Assessment of BoAHV-1 Seronegative Latent Carrier by the Administration of Two Infectious Bovine Rhinotracheitis Live Marker Vaccines in Calves
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Designs
| Group | No. of Calves | Age (Months) | IBR Passive Immunity | Vaccine Identification | Type (Deletion) | Virus Concentration (TCID50/mL) c | Inoculation Route |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 3 | Negative a | A | gE− | 106.24 | Intranasal |
| 2 | 3 | 3 | Positive b | A | gE− | 106.24 | Intranasal |
| 3 | 3 | 3 | Negative a | B | gE−/tk− | 105.74 | Intramuscular |
| 4 | 3 | 3 | Positive b | B | gE−/tk− | 105.74 | Intramuscular |
2.2. Immunization
2.3. Vaccine Reactivation
2.4. Samples Collection
2.5. Virus Isolation
2.6. ELISA Tests
2.7. Virus Neutralization Test (VNT)
2.8. gB-Specific Real-Time PCR
2.9. Statistical Analysis
3. Results
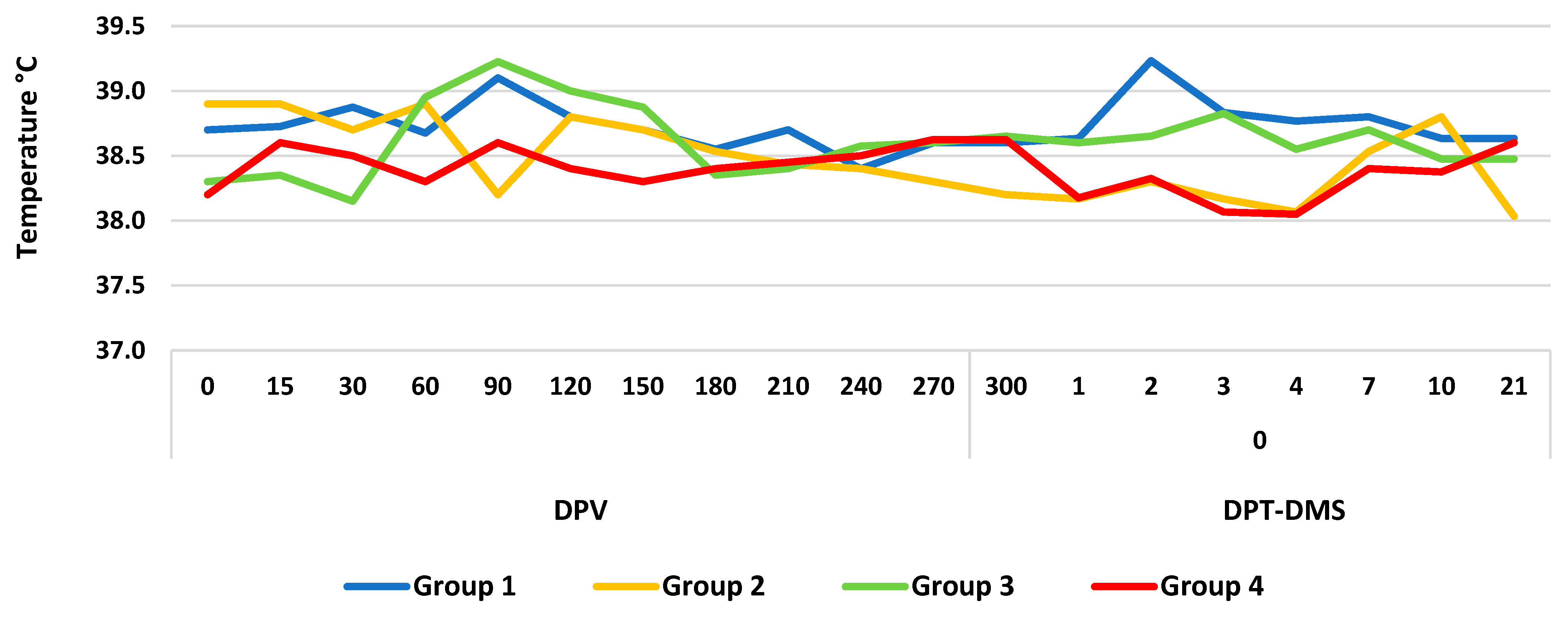
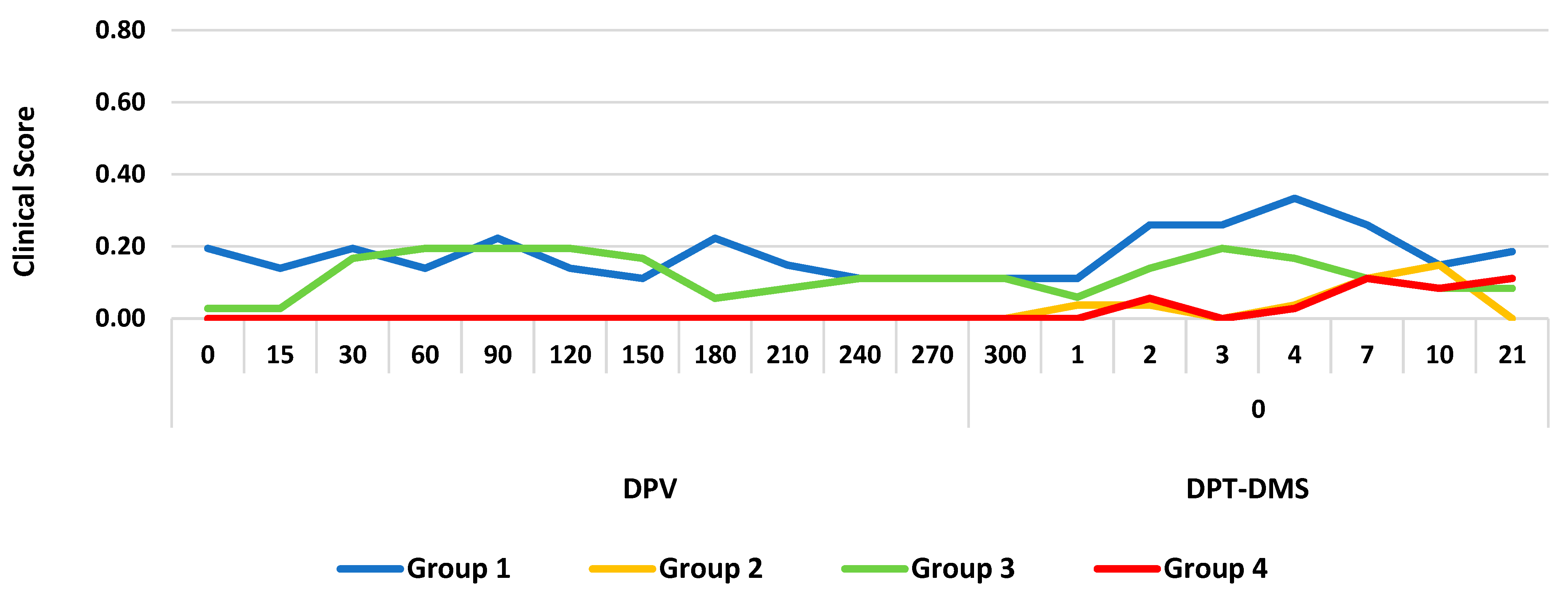
3.1. Clinical Response
3.2. Serological Investigations
3.3. Virological Investigations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV Report. Herpesviridae. 2022. Available online: https://ictv.global/taxonomy/taxondetails?taxnode_id=202201440/ (accessed on 31 July 2023).
- Righi, C.; Franzoni, G.; Jones, C.; Petrini, S. The cell-mediated immune response against Bovine alphaherpesvirus 1 (BoHV-1) infection and vaccination. Vaccines 2023, 11, 785. [Google Scholar] [CrossRef]
- Raaperi, K.; Orro, T.; Viltrop, A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet. J. 2014, 201, 249–256. [Google Scholar] [CrossRef]
- Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016, on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law). Off. J. Eur. Union 2016, 84, 1–208.
- European Commission. Commission Implementing Regulation (EU) 2018/1882 of 3 December 2018, on the application of certain disease prevention and control rules to categories of listed diseases and establishing a list of species and groups of species posing a considerable risk for the spread of these diseases. Off. J. Eur. Union 2018, 308, 1–9. [Google Scholar]
- Mananteau-Horta, A.M.; Ames, T.R.; Johnson, D.W.; Meiske, J.C. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine virus diarrhea vaccines. Can. J. Comp. Med. 1985, 49, 10–14. [Google Scholar]
- Bradshaw, B.J.; Edwards, S. Antibody isotype responses to experimental infection with bovine herpesvirus 1 in calves with colostrally derived antibody. Vet. Microbiol. 1996, 53, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Discontools IBR. Available online: https://www.discontools.eu/database/80-bhv-i-ibr.html (accessed on 31 July 2023).
- Lemaire, M.; Meyer, G.; Baranowski, E.; Schynts, F.; Wellemans, G.; Kerkhofs, P.; Thiry, E. Production of bovine herpesvirus type 1-seronegative latent carriers by administration of a live-attenuated vaccine in passively immunized calves. J. Clin. Microbiol. 2000, 38, 4233–4238. [Google Scholar] [CrossRef]
- Directive 2010/63/EC of the Parliament and of the Council of 22 September 2010, on the protection of the animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 1–47.
- Reed, L.J.; Muench, H.A. A simple method for estimating 50% end points. Am. J. Hyg. 1933, 27, 493–497. [Google Scholar]
- WOAH—Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2018. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.11_IBR_IPV.pdf (accessed on 29 December 2023).
- Commission Delegated Regulation (EU) 2018/1629 of 25 July 2018, amending the list of diseases set out in Annex II to Regulation (EU) 2016/429 of the European Parliament and of the Council on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law). Off. J. Eur. Union 2018, 272, 1–5.
- Zhang, M.; Fu, S.; Deng, M.; Xie, Q.; Xu, H.; Liu, Z.; Hu, C.; Chen, H.; Guo, A. Attenuation of bovine herpesvirus type 1 by deletion of its glycoprotein G and tk genes and protection against virulent viral challenge. Vaccine 2011, 29, 8943–8950. [Google Scholar] [CrossRef]
- Strube, W.; Bergle, A.R.D.; Block, W.; Heinen, E.; Kretzdorn, D.; Rodenbach, C.; Schmeer, N. Safety aspect in the development of an infectious bovine rhinotracheitis marker vaccine. Dev. Biol. Stand. 1995, 84, 75–81. [Google Scholar] [PubMed]
- Castrucci, G.; Frigeri, F.; Salvatori, D.; Ferrari, M.; Sardonini, Q.; Cassai, E.; Lo Dico, M.; Rotola, A.; Angelini, R. Vaccination of calves against bovine herpesvirus-1: Assessment of the protective value of eight vaccines. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Makoschey, B.; Beer, M. A live bovine herpesvirus-1 marker vaccine is not shed after intramuscular vaccination. Berl. Munch. Tierarztl. Wochenschr. 2007, 120, 480–482. [Google Scholar] [PubMed]
- Montagnaro, S.; De Martinis, C.; Iovane, V.; Ciarcia, R.; Damiano, S.; Nizza, S.; De Martino, L.; Iovane, G.; Pagnini, U. Bovine herpesvirus type 1 marker vaccine induces cross-protection against bubaline herpesvirus type 1 in water buffalo. Prev. Vet. Med. 2014, 116, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Martucciello, A.; Balestrieri, A.; Righi, C.; Cappelli, G.; Scoccia, E.; Grassi, C.; Brandi, S.; Rossi, E.; Galiero, G.; Gioia, D.; et al. Evaluation of an Immunization Protocol Using Bovine Alphaherpesvirus 1 gE-Deleted Marker Vaccines against Bubaline Alphaherpesvirus 1 in Water Buffaloes. Vaccines 2023, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Allcock, J.; May, B.; Wright, T.; Otter, A. Suspected lack of efficacy of a live IBR marker vaccine in two dairy herds. Vet. Rec. 2010, 6, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Bacilli, C.; Martin, C.; Silva, K.N.; Nichi, M.; Flores, E.; Vercesi Filho, A.; Pituco, E.; Gomes, V. Serological response against bovine herpesvirus and bovine viral diarrhea virus induced by commercial vaccines in Holstein heifers. Pasq. Vet. Bras. 2019, 39, 870–878. [Google Scholar] [CrossRef]
- Wernike, K.; Hoffmann, B.; Kalthoff, D.; Konig, P.; Beer, M. Development and Validation of a Triplex Real-Time PCR Assay for the rapid detection and differentiation of wild-type and glycoprotein E-deleted vaccine strains of Bovine herpesvirus type 1. J. Virol. Method. 2011, 174, 77–84. [Google Scholar] [CrossRef]
- Strube, W.; Auer, S.; Block, W.; Heinen, E.; Kretzdorn, D.; Rodenbach, C.; Schmeer, N. A gE-deleted infectious bovine rhinotracheitis marker vaccine for use in improved bovine herpesvirus 1 control programs. Vet. Microbiol. 1996, 53, 181–189. [Google Scholar] [CrossRef]
- Petrini, S.; Martucciello, A.; Righi, C.; Cappelli, G.; Torresi, C.; Grassi, C.; De Carlo, E.; Feliziani, F. Assessment of Different Infectious Bovine Rhinotracheitis Marker Vaccines in Calves. Vaccines 2022, 10, 1204. [Google Scholar] [CrossRef]
- Agrawal, P.; Nawadkar, R.; Ojha, H.; Kumar, J.; Sahu, A. Complement Evasion Strategies of Viruses: An Overview. Front. Microbiol. 2017, 8, 1117. [Google Scholar] [CrossRef]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bandyopadhyay, S.; Dimri, U.; Patra, P.H. Bovine herpesvirus-1 a re-emerging concern in livestock: A revisit to its biology, epidemiology, diagnosis, and prophylaxis. Vet. Q. 2013, 33, 68–81. [Google Scholar] [CrossRef]
- Lemaire, M.; Hanon, E.; Schynts, G.; Meyer, G.; Thiry, E. Specific passive immunity reduces the excretion of the glycoprotein E-negative bovine herpes virus type 1 vaccine strain in calves. Vaccine 2000, 19, 1013–1017. [Google Scholar] [CrossRef]
- De Brun, L.; Leites, M.; Furtado, A.; Campos, F.; Roehe, P.; Puentes, R. Field Evaluation of commercial vaccines against infectious bovine rhinotracheitis (IBR) virus using different immunization protocols. Vaccines 2021, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Kaashoek, M.J.; Rijsewijk, F.A.; Oirschot, J.T. Persistence of antibodies against bovine herpesvirus 1 and virus reactivation two to three years after infection. Vet. Microbiol. 1996, 53, 103–110. [Google Scholar] [CrossRef]
- Kaashoek, M.J.; Rijsewijk, F.A.; Ruuls, R.C.; Keil, G.M.; Thiry, E.; Pastoret, P.P.; van Oirschot, J.T. Virulence, immunogenicity and reactivation of bovine herpesvirus 1 mutants with a deletion in the gC, gG, gI, gE, or in both the gI and gE gene. Vaccine 1998, 16, 802–809. [Google Scholar] [CrossRef]
- Kaashoek, M.J.; Kaashoek, M.J.; Moerman, A.; Madic, J.; Rijsewijk, F.A.; Quak, J.; Gielkens, A.L.; van Oirschot, J.T. A Conventionally attenuated glycoprotein E-negative strain of bovine herpesvirus type 1 is an efficacious and safe vaccine. Vaccine 1994, 12, 239–444. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.C.; Kaashoek, M.J.; Kroese, A.H.; van Oirschot, J.T. An attenuated bovine herpesvirus 1 marker vaccine induced a better protection than two inactivated marker vaccines. Vet. Microbiol. 1996, 52, 223–234. [Google Scholar] [CrossRef]
- Choudry, S.I.; Ross, C.S.; Lee, B.J.; Hall, V.; Chu, H.J. Construction and characterization of a glycoprotein E gene-deleted bovine herpesvirus type 1 recombinant. Am. J. Vet. Res. 1999, 60, 227–232. [Google Scholar]
- Belknap, E.B.; Walters, L.M.; Kelling, C.; Ayers, V.K.; Norris, J.; McMillen, J.; Hayhow, C.; Cochran, M.; Reddy, D.N.; Wright, J.; et al. Immunogenicity and protective efficacy of a gE, gG, and US2 gene-deleted bovine herpesvirus-1 (BHV-1) vaccine. Vaccine 1999, 17, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Romera, S.A.; Puntel, M.; Quattrocchi, V.; Del Medico Zajac, P.; Zamorano, P.; Viera, J.B.; Carrillo, C.; Chowdhury, S.; Borca, M.V.; Sadir, A.M. Protection induced by a glycoprotein E-deleted bovine herpesvirus type 1 marker strain used either as an inactivated or live attenuated vaccine in cattle. BMC Vet. Res. 2014, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Castrucci, G.; Frigeri, F.; Salvatori, D.; Ferrari, M.; Lo Dico, M.; Rotola, A.; Sardonini, Q.; Petrini, S.; Cassai, E. A study on latency in calves by five vaccines against bovine herpesvirus-1 infection. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 205–215. [Google Scholar] [CrossRef]
- Forsgren, M.; Klapper, P.E. Herpes Simplex Virus Type 1 and type 2. In Principle & Practice of Clinical Virology, 6th ed.; Zuckerman, A.J., Banatvala, J.E., Scoub, B.D., Griffiths, P.D., Mortimer, P., Eds.; Wiley-Blackwell: West Sussex, UK, 2009; pp. 96–131. ISBN 978-0-470-51799-4. [Google Scholar]
- Lecchi, C.; Ceciliani, F.; Petrini, S.; Cappelli, G.; Grassi, C.; Balestrieri, A.; Galiero, G.; De Carlo, E.; Salvi, G.; Panzeri, F.; et al. Endogenous and viral microRNAs in nasal secretions of water buffaloes (Bubalus bubalis) after Bubaline alphaherpesvirus 1 (BuHV-1) challenge infection. Vet. Res. 2023, 54, 44. [Google Scholar] [CrossRef]
| Group | Test | DPV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | ||
| gE-ELISA a | − | − | − | − | − | − | − | − | − | − | − | − | |
| 1 | gB-ELISA b | − | + | + | + | + | + | + | + | + | + | − | − |
| NA c,d | 0.000 | 0.401 | 1.806 | 2.408 | 2.709 | 2.509 | 2.208 | 1.806 | 1.204 | 0.401 | 0.000 | 0.000 | |
| gE-ELISA a | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2 | gB-ELISA b | + | + | + | + | + | + | + | − | − | − | − | − |
| NA c,d | 2.308 | 2.107 | 2.107 | 1.706 | 1.706 | 1.003 | 0.702 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| p-value | 0.0039 | 0.0431 | 0.1213 | 0.1213 | 0.0431 | 0.0431 | 0.0463 | 0.0369 | 0.0369 | 0.0369 | − | − | |
| Group | Test | DPV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | ||
| gE-ELISA a | − | − | − | − | − | − | − | − | − | − | − | − | |
| 3 | gB-ELISA b | − | + | + | + | + | + | + | + | + | + | − | − |
| NA c,d | 0.000 | 0.903 | 1.505 | 1.505 | 2.007 | 2.208 | 2.107 | 1.806 | 1.505 | 0.903 | 0.000 | 0.000 | |
| gE-ELISA a | − | − | − | − | − | − | − | − | − | − | − | − | |
| 4 | gB-ELISA b | + | + | + | + | + | + | + | + | + | + | − | − |
| NA c,d | 1.505 | 1.505 | 1.505 | 1.505 | 1.505 | 1.405 | 1.204 | 0.602 | 0.502 | 0.401 | 0.000 | 0.000 | |
| p-value | 0.0369 | 0.6579 | 0.8166 | 0.8166 | 0.2463 | 0.0463 | 0.0369 | 0.0369 | 0.0369 | 0.4795 | − | − | |
| Group | Test | DPT-DMS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | 10 | 15 | 21 | 28 | 60 | 90 | 120 | ||
| 3 | gE-ELISA a | − | − | − | − | − | − | − | − | − | − | − | − |
| gB-ELISA b | − | − | − | − | − | − | − | − | − | − | − | − | |
| NA c,d | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 4 | gE-ELISA a | − | − | − | − | − | − | − | − | − | − | − | − |
| gB-ELISA b | − | − | − | − | − | + | + | + | + | + | + | + | |
| NA c,d | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.401 | 0.602 | 0.803 | 0.903 | 0.903 | 0.903 | 1.003 | |
| p-value | − | − | − | − | − | 0.3173 | 0.1138 | 0.1138 | 0.1213 | 0.1213 | 0.1213 | 0.0369 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrini, S.; Righi, C.; Costantino, G.; Scoccia, E.; Gobbi, P.; Pellegrini, C.; Pela, M.; Giammarioli, M.; Viola, G.; Sabato, R.; et al. Assessment of BoAHV-1 Seronegative Latent Carrier by the Administration of Two Infectious Bovine Rhinotracheitis Live Marker Vaccines in Calves. Vaccines 2024, 12, 161. https://doi.org/10.3390/vaccines12020161
Petrini S, Righi C, Costantino G, Scoccia E, Gobbi P, Pellegrini C, Pela M, Giammarioli M, Viola G, Sabato R, et al. Assessment of BoAHV-1 Seronegative Latent Carrier by the Administration of Two Infectious Bovine Rhinotracheitis Live Marker Vaccines in Calves. Vaccines. 2024; 12(2):161. https://doi.org/10.3390/vaccines12020161
Chicago/Turabian StylePetrini, Stefano, Cecilia Righi, Giulia Costantino, Eleonora Scoccia, Paola Gobbi, Claudia Pellegrini, Michela Pela, Monica Giammarioli, Giulio Viola, Roberto Sabato, and et al. 2024. "Assessment of BoAHV-1 Seronegative Latent Carrier by the Administration of Two Infectious Bovine Rhinotracheitis Live Marker Vaccines in Calves" Vaccines 12, no. 2: 161. https://doi.org/10.3390/vaccines12020161
APA StylePetrini, S., Righi, C., Costantino, G., Scoccia, E., Gobbi, P., Pellegrini, C., Pela, M., Giammarioli, M., Viola, G., Sabato, R., Tinelli, E., & Feliziani, F. (2024). Assessment of BoAHV-1 Seronegative Latent Carrier by the Administration of Two Infectious Bovine Rhinotracheitis Live Marker Vaccines in Calves. Vaccines, 12(2), 161. https://doi.org/10.3390/vaccines12020161





