Protective Efficacy of a Novel DNA Vaccine with a CL264 Molecular Adjuvant against Toxoplasma gondii in a Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse and Parasite
2.2. Construction of Recombinant pEGFP-TgIST
2.3. Expression of TgIST In Vitro
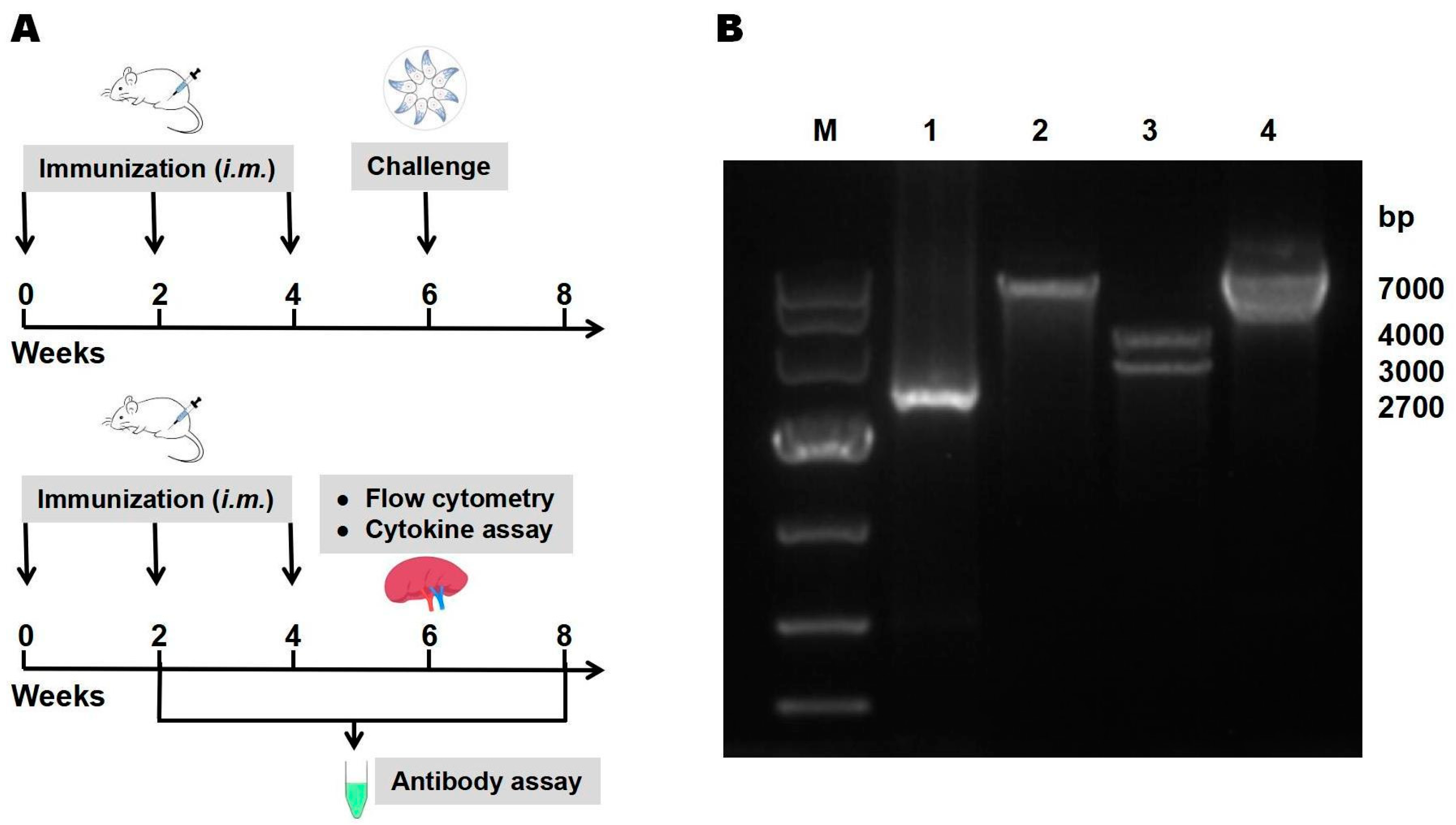
2.4. Immunization and Challenge
2.5. IgG Antibody Assay
2.6. Flow Cytometry
2.7. Cytokine Assays
2.8. Statistical Analysis
3. Results
3.1. Identification of Recombinant pEGFP-TgIST
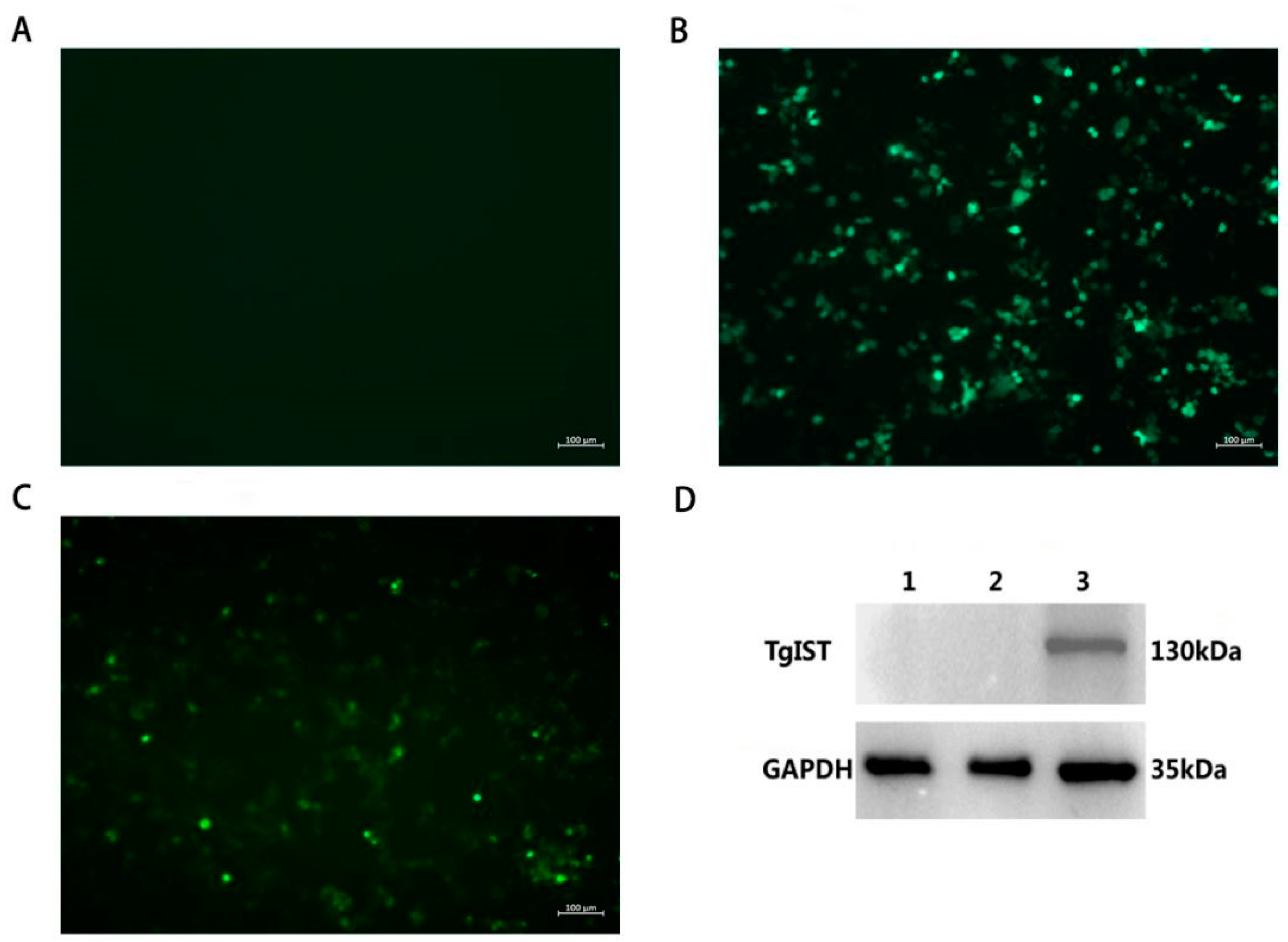
3.2. Identification of pEGFP-TgIST Expression In Vitro
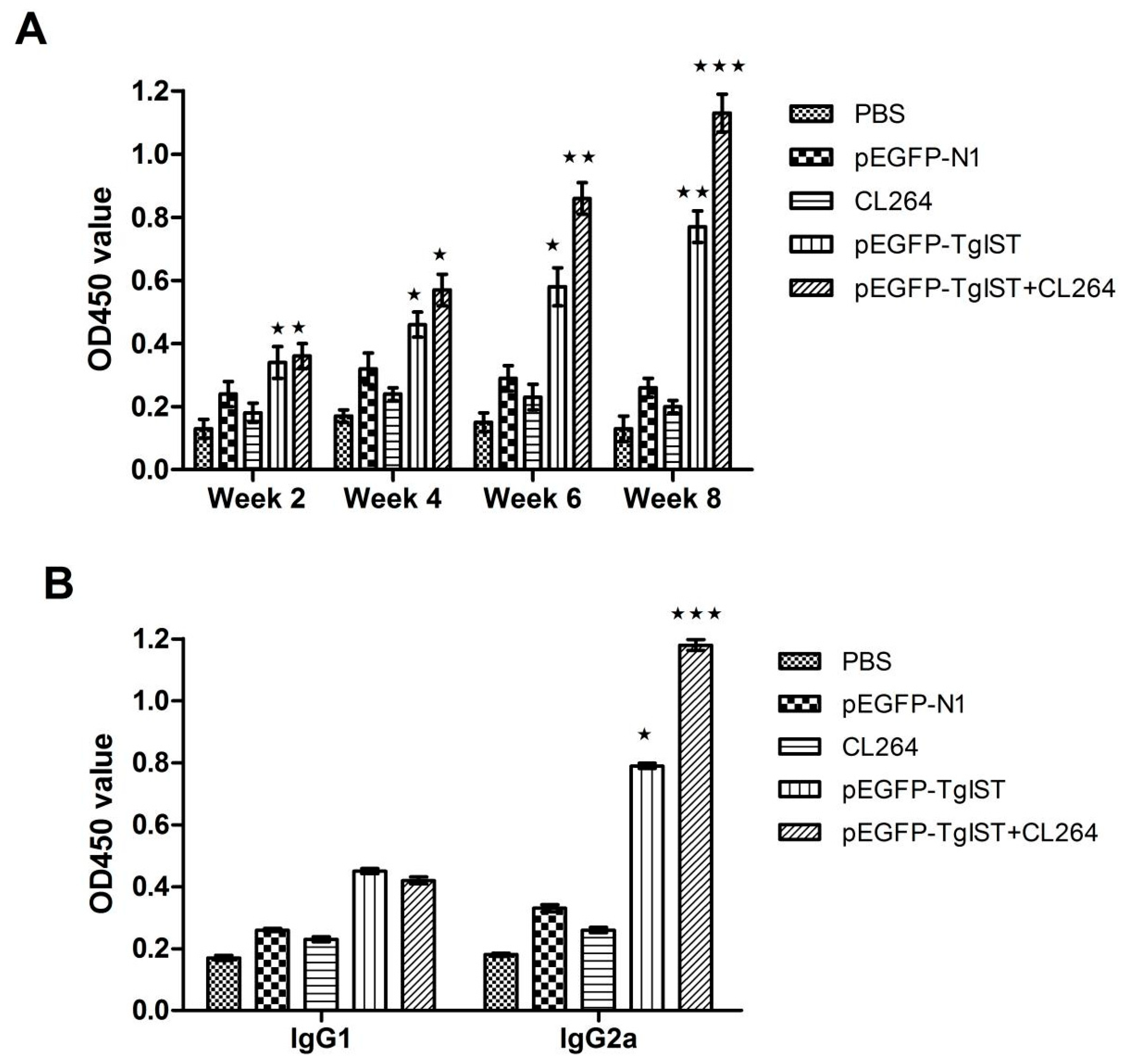
3.3. Detection of IgG and Subtype IgG1, IgG2a
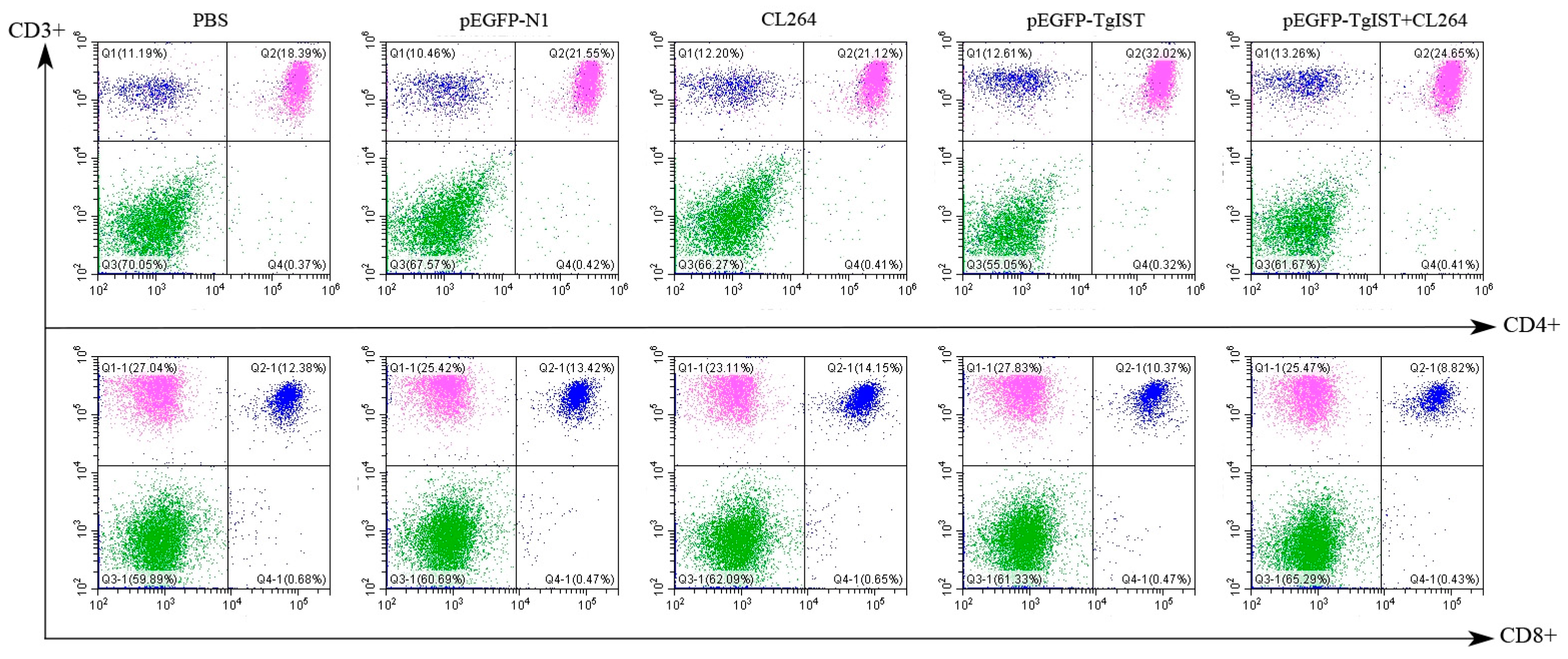
3.4. Flow Cytometry Analysis of CD4+ and CD8+ T Cell Levels
3.5. Detection of Cytokines (IL-4, IL-10 and IFN-γ)
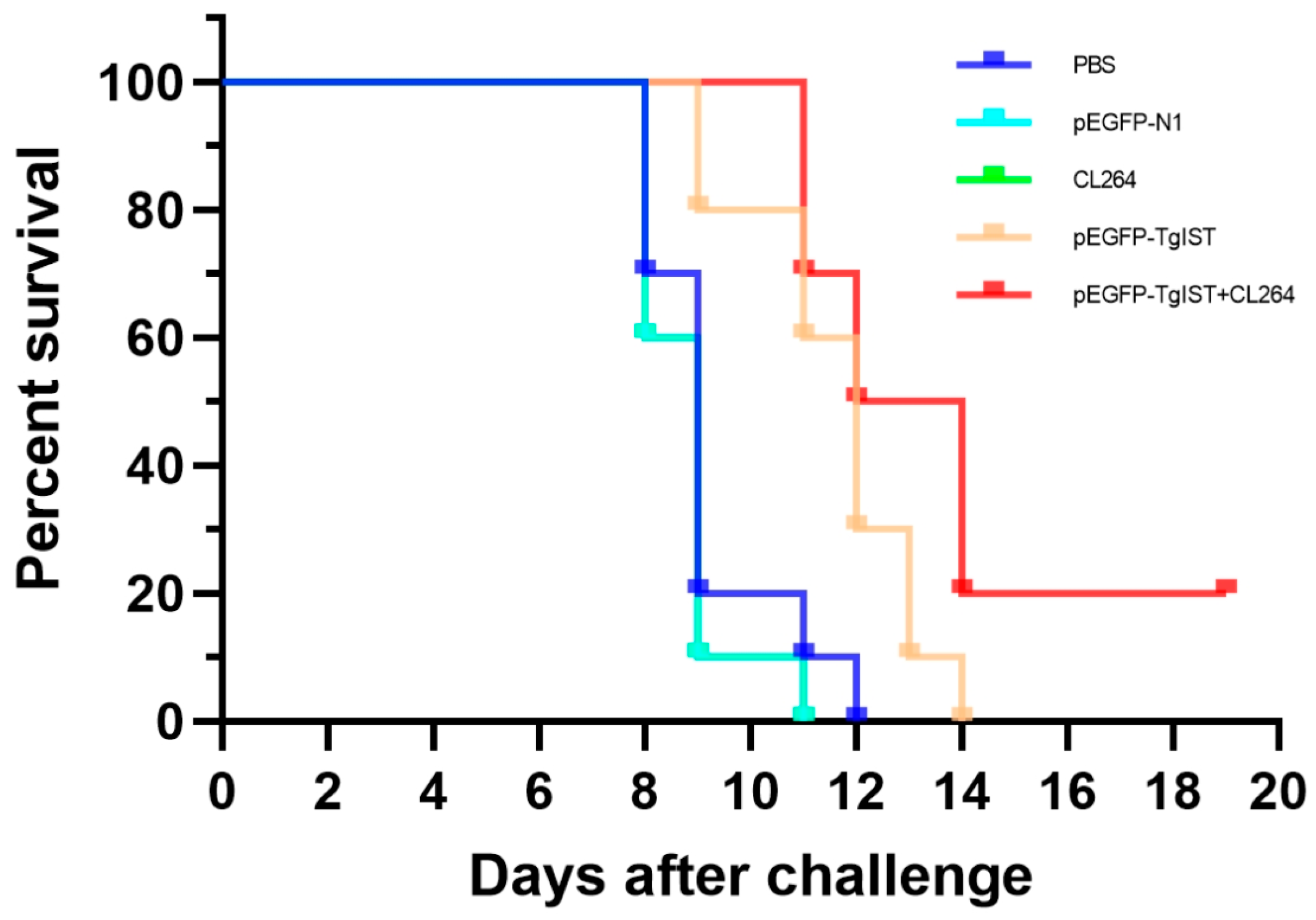
3.6. Protective Efficacy Elicited by pEGFP-TgIST Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaji, R.Y.; Sharp, A.K.; Brown, A.M. Protein kinases in Toxoplasma gondii. Int. J. Parasitol. 2021, 51, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.K.; Rinkenberger, N.; Dunay, I.R.; Sibley, L.D. Toxoplasma gondii infection and its implications within the central nervous system. Nat. Rev. Microbiol. 2021, 19, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Arias, J.C.; Gómez-Marin, J.E.; Bobić, B.; Naranjo-Galvis, C.A.; Djurković-Djaković, O. Toxoplasmosis as a travel risk. Travel. Med. Infect. Dis. 2014, 12 Pt A, 592–601. [Google Scholar] [CrossRef]
- Karakavuk, M.; Can, H.; Gül, A.; Döşkaya, A.D.; Alak, S.E.; Ün, C.; Gürüz, A.Y.; Döşkaya, M. GRA8 DNA vaccine formulations protect against chronic toxoplasmosis. Microb. Pathog. 2021, 158, 105016. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.C.; Huang, J.; Fu, Y.; Hao, L.L.; Liu, X.; Shi, T.Y. Enhancing Immune Responses to a DNA Vaccine Encoding Toxoplasma gondii GRA7 Using Calcium Phosphate Nanoparticles as an Adjuvant. Front. Cell. Infect. Microbiol. 2021, 11, 787635. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Eza, D.E.; Lucas, S.B. Fulminant toxoplasmosis causing fatal pneumonitis and myocarditis. HIV Med. 2006, 7, 415–420. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, N.Z.; Li, T.T.; He, J.J.; Elsheikha, H.M.; Zhu, X.Q. Advances in the Development of Anti-Toxoplasma gondii Vaccines: Challenges, Opportunities, and Perspectives. Trends Parasitol. 2019, 35, 239–253. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Huang, S.Y.; Xu, Y.; Chen, J.; Wang, J.L.; Tian, W.P.; Zhu, X.Q. Evaluation of immune responses in mice after DNA immunization with putative Toxoplasma gondii calcium-dependent protein kinase 5. Clin. Vaccine Immunol. CVI 2014, 21, 924–929. [Google Scholar] [CrossRef]
- Zhu, Y.C.; He, Y.; Liu, J.F.; Chen, J. Adjuvantic cytokine IL-33 improves the protective immunity of cocktailed DNA vaccine of ROP5 and ROP18 against toxoplasma gondii infection in mice. Parasite 2020, 27, 26. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, Y.; Cao, W.; Aleem, M.T.; Liu, J.; Luo, J.; Yan, R.; Xu, L.; Song, X.; Li, X. Nano DNA Vaccine Encoding Toxoplasma gondii Histone Deacetylase SIR2 Enhanced Protective Immunity in Mice. Pharmaceutics 2021, 13, 1582. [Google Scholar] [CrossRef] [PubMed]
- Gay, G.; Braun, L.; Brenier-Pinchart, M.P.; Vollaire, J.; Josserand, V.; Bertini, R.L.; Varesano, A.; Touquet, B.; De Bock, P.J.; Coute, Y.; et al. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-γ-mediated host defenses. J. Exp. Med. 2016, 213, 1779–1798. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, H.; Nix, J.; Xu, R.; Knoverek, C.R.; Bowman, G.R.; Amarasinghe, G.K.; Sibley, L.D. The intrinsically disordered protein TgIST from Toxoplasma gondii inhibits STAT1 signaling by blocking cofactor recruitment. Nat. Commun. 2022, 13, 4047. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.E.; Lavelle, E.C. Trends in vaccine adjuvants. Expert. Rev. Vaccines 2011, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.O.; Wise, M.C.; Walters, J.N.; Reuschel, E.L.; Choi, M.J.; Obeng-Adjei, N.; Yan, J.; Morrow, M.P.; Weiner, D.B. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014, 74, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Tran, T.T.P.; Truong, D.H.; Nguyen, H.T.; Pham, T.T.; Yong, C.S.; Kim, J.O. Toll-like receptor-targeted particles: A paradigm to manipulate the tumor microenvironment for cancer immunotherapy. Acta Biomater. 2019, 94, 82–96. [Google Scholar] [CrossRef]
- Butterfield, J.S.S.; Biswas, M.; Shirley, J.L.; Kumar, S.R.P.; Sherman, A.; Terhorst, C.; Ling, C.; Herzog, R.W. TLR9-Activating CpG-B ODN but Not TLR7 Agonists Triggers Antibody Formation to Factor IX in Muscle Gene Transfer. Hum. Gene Ther. Methods 2019, 30, 81–92. [Google Scholar] [CrossRef]
- Hilbert, T.; Steinhagen, F.; Weisheit, C.; Baumgarten, G.; Hoeft, A.; Klaschik, S. Synergistic Stimulation with Different TLR7 Ligands Modulates Gene Expression Patterns in the Human Plasmacytoid Dendritic Cell Line CAL-1. Mediat. Inflamm. 2015, 2015, 948540. [Google Scholar] [CrossRef]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef]
- Laiño, J.; Wangorsch, A.; Blanco, F.; Wolfheimer, S.; Krause, M.; Flaczyk, A.; Möller, T.M.; Tsai, M.; Galli, S.; Vieths, S.; et al. Targeting of Immune Cells by Dual TLR2/7 Ligands Suppresses Features of Allergic Th2 Immune Responses in Mice. J. Immunol. Res. 2017, 2017, 7983217. [Google Scholar] [CrossRef]
- Stanley, A.C.; Buxton, D.; Innes, E.A.; Huntley, J.F. Intranasal immunisation with Toxoplasma gondii tachyzoite antigen encapsulated into PLG microspheres induces humoral and cell-mediated immunity in sheep. Vaccine 2004, 22, 3929–3941. [Google Scholar] [CrossRef]
- Saavedra, R.; Leyva, R.; Tenorio, E.P.; Haces, M.L.; Rodríguez-Sosa, M.; Terrazas, L.I.; Hérion, P. CpG-containing ODN has a limited role in the protection against Toxoplasma gondii. Parasite Immunol. 2004, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, J.; Shamamian, P., Jr.; Weiss, L.M. In Vitro Characterization of Protein Effector Export in the Bradyzoite Stage of Toxoplasma gondii. mBio 2020, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ducournau, C.; Nguyen, T.T.; Carpentier, R.; Lantier, I.; Germon, S.; Précausta, F.; Pisella, P.J.; Leroux, H.; Van Langendonck, N.; Betbeder, D.; et al. Synthetic parasites: A successful mucosal nanoparticle vaccine against Toxoplasma congenital infection in mice. Future Microbiol. 2017, 12, 393–405. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Loré, K.; Betts, M.R.; Brenchley, J.M.; Kuruppu, J.; Khojasteh, S.; Perfetto, S.; Roederer, M.; Seder, R.A.; Koup, R.A. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 2003, 171, 4320–4328. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, L.C.; Costa, P.A.C.; Scalzo Júnior, S.R.A.; Ferreira, H.A.S.; Braga, A.C.S.; de Oliveira, L.C.; Figueiredo, M.M.; Shepherd, S.; Hamilton, A.; Queiroz-Junior, C.M.; et al. Nanoparticle-based DNA vaccine protects against SARS-CoV-2 variants in female preclinical models. Nat. Commun. 2024, 15, 590. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zhang, X.; Zhang, Z.; Ding, Y.; Wang, L.; Xu, X.; Pan, Y.; Gong, F.Y.; Jiang, L.; Kang, L.; et al. Potent immunogenicity and broad-spectrum protection potential of microneedle array patch-based COVID-19 DNA vaccine candidates encoding dimeric RBD chimera of SARS-CoV and SARS-CoV-2 variants. Emerg. Microbes Infect. 2023, 12, 2202269. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Borbone, N.; Roviello, V.; Roviello, G.N.; Oliviero, G. Summary of the Current Status of DNA Vaccination for Alzheimer Disease. Vaccines 2023, 11, 1706. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H. Moving towards improved vaccines for Toxoplasma gondii. Expert. Opin. Biol. Ther. 2018, 18, 273–280. [Google Scholar] [CrossRef]
- Derouin, F. Pathogeny and immunological control of toxoplasmosis. Braz. J. Med. Biol. Res. 1992, 25, 1163–1169. [Google Scholar] [PubMed]
- Kemball, C.C.; Pack, C.D.; Guay, H.M.; Li, Z.N.; Steinhauer, D.A.; Szomolanyi-Tsuda, E.; Lukacher, A.E. The antiviral CD8+ T cell response is differentially dependent on CD4+ T cell help over the course of persistent infection. J. Immunol. 2007, 179, 1113–1121. [Google Scholar] [CrossRef]
- Ely, K.H.; Kasper, L.H.; Khan, I.A. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J. Immunol. 1999, 162, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli, R.T.; Wysocka, M.; Hayashi, S.; Denkers, E.Y.; Hieny, S.; Caspar, P.; Trinchieri, G.; Sher, A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994, 153, 2533–2543. [Google Scholar] [CrossRef]
- Suzuki, Y.; Conley, F.K.; Remington, J.S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 1989, 143, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Aline, F.; Bout, D.; Dimier-Poisson, I. Dendritic cells as effector cells: Gamma interferon activation of murine dendritic cells triggers oxygen-dependent inhibition of Toxoplasma gondii replication. Infect. Immun. 2002, 70, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Däubener, W.; Posdziech, V.; Hadding, U.; MacKenzie, C.R. Inducible anti-parasitic effector mechanisms in human uroepithelial cells: Tryptophan degradation vs. NO production. Med. Microbiol. Immunol. 1999, 187, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Pardhasaradhi, K.; Martins, M.C.; Detrick, B.; Hooks, J.J. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect. Immun. 1996, 64, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Deckert-Schlüter, M.; Rang, A.; Weiner, D.; Huang, S.; Wiestler, O.D.; Hof, H.; Schlüter, D. Interferon-gamma receptor-deficiency renders mice highly susceptible to toxoplasmosis by decreased macrophage activation. Lab. Investig. A J. Tech. Methods Pathol. 1996, 75, 827–841. [Google Scholar]
- Gavrilescu, L.C.; Butcher, B.A.; Del Rio, L.; Taylor, G.A.; Denkers, E.Y. STAT1 is essential for antimicrobial effector function but dispensable for gamma interferon production during Toxoplasma gondii infection. Infect. Immun. 2004, 72, 1257–1264. [Google Scholar] [CrossRef]
- Lieberman, L.A.; Banica, M.; Reiner, S.L.; Hunter, C.A. STAT1 plays a critical role in the regulation of antimicrobial effector mechanisms, but not in the development of Th1-type responses during toxoplasmosis. J. Immunol. 2004, 172, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Andrabi, S.B.; Matsubara, R.; Aonuma, H.; Nagamune, K. A host cell membrane microdomain is a critical factor for organelle discharge by Toxoplasma gondii. Parasitol. Int. 2016, 65, 378–388. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Hong, L.; Zhou, C.; Chen, J. Immunization With a DNA Vaccine Encoding the Toxoplasma gondii’ s GRA39 Prolongs Survival and Reduce Brain Cyst Formation in a Murine Model. Front. Microbiol. 2021, 12, 630682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Xie, Q.; Li, P.; Nan, X.; Kong, L.; Zeng, D.; Ding, Z.; Wang, S. The Molecular Characterization and Immunity Identification of Rhoptry Protein 22 of Toxoplasma gondii as a DNA Vaccine Candidate Against Toxoplasmosis. J. Eukaryot. Microbiol. 2019, 66, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Hayashi, T.; Datta, S.K.; Takabayashi, K.; Van Uden, J.H.; Horner, A.; Corr, M.; Raz, E. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J. Immunol. 2002, 168, 4907–4913. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Chen, Q.; Zhang, J.; Li, W.; Hu, H.; Zhao, X.; Qiao, M.; Chen, D. Synthetic Polymeric Mixed Micelles Targeting Lymph Nodes Trigger Enhanced Cellular and Humoral Immune Responses. ACS Appl. Mater. Interfaces 2018, 10, 2874–2889. [Google Scholar] [CrossRef]
| Group | Administration | Immunization Protocol |
|---|---|---|
| PBS | Control | 100 μL 1 × PBS |
| pEGFP-N1 | Control | 100 μg pEGFP-N1 in 100 μL |
| CL264 | Control | 40 μg CL264 in 100 μL |
| pEGFP-TgIST | Vaccination | 100 μg pEGFP-TgIST in 100 μL |
| pEGFP-TgIST + CL264 | Vaccination | 100 μg pEGFP-TgIST plus 40 μg CL264 in 100 μL |
| Group | Cytokine Concentration (pg/mL) | ||
|---|---|---|---|
| IFN-γ | IL-4 | IL-10 | |
| PBS | 175.24 ± 22.63 | 35.58 ± 4.22 | 38.30 ± 3.12 |
| pEGFP-N1 | 189.16 ± 32.81 | 41.31 ± 4.47 | 40.08 ± 3.89 |
| CL264 | 163.82 ± 17.85 | 29.96 ± 3.01 | 36.69 ± 2.92 |
| pEGFP-TgIST | 1084.38 ± 194.23 * | 35.13 ± 3.60 | 42.72 ± 4.65 |
| PEGFP-TgIST + CL264 | 2163.14 ± 331.66 ** | 30.00 ± 3.46 | 41.92 ± 4.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, K.; Zhang, Y.; Xu, Z.; Li, L.; Zhao, X.; Zhou, H. Protective Efficacy of a Novel DNA Vaccine with a CL264 Molecular Adjuvant against Toxoplasma gondii in a Murine Model. Vaccines 2024, 12, 577. https://doi.org/10.3390/vaccines12060577
Ju K, Zhang Y, Xu Z, Li L, Zhao X, Zhou H. Protective Efficacy of a Novel DNA Vaccine with a CL264 Molecular Adjuvant against Toxoplasma gondii in a Murine Model. Vaccines. 2024; 12(6):577. https://doi.org/10.3390/vaccines12060577
Chicago/Turabian StyleJu, Kunping, Yunnan Zhang, Zhaolin Xu, Lingyu Li, Xiaoyan Zhao, and Huaiyu Zhou. 2024. "Protective Efficacy of a Novel DNA Vaccine with a CL264 Molecular Adjuvant against Toxoplasma gondii in a Murine Model" Vaccines 12, no. 6: 577. https://doi.org/10.3390/vaccines12060577
APA StyleJu, K., Zhang, Y., Xu, Z., Li, L., Zhao, X., & Zhou, H. (2024). Protective Efficacy of a Novel DNA Vaccine with a CL264 Molecular Adjuvant against Toxoplasma gondii in a Murine Model. Vaccines, 12(6), 577. https://doi.org/10.3390/vaccines12060577






