Innate and Adaptive Immune Parameters following mRNA Vaccination in Mice
Abstract
1. Introduction
2. Materials and Methods
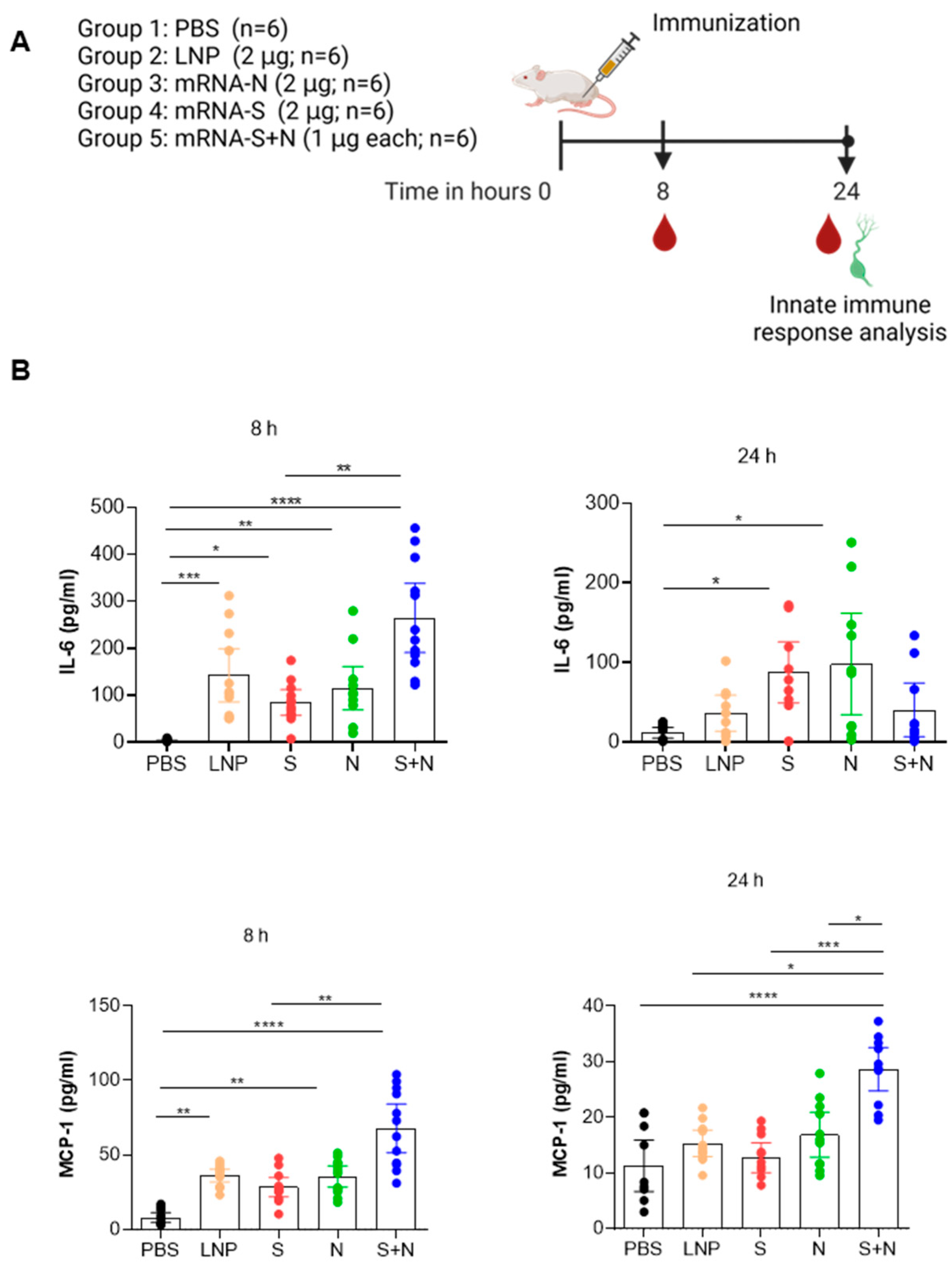
2.1. Study Design
2.2. mRNA Synthesis, LNP Formulation, and Confirmation of Protein Expression
2.3. Immunization
2.4. Cytokine/Chemokine Assay
2.5. In Vivo
2.6. Lymph Node Immunophenotyping
2.7. RNA Extraction, Library Construction, High-Throughput Sequencing, and Transcriptome Analysis
2.8. Statistical Analysis
3. Results
3.1. mRNA-LNP Vaccination in Mice Induced Innate Immune Response
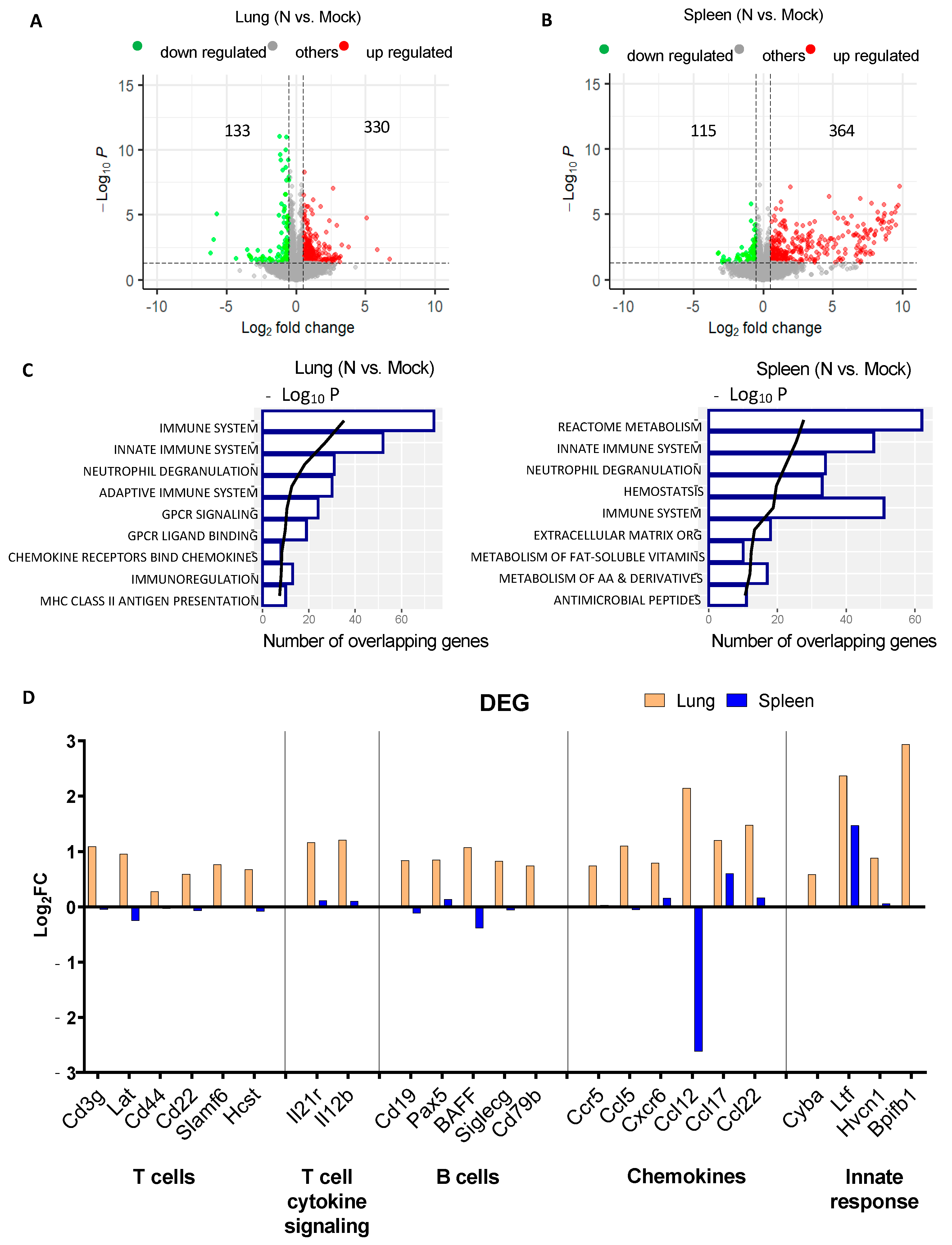
3.2. mRNA-N Vaccination Regulates both Innate and Adaptive Immune Transcripts
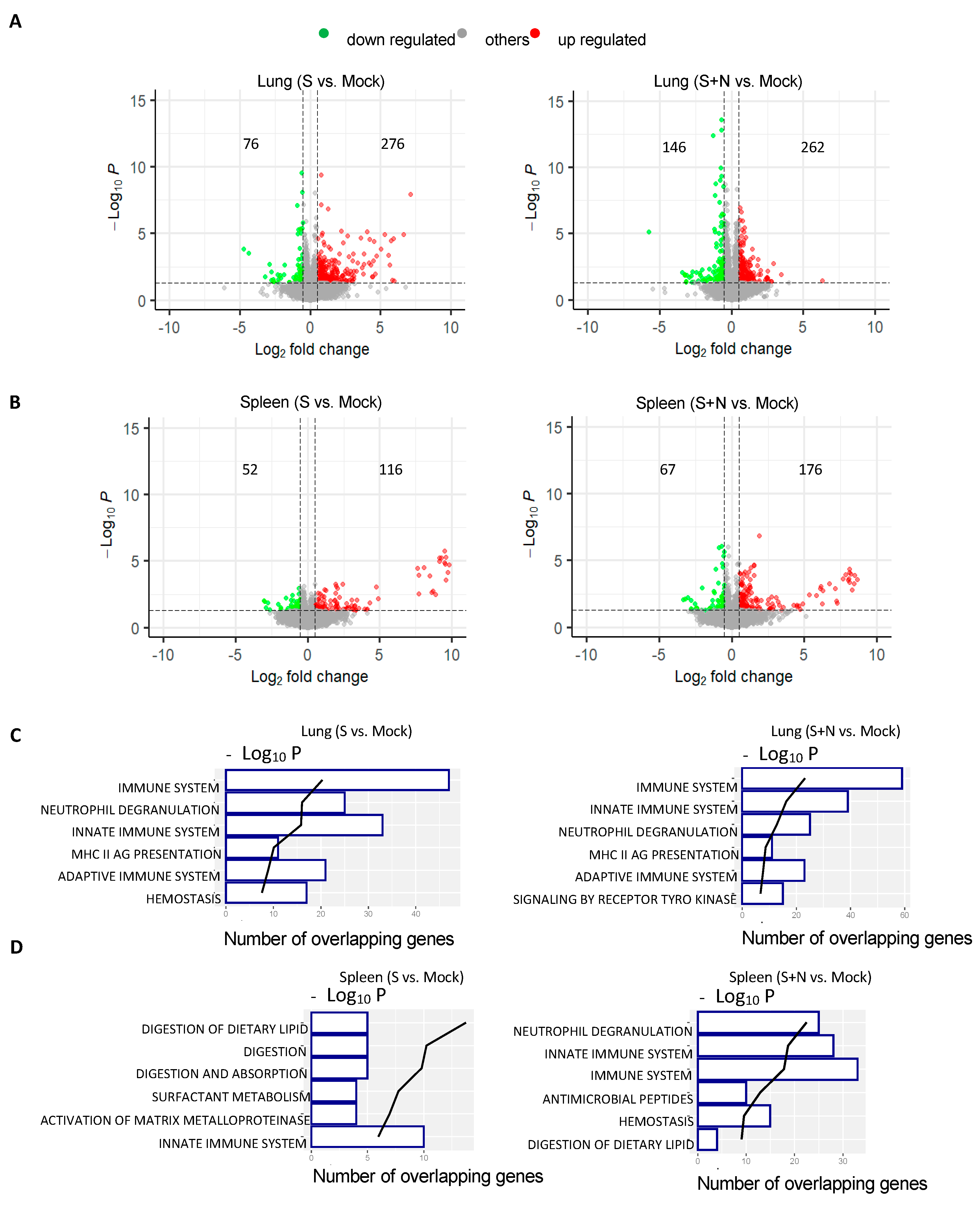
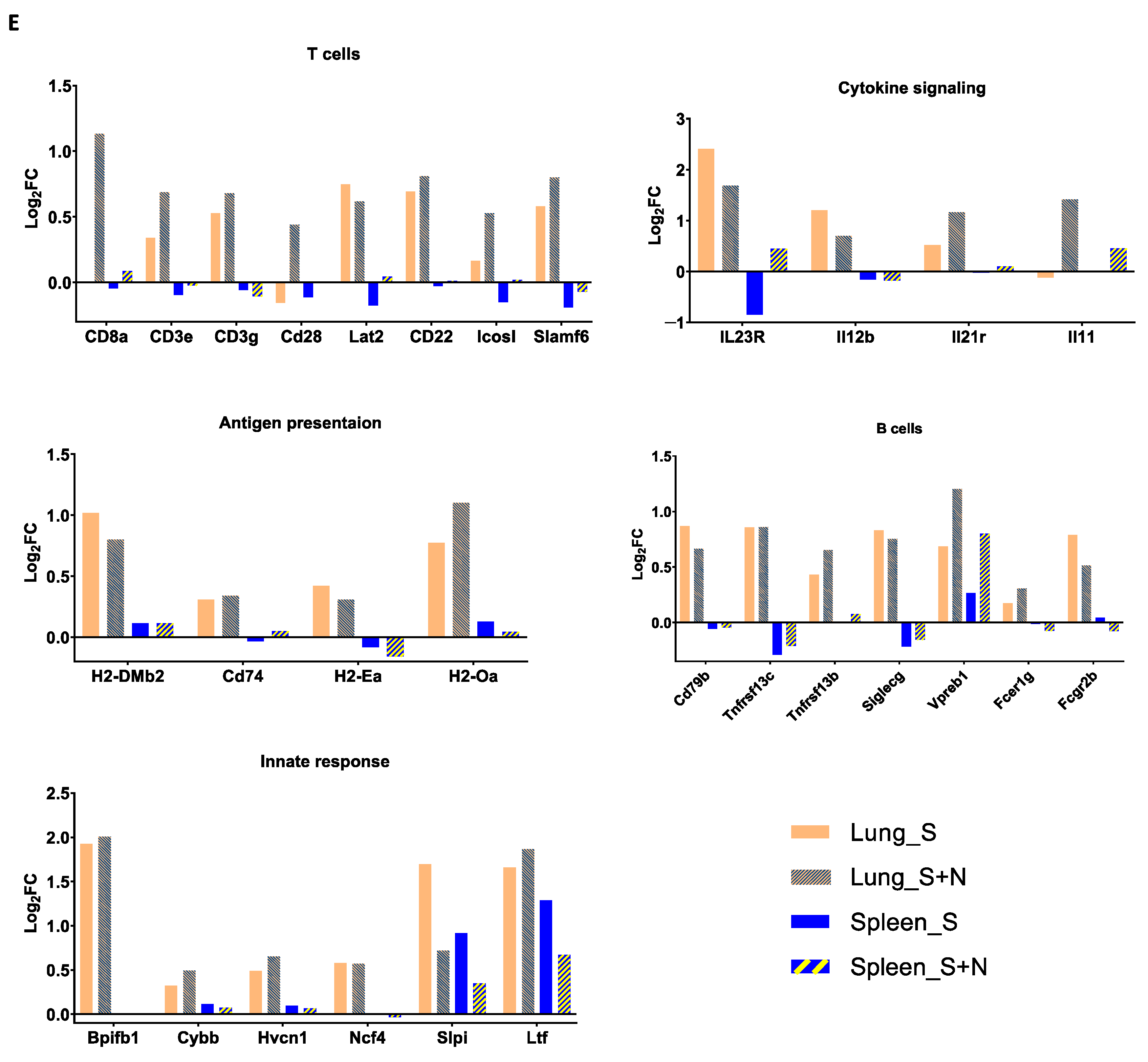
3.3. mRNA-S+N Vaccination Induces Stronger Innate and Adaptive Immune Profiles than mRNA-S
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyarto, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef] [PubMed]
- Hajnik, R.L.; Plante, J.A.; Liang, Y.; Alameh, M.G.; Tang, J.; Bonam, S.R.; Zhong, C.; Adam, A.; Scharton, D.; Rafael, G.H.; et al. Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Sci. Transl. Med. 2022, 14, eabq1945. [Google Scholar] [CrossRef]
- Saresella, M.; Piancone, F.; Marventano, I.; Hernis, A.; Trabattoni, D.; Invernizzi, M.; La Rosa, F.; Clerici, M. Innate immune responses to three doses of the BNT162b2 mRNA SARS-CoV-2 vaccine. Front. Immunol. 2022, 13, 947320. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Hu, H. Next-Generation Vaccines against COVID-19 Variants: Beyond the Spike Protein. Zoonoses 2023, 3, 18. [Google Scholar] [CrossRef]
- Connors, J.; Joyner, D.; Mege, N.J.; Cusimano, G.M.; Bell, M.R.; Marcy, J.; Taramangalam, B.; Kim, K.M.; Lin, P.J.C.; Tam, Y.K.; et al. Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals. Commun. Biol. 2023, 6, 188. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Zhivaki, D.; Gosselin, E.A.; Sengupta, D.; Concepcion, H.; Arinze, C.; Chow, J.; Nikiforov, A.; Komoroski, V.; MacFarlane, C.; Sullivan, C.; et al. mRNAs encoding self-DNA reactive cGAS enhance the immunogenicity of lipid nanoparticle vaccines. mBio 2023, 14, e02506–e02523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118. [Google Scholar] [CrossRef] [PubMed]
- Anindita, J.; Tanaka, H.; Yamakawa, T.; Sato, Y.; Matsumoto, C.; Ishizaki, K.; Oyama, T.; Suzuki, S.; Ueda, K.; Higashi, K.; et al. The Effect of Cholesterol Content on the Adjuvant Activity of Nucleic-Acid-Free Lipid Nanoparticles. Pharmaceutics 2024, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, G.; Picozza, M.; D’Orso, S.; Placido, R.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Giannessi, F.; Sambucci, M.; et al. BNT162b2 vaccination induces durable SARS-CoV-2–specific T cells with a stem cell memory phenotype. Sci. Immunol. 2021, 6, eabl5344. [Google Scholar] [CrossRef] [PubMed]
- Reinscheid, M.; Luxenburger, H.; Karl, V.; Graeser, A.; Giese, S.; Ciminski, K.; Reeg, D.B.; Oberhardt, V.; Roehlen, N.; Lang-Meli, J.; et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat. Commun. 2022, 13, 4631. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Isakova-Sivak, I.; Rudenko, L. Overview of Nucleocapsid-Targeting Vaccines against COVID-19. Vaccines 2023, 11, 1810. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.W.; Zhu, C.; Jolivet, P.; Zhu, S.X.; Lu, P.; Meaney, M.J.; West, R.B. Gene expression profiling of single cells from archival tissue with laser-capture microdissection and Smart-3SEQ. Genome Res. 2019, 29, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Föhse, K.; Geckin, B.; Zoodsma, M.; Kilic, G.; Liu, Z.; Röring, R.J.; Overheul, G.J.; van de Maat, J.; Bulut, O.; Hoogerwerf, J.J.; et al. The impact of BNT162b2 mRNA vaccine on adaptive and innate immune responses. Clin. Immunol. 2023, 255, 109762. [Google Scholar] [CrossRef]
- Wei, L.; Dong, C.; Zhu, W.; Wang, B.-Z. mRNA Vaccine Nanoplatforms and Innate Immunity. Viruses 2024, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Oyama, R.; Ishigame, H.; Tanaka, H.; Tateshita, N.; Itazawa, M.; Imai, R.; Nishiumi, N.; Kishikawa, J.-i.; Kato, T.; Anindita, J.; et al. An Ionizable Lipid Material with a Vitamin E Scaffold as an mRNA Vaccine Platform for Efficient Cytotoxic T Cell Responses. ACS Nano 2023, 17, 18758–18774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; More, K.R.; Ojha, A.; Jackson, C.B.; Quinlan, B.D.; Li, H.; He, W.; Farzan, M.; Pardi, N.; Choe, H. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. NPJ Vaccines 2023, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Meschino, S.; Dubey, S.A.; Vora, K.A.; Celano, R.; Gindy, M.; Casimiro, D.R.; Bett, A.J. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine 2016, 34, 110–119. [Google Scholar] [CrossRef]
- Filipić, B.; Pantelić, I.; Nikolić, I.; Majhen, D.; Stojić-Vukanić, Z.; Savić, S.; Krajišnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ryu, J.-H. Influenza Viruses: Innate Immunity and mRNA Vaccines. Front. Immunol. 2021, 12, 710647. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e2877. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, A.; Pouton, C.W.; Al-Wassiti, H. Delivery and Expression of mRNA in the Secondary Lymphoid Organs Drive Immune Responses to Lipid Nanoparticle-mRNA Vaccines after Intramuscular Injection. Mol. Pharm. 2023, 20, 3876–3885. [Google Scholar] [CrossRef]
- Clemente, B.; Denis, M.; Silveira, C.P.; Schiavetti, F.; Brazzoli, M.; Stranges, D. Straight to the point: Targeted mRNA-delivery to immune cells for improved vaccine design. Front. Immunol. 2023, 14, 1294929. [Google Scholar] [CrossRef]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kingstad-Bakke, B.; Cleven, T.; Bussan, H.; Yount, B.L., Jr.; Uraki, R.; Iwatsuki-Horimoto, K.; Koga, M.; Yamamoto, S.; Yotsuyanagi, H.; Park, H.; et al. Airway surveillance and lung viral control by memory T cells induced by COVID-19 mRNA vaccine. JCI Insight 2023, 8, e172510. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonam, S.R.; Hazell, N.C.; Mathew, M.J.; Liang, Y.; Zhang, X.; Wei, Z.; Alameh, M.-G.; Weissman, D.; Hu, H. Innate and Adaptive Immune Parameters following mRNA Vaccination in Mice. Vaccines 2024, 12, 543. https://doi.org/10.3390/vaccines12050543
Bonam SR, Hazell NC, Mathew MJ, Liang Y, Zhang X, Wei Z, Alameh M-G, Weissman D, Hu H. Innate and Adaptive Immune Parameters following mRNA Vaccination in Mice. Vaccines. 2024; 12(5):543. https://doi.org/10.3390/vaccines12050543
Chicago/Turabian StyleBonam, Srinivasa Reddy, Nicholas C. Hazell, Mano Joseph Mathew, Yuejin Liang, Xuxiang Zhang, Zhi Wei, Mohamad-Gabriel Alameh, Drew Weissman, and Haitao Hu. 2024. "Innate and Adaptive Immune Parameters following mRNA Vaccination in Mice" Vaccines 12, no. 5: 543. https://doi.org/10.3390/vaccines12050543
APA StyleBonam, S. R., Hazell, N. C., Mathew, M. J., Liang, Y., Zhang, X., Wei, Z., Alameh, M.-G., Weissman, D., & Hu, H. (2024). Innate and Adaptive Immune Parameters following mRNA Vaccination in Mice. Vaccines, 12(5), 543. https://doi.org/10.3390/vaccines12050543








