IgG Subclass Switch in Volunteers Repeatedly Immunized with the Full-Length Plasmodium falciparum Merozoite Surface Protein 1 (MSP1)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection Burkina Faso
2.2. Purification of Human Sera and PBMCs from Phase Ia Trial
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) for Anti-MSP1FL IgG Subclasses
2.4. Growth Inhibition Assay (GIA) for P. falciparum
2.5. Avidity Assay
2.6. PBMCs Stimulation
2.7. Real-Time Reverse-Transcriptase (RT)-PCR
2.8. Flow Cytometric Analysis of Surface Markers and Intracellular Cytokines
2.9. Enzyme-Linked Immunosorbent Assay (ELISA) for IL-4
2.10. Statistical Analysis
3. Results
3.1. IgG Subclass Profile in Response to Repeated Immunization with MSP1FL
3.2. IgG Subclass Profile of Malaria Exposed Populations from Africa
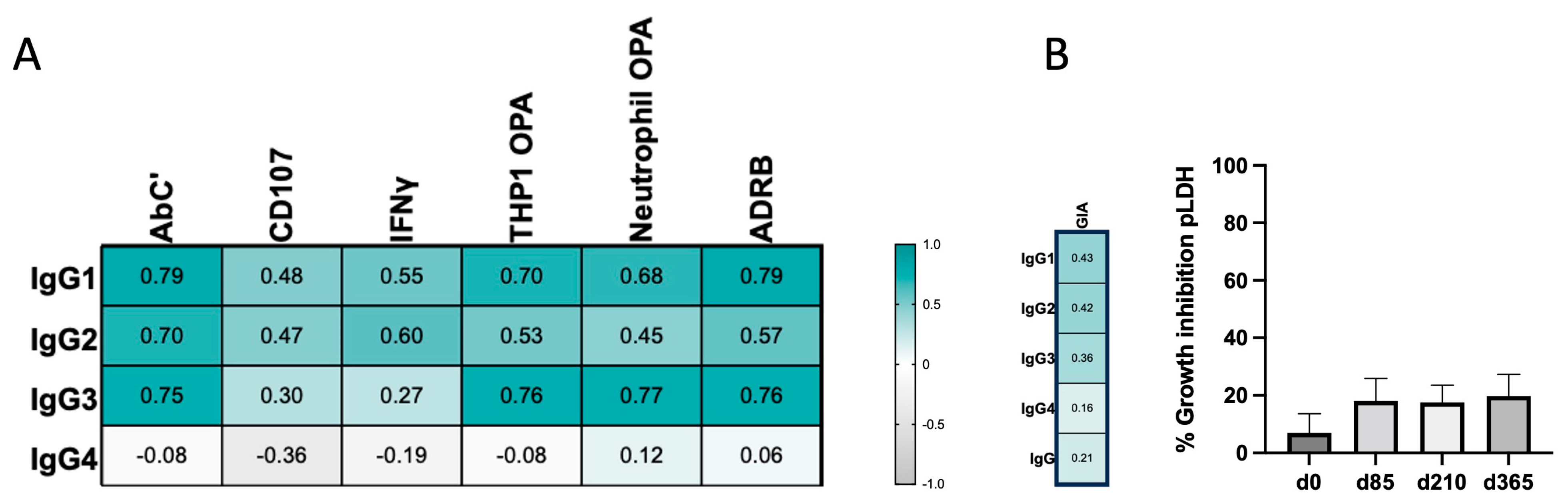
3.3. IgG4 Occurrence Is Weakly Correlated with Fc-Mediated Effector Functions
3.4. IgG Avidity Increases after Vaccination and Is Associated with IgG4 Levels
3.5. Progressive Production of Interleukin-4 during Vaccination Parallels with the Late Increase in IgG4
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Swizterland, 2021. [Google Scholar]
- Achan, J.; Serwanga, A.; Wanzira, H.; Kyagulanyi, T.; Nuwa, A.; Magumba, G.; Kusasira, S.; Sewanyana, I.; Tetteh, K.; Drakeley, C.; et al. Current malaria infection, previous malaria exposure, and clinical profiles and outcomes of COVID-19 in a setting of high malaria transmission: An exploratory cohort study in Uganda. Lancet Microbe 2022, 3, e62–e71. [Google Scholar] [CrossRef]
- Penny, M.A.; Camponovo, F.; Chitnis, N.; Smith, T.A.; Tanner, M. Future use-cases of vaccines in malaria control and elimination. Parasite Epidemiol. Control 2020, 10, e00145. [Google Scholar] [CrossRef]
- Keating, S.M.; Bejon, P.; Berthoud, T.; Vuola, J.M.; Todryk, S.; Webster, D.P.; Dunachie, S.J.; Moorthy, V.S.; McConkey, S.J.; Gilbert, S.C.; et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J. Immunol. 2005, 175, 5675–5680. [Google Scholar] [CrossRef]
- Adepoju, P. RTS,S malaria vaccine pilots in three African countries. Lancet 2019, 393, 1685. [Google Scholar] [CrossRef]
- Genton, B. R21/Matrix-M™ malaria vaccine: A new tool to achieve WHO’s goal to eliminate malaria in 30 countries by 2030? J. Travel. Med. 2023, 30, taad140. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, H.M.; Somé, A.; Bellamy, D.; Traoré, O.; Rouamba, T.; Tahita, M.C.; Ido, N.F.A.; Yameogo, P.; Valia, D.; et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: A phase 1/2b randomised controlled trial. Lancet Infect. Dis. 2022, 22, 1728–1736. [Google Scholar] [CrossRef]
- Bustreo, F.; Okwo-Bele, J.M.; Kamara, L. World Health Organization perspectives on the contribution of the Global Alliance for Vaccines and Immunization on reducing child mortality. Arch. Dis. Child. 2015, 100 (Suppl. S1), S34–S37. [Google Scholar] [CrossRef]
- Portugal, S.; Tipton, C.M.; Sohn, H.; Kone, Y.; Wang, J.; Li, S.; Skinner, J.; Virtaneva, K.; Sturdevant, D.E.; Porcella, S.F.; et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 2015, 4, e07218. [Google Scholar] [CrossRef]
- Cohen, S.; Mc, G.I.; Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature 1961, 192, 733–737. [Google Scholar] [CrossRef]
- Palm, A.-K.E.; Henry, C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Males, S.; Gaye, O.; Garcia, A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: A prospective study among Senegalese children. Clin. Infect. Dis. 2008, 46, 516–522. [Google Scholar] [CrossRef]
- Cockburn, I.A.; Seder, R.A. Malaria prevention: From immunological concepts to effective vaccines and protective antibodies. Nat. Immunol. 2018, 19, 1199–1211. [Google Scholar] [CrossRef]
- Mugo, R.M.; Mwai, K.; Mwacharo, J.; Shee, F.M.; Musyoki, J.N.; Wambua, J.; Otieno, E.; Bejon, P.; Ndungu, F.M. Seven-year kinetics of RTS, S/AS01-induced anti-CSP antibodies in young Kenyan children. Malar. J. 2021, 20, 452. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Meyer, N.; Moniuszko, M.; Sokolowska, M.; Eljaszewicz, A.; Wirz, O.F.; Tomasiak-Lozowska, M.M.; Bodzenta-Lukaszyk, A.; Ruxrungtham, K.; van de Veen, W. High-dose bee venom exposure induces similar tolerogenic B-cell responses in allergic patients and healthy beekeepers. Allergy 2017, 72, 407–415. [Google Scholar] [CrossRef]
- Damelang, T.; Rogerson, S.J.; Kent, S.J.; Chung, A.W. Role of IgG3 in Infectious Diseases. Trends Immunol. 2019, 40, 197–211. [Google Scholar] [CrossRef]
- Bouharoun-Tayoun, H.; Druilhe, P. Plasmodium falciparum malaria: Evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 1992, 60, 1473–1481. [Google Scholar] [CrossRef]
- Bouharoun-Tayoun, H.; Druilhe, P. Antibodies in falciparum malaria: What matters most, quantity or quality? Mem. Inst. Oswaldo Cruz 1992, 87 (Suppl. S3), 229–234. [Google Scholar] [CrossRef]
- Ndungu, F.M.; Bull, P.C.; Ross, A.; Lowe, B.S.; Kabiru, E.; Marsh, K. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: Evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 2002, 24, 77–82. [Google Scholar] [CrossRef]
- Lusingu, J.P.; Vestergaard, L.S.; Alifrangis, M.; Mmbando, B.P.; Theisen, M.; Kitua, A.Y.; Lemnge, M.M.; Theander, T.G. Cytophilic antibodies to Plasmodium falciparum glutamate rich protein are associated with malaria protection in an area of holoendemic transmission. Malar. J. 2005, 4, 48. [Google Scholar] [CrossRef]
- Roussilhon, C.; Oeuvray, C.; Müller-Graf, C.; Tall, A.; Rogier, C.; Trape, J.F.; Theisen, M.; Balde, A.; Pérignon, J.L.; Druilhe, P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007, 4, e320. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Richards, J.S.; McCallum, F.J.; Michon, P.; King, C.L.; Schoepflin, S.; Gilson, P.R.; Murphy, V.J.; Anders, R.F.; Mueller, I.; et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 2009, 77, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Dobaño, C.; Quelhas, D.; Quintó, L.; Puyol, L.; Serra-Casas, E.; Mayor, A.; Nhampossa, T.; Macete, E.; Aide, P.; Mandomando, I.; et al. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin. Vaccine Immunol. 2012, 19, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.; Reiling, L.; Feng, G.; Drew, D.R.; Mueller, I.; Siba, P.M.; Tsuboi, T.; Richards, J.S.; Fowkes, F.J.I.; Beeson, J.G. The association between naturally acquired IgG subclass specific antibodies to the PfRH5 invasion complex and protection from Plasmodium falciparum malaria. Sci. Rep. 2016, 6, 33094. [Google Scholar] [CrossRef] [PubMed]
- Kana, I.H.; Garcia-Senosiain, A.; Singh, S.K.; Tiendrebeogo, R.W.; Chourasia, B.K.; Malhotra, P.; Sharma, S.K.; Das, M.K.; Singh, S.; Adu, B.; et al. Cytophilic Antibodies Against Key Plasmodium falciparum Blood Stage Antigens Contribute to Protection Against Clinical Malaria in a High Transmission Region of Eastern India. J. Infect. Dis. 2018, 218, 956–965. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Boyle, M.J.; Reiling, L.; Feng, G.; Langer, C.; Osier, F.H.; Aspeling-Jones, H.; Cheng, Y.S.; Stubbs, J.; Tetteh, K.K.; Conway, D.J.; et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015, 42, 580–590. [Google Scholar] [CrossRef]
- Hill, D.L.; Eriksson, E.M.; Li Wai Suen, C.S.; Chiu, C.Y.; Ryg-Cornejo, V.; Robinson, L.J.; Siba, P.M.; Mueller, I.; Hansen, D.S.; Schofield, L. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS ONE 2013, 8, e74627. [Google Scholar] [CrossRef]
- Osier, F.H.; Feng, G.; Boyle, M.J.; Langer, C.; Zhou, J.; Richards, J.S.; McCallum, F.J.; Reiling, L.; Jaworowski, A.; Anders, R.F.; et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: Mechanism in human immunity and a correlate of protection against malaria. BMC Med. 2014, 12, 108. [Google Scholar] [CrossRef]
- Hill, D.L.; Wilson, D.W.; Sampaio, N.G.; Eriksson, E.M.; Ryg-Cornejo, V.; Harrison, G.L.A.; Uboldi, A.D.; Robinson, L.J.; Beeson, J.G.; Siba, P.; et al. Merozoite Antigens of Plasmodium falciparum Elicit Strain-Transcending Opsonizing Immunity. Infect. Immun. 2016, 84, 2175–2184. [Google Scholar] [CrossRef]
- Joos, C.; Varela, M.L.; Mbengue, B.; Mansourou, A.; Marrama, L.; Sokhna, C.; Tall, A.; Trape, J.F.; Toure, A.; Mercereau-Puijalon, O.; et al. Antibodies to Plasmodium falciparum merozoite surface protein-1p19 malaria vaccine candidate induce antibody-dependent respiratory burst in human neutrophils. Malar. J. 2015, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Joos, C.; Marrama, L.; Polson, H.E.; Corre, S.; Diatta, A.M.; Diouf, B.; Trape, J.F.; Tall, A.; Longacre, S.; Perraut, R. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS ONE 2010, 5, e9871. [Google Scholar] [CrossRef] [PubMed]
- Richie, T.L.; Billingsley, P.F.; Sim, B.K.; James, E.R.; Chakravarty, S.; Epstein, J.E.; Lyke, K.E.; Mordmuller, B.; Alonso, P.; Duffy, P.E.; et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 2015, 33, 7452–7461. [Google Scholar] [CrossRef] [PubMed]
- Dobaño, C.; Santano, R.; Vidal, M.; Jiménez, A.; Jairoce, C.; Ubillos, I.; Dosoo, D.; Aguilar, R.; Williams, N.A.; Díez-Padrisa, N.; et al. Differential Patterns of IgG Subclass Responses to Plasmodium falciparum Antigens in Relation to Malaria Protection and RTS,S Vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef]
- Blank, A.; Fürle, K.; Jäschke, A.; Mikus, G.; Lehmann, M.; Hüsing, J.; Heiss, K.; Giese, T.; Carter, D.; Böhnlein, E.; et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. NPJ Vaccines 2020, 5, 10. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Fürle, K.; Hibbert, J.; Ulmer, A.; Ali, A.; Giese, T.; Blank, A.; Haefeli, W.E.; Böhnlein, E.; Lanzer, M.; et al. Multifunctional IgG/IgM antibodies and cellular cytotoxicity are elicited by the full-length MSP1 SumayaVac-1 malaria vaccine. NPJ Vaccines 2023, 8, 112. [Google Scholar] [CrossRef]
- Jaschke, A.; Coulibaly, B.; Remarque, E.J.; Bujard, H.; Epp, C. Merozoite Surface Protein 1 from Plasmodium falciparum Is a Major Target of Opsonizing Antibodies in Individuals with Acquired Immunity against Malaria. Clin. Vaccine Immunol. 2017, 24, 11. [Google Scholar] [CrossRef]
- Kauth, C.W.; Woehlbier, U.; Kern, M.; Mekonnen, Z.; Lutz, R.; Mucke, N.; Langowski, J.; Bujard, H. Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 2006, 281, 31517–31527. [Google Scholar] [CrossRef] [PubMed]
- Kauth, C.W.; Epp, C.; Bujard, H.; Lutz, R. The merozoite surface protein 1 complex of human malaria parasite Plasmodium falciparum: Interactions and arrangements of subunits. J. Biol. Chem. 2003, 278, 22257–22264. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K. Post-COVID mRNA-vaccine IgG4 shift: Worrisome? mSphere 2023, 8, e0008523. [Google Scholar] [CrossRef]
- Teo, A.; Feng, G.; Brown, G.V.; Beeson, J.G.; Rogerson, S.J. Functional Antibodies and Protection against Blood-stage Malaria. Trends Parasitol. 2016, 32, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Moormann, A.M.; Nixon, C.E.; Forconi, C.S. Immune effector mechanisms in malaria: An update focusing on human immunity. Parasite Immunol. 2019, 41, e12628. [Google Scholar] [CrossRef] [PubMed]
- Nkumama, I.N.; Odera, D.; Musasia, F.; Mwai, K.; Nyamako, L.; Murungi, L.; Tuju, J.; Fürle, K.; Rosenkranz, M.; Kimathi, R.; et al. Breadth of Fc-mediated effector function delineates grades of clinical immunity following human malaria challenge. bioRxiv 2022. [Google Scholar] [CrossRef]
- Minassian, A.M.; Silk, S.E.; Barrett, J.R.; Nielsen, C.M.; Miura, K.; Diouf, A.; Loos, C.; Fallon, J.K.; Michell, A.R.; White, M.T.; et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med 2021, 2, 701–719.e19. [Google Scholar] [CrossRef]
- Rispens, T.; Huijbers, M.G. The unique properties of IgG4 and its roles in health and disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef]
- Kurniawan, A.; Yazdanbakhsh, M.; van Ree, R.; Aalberse, R.; Selkirk, M.E.; Partono, F.; Maizels, R.M. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J. Immunol. 1993, 150, 3941–3950. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Ooijevaar-de Heer, P.; Bende, O.; Aalberse, R.C. Mechanism of Immunoglobulin G4 Fab-arm Exchange. J. Am. Chem. Soc. 2011, 133, 10302–10311. [Google Scholar] [CrossRef]
- Aalberse, R.C.; van der Gaag, R.; van Leeuwen, J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J. Immunol. 1983, 130, 722–726. [Google Scholar] [CrossRef]
- de Jong, B.G.; IJspeert, H.; Marques, L.; van der Burg, M.; van Dongen, J.J.; Loos, B.G.; van Zelm, M.C. Human IgG2- and IgG4-expressing memory B cells display enhanced molecular and phenotypic signs of maturity and accumulate with age. Immunol. Cell Biol. 2017, 95, 744–752. [Google Scholar] [CrossRef]
- Horns, F.; Vollmers, C.; Croote, D.; Mackey, S.F.; Swan, G.E.; Dekker, C.L.; Davis, M.M.; Quake, S.R. Correction: Lineage tracing of human B cells reveals the in vivo landscape of human antibody class switching. Elife 2016, 5, e23066. [Google Scholar] [CrossRef]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.M.; Barrett, J.R.; Davis, C.; Fallon, J.K.; Goh, C.; Michell, A.R.; Griffin, C.; Kwok, A.; Loos, C.; Darko, S.; et al. Delayed boosting improves human antigen-specific Ig and B cell responses to the RH5.1/AS01B malaria vaccine. JCI Insight 2023, 8, e163859. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Duncan, C.J.; Hill, A.V.; Ellis, R.D. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum. Vaccin. Immunother. 2012, 8, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.F.; Burghaus, P.; Druilhe, P.; Holder, A.A.; Riley, E.M. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999, 21, 133–139. [Google Scholar] [CrossRef]
- Murungi, L.M.; Sonden, K.; Llewellyn, D.; Rono, J.; Guleid, F.; Williams, A.R.; Ogada, E.; Thairu, A.; Farnert, A.; Marsh, K.; et al. Targets and Mechanisms Associated with Protection from Severe Plasmodium falciparum Malaria in Kenyan Children. Infect. Immun. 2016, 84, 950–963. [Google Scholar] [CrossRef]
- Bejon, P.; Cook, J.; Bergmann-Leitner, E.; Olotu, A.; Lusingu, J.; Mwacharo, J.; Vekemans, J.; Njuguna, P.; Leach, A.; Lievens, M.; et al. Effect of the pre-erythrocytic candidate malaria vaccine RTS,S/AS01E on blood stage immunity in young children. J. Infect. Dis. 2011, 204, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.E.; Bergmann-Leitner, E.S.; Wilson, D.W.; Tisch, D.J.; Kimmel, R.; Vulule, J.; Sumba, P.O.; Beeson, J.G.; Angov, E.; Moormann, A.M.; et al. Antibody-mediated growth inhibition of Plasmodium falciparum: Relationship to age and protection from parasitemia in Kenyan children and adults. PLoS ONE 2008, 3, e3557. [Google Scholar] [CrossRef]
- McCallum, F.J.; Persson, K.E.; Mugyenyi, C.K.; Fowkes, F.J.; Simpson, J.A.; Richards, J.S.; Williams, T.N.; Marsh, K.; Beeson, J.G. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS ONE 2008, 3, e3571. [Google Scholar] [CrossRef]
- Marsh, K.; Otoo, L.; Hayes, R.J.; Carson, D.C.; Greenwood, B.M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Courtin, D.; Oesterholt, M.; Huismans, H.; Kusi, K.; Milet, J.; Badaut, C.; Gaye, O.; Roeffen, W.; Remarque, E.J.; Sauerwein, R.; et al. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS ONE 2009, 4, e7590. [Google Scholar] [CrossRef] [PubMed]
- Crompton, P.D.; Miura, K.; Traore, B.; Kayentao, K.; Ongoiba, A.; Weiss, G.; Doumbo, S.; Doumtabe, D.; Kone, Y.; Huang, C.Y.; et al. In vitro growth-inhibitory activity and malaria risk in a cohort study in mali. Infect. Immun. 2010, 78, 737–745. [Google Scholar] [CrossRef]
- Aguilar, R.; Ubillos, I.; Vidal, M.; Balanza, N.; Crespo, N.; Jiménez, A.; Nhabomba, A.; Jairoce, C.; Dosoo, D.; Gyan, B.; et al. Antibody responses to α-Gal in African children vary with age and site and are associated with malaria protection. Sci. Rep. 2018, 8, 9999. [Google Scholar] [CrossRef]
- Zong, S.; Kron, M.W.; Epp, C.; Engler, T.; Bujard, H.; Kochanek, S.; Kreppel, F. ΔE1 and high-capacity adenoviral vectors expressing full-length codon-optimized merozoite surface protein 1 for vaccination against Plasmodium falciparum. J. Gene Med. 2011, 13, 670–679. [Google Scholar] [CrossRef]
- Coler, R.N.; Day, T.A.; Ellis, R.; Piazza, F.M.; Beckmann, A.M.; Vergara, J.; Rolf, T.; Lu, L.; Alter, G.; Hokey, D.; et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines 2018, 3, 34. [Google Scholar] [CrossRef]
- Coler, R.N.; Duthie, M.S.; Hofmeyer, K.A.; Guderian, J.; Jayashankar, L.; Vergara, J.; Rolf, T.; Misquith, A.; Laurance, J.D.; Raman, V.S.; et al. From mouse to man: Safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin. Transl. Immunol. 2015, 4, e35. [Google Scholar] [CrossRef] [PubMed]
- Windish, H.P.; Duthie, M.S.; Misquith, A.; Ireton, G.; Lucas, E.; Laurance, J.D.; Bailor, R.H.; Coler, R.N.; Reed, S.G. Protection of mice from Mycobacterium tuberculosis by ID87/GLA-SE, a novel tuberculosis subunit vaccine candidate. Vaccine 2011, 29, 7842–7848. [Google Scholar] [CrossRef] [PubMed]
- Coler, R.N.; Bertholet, S.; Moutaftsi, M.; Guderian, J.A.; Windish, H.P.; Baldwin, S.L.; Laughlin, E.M.; Duthie, M.S.; Fox, C.B.; Carter, D.; et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE 2011, 6, e16333. [Google Scholar] [CrossRef]
- Stephens, R.; Langhorne, J. Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol. 2006, 28, 25–30. [Google Scholar] [CrossRef]
- Chan, J.A.; Loughland, J.R.; de Labastida Rivera, F.; SheelaNair, A.; Andrew, D.W.; Dooley, N.L.; Wines, B.D.; Amante, F.H.; Webb, L.; Hogarth, P.M.; et al. Th2-like T Follicular Helper Cells Promote Functional Antibody Production during Plasmodium falciparum Infection. Cell Rep. Med. 2020, 1, 100157. [Google Scholar] [CrossRef]
- Ullah, I.; Beaudoin-Bussières, G.; Symmes, K.; Cloutier, M.; Ducas, E.; Tauzin, A.; Laumaea, A.; Grunst, M.W.; Dionne, K.; Richard, J.; et al. The Fc-effector function of COVID-19 convalescent plasma contributes to SARS-CoV-2 treatment efficacy in mice. Cell Rep. Med. 2023, 4, 100893. [Google Scholar] [CrossRef]
- Zohar, T.; Hsiao, J.C.; Mehta, N.; Das, J.; Devadhasan, A.; Karpinski, W.; Callahan, C.; Citron, M.P.; DiStefano, D.J.; Touch, S.; et al. Upper and lower respiratory tract correlates of protection against respiratory syncytial virus following vaccination of nonhuman primates. Cell Host Microbe 2022, 30, 41–52.e5. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.M.; Alter, G. Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine. Front. Immunol. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Gorman, M.J.; Yuan, D.; Yu, J.; Mercado, N.B.; McMahan, K.; Borducchi, E.N.; Lifton, M.; Liu, J.; Nampanya, F.; et al. Defining the determinants of protection against SARS-CoV-2 infection and viral control in a dose-down Ad26.CoV2.S vaccine study in nonhuman primates. PLoS Biol. 2022, 20, e3001609. [Google Scholar] [CrossRef] [PubMed]
- Odera, D.O.; Tuju, J.; Mwai, K.; Nkumama, I.N.; Fürle, K.; Chege, T.; Kimathi, R.; Diehl, S.; Musasia, F.K.; Rosenkranz, M.; et al. Anti-merozoite antibodies induce natural killer cell effector function and are associated with immunity against malaria. Sci. Transl. Med. 2023, 15, eabn5993. [Google Scholar] [CrossRef] [PubMed]
- Osier, F.H.; Mackinnon, M.J.; Crosnier, C.; Fegan, G.; Kamuyu, G.; Wanaguru, M.; Ogada, E.; McDade, B.; Rayner, J.C.; Wright, G.J.; et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci. Transl. Med. 2014, 6, 247ra102. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef]
- Groux, H.; Gysin, J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: Functional role of IgG subclasses. Res. Immunol. 1990, 141, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Jackson, K.J. A Temporal Model of Human IgE and IgG Antibody Function. Front. Immunol. 2013, 4, 235. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathay, V.; Fürle, K.; Kiehl, V.; Ulmer, A.; Lanzer, M.; Thomson-Luque, R. IgG Subclass Switch in Volunteers Repeatedly Immunized with the Full-Length Plasmodium falciparum Merozoite Surface Protein 1 (MSP1). Vaccines 2024, 12, 208. https://doi.org/10.3390/vaccines12020208
Rathay V, Fürle K, Kiehl V, Ulmer A, Lanzer M, Thomson-Luque R. IgG Subclass Switch in Volunteers Repeatedly Immunized with the Full-Length Plasmodium falciparum Merozoite Surface Protein 1 (MSP1). Vaccines. 2024; 12(2):208. https://doi.org/10.3390/vaccines12020208
Chicago/Turabian StyleRathay, Veronika, Kristin Fürle, Viktoria Kiehl, Anne Ulmer, Michael Lanzer, and Richard Thomson-Luque. 2024. "IgG Subclass Switch in Volunteers Repeatedly Immunized with the Full-Length Plasmodium falciparum Merozoite Surface Protein 1 (MSP1)" Vaccines 12, no. 2: 208. https://doi.org/10.3390/vaccines12020208
APA StyleRathay, V., Fürle, K., Kiehl, V., Ulmer, A., Lanzer, M., & Thomson-Luque, R. (2024). IgG Subclass Switch in Volunteers Repeatedly Immunized with the Full-Length Plasmodium falciparum Merozoite Surface Protein 1 (MSP1). Vaccines, 12(2), 208. https://doi.org/10.3390/vaccines12020208





