD), and the development of any AR after a second vaccine dose (Table S11 in the Supplementary Materials).
As for specific ARs, we observed a significantly higher proportion of chills after a second dose (35 (8.0%) vs. 352 (11.3%), p = 0.040) as well as severe headache after any dose (39 (8.9%) vs. 383 (12.3%), p = 0.040) and severe headache after a second dose (28 (6.4%) vs. 305 (9.8%), p = 0.020) in vaccinees with antibody titres above the median cut-off (Table 3 and Tables S12 and S13 in the Supplementary Materials).
Cellular Immunogenicity
A positive trend of a higher frequency of vaccinees with positive cellular immunogenicity was observed for some solicited ARs such as malaise, chills, arm pain, myalgia, and severe headache (Figure 3 and Tables S14–S16 in the Supplementary Materials). However, none of these associations were statistically significant. The analysis of intensity did not show any differences between vaccines with negative and positive cellular immunogenicity.
Descriptive Analysis According to Type of Vaccine and Population
Regarding vaccine type (mRNA-1273 vs. BNT162b2), data obtained from HCPs vaccinated with both types of vaccine showed similar immunogenicity rates (98.5% vs. 99% of vaccinees with positive antibody titres, respectively). However, the reactogenicity seemed to be greater with mRNA-1273 vaccines than with BNT162b2 (the proportion of any AR after vaccination was 97.4% vs. 81.7%, respectively), especially concerning severe AR rates (61.8% vs. 34.2%, respectively). Increased reactogenicity with mRNA-1273 vaccines was also observed in the analysis of solicited ARs (Table S24 and Figure S1).
We also observed a difference in terms of immunogenicity and reactogenicity between the HCPs and SOTRs after vaccination with mRNA-1273 vaccines. Accordingly, we observed a decreased rate of immunogenicity in SOTRs as opposed to HCPs (57% vs. 98.5% of vaccinees with positive antibody titres, respectively) and a decreased reactogenicity rate (proportion of vaccinees with at least one AR, 87.4% vs. 97.4%, respectively; proportion of vaccinees with at least one severe AR, 28.2% vs. 61.8%, respectively). The same trend was observed with the solicited ARs (Table S24, Figure S2).
3.1.3. Sensitivity Analysis 2 (N = 597)
Immunogenicity Assessed as Positive/Negative Antibody Titres
Although there was a positive trend, we did not find a significant association with ARs of grades 1–3 after the first vaccine dose. There was a significant association with intensity measured as the LS after a second dose (3.63 (3.31%) vs. 3.01 (3.23%), p = 0.001). In addition, we observed a positive association between the development of any AR after a second dose (187 (68.5%) vs. 188 (58.0%), p = 0.008) and the development of any severe AR after the first dose (53 (19.4%) vs. 90 (27.8%), p = 0.017) (Table 4 and Table S18 in the Supplementary Materials).
As for the distribution of specific ARs, we observed a significant association with fever after a first dose (16 (5.9%) vs. 73 (22.5%), p ≤ 0.001), malaise after any dose (37 (13.6%) vs. 76 (23.5%), p ≤ 0.002) and after a first dose (5 (1.8%) vs. 36 (11.1%), p ≤ 0.001), chills after a first dose (2 (0.7%) vs. 25 (7.7%), p ≤ 0.001), myalgia after a first dose (9 (3.3%) vs. 36 (11.1%), p = 0.003), arthralgia after a first dose (0 (0.0%) vs. 10 (3.1%), p = 0.034), and headache after a first dose (24 (8.8%) vs. 48 (14.8%), p = 0.024) (Table 2 and Table S19 in the Supplementary Materials). Regarding severe ARs, severe fever after a first dose (0 (0.0%) vs. 11 (3.4%), p = 0.002), severe malaise after any dose (17 (6.2%) vs. 42 (13.0%), p = 0.006) and after a first dose (3 (1.1%) vs. 18 (5.6%), p = 0.003), severe myalgia after a first dose (3 (1.0%) vs. 18 (5.8%), p = 0.003), severe arthralgia after a first dose (0 (0.0%) vs. 6 (1.9%), p = 0.024), and severe headache after a first dose (6 (2.2%) vs. 20 (6.2%), p = 0.018) showed a significant association with the development of positive antibody titres (Figure 4, Table 4, and Table S20 in the Supplementary Materials).
Immunogenicity Assessed as Antibody Titres above/below Median
We did not observe any significant association with the maximum intensity grades or with LS (mean, SD). We observed a significant association with the proportion of ARs after a second dose (193 (67.0%) vs. 182 (58.9%), p = 0.040) and the proportion of any moderate AR after the first dose (103 (35.8%) vs. 135 (43.7%), p = 0.048) (Table 4 and Table S21 in the Supplementary Materials).
A positive association was also observed for some specific ARs such as fatigue after the first dose (24 (8.3%) vs. 43 (13.9%), p = 0.031), fever after the first dose (19 (6.6%) vs. 70 (22.7%), p ≤ 0.001), malaise after any dose (41 (14.2%) vs. 72 (23.3%), p = 0.004) and after the first dose (7 (2.4%) vs. 34 (11.0%), p ≤ 0.001), chills after a first dose (4 (1.4%) vs. 23 (7.4%), p = 0.004), myalgia after a first dose (9 (3.1%) vs. 36 (11.7%), p ≤ 0.001), arthralgia after the first dose (1 (0.3%) vs. 9 (2.9%), p = 0.015), and headache after a first dose (26 (9.0%) vs. 46 (14.9%), p = 0.028). Regarding severe ARs, we observed a significant association for severe fever after the first dose (1 (0.3%) vs. 10 (3.2%), p = 0.009), severe malaise after any dose (19 (6.6%) vs. 40 (12.9%), p = 0.009) and after the first dose (5 (1.7%) vs. 16 (5.2%), p = 0.023), severe myalgia after the first dose (3 (1.0%) vs. 18 (5.8%), p = 0.002), severe arthralgia after the first dose (1 (0.3%) vs. 5 (1.6%), p = 0.120), and severe headache after a first dose (6 (2.1%) vs. 20 (6.5%), p = 0.009) (Table 4 and Tables S22–S23 in the Supplementary Materials).
3.1.4. Overall Reactogenicity and Immunogenicity Analysis
Table 2, Table 3 and Table 4 depict ARs for which statistical significance or a trend towards significance (with a more conservative alpha level of 10%) was observed. After a consistency assessment among the different analyses, a higher proportion of fever, malaise, and myalgia including severe episodes of these ARs was observed among vaccinees that developed positive antibody titres in the three analyses. The proportion of vaccinees with myalgia was statistically significant in sensitivity analyses 1 and 2, and a trend towards significance (alpha level of <10%) was observed for severe myalgia when antibody positiveness was considered in the main analysis. These ARs developed after any vaccine dose in the primary analysis and sensitivity analysis 1 and did not demonstrate a specific predominance in frequency over the first or second doses, whereas most of the statistically significant ARs in sensitivity analysis 2 were observed after any dose or after a second vaccine dose. Figure 2, Figure 3 and Figure 4 show an association between reactogenicity (as expressed by solicited ARs) and immunogenicity that seemed consistent across the three performed analyses.
Some ARs such as chills and headache were significant in sensitivity analyses 1 and 2 but were not significant in the main analysis. Similarly, the association between the intensity of ARs and immunogenicity was not consistent either.
Of note, in some of these ARs (e.g., injection site pain, injection site swelling, fatigue, hypersensitivity, gastrointestinal disorders), a significant association was observed, but this association was observed in a higher proportion of vaccinees from the negative immunogenicity group, thereby showing a negative association. The individual absolute and relative numbers of these ARs can be found in the tables in the Supplementary Materials (Tables S1–S23).
4. Discussion
We believe our study provides novel insights into the enquiry of the hypothesis of the potential link between immunogenicity and reactogenicity in mRNA vaccines including the following: (i) an exploration of both humoral and cellular immunogenicity, (ii) a methodology for assessing both the development and quantity of immunogenicity, (iii) the inclusion of a large vaccinated cohort composed of HCPs and immunocompromised patients that can be more reflective of typical vaccination practices, (iv) the assessment of two mRNA vaccines, and (v) the evaluation of a wide range of local and systemic reactions with the additional consideration of symptom intensity.
Overall, we observed a consistent significant association between fever, malaise, and myalgia and the development of elicited humoral immunogenicity. According to our data, these results also seem to be associated with the quantity of immunogenicity developed. In the sensitivity analyses performed, we also observed a significant association between chills, headache, injection site pain, arm pain, arthralgia, and elicited immunogenicity.
On the whole, our results are consistent with those reported in other studies by various authors. Interestingly, most of these previous works studied HCPs vaccinated with BNT162b2 vaccines. Speletas et al. studied the intensity and duration of immunogenicity in 511 individuals who were vaccinated with two doses of the BNT162b2 vaccine. Taking into account the impact of adverse side effects on the strength of IgG antibody responses, a multivariate analysis showed that on day 21, only increased age had a significant effect, with no impact observed from AR. However, individuals who experienced fever and muscle pain after the second dose had notably higher IgG levels on day 42. Interestingly, this effect had disappeared by day 90 and day 180 following vaccination [23].
Yamamoto et al. assessed the immunogenicity and reactogenicity in 100 HCPs who had been vaccinated with BNT162b2. Participants who exhibited fever showed notably elevated spike IgG titres 7 days after the second vaccination dose compared to those who reported no fever or only mild fever. Although spike IgG titres tended to decline over time for all participants, those who had a high fever still maintained higher titres than those who experienced no or mild fever, even at both 39 and 60–74 days post-vaccination [24]. Other studies have also found a positive association between local and systemic ARs and the development of immunogenicity after vaccination with BNT162b2 [25,26].
Immunogenicity and reactogenicity after vaccination with mRNA-1273 vaccines have been explored less than with BNT162b2. Matsumoto et al. assessed the dynamics of immunogenicity in 47 healthy individuals vaccinated with a third dose of mRNA-1273 at 3 days, 7 days, and 1 month post-vaccination. The investigators found a significant association between fever and the development of antibody titres [27]. Other SARS-CoV-2 vaccines have also been studied in terms of immunogenicity and reactogenicity, without consistent results. Yun Lim et al. assessed a prospective cohort of HCPs vaccinated with ChAdOx1 and BNT162b2. In their study, only a positive association with local ARs was observed [28].
Alternatively, other authors have found negative results after vaccination with ChAdOx1 and BNT162b2, with sample sizes of 67 HCP [29], 80 HCP [15], and 135 healthy adults [30], while others have only observed some association with AR intensity but not with AR frequency [14], a weak correlation [31], or an association only in males but not in female vaccinees [32].
With regard to AR intensity, while we found some significant differences in the sensitivity analyses, we did not observe any difference in the main population of the study, as was the case in most of the previously described studies. Hence, a positive association with AR intensity cannot be firmly inferred.
Compared to previously reported results, our study analysed a wider study population (HCP and SOTR), two mRNA vaccines (BNT162b2 vs. mRNA-1273), and both immunity arms (humoral and cellular)). In the explanatory analysis, we observed decreased immunogenicity and reactogenicity in SOTRs vaccinated with mRNA-1273 in contrast to HCPs who had also been vaccinated with mRNA-1273. While reactogenicity was higher with mRNA-1273 in the vaccinated HCPs, and taking into consideration that certain reactogenicities may stem from vaccine composition rather than immunogenicity itself, the differences observed in reactogenicity between HCPs and SOTRs provide evidence for the role of immunogenicity. These data, together with the results in the overall population, provide support for the confirmation of our initial hypothesis formulated following the results obtained in our first study.
Notwithstanding, our study has several limitations. Firstly, our study was based on reactogenicity data from the VigilVacCOVID study. Therefore, the AR data may have limitations inherent to the observational and non-randomised nature of the VigilVacCOVID study such as recall bias and potential confounders. In addition, AR data were obtained through a structured questionnaire. This can be a subjective method for the assessment of certain ARs such as fatigue or malaise. Furthermore, the method employed for assessing humoral immunogenicity in clinical practice was semiquantitative and did not differentiate between infection-related and vaccine-induced neutralising antibodies. Routine SARS-CoV-2 testing was not conducted pre- and post-vaccination. Additionally, some immunogenicity measurements were obtained months after vaccination, while others were obtained prior to vaccination, potentially not reflecting ARs immediately following vaccination. Therefore, we cannot exclude the possibility that some participants may have developed immunogenicity due to SARS-CoV-2 infection rather than vaccination. However, we performed two sensitivity analyses to account for these potential biases; this reinforced the consistency of our main results.
Of note, the first sensitivity analysis included all vaccinees followed-up in the VigilVacCOVID study with immunogenicity and reactogenicity data (3563 vaccines); these data were collected in a 4-month timeframe. Despite the limitations related to the 4-month study timeframe, one should take into consideration that previous authors have found an association between ARs and immunogenicity even at 74 days post-vaccination [24]. Therefore, we consider that the results obtained in the 4-month timeframe, with a higher sample size of 3563 vaccinees, are still informative. The results of cellular immunogenicity were negative, both in the rate and intensity of ARs and in the assessment of solicited and unsolicited AR. We acknowledge that our sample size for this assessment was rather small (n = 59 vaccinees with testing performed), given that cellular immunogenicity was not standard practice for all vaccinees at the time of the vaccination campaign. Most likely because of the sample size, we could observe some positive trends in some solicited ARs, but none were statistically significant. Although Speletas et al. elucidated a clear correlation between humoral and cellular responses [23], few studies have assessed both humoral and cellular immunogenicity, and those that did measure the T cell responses included similar sample sizes [15,28,32] with negative results. Our results, in terms of cellular immunogenicity, were statistically non-significant. However, we observed a positive trend, with 86% of vaccinees testing positive for cellular immunogenicity amongst those with positive antibody titres. A relevant aspect to consider is that humoral immunogenicity is a surrogate marker of the T cell response; in fact, there is no immune memory without T lymphocytes and, although it is not frequent, there can be one without antibodies or B lymphocytes [33]. Although the humoral response can be considered a valid marker of the association between reactogenicity and immunogenicity, it is relevant to assess the two arms of adaptive immunity (humoral and cellular immunogenicity) whenever possible, especially in those patients without an antibody response and/or at risk of severe COVID-19. Lack of standardised protocols for the concomitant assessment of the two arms of adaptive immunity in the standard practice of clinical centres can be a limitation for the collection of RWD regarding cellular immunogenicity, and humoral immunogenicity can be a valid marker in these scenarios. Nonetheless, further studies with larger sample sizes and where reactogenicity and cellular immunogenicity are assessed are necessary, especially with the likely development of new vaccines based on the mRNA platform.
On the other hand, a significant association was observed for some ARs, but this association was found among a higher proportion of vaccinees in the negative immunogenicity group. The clinical meaning of this finding is unclear to us, and it should be explored in future studies where systemic and local ARs are thoroughly assessed.
Moreover, immunogenicity assessments were based solely on the total antibody titres (IgM + IgG), and specific IgG titres were only measured in a subset of vaccinees. While we are aware of the limitations related to data sources, data type, and the timing of determinations, we would like to highlight that these limitations are due to RWD acquisition processes. Our data are reflective of the standard practice during the period when the vaccination campaign was established rapidly amid the pressures of SARS-CoV-2 fears, and safety follow-ups were also organised rapidly to provide vaccinees with exhaustive vaccine surveillance. Immunogenicity assessments were not standard practice at the time and, as such, could not be carried out during the vaccinations. Because the data were obtained within the limitations of standard practice, we consider the results to be representative, and that these as results can be generalised to the overall population who are routinely vaccinated at healthcare centres. The methodology of our study reflects data acquisition and processing within a real-world context in standard clinical practice, which should be instructive for the development of further RWD studies, especially those that are performed within the context of mass vaccination campaigns during emergency periods with the use of data from clinical practices.
Translating our results into clinical practice, fever, myalgia, and malaise are systemic symptoms that develop rapidly after vaccination and can be relatively easily detected. Furthermore, these symptoms are relatively frequent (the European Medicines Agency has identified 1.7 million spontaneous reports of suspected ARs in almost 768 million vaccine doses administered in Europe [34], of which fever and myalgia are two of the most reported ARs in the European database). The reporting of these symptoms may be indicative of the prospective development of immunogenicity after vaccination. Likewise, SOTRs may be asymptomatic after vaccination and may have lower chances of developing immunogenicity. The main focus of our study was to assess a potential link between reactogenicity and immunogenicity. These are preliminary results that cannot be translated directly into clinical practice. That is to say, immunogenicity is a heterogenous multimodal clinical entity that can be explained by several factors other than reactogenicity such as genomic data or other types of clinical data, among others. The construction and validation of a future prediction signature may be helpful when planning immunogenicity testing programs, vaccination campaigns, and closer monitoring programs in special patient subgroups in clinical care centres. Therefore, we consider that the data of our study, which revealed an association between immunogenicity and reactogenicity, can contribute to the creation of this signature in further dedicated studies.
Conversely, the EMA Pharmacovigilance Risk Assessment Committee (PRAC) began an analysis of rare cases of myocarditis and pericarditis after vaccination with BNT162b2 [35] following early reports of cases of myocarditis after vaccination with BNT162b2 [36]. The safety signal of myocarditis and pericarditis was finally validated and included in the Summary of Product Characteristics (SmPC) of the vaccines [37]. The overall incidence of myocarditis and pericarditis is very low. Although figures varied according to the different series [38,39,40], published evidence shows that these events tend to be rare or very rare. Therefore, with 5088 subjects, the VigilVacCOVID study was not powered enough to detect rare cases such as myocarditis or pericarditis, but adequate for obtaining a short-term reactogenicity profile. The current study, in line with the VigilVacCOVID study, aimed to characterise the association between short-term reactogenicity and developed immunogenicity.
In addition, apart from myocarditis and pericarditis, no other safety signals related to cardiovascular complications have been validated to date. Cardiovascular events such as myocardial infarctions, strokes, embolism, and others have been studied, and while some studies have suggested increased reporting rates of these events [41,42], others have not found such an association [36,43,44,45,46,47]. While some authors have previously suggested that mRNA vaccines have been associated with increased reports of adverse cardiovascular reactions than other types of SARS-CoV-2 vaccines, it is true that the strongest association remains thus far with myocarditis and pericarditis, and the evidence regarding the association of SARs-CoV-2 vaccines with cardiovascular outcomes still remains to be elucidated [48]. In our previous VigilVacCOVID study, we detected some cases of syncope, hypertension, hypotension, chest pain, cold sweat, and tachycardia as unsolicited ARs; similar findings were previously reported as potential cardiovascular adverse effects in other studies [41]. These results confirm the sensitivity of our study to detect and report ARs developed after vaccination, in line with what has been previously published. However, since the study was designed to obtain a short-term reactogenicity profile, we were not able to confirm whether these symptoms were finally confirmed through a cardiovascular diagnosis, and whether there was a causal link with the vaccine. Therefore, in the current analysis, we did not observe an association between these events and immunogenicity.
Finally, our study suggests a positive association between reactogenicity and the development and quantity of immunogenicity after vaccination. This association seems to be linked to the development of anti-spike protective immunogenicity and should not be generalised to other types of adaptive immunity. For instance, recent reports have suggested that autoantibodies targeting type I IFNs develop after vaccination with mRNA SARS-CoV-2 vaccines but not with viral vector-based vaccines [49,50]. While these autoantibodies have been associated with adverse reactions in other vaccines such as the live-attenuated yellow vaccine [51], such an association has not been observed in SARS-CoV-2 vaccines [52,53].
5. Conclusions
In conclusion, our study detected a positive association between reactogenicity (defined as the onset of systemic symptoms such as fever, myalgia, and malaise) and the development and quantity of immunogenicity after vaccination. In addition to their high prevalence, these ARs are early, easily detectable symptoms post-vaccination and are indicative of the development of potential immunogenicity. These results can be valuable for the future construction of immunogenicity prediction signatures. Moreover, we believe that our results can be impactful in advancing our understanding of the immunological correlates of mRNA vaccines and, consequently, in the design of future mRNA vaccine constructs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12060665/s1, Measurement of antibodies and T cell response. Analytical methods; Table S1: Assessment of antibody positiveness (positive/negative titres); Table S2: Assessment of antibody positiveness (positive/negative titres): solicited and unsolicited ARs; Table S3: Assessment of antibody positiveness (positive/negative titres): severe solicited and unsolicited ARs; Table S4: Assessment of antibody titres (antibody titres above/below median); Table S5: Assessment of antibody titres (antibody titres above/below median): solicited and unsolicited ARs; Table S6: Assessment of antibody titres (antibody titres above/below median): severe solicited ARs; Figure S1: Summary of results of immunogenicity and reactogenicity of the main population; Table S7: Characteristics of subpopulation 1; Table S8: Assessment of antibody positiveness (positive/negative titres); Table S9: Assessment of antibody positiveness (positive/negative titres): solicited and unsolicited ARs; Table S10: Assessment of antibody positiveness (positive/negative titres): severe solicited ARs; Table S11: Assessment of antibody titres (antibody titres above/below median); Table S12: Assessment of antibody titres (antibody titres above/below median): solicited and unsolicited ARs; Table S13: Assessment of antibody titres (antibody titres above/below median): severe solicited ARs; Table S14: Cellular immunogenicity assessment; Table S15: Cellular immunogenicity assessment: solicited and unsolicited ARs; Table S16: Cellular immunogenicity assessment: severe solicited ARs; Figure S2: Summary of results of the immunogenicity and reactogenicity of sensitivity analysis 1; Table S17: Characteristics of subpopulation 2; Table S18: Assessment of antibody positiveness (positive/negative titres); Table S19: Assessment of antibody positiveness (positive/negative titres): solicited and unsolicited ARs; Table S20: Assessment of antibody positiveness (positive/negative titres): severe solicited ARs; Table S21: Assessment of antibody titres (antibody titres above/below median); Table S22: Assessment of antibody titres (antibody titres above/below median): solicited and unsolicited ARs; Table S23: Assessment of antibody titres (antibody titres above/below median): severe solicited ARs; Figure S3: Summary of results of the immunogenicity and reactogenicity of sensitivity analysis 2; Table S24: Reactogenicity and immunogenicity by vaccine and population types; Figure S4: Comparison of vaccine types; Figure S5: Comparison of population types; Table S25: STROBE Checklist.
Author Contributions
The corresponding author (J.S.-P.) attests that all of the listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J.S.-P., F.T., J.B. and G.C. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: J.S.-P., F.T., J.B. and G.C. Acquisition, analysis, or interpretation of data: J.S.-P., G.C., F.T., J.B., M.Á.M., M.M.M. and N.E. Drafting of the manuscript: J.S.-P., G.C., F.T., J.B., M.Á.M., M.M.M. and N.E. Critical revision of the manuscript for significant intellectual content: J.S.-P., G.C., F.T., J.B., M.Á.M., M.M.M. and N.E. Statistical analysis: F.T. The lead author (J.S.-P.) confirms that the manuscript is an honest, accurate, and transparent account of the study being reported, there are no important aspects of the study that have been omitted, and that any discrepancies from the study as planned have been reported and explained. All authors have read and agreed to the published version of the manuscript.
Funding
The study was performed Hospital Clínic’s own resources. No grants/funding were received for this study.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Research Ethics Committee of Hospital Clínic (HCB/2021/0684-amendment 1 and HCB/2021/0685-amendment 1, approval date: 7 March 2022).
Informed Consent Statement
An informed consent waiver was approved by the Ethics Committee given the retrospective nature of data collection and review, with no interventions performed on patients.
Data Availability Statement
Complete data are available in the Supplementary Materials. Raw data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors confirm that all persons named in the Acknowledgements section have provided written permission to be included in the manuscript. We are thankful to the valuable support provided by Sandra Serrano, Victor Sapena, and Juan Bascuas in the data extraction. We are also thankful to the interviewer’s team (IT) that made this work possible by their support in the VigilVacCOVID study: Juan Bascuas, Coordinator of the IT; Luis Aparicio; Susanna Bañuelos; Pilar Cano; Vanessa de Dios; Anna Massó; Celia Puente; Carlos Sánchez; and Martina Villarreal.
Conflicts of Interest
None of the authors have any conflicts of interest to disclose.
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef]
- CDC Morbidity and Mortality Weekly Report (MMWR) [Internet]. 2021. Available online: https://www.cdc.gov/mmwr/index.html (accessed on 7 June 2024).
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Lee, J.; Woodruff, M.C.; Kim, E.H.; Nam, J.-H. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 2023, 55, 1305–1313. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Casella, C.R. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr. Opin. Immunol. 2017, 47, 17–25. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P.; et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Ciccimarra, F.; Luxi, N.; Bellitto, C.; L’Abbate, L.; Raethke, M.; van Hunsel, F.; Lieber, T.; Mulder, E.; Riefolo, F.; Dureau-Pournin, C.; et al. Safety Monitoring of COVID-19 Vaccines in Persons with Prior SARS-CoV-2 Infection: A European Multi-Country Study. Vaccines 2024, 12, 241. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Debes, A.K.; Xiao, S.; Colantuoni, E.; Egbert, E.R.; Caturegli, P.; Gadala, A.; Milstone, A.M. Association of Vaccine Type and Prior SARS-CoV-2 Infection with Symptoms and Antibody Measurements Following Vaccination among Health CareWorkers. JAMA Intern. Med. 2021, 181, 1660–1662. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.H.; Chung, J.W.; Hwang, M.-H.; Kim, M.-C. Systemic Adverse Events and Use of Antipyretics Predict the Neutralizing Antibody Positivity Early after the First Dose of ChAdOx1 Coronavirus Disease Vaccine. J. Clin. Med. 2021, 10, 2844. [Google Scholar] [CrossRef]
- Lee, S.W.; Moon, J.Y.; Lee, S.K.; Lee, H.; Moon, S.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Kim, T.-H.; et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021, 12, 779212. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S.M.; Lim, S.; Shin, K.-H.; Kim, T.; Kim, W.-J.; Yun, M.; Oh, S.-H. Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle. Vaccines 2021, 9, 1473. [Google Scholar] [CrossRef]
- Son, S.; Lee, K. Development of mRNA Vaccines/Therapeutics and Their Delivery System. Mol. Cells 2023, 46, 41–47. [Google Scholar] [CrossRef]
- Sáez-Peñataro, J.; Torres, F.; Bartra, J.; Bascuas, J.; Vilella, A.; Tortajada, M.; Quesada, S.; González, E.; López-Suñé, E.; Castells, C.; et al. Tolerability and Reactogenicity Profile of mRNA SARS-Cov-2 Vaccines from a Mass Vaccination Campaign in a Tertiary Hospital: Between-Vaccine and Between-Population Prospective Observational Study (VigilVacCOVID Study). BioDrugs 2022, 36, 509–520. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Akashi, Y.; Horie, M.; Kiyotaki, J.; Takeuchi, Y.; Togashi, K.; Adachi, Y.; Ueda, A.; Notake, S.; Nakamura, K.; Terada, N.; et al. Clinical Performance of the cobas Liat SARS-CoV-2 & Influenza A/B Assay in Nasal Samples. Mol. Diagn. Ther. 2022, 26, 323–331. [Google Scholar]
- Florin, L.; Maelegheer, K.; Vandewal, W.; Bernard, D.; Robbrecht, J. Performance Evaluation of the Siemens SARS-CoV-2 Total Antibody and IgG Antibody Test. Lab. Med. 2021, 52, e147–e153. [Google Scholar] [CrossRef]
- Egri, N.; Olivé, V.; Hernández-Rodríguez, J.; Castro, P.; De Guzman, C.; Heredia, L.; Segura, A.C.; Fernandez, M.D.; de Moner, N.; Torradeflot, M.; et al. CoVITEST: A Fast and Reliable Method to Monitor Anti-SARS-CoV-2 Specific T Cells From Whole Blood. Front. Immunol. 2022, 13, 848586. [Google Scholar] [CrossRef]
- Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M.; Kyritsi, M.A.; Peristeri, A.-M.; Avakian, I.; et al. Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines 2022, 10, 316. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukunaga, A.; Tanaka, A.; Takeuchi, J.S.; Inoue, Y.; Kimura, M.; Maeda, K.; Ueda, G.; Mizoue, T.; Ujiie, M.; et al. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine 2022, 40, 1924–1927. [Google Scholar] [CrossRef]
- Rechavi, Y.; Shashar, M.; Lellouche, J.; Yana, M.; Yakubovich, D.; Sharon, N. Occurrence of BNT162b2 Vaccine Adverse Reactions Is Associated with Enhanced SARS-CoV-2 IgG Antibody Response. Vaccines 2021, 9, 977. [Google Scholar] [CrossRef]
- Oyebanji, O.A.; Wilson, B.; Keresztesy, D.; Carias, L.; Wilk, D.; Payne, M.; Aung, H.; St Denis, K.; Lam, E.C.; Rowley, C.F.; et al. Does a lack of vaccine side effects correlate with reduced BNT162b2 mRNA vaccine response among healthcare workers and nursing home residents? Aging Clin. Exp. Res. 2021, 33, 3151–3160. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kadowaki, T.; Matsuo, R.; Sasaki, A.; Miyaji, C.; Higuchi, C.; Nakayama, M.; Sakurada, Y.; Hagiya, H.; Takao, S.; et al. Association Between Fever and Antibody Titer Trends After a Third Dose of the mRNA-1273 Vaccine. J. Epidemiol. 2022, 32, 567–569. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, J.Y.; Park, S.; Kwon, J.-S.; Park, J.Y.; Cha, H.H.; Suh, M.H.; Lee, H.J.; Lim, J.S.; Bae, S.; et al. Correlation between Reactogenicity and Immunogenicity after the ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccination. Immune Netw. 2021, 21, e41. [Google Scholar] [CrossRef]
- Takeuchi, M.; Higa, Y.; Esaki, A.; Nabeshima, Y.; Nakazono, A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLoS ONE 2021, 16, e0257668. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Song, K.H.; Choi, Y.; Go, S.; Choi, S.-J.; Jung, J.; Kang, C.K.; Choe, P.G.; Kim, N.-J.; Park, W.B.; et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021, 36, 1486–1491. [Google Scholar] [CrossRef]
- Held, J.; Esse, J.; Tascilar, K.; Steininger, P.; Schober, K.; Irrgang, P.; Alsalameh, R.; Tenbusch, M.; Seggewies, C.; Bogdan, C. Reactogenicity Correlates Only Weakly with Humoral Immunogenicity after COVID-19 Vaccination with BNT162b2 mRNA (Comirnaty®). Vaccines 2021, 9, 1063. [Google Scholar] [CrossRef]
- Bauernfeind, S.; Salzberger, B.; Hitzenbichler, F.; Scigala, K.; Einhauser, S.; Wagner, R.; Gessner, A.; Koestler, J.; Peterhoff, D. Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines 2021, 9, 1089. [Google Scholar] [CrossRef]
- Egri, N.; Juan, M. Immunology in COVID-19; more than diagnosis of infection or the basis of vaccination. Med. Clin. (Barc.) 2022, 158, 324–326. [Google Scholar] [CrossRef]
- European Medicines Agency. Safety of COVID-19 Vaccines. 2023. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines/safety-covid-19-vaccines (accessed on 7 June 2024).
- Pharmacovigilance Risk Assessment Committee (PRAC) Meeting Highlights. 3–6 May 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-3-6-may-2021 (accessed on 7 June 2024).
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Pharmacovigilance Risk Assessment Committee (PRAC) Meeting Highlights, 29 November–2 Deacember 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-29-november-2-december-2021 (accessed on 7 June 2024).
- Elizalde, M.U.; Eguinoa, F.J.G.; de Las Huertas, A.G.L.; Jiménez-González, M.; Ramírez, E. Myocarditis and pericarditis risk with mRNA COVID-19 vaccination compared to unvaccinated individuals: A retrospective cohort study in a Spanish Tertiary Hospital. Biomed. Pharmacother. 2024, 171, 116181. [Google Scholar] [CrossRef]
- Bouchlarhem, A.; Boulouiz, S.; Bazid, Z.; Ismaili, N.; El ouafi, N. Is There a Causal Link Between Acute Myocarditis and COVID-19 Vaccination: An Umbrella Review of Published Systematic Reviews and Meta-Analyses. Clin. Med. Insights Cardiol. 2024, 18, 11795468231221406. [Google Scholar] [CrossRef]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID-19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [CrossRef]
- Jeet Kaur, R.; Dutta, S.; Charan, J.; Bhardwaj, P.; Tandon, A.; Yadav, D.; Islam, S.; Haque, M. Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. Int. J. Gen. Med. 2021, 14, 3909–3927. [Google Scholar] [CrossRef]
- Sun, C.L.F.; Jaffe, E.; Levi, R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci. Rep. 2022, 12, 6978, Erratum in Sci Rep. 2023, 13, 13276. [Google Scholar] [CrossRef]
- Chang, Y.; Lv, G.; Liu, C.; Huang, E.; Luo, B. Cardiovascular safety of COVID-19 vaccines in real-world studies: A systematic review and meta-analysis. Expert Rev. Vaccines 2023, 22, 25–34. [Google Scholar] [CrossRef]
- Ho, J.S.Y.; Sia, C.H.; Ngiam, J.N.; Loh, P.H.; Chew, N.W.S.; Kong, W.K.-F.; Poh, K.-K. A review of COVID-19 vaccination and the reported cardiac manifestations. Singapore Med. J. 2023, 64, 543–549. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Urdaneta, V.; Esposito, D.B.; Dharia, P.; Moraga, M.S.; Anteyi, K.; Oduyebo-Omotosho, T.; Rossi, M.; Burton, P.; Vega, J.M.; Dawson, R.; et al. Global Safety Assessment of Adverse Events of Special Interest Following 2 Years of Use and 772 Million Administered Doses of mRNA-1273. Open Forum Infect Dis. 2024, 11, ofae067. [Google Scholar] [CrossRef]
- Chen, C.Y.; Su, T.C. Benefits and Harms of COVID-19 Vaccines in Cardiovascular Disease: A Comprehensive Review. J. Lipid Atheroscler. 2023, 12, 119–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, W.; Wen, X.; Cong, X.; Jiang, W. COVID-19 mRNA vaccine, but not a viral vector-based vaccine, promotes neutralizing anti-type I interferon autoantibody production in a small group of healthy individuals. J. Med. Virol. 2023, 95, e29137. [Google Scholar] [CrossRef]
- Ning, W.; Xu, W.; Cong, X.; Fan, H.; Gilkeson, G.; Wu, X.; Hughes, H.; Jiang, W. COVID-19 mRNA vaccine BNT162b2 induces autoantibodies against type I interferons in a healthy woman. J. Autoimmun. 2022, 132, 102896. [Google Scholar] [CrossRef]
- Bastard, P.; Michailidis, E.; Hoffmann, H.H.; Chbihi, M.; Voyer, T.L.; Rosain, J.; Philippot, Q.; Seeleuthner, Y.; Gervais, A.; Materna, M.; et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 2021, 218, e20202486. [Google Scholar] [CrossRef]
- Wolff, A.S.B.; Hansen, L.; Grytaas, M.A.; Oftedal, B.E.; Breivik, L.; Zhou, F.; Hufthammer, K.O.; Sjøgren, T.; Olofsson, J.S.; Trieu, M.C.; et al. Vaccination prevents severe COVID-19 outcome in patients with neutralizing type 1 interferon autoantibodies. iScience 2023, 26, 107084. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Cavalli, M.; Cederholm, A.; Aranda-Guillén, M.; Behere, A.; Mildner, H.; Lakshmikanth, T.; Gonzalez, L.; Mugabo, C.H.; Johnsson, A.; et al. No link between type I interferon autoantibody positivity and adverse reactions to COVID-19 vaccines. NPJ Vaccines 2024, 9, 42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).