High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Laboratory Testing
2.3. Statistical Analysis
3. Results
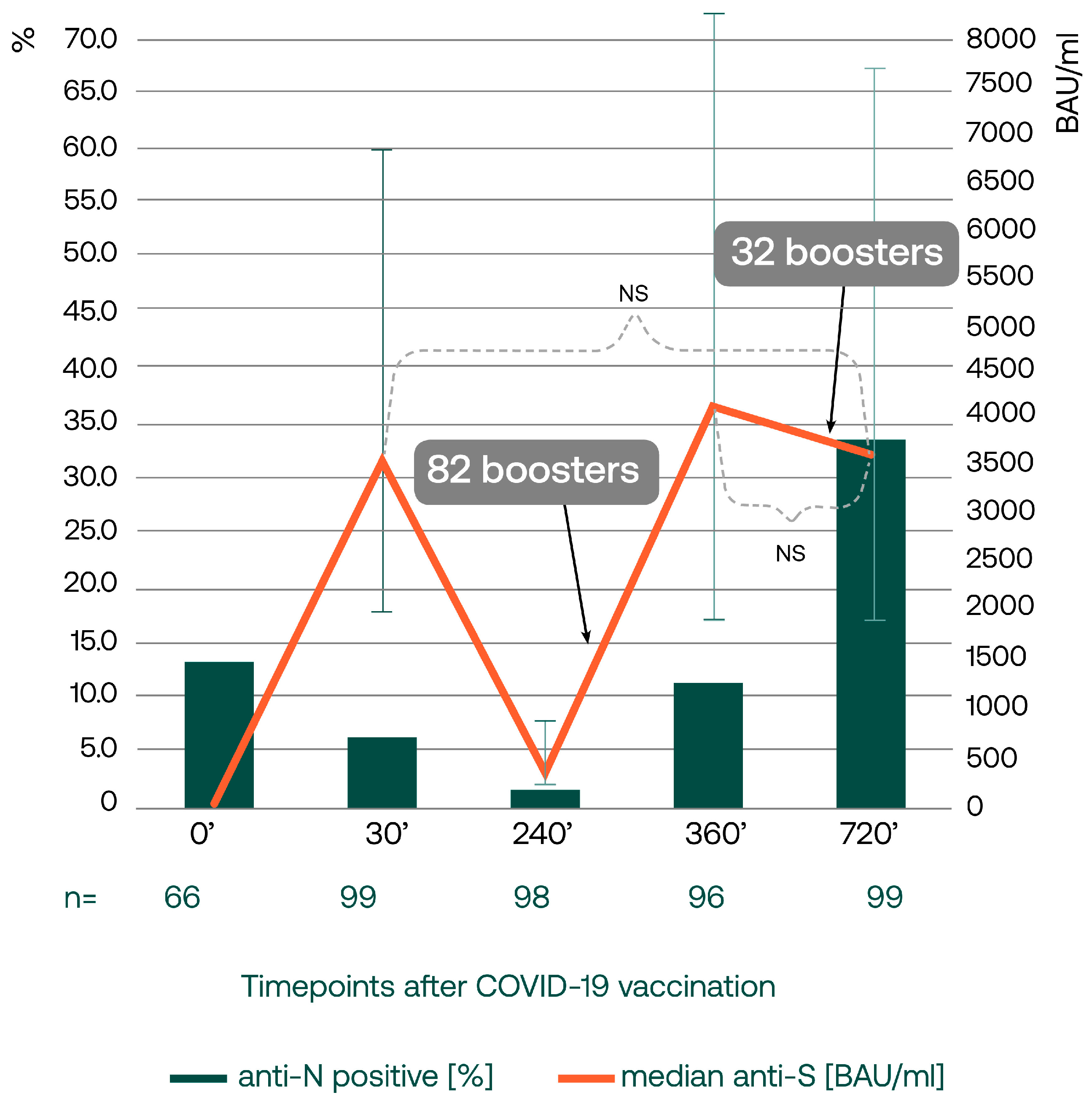
3.1. Anti-Spike and Anti-Nucleocapsid SARS-CoV-2 IgG Concentrations Two Years after the Primary Vaccination
3.2. Relationship between Anti-Nucleocapsid SARS-CoV-2 IgG and the Reported COVID-19 Incidence
3.3. Factors Influencing Anti-Spike SARS-CoV-2 IgG Two Years after Vaccination
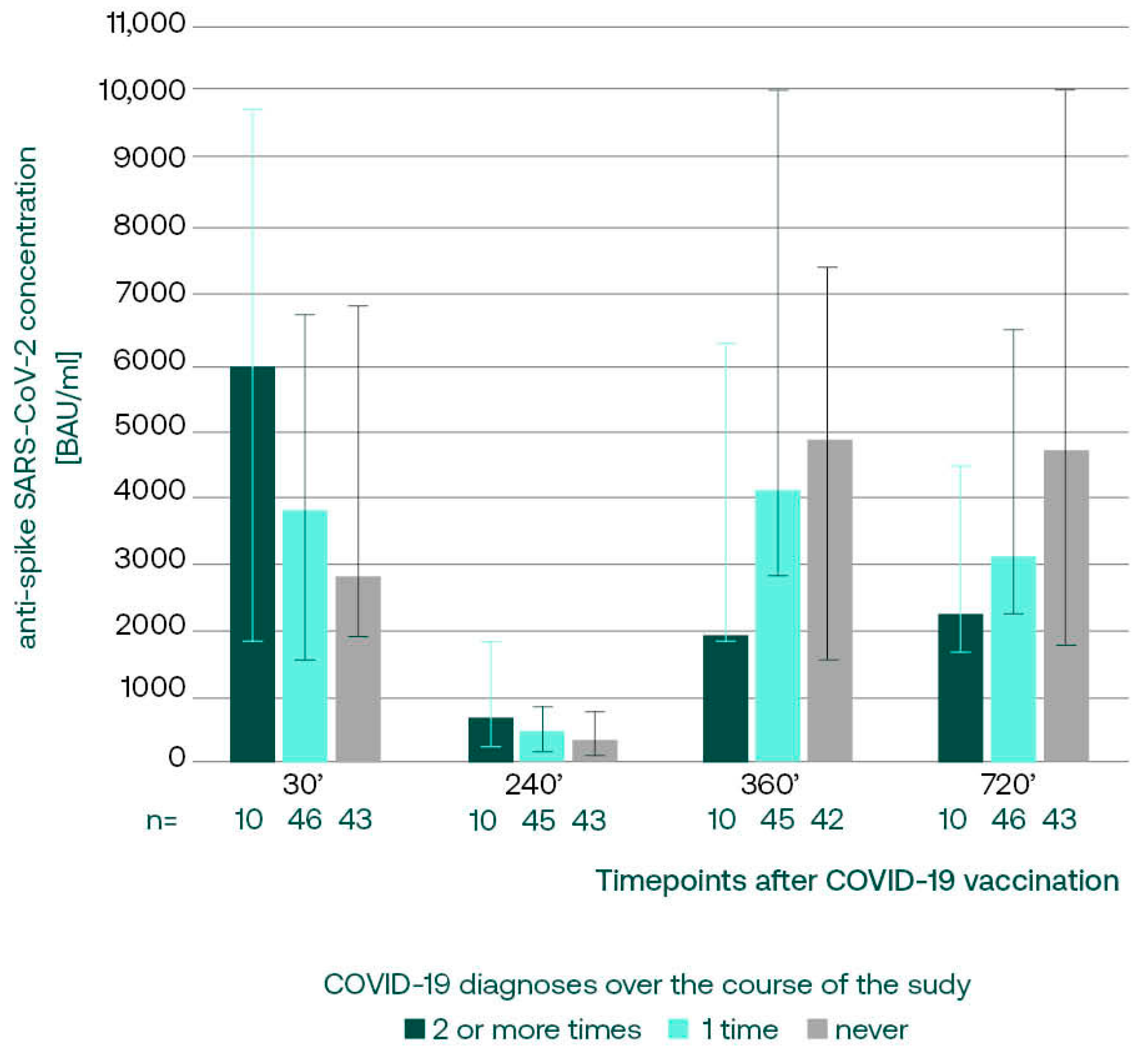
3.3.1. Anti-S SARS-CoV-2 IgG in Subgroups with Different COVID-19 Incidence
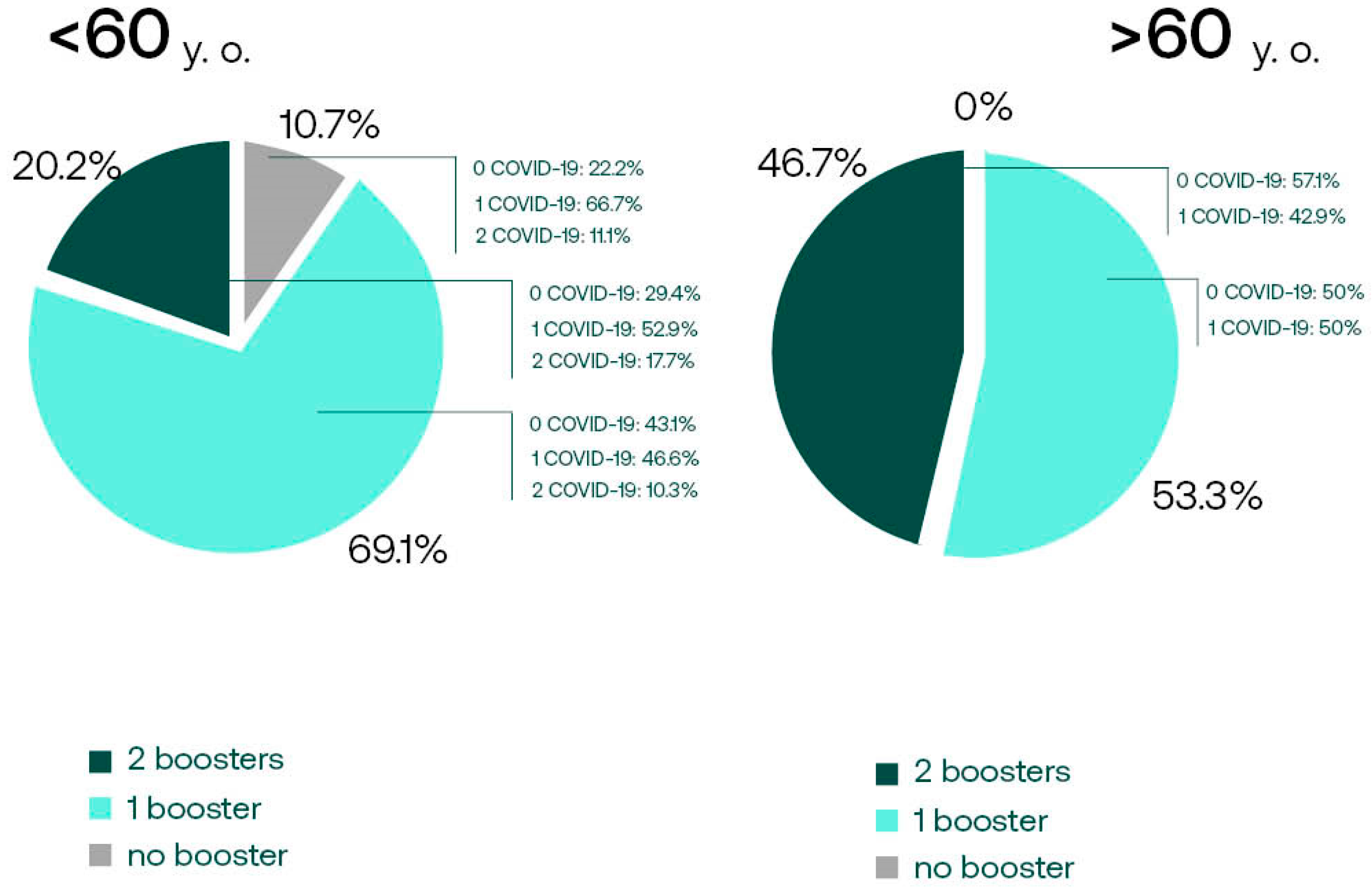
3.3.2. Anti-S SARS-CoV-2 IgG, Booster Acceptance, and COVID-19 Incidence in Age Subgroups
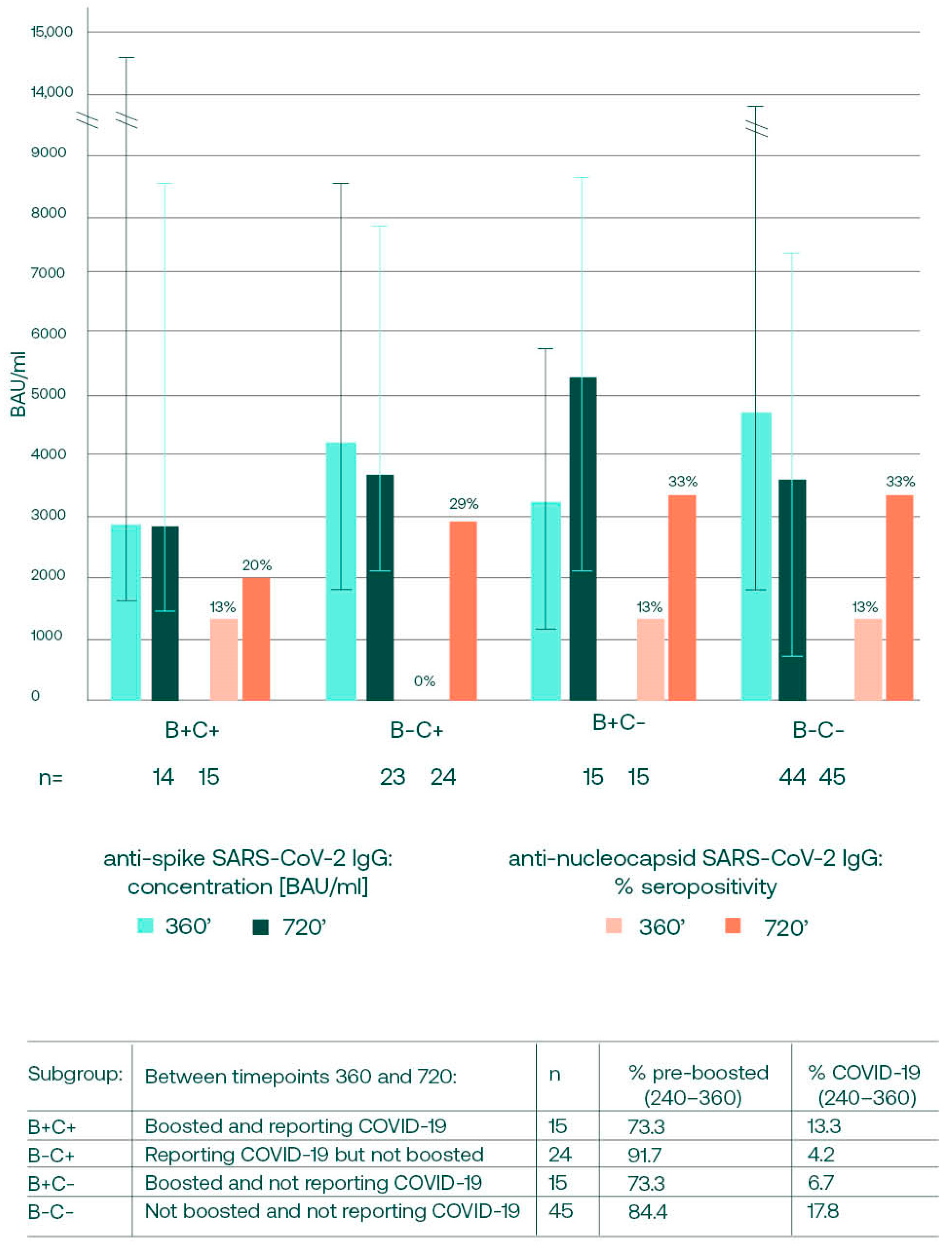
3.4. Anti-S SARS-CoV-2 Changes between Days 360 and 720, in Relation to Booster and Convalescence Status
3.5. Anti-S SARS-CoV-2 IgG Concentrations on Day 360 and the Future Infections
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO—COVID-19 Epidemiological Update—16 February 2024, Edition 164. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update-16-february-2024 (accessed on 27 February 2024).
- Coronavirus (COVID-19) Cases—Our World in Data. Available online: https://ourworldindata.org/covid-cases#confirmed-deaths-and-cases-our-data-source (accessed on 27 February 2024).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Published online at OurWorldInData.org. 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 27 February 2024).
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Influenza and Other Viruses in the Acutely Ill (IVY) Network. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brüssow, H. COVID-19: Omicron—The latest, the least virulent, but probably not the last variant of concern of SARS-CoV-2. Microb. Biotechnol. 2022, 15, 1927–1939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamed, K.; Rzymski, P.; Islam, M.S.; Makuku, R.; Mushtaq, A.; Khan, A.; Ivanovska, M.; Makka, S.A.; Hashem, F.; Marquez, L.; et al. COVID-19 vaccinations: The unknowns, challenges, and hopes. J. Med. Virol. 2022, 94, 1336–1349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- 2023 Timeline of EU Action in the Fight against Coronavirus. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/timeline-eu-action_en (accessed on 27 February 2024).
- Chivu-Economescu, M.; Vremera, T.; Ruta, S.M.; Grancea, C.; Leustean, M.; Chiriac, D.; David, A.; Matei, L.; Diaconu, C.C.; Gatea, A.; et al. Assessment of the Humoral Immune Response Following COVID-19 Vaccination in Healthcare Workers: A One Year Longitudinal Study. Biomedicines 2022, 10, 1526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, D.; Xiao, S.; Debes, A.K.; Egbert, E.R.; Caturegli, P.; Colantuoni, E.; Milstone, A.M. Durability of Antibody Levels After Vaccination With mRNA SARS-CoV-2 Vaccine in Individuals With or Without Prior Infection. JAMA 2021, 326, 2524–2526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lippi, G.; Henry, B.M.; Plebani, M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics 2021, 11, 941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swadźba, J.; Anyszek, T.; Panek, A.; Martin, E. Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination. Vaccines 2021, 9, 1367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrari, D.; Clementi, N.; Criscuolo, E.; Ambrosi, A.; Corea, F.; Di Resta, C.; Tomaiuolo, R.; Mancini, N.; Locatelli, M.; Plebani, M.; et al. Antibody Titer Kinetics and SARS-CoV-2 Infections Six Months after Administration with the BNT162b2 Vaccine. Vaccines 2021, 9, 1357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rastawicki, W.; Rokosz-Chudziak, N. Characteristics and assessment of the usefulness of serological tests in the diagnostic of infections caused by coronavirus SARS-CoV-2 on the basis of available manufacturer’s data and literature review. Prz. Epidemiol. 2020, 74, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Swadźba, J.; Kozłowska, D.; Anyszek, T.; Dorycka, M.; Martin, E.; Piotrowska-Mietelska, A. Atypical pneumonia diagnosed as coronavirus disease 2019 by a serologic test (patient-1 in Poland). Pol. Arch. Intern. Med. 2020, 130, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Swadźba, J.; Bednarczyk, M.; Anyszek, T.; Martin, E. A comparison of 7 commercial anti-SARS-CoV-2 antibody immunoassays. Arch. Med. Sci. 2020, 19, 1281–1288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swadźba, J.; Bednarczyk, M.; Anyszek, T.; Kozlowska, D.; Panek, A.; Martin, E. The real life performance of 7 automated anti-SARS-CoV-2 IgG and IgM/IgA immunoassays. Pract. Lab. Med. 2021, 25, e00212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- First WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin (Human). NIBSC Code: 20/136. Instructions for Use (Version 2.0, Dated 17 December 2020). Available online: https://www.nibsc.org/documents/ifu/20-136.pdf (accessed on 4 March 2022).
- Giavarina, D.; Carta, M. Improvements and limits of anti SARS-CoV-2 antibodies assays by WHO (NIBSC 20/136) standardization. Diagnosis 2021, 9, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Cupaiolo, R.; Wilmet, A.; Antoine-Moussiaux, T.; Della Vecchia, A.; Horeanga, A.; Papleux, E.; Vekemans, M.; Beukinga, I.; Blairon, L. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: A third dose is expected. J. Infect. 2021, 83, 559–564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carta, M.; Marinello, I.; Cappelletti, A.; Rodolfi, A.; Cerrito, E.; Bernasconi, C.; Gottardo, M.; Dal Lago, F.; Rizzetto, D.; Barzon, E.; et al. Comparison of Anti-SARS-CoV-2 S1 Receptor-Binding Domain Antibody Immunoassays in Health Care Workers Before and After the BNT162b2 mRNA Vaccine. Am. J. Clin. Pathol. 2022, 157, 212–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hibino, M.; Watanabe, S.; Kamada, R.; Tobe, S.; Maeda, K.; Horiuchi, S.; Kondo, T. Antibody Responses to the BNT162b2 mRNA Vaccine in Healthcare Workers in a General Hospital in Japan: A Comparison of Two Assays for Anti-spike Protein Immunoglobulin G. Intern. Med. 2021, 61, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Lukaszuk, K.; Kiewisz, J.; Rozanska, K.; Dabrowska, M.; Podolak, A.; Jakiel, G.; Woclawek-Potocka, I.; Lukaszuk, A.; Rabalski, L. Usefulness of IVD Kits for the Assessment of SARS-CoV-2 Antibodies to Evaluate the Humoral Response to Vaccination. Vaccines 2021, 9, 840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvagno, G.L.; Henry, B.M.; di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogrič, M.; Žigon, P.; Podovšovnik, E.; Lakota, K.; Sodin-Semrl, S.; Rotar, Ž.; Čučnik, S. Differences in SARS-CoV-2-Specific Antibody Responses After the First, Second, and Third Doses of BNT162b2 in Naïve and Previously Infected Individuals: A 1-Year Observational Study in Healthcare Professionals. Front. Immunol. 2022, 13, 876533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zurac, S.; Vladan, C.; Dinca, O.; Constantin, C.; Neagu, M. Immunogenicity evaluation after BNT162b2 booster vaccination in healthcare workers. Sci. Rep. 2022, 12, 12716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease 2019 Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Movsisyan, M.; Chopikyan, A.; Kasparova, I.; Hakobjanyan, G.; Carrat, F.; Sukiasyan, M.; Rushanyan, M.; Chalabyan, M.; Shariff, S.; Kantawala, B.; et al. Kinetics of anti-nucleocapsid IgG response in COVID-19 immunocompetent convalescent patients. Sci. Rep. 2022, 12, 12403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Yansouni, C.P.; Basta, N.E.; Desjardins, M.; Kanjilal, S.; Paquette, K.; Caya, C.; Semret, M.; Quach, C.; Libman, M.; et al. Serodiagnostics for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020, 173, 450–460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Paul Chourdhury, R.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, D.; Yang, L.; Jin, C.; Feng, J.; Cao, M.; Liu, Y. Effect of second booster vaccination on clinical outcomes of Omicron-variant breakthrough infection: A propensity score matching cohort study. Heliyon 2023, 10, e23344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marklund, E.; Leach, S.; Nyström, K.; Lundgren, A.; Liljeqvist, J.Å.; Nilsson, S.; Yilmaz, A.; Andersson, L.M.; Bemark, M.; Gisslén, M. Longitudinal Follow Up of Immune Responses to SARS-CoV-2 in Health Care Workers in Sweden With Several Different Commercial IgG-Assays, Measurement of Neutralizing Antibodies and CD4+ T-Cell Responses. Front. Immunol. 2021, 12, 750448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, N.; Jeong, S.; Lee, S.K.; Cho, E.J.; Hyun, J.; Park, M.J.; Song, W.; Kim, H.S. Quantitative Analysis of Anti-N and Anti-S Antibody Titers of SARS-CoV-2 Infection after the Third Dose of COVID-19 Vaccination. Vaccines 2022, 10, 1143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coronavirus (COVID-19) Cases—Our World in Data. Available online: https://ourworldindata.org/covid-cases (accessed on 1 December 2023).
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Y.; Esposito, D.; Kang, Z.; Lu, J.; Remaley, A.T.; De Giorgi, V.; Chen, L.N.; West, K.; Cao, L. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci. Rep. 2022, 12, 2628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Focosi, D.; Baj, A.; Maggi, F. Is a single COVID-19 vaccine dose enough in convalescents? Hum. Vaccines Immunother. 2021, 17, 2959–2961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swadźba, J.; Anyszek, T.; Panek, A.; Chojęta, A.; Wyrzykowska, K.; Martin, E. Head-to-Head Comparison of 5 Anti-SARS-CoV-2 Assays Performance in One Hundred COVID-19 Vaccinees, over an 8-Month Course. Diagnostics 2022, 12, 1426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swadźba, J.; Anyszek, T.; Panek, A.; Chojęta, A.; Piotrowska-Mietelska, A.; Martin, E. The Influence of Booster Shot and SARS-CoV-2 Infection on the Anti-Spike Antibody Concentration One Year after the First COVID-19 Vaccine Dose Administration. Vaccines 2023, 11, 278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasso, B.L.; Giglio, R.V.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.M.; Ciaccio, A.M.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.D.; Liacos, C.I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stærke, N.B.; Reekie, J.; Nielsen, H.; Benfield, T.; Wiese, L.; Knudsen, L.S.; Iversen, M.B.; Iversen, K.; Fogh, K.; Bodilsen, J.; et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat. Commun. 2022, 13, 4466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Newton, J.N.; Diamond, I.; Studley, R.; Taylor, N.; Bell, J.I.; Farrar, J.; Kolenchery, J.; et al. Protection against SARS-CoV-2 Omicron BA.4/5 variant following booster vaccination or breakthrough infection in the UK. Nat. Commun. 2023, 14, 2799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C. COVID-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021, 385, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Fiore-Gartland, A.; Johnson, M.; Hunt, A.; Bengt, C.; Zavadska, D.; Snipe, H.D.; Brown, J.S.; Workman, L.; Zar, H.J.; et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine 2022, 40, 306–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody titers and protection against a SARS-CoV-2 infection. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, E.; Sawabuchi, T.; Homma, T.; Fukuda, Y.; Sagara, H.; Kinjo, T.; Fujita, K.; Suga, S.; Kimoto, T.; Sakai, S.; et al. Clinical Utility of SARS-CoV-2 Antibody Titer Multiplied by Binding Avidity of Receptor-Binding Domain (RBD) in Monitoring Protective Immunity and Clinical Severity. Viruses 2023, 15, 1662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perry, J.; Osman, S.; Wright, J.; Richard-Greenblatt, M.; Buchan, S.A.; Sadarangani, M.; Bolotin, S. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS ONE 2022, 17, e0266852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sobhani, K.; Cheng, S.; Binder, R.A.; Mantis, N.J.; Crawford, J.M.; Okoye, N.; Braun, J.G.; Joung, S.; Wang, M.; Lozanski, G.; et al. Clinical Utility of SARS-CoV-2 Serological Testing and Defining a Correlate of Protection. Vaccines 2023, 11, 1644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Subgroups | N | Median Anti-S SARS-CoV-2 IgG [BAU/mL] 2 Years after the Vaccination | Significance | |
|---|---|---|---|---|
| Sex | Female | 85 | 3980 | NS (p = 0.4454) U Mann–Whitney test |
| Male | 14 | 3010 | ||
| Age | <60 | 84 | 3090 | p = 0.001 U Mann–Whitney test |
| >60 | 15 | 6460 | ||
| Number of vaccine doses received | 2 | 9 | 3520 | NS (p = 0.4175) Kruskal–Wallis test |
| 3 | 66 | 3140 | ||
| 4 | 24 | 4950 | ||
| Timing of the last booster | No booster | 9 | 3520 | NS (p = 0.5578) Kruskal–Wallis test |
| Before 360 | 60 | 3660 | ||
| After 360 | 30 | 3880 | ||
| COVID-19 history (self-reported) | No | 39 | 4640 | NS (p = 0.0642) Kruskal–Wallis test |
| 1 | 50 | 3090 | ||
| ≥2 | 10 | 2190 | ||
| COVID-19 history between days 360 and 720 | Yes (self-reported) | 39 | 3140 | NS (p = 0.8467) U Mann–Whitney test |
| No | 60 | 3850 | ||
| Anti-nucleocapsid seropositivity on day 720 | Anti-N positive | 30 | 3010 | NS (p = 0.8088) U Mann–Whitney test |
| Anti-N negative | 67 | 4180 | ||
| Other respiratory infections over the past 3 months | Yes | 31 | 3140 | NS (p = 1.0000) U Mann–Whitney test |
| No | 68 | 3850 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swadźba, J.; Panek, A.; Wąsowicz, P.; Anyszek, T.; Martin, E. High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus. Vaccines 2024, 12, 471. https://doi.org/10.3390/vaccines12050471
Swadźba J, Panek A, Wąsowicz P, Anyszek T, Martin E. High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus. Vaccines. 2024; 12(5):471. https://doi.org/10.3390/vaccines12050471
Chicago/Turabian StyleSwadźba, Jakub, Andrzej Panek, Paweł Wąsowicz, Tomasz Anyszek, and Emilia Martin. 2024. "High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus" Vaccines 12, no. 5: 471. https://doi.org/10.3390/vaccines12050471
APA StyleSwadźba, J., Panek, A., Wąsowicz, P., Anyszek, T., & Martin, E. (2024). High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus. Vaccines, 12(5), 471. https://doi.org/10.3390/vaccines12050471






