SARS-CoV-2 Symptoms during the Omicron Surge Differ between Boosted and Vaccinated Non-Boosted Persons
Abstract
1. Introduction
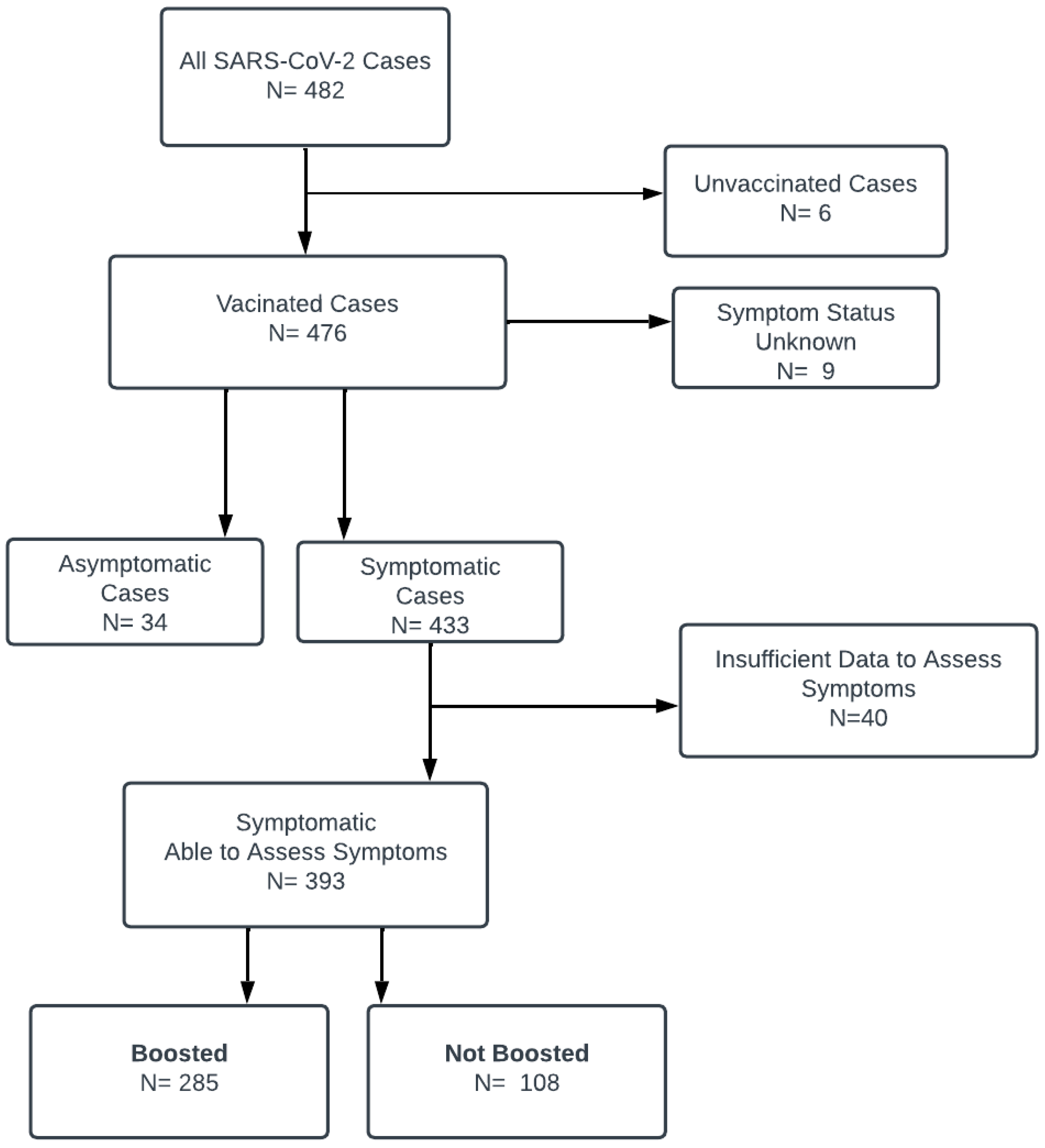
2. Methods
Statistical Methods
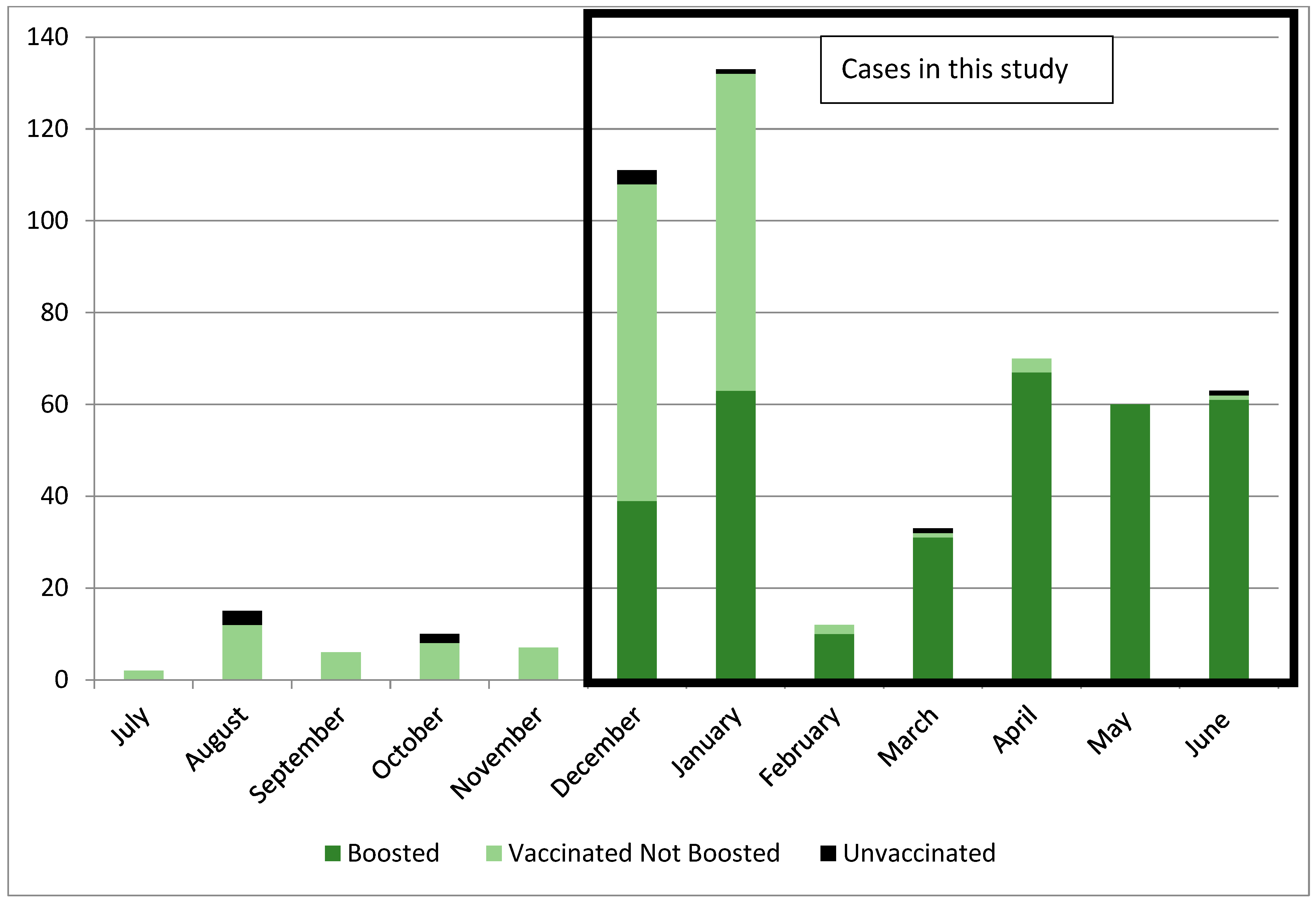
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts. Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Keehner, J.; Horton, L.E.; Binkin, N.J.; Laurent, L.C.; Pride, D.; Longhurst, C.A.; Abeles, S.R.; Torriani, F.J. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N. Engl. J. Med. 2021, 385, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. American College of Immunization Practices Meeting. 23 September 2021. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-9-23/03-COVID-Oliver.pdf (accessed on 5 October 2022).
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA 2022, 327, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, A.C.; Buchan, S.A.; Daneman, N.; Brown, K.A. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA 2022, 327, 1286–1287. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat. Med. 2022, 28, 1933–1943. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by SARS-CoV-2 Omicron and Delta variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef]
- Yoon, S.K.; Hegmann, K.T.; Thiese, M.S.; Burgess, J.L.; Ellingson, K.; Lutrick, K.; Olsho, L.E.W.; Edwards, L.J.; Sokol, B.; Caban-Martinez, A.J.; et al. Protection with a third dose of mRNA vaccine against SARS-CoV-2 variants in frontline workers. N. Engl. J. Med. 2022, 386, 1855–1857. [Google Scholar] [CrossRef]
- New York State Department of Health. Health Advisory: COVID-19 Vaccine Boosters Recommended for All Adults; New York State Department of Health: New York, NY, USA, 2021.
- New York State Department of Health. Interim Updated Isolation & Quarantine Guidance, 4 January 2022. Available online: https://coronavirus.health.ny.gov/system/files/documents/2022/01/nys_updated_isolation_quarantine_guidance_01042022.pdf (accessed on 20 December 2022).
- Centers for Disease Control and Prevention. Use of COVID-19 Vaccines in the United States, Interim Clinical Considerations. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#covid-vaccines (accessed on 1 February 2022).
- Reddy, S. Interim Clinical Considerations for Moderna and Janssen COVID-19 Vaccine Booster Doses, Presented at the American College of Immunization Practices Meeting, 21 October 2021. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/12-COVID-Reddy-508.pdf (accessed on 29 May 2023).
- Laracy, J.C.; Robilotti, E.V.; Yan, J.; Lucca, A.; Aslam, A.; Babady, N.E.; Kamboj, M. Comparison of coronavirus disease 2019 (COVID-19) symptoms at diagnosis among healthcare personnel before and after the emergence of the omicron variant. Infect. Control. Hosp. Epidemiol. 2022, 44, 821–823. [Google Scholar] [CrossRef]
- Akaishi, T.; Kushimoto, S.; Katori, Y.; Sugawara, N.; Egusa, H.; Igarashi, K.; Fujita, M.; Kure, S.; Takayama, S.; Abe, M.; et al. COVID-19 related symptoms during the SARS-CoV-2 Omicron (B.1.1.529) variant surge in Japan. Tohoku J. Exp. Med. 2022, 258, 103–110. [Google Scholar] [CrossRef]
- Marquez, C.; Kerkhoff, A.D.; Schrom, J.; Rojas, S.; Black, D.; Mitchell, A.; Wang, C.-Y.; Pilarowski, G.; Ribeiro, S.; Jones, D.; et al. COVID-19 symptoms and duration of rapid antigen test positivity at a community testing and surveillance site during pre-Delta, Delta, and Omicron BA.1 periods. JAMA Netw. Open 2022, 5, e2235844. [Google Scholar] [CrossRef]
- Vihta, K.D.; Pouwels, K.B.; Peto, T.E.A.; Pritchard, E.; House, T.; Studley, R.; Rourke, E.; Cook, D.; Diamond, I.; Crook, D.; et al. Omicron-associated changes in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) symptoms in the United Kingdom. Clin. Infect. Dis. 2023, 76, e133–e141. [Google Scholar]
- Fowle, N.; Garrett, B.; Floyd, O.L.; Collins, J.; Krasnow, A.D.; Islas, M.; Holland, S.C.; Smith, M.F.; Lim, E.S.; Jarrett, N.M.; et al. University-associated SARS-CoV-2 Omicron BA.2 infections, Maricopa County, Arizona, USA, 2022. Emerg. Infect. Dis. 2022, 28, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Bilińska, K.; von Bartheld, C. Why does the Omicron variant largely spare olfactory function? Implications for the pathogenesis of anosmia in coronavirus disease 2019. J. Infect. Dis. 2022, 226, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Hiyama, T.; Nakano, Y.; Yoshida, M.; Yoshino, A.; Miyake, Y.; Okamoto, Y. Real world effectiveness of a booster dose of the COVID-19 vaccines among Japanese university students. Vaccines 2022, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Flury, B.B.; Güsewell, S.; Egger, T.; Leal, O.; Brucher, A.; Lemmenmeier, E.; Meier Kleeb, D.; Möller, J.C.; Rieder, P.; Rütti, M.; et al. Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland- A multicenter cohort study. PLoS Med. 2022, 19, e1004125. [Google Scholar]
- The Heroes Recover Network. Association of mRNA vaccination with clinical and virologic features of COVID-19 among US essential and frontline workers. JAMA 2022, 328, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.G.; Pierson, L.; Doernberg, S. The role of medical students during the COVID-19 pandemic. Ann. Intern. Med. 2020, 173, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. COVID-19 Vaccines—Immunity, variants, boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Tubert, J.E.; Lee, G.S.; Ku, J.H.; Bruxvoort, K.J.; Talarico, C.A.; et al. Effectiveness of messenger RNA-1273 booster against coronavirus disease 2019 in immunocompetent adults. Clin. Infect. Dis. 2023, 76, 252–262. [Google Scholar] [CrossRef]
- Laake, I.; Skodvin, S.N.; Blix, K.; Caspersen, I.H.; Gjessing, H.K.; Juvet, L.K.; Magnus, P.; Mjaaland, S.; Robertson, A.H.; Starrfelt, J.; et al. Effectiveness of mRNA booster vaccination against mild, moderate and severe COVID-19 caused by Omicron variant in a large, population-based Norwegian cohort. J. Infect. Dis. 2022, 226, 1924–1933. [Google Scholar] [CrossRef]

| Symptom | Definition/Terms Used to Capture Symptom | Number (%) Cases N = 393 |
|---|---|---|
| Symptoms Specifically Requested on Symptom Log | ||
| Fever | Temp ≥ 100° Fahrenheit or “fever” recorded on log | 211 (53.6) |
| Cough | Cough, chest congestion | 327 (83.2) |
| Shortness of breath | Shortness of breath | 55 (13.9) |
| Symptoms Not Specifically Requested on Symptom Log | ||
| Nasal congestion | Nasal congestion, runny nose, stuffy nose, sneezing, post nasal drip, head congestion, head cold | 213 (54.1) |
| Sore throat/pharyngeal | Sore throat, scratchy throat, itchy throat, tingling in throat, dry throat, pharyngitis, tonsillitis, swollen tonsils | 183 (46.5) |
| Headache | Headache, migraine | 110 (27.9) |
| Fatigue | Fatigue, lethargy, tired, exhausted | 100 (25.4) |
| Body/muscle aches | Body aches, muscle aches, myalgias, achy, back pain, leg pain, joint pain | 98 (24.9) |
| Gastrointestinal | Nausea, vomiting, diarrhea, stomach upset, stomach cramping, abdominal pain | 28 (7.1) |
| Change in taste or smell | Loss of taste, loss of smell, change in taste or smell | 13 (3.3) |
| Other * | 73 (18.5) | |
| Not Boosted | Boosted | p Value | |
|---|---|---|---|
| N = 108 | N = 285 | ||
| Gender = Female, No. (%) | 64 (59.3) | 163 (57.2) | 0.733 |
| Age in years, Mean (SD) * | 31.6 (10.7) | 31.3 (12.1) | 0.813 |
| Student versus Employee Status, No. (%) | 0.032 | ||
| Student | 69 (63.9) | 214 (75.1) | |
| Employee | 39 (36.1) | 71 (24.9) | |
| Days Since Last Vaccine Until Infection, Mean (SD) | 244.9 (69.2) | 123.7 (64.4) | <0.001 |
| Primary Symptoms Present, No. (%) | |||
| Cough | 87 (80.6) | 240 (84.2) | 0.450 |
| Fever—present or temperature ≥ 100 °F | 57 (52.8) | 154 (54.0) | 0.822 |
| Nasal congestion | 48 (44.4) | 165 (57.9) | 0.018 |
| Sore throat | 44 (40.7) | 138 (48.4) | 0.176 |
| Headache | 37 (34.3) | 73 (25.6) | 0.102 |
| Body/muscle aches | 35 (32.4) | 63 (22.1) | 0.038 |
| Fatigue | 23 (21.3) | 77 (27.0) | 0.299 |
| Shortness of breath | 21 (19.4) | 34 (11.9) | 0.072 |
| Gastrointestinal symptoms | 10 (9.3) | 18 (6.3) | 0.379 |
| Change in taste or smell | 7 (6.5) | 6 (2.1) | 0.052 |
| Nasal congestion and/or sore throat | 67 (62.0) | 220 (77.2) | 0.003 |
| Number of Primary Symptoms Reported, Mean (SD) | 3.4 (1.8) | 3.4 (1.5) | 0.911 |
| Other symptoms, No. (%) | 0.427 | ||
| 0 | 95 (88.0) | 245 (86.0) | |
| 1 | 10 (9.3) | 36 (12.6) | |
| 2 | 3 (2.8) | 4 (1.4) | |
| Number of Primary Plus Other Symptoms, Mean (SD) | 3.6 (1.9) | 3.6 (1.6) | 0.943 |
| Days Symptoms Present, Mean (SD) | 7.1 (3.2) | 6.3 (2.9) | 0.014 |
| Cases before 1 January, required isolation = 10 days n = 76 | 7.5 (3.1) | 6.3 (2.5) | 0.112 |
| Cases on/after 1 January, required isolation = 5 days n = 317 | 6.8 (3.1) | 6.3 (2.8) | 0.248 |
| Days Isolated, Mean (SD) | |||
| Cases before 1 January, required isolation = 10 days n = 76 | 10.9 (1.6) | 11.1 (1.7) | 0.661 |
| Cases on/after 1 January, required isolation = 5 days n = 317 | 10.4 (2.9) | 8.4 (2.9) | < 0.001 |
| Not Boosted | Boosted | p Value | |
|---|---|---|---|
| N = 103 | N = 78 | ||
| Gender = Female, No. (%) | 60 (58.3) | 47 (60.3) | 0.879 |
| Age in years, Mean (SD) * | 31.6 (10.8) | 32.1 (12.1) | 0.778 |
| Student versus Employee Status, No. (%) | 0.421 | ||
| Student | 67 (65.0) | 56 (71.8) | |
| Employee | 36 (35.0) | 22 (28.2) | |
| Days Since Last Vaccine Until Infection, Mean (SD) | 244.9 (68.6) | 47.9 (30.6) | <0.001 |
| Primary Symptoms Present, No. (%) | |||
| Cough | 84 (81.6) | 60 (76.9) | 0.462 |
| Fever—present or temperature ≥ 100 °F | 53 (51.5) | 30 (38.5) | 0.098 |
| Nasal congestion | 46 (44.7) | 40 (51.3) | 0.453 |
| Sore throat | 43 (41.7) | 37 (47.4) | 0.455 |
| Headache | 36 (35.0) | 23 (29.5) | 0.522 |
| Body/muscle aches | 33 (32.0) | 22 (28.2) | 0.627 |
| Fatigue | 23 (22.3) | 19 (24.4) | 0.859 |
| Shortness of breath | 20 (19.4) | 10 (12.8) | 0.313 |
| Gastrointestinal symptoms | 9 (8.7) | 6 (7.7) | 1.000 |
| Change in taste or smell | 7 (6.8) | 1 (1.3) | 0.140 |
| Nasal congestion and/or sore throat | 65 (63.1) | 59 (75.6) | 0.078 |
| Number of Primary Symptoms Reported, Mean (SD) | 3.4 (1.8) | 3.2 (1.6) | 0.314 |
| Other symptoms, No. (%) | 0.861 | ||
| 0 | 90 (87.4) | 69 (88.5) | |
| 1 | 10 (9.7) | 8 (10.3) | |
| 2 | 3 (2.9) | 1 (1.3) | |
| Number of Primary Plus Other Symptoms, Mean (SD) | 3.6 (2.0) | 3.3 (1.7) | 0.308 |
| Days Symptoms Present, Mean (SD) | 7.3 (3.1) | 6.7 (3.2) | 0.261 |
| Cases before 1 January, required isolation = 10 days n = 76 | 7.5 (3.1) | 6.3 (2.5) | 0.112 |
| Cases on/after 1 January, required isolation = 5 days n = 105 | 7 (3.1) | 6.8 (3.4) | 0.861 |
| Days Isolated, Mean (SD) | |||
| Cases before 1 January, required isolation = 10 days n = 76 | 10.9 (1.6) | 11.1 (1.7) | 0.661 |
| Cases on/after 1 January, required isolation = 5 days n = 105 | 10.7 (2.8) | 9.8 (3.0) | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montecalvo, M.A.; Visintainer, P.; Drugge, E.; Kowalski, K.; Raffa, R.; McKenna, D.; Moronta, C.; Wormser, G.P. SARS-CoV-2 Symptoms during the Omicron Surge Differ between Boosted and Vaccinated Non-Boosted Persons. Vaccines 2024, 12, 327. https://doi.org/10.3390/vaccines12030327
Montecalvo MA, Visintainer P, Drugge E, Kowalski K, Raffa R, McKenna D, Moronta C, Wormser GP. SARS-CoV-2 Symptoms during the Omicron Surge Differ between Boosted and Vaccinated Non-Boosted Persons. Vaccines. 2024; 12(3):327. https://doi.org/10.3390/vaccines12030327
Chicago/Turabian StyleMontecalvo, Marisa A., Paul Visintainer, Elizabeth Drugge, Katherine Kowalski, Rosemarie Raffa, Donna McKenna, Christine Moronta, and Gary P. Wormser. 2024. "SARS-CoV-2 Symptoms during the Omicron Surge Differ between Boosted and Vaccinated Non-Boosted Persons" Vaccines 12, no. 3: 327. https://doi.org/10.3390/vaccines12030327
APA StyleMontecalvo, M. A., Visintainer, P., Drugge, E., Kowalski, K., Raffa, R., McKenna, D., Moronta, C., & Wormser, G. P. (2024). SARS-CoV-2 Symptoms during the Omicron Surge Differ between Boosted and Vaccinated Non-Boosted Persons. Vaccines, 12(3), 327. https://doi.org/10.3390/vaccines12030327






