Mt10 Vaccine Protects Diversity Outbred Mice from CVB3 Infection by Producing Virus-Specific Neutralizing Antibodies and Diverse Antibody Isotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Proteins
2.3. Infection Studies
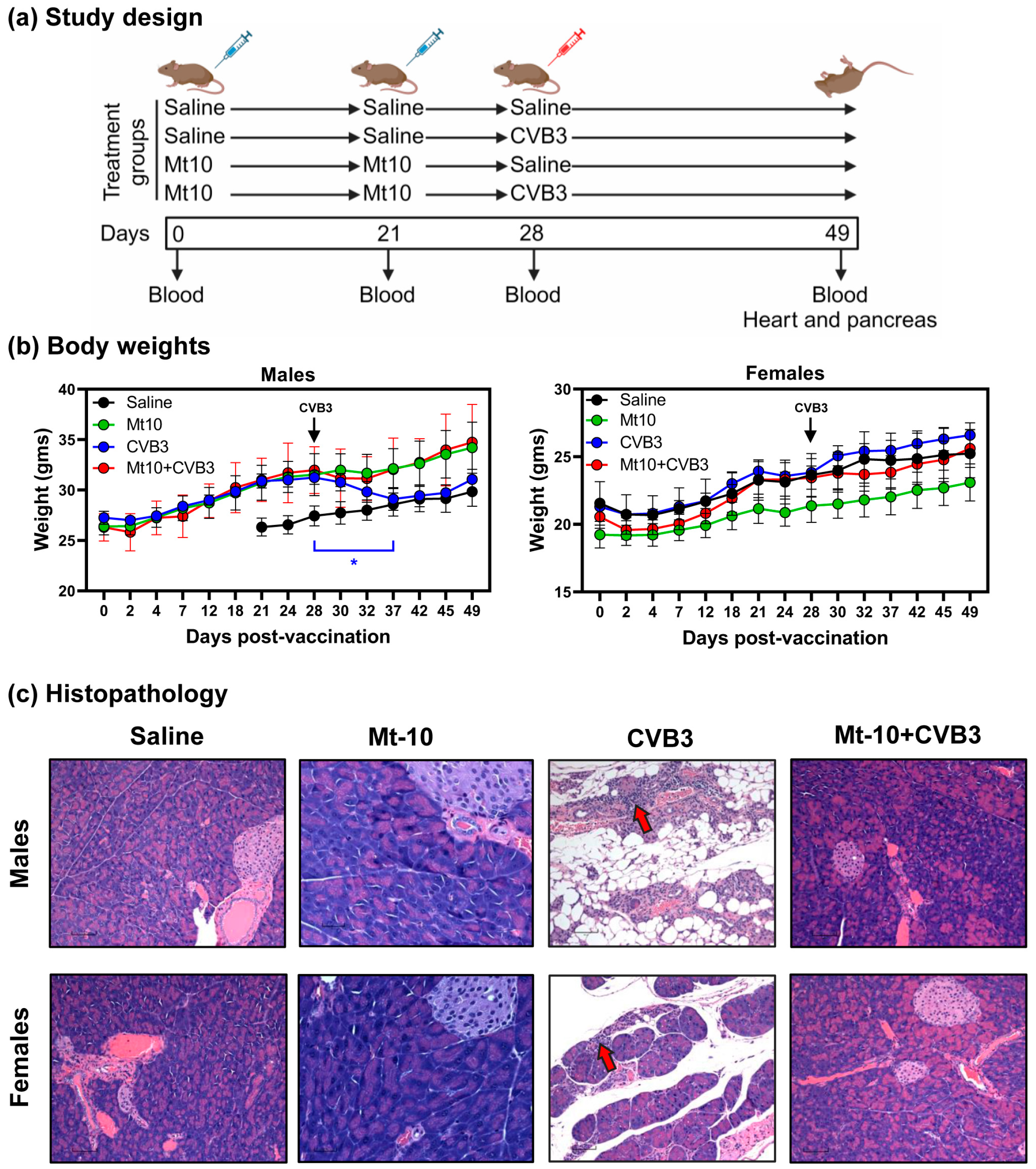
2.4. Vaccination and Challenge Studies
2.5. Histopathology
2.6. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.7. Virus Neutralization Assay
2.8. Determination of CVB-Reactive Antibodies
2.9. Statistical Analysis
3. Results
3.1. DO Mice Infected with CVB3 Developed Mainly Pancreatitis
3.2. Mt-10 Vaccine Virus Offered Complete Protection against CVB3 Infection in Challenge Studies in Both Male and Female DO Mice
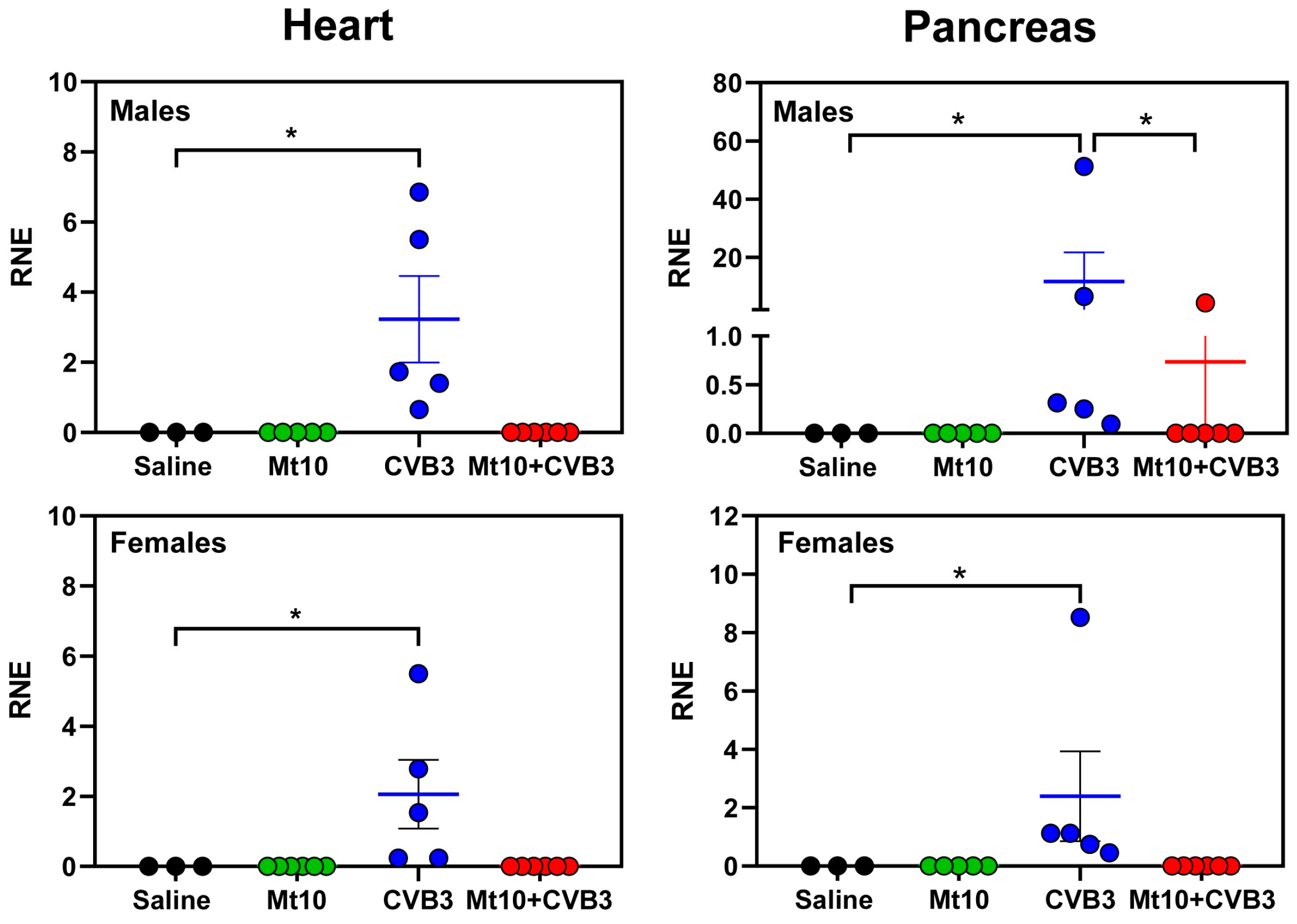
3.3. Viral Nucleic Acid Was Absent in the Tissues of Vaccine Recipients
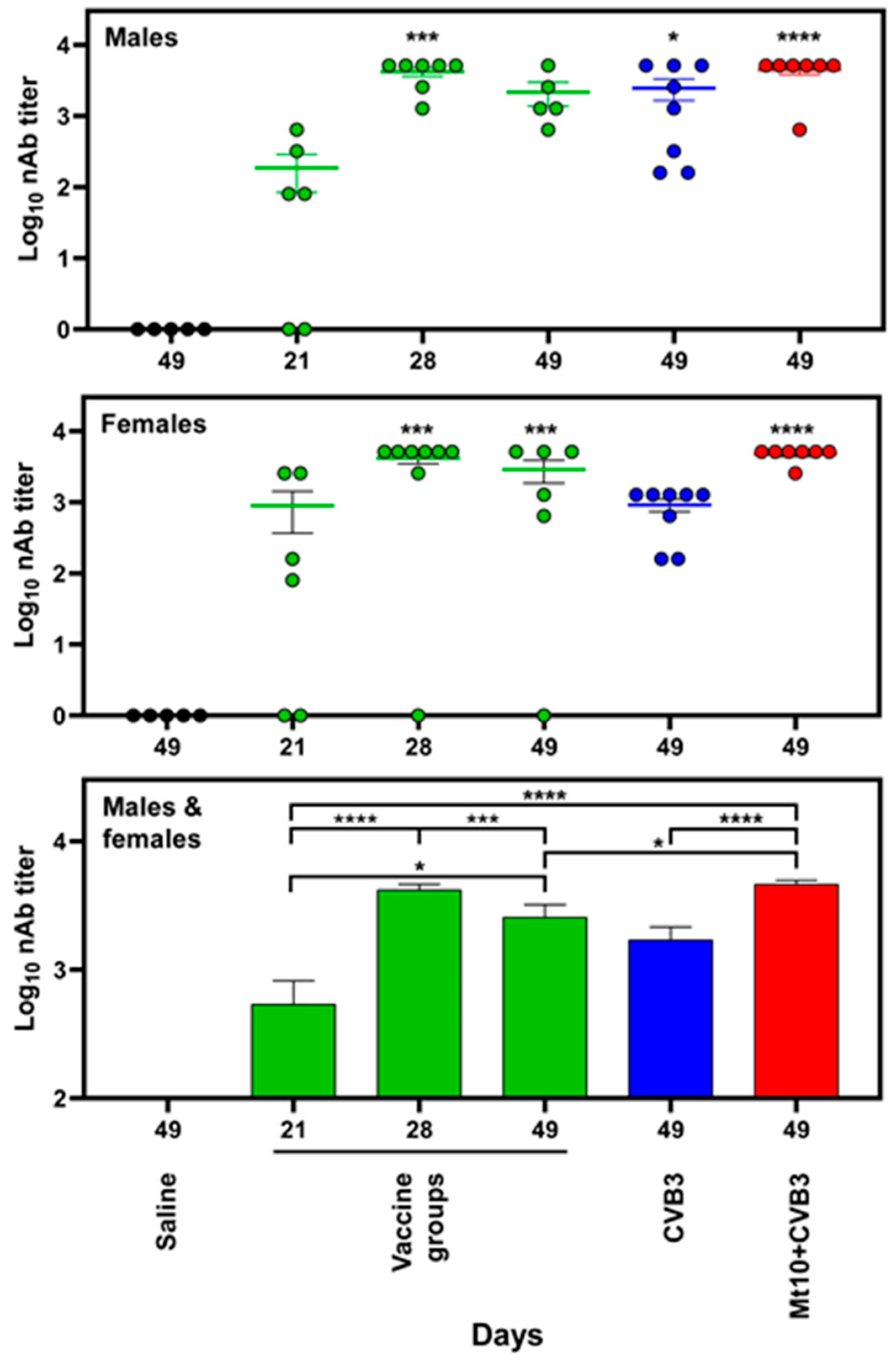
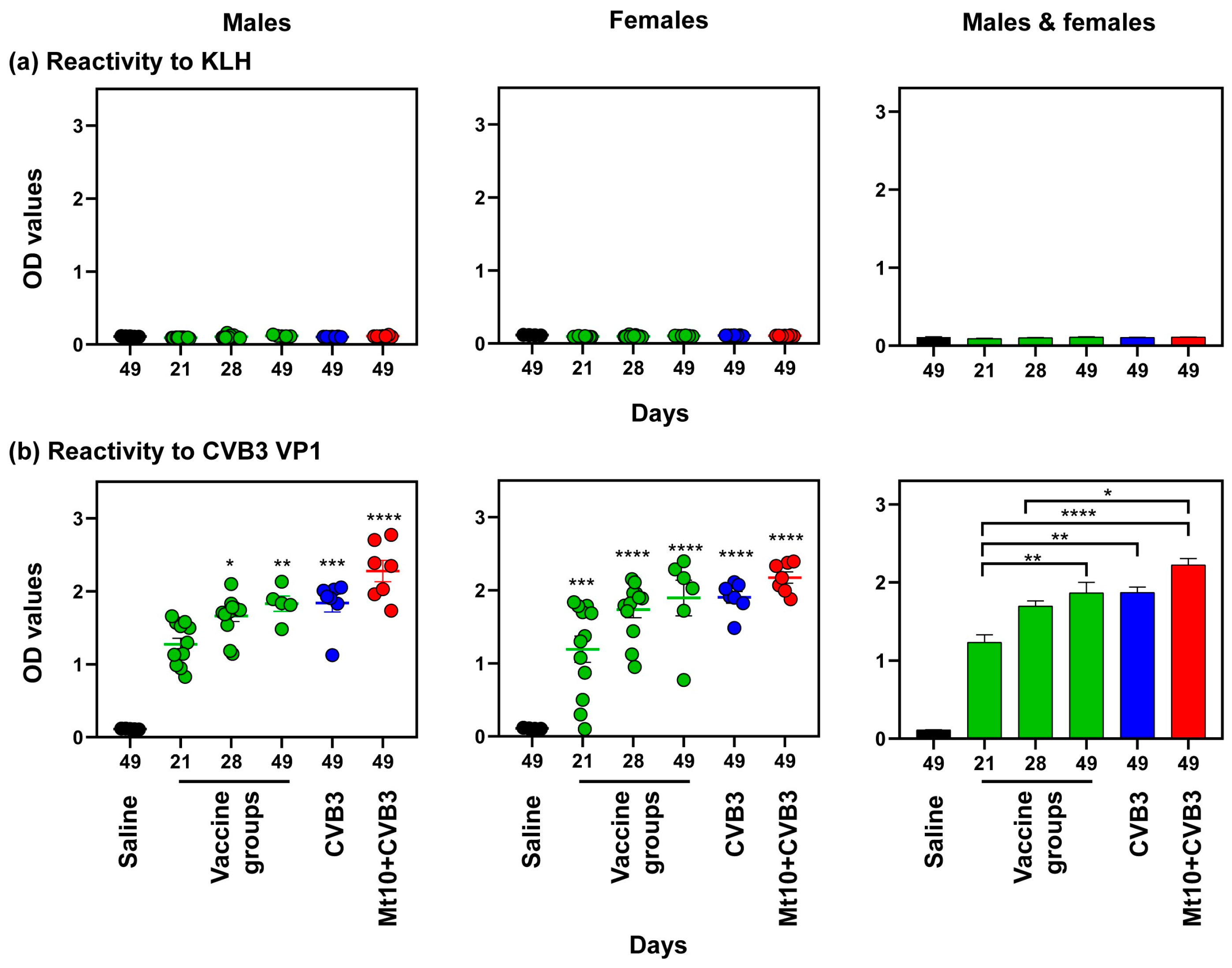
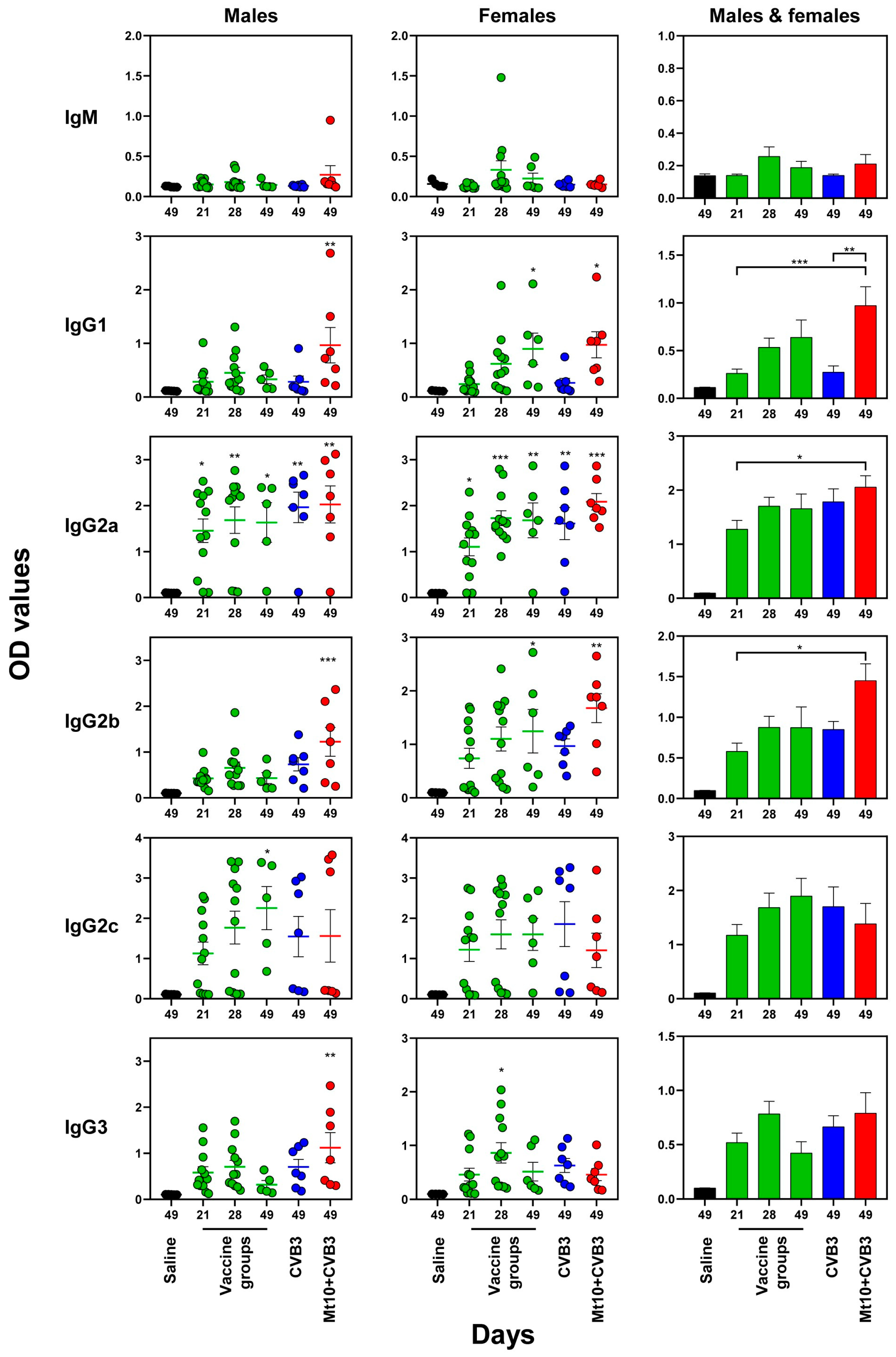
3.4. Vaccine Responses to Mt10 Were Associated with the Induction of nAbs, and the Virus-Reactive Antibodies Were Skewed towards IgG Isotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Poyry, T.; Kinnunen, L.; Hyypia, T.; Brown, B.; Horsnell, C.; Hovi, T.; Stanway, G. Genetic and phylogenetic clustering of enteroviruses. J. Gen. Virol. 1996, 77 Pt 8, 1699–1717. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; Garcia, M.L.; Hendrickson, R.C.; et al. Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses (2023). Arch. Virol. 2023, 168, 175. [Google Scholar] [CrossRef]
- Lo, S.H.; Huang, Y.C.; Huang, C.G.; Tsao, K.C.; Li, W.C.; Hsieh, Y.C.; Chiu, C.H.; Lin, T.Y. Clinical and epidemiologic features of Coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J. Microbiol. Immunol. Infect. 2011, 44, 252–257. [Google Scholar] [CrossRef]
- Mone, K.; Lasrado, N.; Sur, M.; Reddy, J. Vaccines against Group B Coxsackieviruses and Their Importance. Vaccines 2023, 11, 274. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li Lu, F.; Wu, M.H.; Lee, C.Y.; Huang, L.M. Fatal coxsackievirus A16 infection. Pediatr. Infect. Dis. J. 2004, 23, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Clements, G.B.; Galbraith, D.N.; Taylor, K.W. Coxsackie B virus infection and onset of childhood diabetes. Lancet 1995, 346, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.A.; Abdou, M.H.; Hadi, M.A. Coxsackie B2 Virus Infection Causing Multiorgan Failure and Cardiogenic Shock in a 42-Year-Old Man. Tex. Heart Inst. J. 2019, 46, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Hufnagel, G.; Chapman, N.M.; Tracy, S. The group B coxsackieviruses and myocarditis. Rev. Med. Virol. 2001, 11, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Yolken, R.H.; Bishop, C.A.; Townsend, T.R.; Bolyard, E.A.; Bartlett, J.; Santos, G.W.; Saral, R. Infectious gastroenteritis in bone-marrow-transplant recipients. N. Engl. J. Med. 1982, 306, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Cihakova, D.; Rose, N.R. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv. Immunol. 2008, 99, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Dotta, F.; Censini, S.; van Halteren, A.G.; Marselli, L.; Masini, M.; Dionisi, S.; Mosca, F.; Boggi, U.; Muda, A.O.; Del Prato, S.; et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA 2007, 104, 5115–5120. [Google Scholar] [CrossRef]
- Huber, S.A. Viral Myocarditis and Dilated Cardiomyopathy: Etiology and Pathogenesis. Curr. Pharm. Des. 2016, 22, 408–426. [Google Scholar] [CrossRef]
- Laitinen, O.H.; Honkanen, H.; Pakkanen, O.; Oikarinen, S.; Hankaniemi, M.M.; Huhtala, H.; Ruokoranta, T.; Lecouturier, V.; Andre, P.; Harju, R.; et al. Coxsackievirus B1 is associated with induction of beta-cell autoimmunity that portends type 1 diabetes. Diabetes 2014, 63, 446–455. [Google Scholar] [CrossRef]
- Richardson, S.J.; Morgan, N.G. Enteroviral infections in the pathogenesis of type 1 diabetes: New insights for therapeutic intervention. Curr. Opin. Pharmacol. 2018, 43, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Gangaplara, A.; Massilamany, C.; Arumugam, R.; Shelbourn, A.; Rasquinha, M.T.; Basavalingappa, R.H.; Delhon, G.; Xiang, S.H.; Pattnaik, A.K.; et al. Attenuated strain of CVB3 with a mutation in the CAR-interacting region protects against both myocarditis and pancreatitis. Sci. Rep. 2021, 11, 12432. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Arumugam, R.; Rasquinha, M.T.; Sur, M.; Steffen, D.; Reddy, J. Mt10-CVB3 Vaccine Virus Protects against CVB4 Infection by Inducing Cross-Reactive, Antigen-Specific Immune Responses. Microorganisms 2021, 9, 2323. [Google Scholar] [CrossRef]
- Rasquinha, M.T.; Lasrado, N.; Sur, M.; Mone, K.; Qiu, H.; Riethoven, J.J.; Sobel, R.A.; Reddy, J. A Monovalent Mt10-CVB3 Vaccine Prevents CVB4-Accelerated Type 1 Diabetes in NOD Mice. Vaccines 2022, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Lilue, J.; Doran, A.G.; Fiddes, I.T.; Abrudan, M.; Armstrong, J.; Bennett, R.; Chow, W.; Collins, J.; Collins, S.; Czechanski, A.; et al. Sixteen diverse laboratory mouse reference genomes define strain-specific haplotypes and novel functional loci. Nat. Genet. 2018, 50, 1574–1583. [Google Scholar] [CrossRef]
- Marton, J.; Albert, D.; Wiltshire, S.A.; Park, R.; Bergen, A.; Qureshi, S.; Malo, D.; Burelle, Y.; Vidal, S.M. Cyclosporine A Treatment Inhibits Abcc6-Dependent Cardiac Necrosis and Calcification following Coxsackievirus B3 Infection in Mice. PLoS ONE 2015, 10, e0138222. [Google Scholar] [CrossRef] [PubMed]
- Massilamany, C.; Gangaplara, A.; Basavalingappa, R.H.; Rajasekaran, R.A.; Vu, H.; Riethoven, J.J.; Steffen, D.; Pattnaik, A.K.; Reddy, J. Mutations in the 5’ NTR and the Non-Structural Protein 3A of the Coxsackievirus B3 Selectively Attenuate Myocarditogenicity. PLoS ONE 2015, 10, e0131052. [Google Scholar] [CrossRef][Green Version]
- Pearson, J.A.; Wong, F.S.; Wen, L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J. Autoimmun. 2016, 66, 76–88. [Google Scholar] [CrossRef]
- Skinner, C.M.; Miousse, I.R.; Ewing, L.E.; Sridharan, V.; Cao, M.; Lin, H.; Williams, D.K.; Avula, B.; Haider, S.; Chittiboyina, A.G.; et al. Impact of obesity on the toxicity of a multi-ingredient dietary supplement, OxyELITE Pro (New Formula), using the novel NZO/HILtJ obese mouse model: Physiological and mechanistic assessments. Food Chem. Toxicol. 2018, 122, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.P.; Didion, J.P.; Doran, A.G.; Holt, J.M.; McMillan, L.; Keane, T.M.; de Villena, F.P. Whole Genome Sequence of Two Wild-Derived Mus musculus domesticus Inbred Strains, LEWES/EiJ and ZALENDE/EiJ, with Different Diploid Numbers. G3 Genes Genomes Genet. 2016, 6, 4211–4216. [Google Scholar] [CrossRef]
- Svenson, K.L.; Gatti, D.M.; Valdar, W.; Welsh, C.E.; Cheng, R.; Chesler, E.J.; Palmer, A.A.; McMillan, L.; Churchill, G.A. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics 2012, 190, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.; Gibson, H.; Frelinger, J.; Buntzman, A. Using the Collaborative Cross and Diversity Outbred Mice in Immunology. Curr. Protoc. 2022, 2, e547. [Google Scholar] [CrossRef] [PubMed]
- AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (accessed on 20 November 2023).
- Gangaplara, A.; Massilamany, C.; Brown, D.M.; Delhon, G.; Pattnaik, A.K.; Chapman, N.; Rose, N.; Steffen, D.; Reddy, J. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-alpha-reactive CD4 T cells in A/J mice. Clin. Immunol. 2012, 144, 237–249. [Google Scholar] [CrossRef]
- Massilamany, C.; Gangaplara, A.; Steffen, D.; Reddy, J. Identification of novel mimicry epitopes for cardiac myosin heavy chain-alpha that induce autoimmune myocarditis in A/J mice. Cell Immunol. 2011, 271, 438–449. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Watson, S.; Mercier, S.; Bye, C.; Wilkinson, J.; Cunningham, A.L.; Harman, A.N. Determination of suitable housekeeping genes for normalisation of quantitative real time PCR analysis of cells infected with human immunodeficiency virus and herpes viruses. Virol. J. 2007, 4, 130. [Google Scholar] [CrossRef]
- Smallwood, T.L.; Gatti, D.M.; Quizon, P.; Weinstock, G.M.; Jung, K.C.; Zhao, L.; Hua, K.; Pomp, D.; Bennett, B.J. High-resolution genetic mapping in the diversity outbred mouse population identifies Apobec1 as a candidate gene for atherosclerosis. G3 Genes Genomes Genet. 2014, 4, 2353–2363. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.E.; Cutino-Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 372, 1418–1423. [Google Scholar] [CrossRef]
- Stamatatos, L.; Czartoski, J.; Wan, Y.H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef]
- Martin, R.M.; Brady, J.L.; Lew, A.M. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 1998, 212, 187–192. [Google Scholar] [CrossRef]
- Niazi, M.K.; Dhulekar, N.; Schmidt, D.; Major, S.; Cooper, R.; Abeijon, C.; Gatti, D.M.; Kramnik, I.; Yener, B.; Gurcan, M.; et al. Lung necrosis and neutrophils reflect common pathways of susceptibility to Mycobacterium tuberculosis in genetically diverse, immune-competent mice. Dis. Model. Mech. 2015, 8, 1141–1153. [Google Scholar] [CrossRef]
- Recla, J.M.; Robledo, R.F.; Gatti, D.M.; Bult, C.J.; Churchill, G.A.; Chesler, E.J. Precise genetic mapping and integrative bioinformatics in Diversity Outbred mice reveals Hydin as a novel pain gene. Mamm. Genome 2014, 25, 211–222. [Google Scholar] [CrossRef]
- Shorter, J.R.; Huang, W.; Beak, J.Y.; Hua, K.; Gatti, D.M.; de Villena, F.P.; Pomp, D.; Jensen, B.C. Quantitative trait mapping in Diversity Outbred mice identifies two genomic regions associated with heart size. Mamm. Genome 2018, 29, 80–89. [Google Scholar] [CrossRef]
- Mayeux, J.M.; Escalante, G.M.; Christy, J.M.; Pawar, R.D.; Kono, D.H.; Pollard, K.M. Silicosis and Silica-Induced Autoimmunity in the Diversity Outbred Mouse. Front. Immunol. 2018, 9, 874. [Google Scholar] [CrossRef] [PubMed]
- Keenan, B.T.; Galante, R.J.; Lian, J.; Simecek, P.; Gatti, D.M.; Zhang, L.; Lim, D.C.; Svenson, K.L.; Churchill, G.A.; Pack, A.I. High-throughput sleep phenotyping produces robust and heritable traits in Diversity Outbred mice and their founder strains. Sleep 2020, 43, zsz278. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Deighan, A.G.; Di Francesco, A.; Freund, A.; Jojic, V.; Churchill, G.A.; Raj, A. Age and diet shape the genetic architecture of body weight in diversity outbred mice. eLife 2022, 11, e64329. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.L.; Mittereder, L.R.; Lehman, C.C.; Khan, H.; Gould, V.A.; Elkins, K.L. Intravenous BCG Vaccination of Diversity Outbred Mice Results in Moderately Enhanced Protection against Challenge with Mycobacterium tuberculosis Compared to Intradermal Vaccination. Infect. Immun. 2023, 91, e0016823. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.L.; Rossi, A.P.; Beamer, G.L.; Gatti, D.M.; Kramnik, I.; Elkins, K.L. The Diversity Outbred Mouse Population Is an Improved Animal Model of Vaccination against Tuberculosis That Reflects Heterogeneity of Protection. mSphere 2020, 5, e00097-20. [Google Scholar] [CrossRef]
- Binh Tran, T.D.; Nguyen, H.; Sodergren, E.; Addiction, C.; Dickson, P.E.; Wright, S.N.; Philip, V.M.; Weinstock, G.M.; Chesler, E.J.; Zhou, Y.; et al. Microbial glutamate metabolism predicts intravenous cocaine self-administration in diversity outbred mice. Neuropharmacology 2023, 226, 109409. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; Robledo, R.F.; Recla, J.M.; Philip, V.M.; Bubier, J.A.; Jay, J.J.; Harwood, C.; Wilcox, T.; Gatti, D.M.; Bult, C.J.; et al. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav. 2013, 12, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Church, R.J.; Gatti, D.M.; Urban, T.J.; Long, N.; Yang, X.; Shi, Q.; Eaddy, J.S.; Mosedale, M.; Ballard, S.; Churchill, G.A.; et al. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef] [PubMed]
- French, J.E.; Gatti, D.M.; Morgan, D.L.; Kissling, G.E.; Shockley, K.R.; Knudsen, G.A.; Shepard, K.G.; Price, H.C.; King, D.; Witt, K.L.; et al. Diversity Outbred Mice Identify Population-Based Exposure Thresholds and Genetic Factors that Influence Benzene-Induced Genotoxicity. Environ. Health Perspect. 2015, 123, 237–245. [Google Scholar] [CrossRef]
- Schmidt, C.W. Diversity outbred: A new generation of mouse model. Environ. Health Perspect. 2015, 123, A64–A67. [Google Scholar] [CrossRef] [PubMed]
- Gatti, D.M.; Svenson, K.L.; Shabalin, A.; Wu, L.Y.; Valdar, W.; Simecek, P.; Goodwin, N.; Cheng, R.; Pomp, D.; Palmer, A.; et al. Quantitative trait locus mapping methods for diversity outbred mice. G3 Genes Genomes Genet. 2014, 4, 1623–1633. [Google Scholar] [CrossRef]
- Churchill, G.A.; Gatti, D.M.; Munger, S.C.; Svenson, K.L. The Diversity Outbred mouse population. Mamm. Genome 2012, 23, 713–718. [Google Scholar] [CrossRef]
- Zeiss, C.J.; Brayton, C.F. Immune responses to the real world. Lab. Anim. 2017, 47, 13–14. [Google Scholar] [CrossRef]
- Huber, S.; Ramsingh, A.I. Coxsackievirus-induced pancreatitis. Viral Immunol. 2004, 17, 358–369. [Google Scholar] [CrossRef]
- Leipner, C.; Grun, K.; Schneider, I.; Gluck, B.; Sigusch, H.H.; Stelzner, A. Coxsackievirus B3-induced myocarditis: Differences in the immune response of C57BL/6 and Balb/c mice. Med. Microbiol. Immunol. 2004, 193, 141–147. [Google Scholar] [CrossRef]
- Drescher, K.M.; Kono, K.; Bopegamage, S.; Carson, S.D.; Tracy, S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: Insulitis determines susceptibility of pancreatic islets to virus infection. Virology 2004, 329, 381–394. [Google Scholar] [CrossRef]
- Kanno, T.; Kim, K.; Kono, K.; Drescher, K.M.; Chapman, N.M.; Tracy, S. Group B coxsackievirus diabetogenic phenotype correlates with replication efficiency. J. Virol. 2006, 80, 5637–5643. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.; Drescher, K.M.; Chapman, N.M.; Kim, K.S.; Carson, S.D.; Pirruccello, S.; Lane, P.H.; Romero, J.R.; Leser, J.S. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: Inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 2002, 76, 12097–12111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramsingh, A.I.; Lee, W.T.; Collins, D.N.; Armstrong, L.E. T cells contribute to disease severity during coxsackievirus B4 infection. J. Virol. 1999, 73, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Chrysos, G.; Kokkoris, S.; Protopsaltis, J.; Korantzopoulos, P.; Giannoulis, G. Coxsackievirus infection associated with acute pancreatitis. JOP 2004, 5, 384–387. [Google Scholar]
- Selinka, H.C.; Wolde, A.; Sauter, M.; Kandolf, R.; Klingel, K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med. Microbiol. Immunol. 2004, 193, 127–131. [Google Scholar] [CrossRef]
| Parameters | Saline | CVB3 | Mt10 | Mt10 + CVB3 | ||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | |
| Mortality | 0/9 (0.0) | 0/9 (0.0) | 0/6 (0.0) | 0/8 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
| Heart | ||||||||
| Incidence | 1/9 (11.1) | 1/9 (11.1) | 0/6 (0.0) | 1/8 (12.5) | 0/6 (0.0) | 0/6 (0.0) | 2/7 (28.6) | 0/7 (0.0) |
| Myocardial lesions | 1/9 (11.1) | 1/9 (11.1) | 0/6 (0.0) | 1/8 (12.5) | 0/6 (0.0) | 0/6 (0.0) | 2/7 (28.6) | 0/7 (0.0) |
| Pancreas | ||||||||
| Incidence | 0/9 (0.0) | 0/9 (0.0) | 4/6 (66.7) ** | 4/8 (50.0) * | 0/6 (0.0) | 0/6 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
| Atrophy | 0/9 (0.0) | 0/9 (0.0) | 4/6 (66.7) ** | 4/8 (50.0) * | 0/6 (0.0) | 0/6 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
| Infiltration | 0/9 (0.0) | 0/9 (0.0) | 4/6 (66.7) ** | 4/8 (50.0) * | 0/6 (0.0) | 0/6 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
| Necrosis | 0/9 (0.0) | 0/9 (0.0) | 0/6 (0.0) | 0/8 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasquinha, M.T.; Mone, K.; Sur, M.; Lasrado, N.; Massilamany, C.; Kachman, S.D.; Steffen, D.; Reddy, J. Mt10 Vaccine Protects Diversity Outbred Mice from CVB3 Infection by Producing Virus-Specific Neutralizing Antibodies and Diverse Antibody Isotypes. Vaccines 2024, 12, 266. https://doi.org/10.3390/vaccines12030266
Rasquinha MT, Mone K, Sur M, Lasrado N, Massilamany C, Kachman SD, Steffen D, Reddy J. Mt10 Vaccine Protects Diversity Outbred Mice from CVB3 Infection by Producing Virus-Specific Neutralizing Antibodies and Diverse Antibody Isotypes. Vaccines. 2024; 12(3):266. https://doi.org/10.3390/vaccines12030266
Chicago/Turabian StyleRasquinha, Mahima T., Kiruthiga Mone, Meghna Sur, Ninaad Lasrado, Chandirasegaran Massilamany, Stephen D. Kachman, David Steffen, and Jay Reddy. 2024. "Mt10 Vaccine Protects Diversity Outbred Mice from CVB3 Infection by Producing Virus-Specific Neutralizing Antibodies and Diverse Antibody Isotypes" Vaccines 12, no. 3: 266. https://doi.org/10.3390/vaccines12030266
APA StyleRasquinha, M. T., Mone, K., Sur, M., Lasrado, N., Massilamany, C., Kachman, S. D., Steffen, D., & Reddy, J. (2024). Mt10 Vaccine Protects Diversity Outbred Mice from CVB3 Infection by Producing Virus-Specific Neutralizing Antibodies and Diverse Antibody Isotypes. Vaccines, 12(3), 266. https://doi.org/10.3390/vaccines12030266






