Assessing the Implementation Determinants of Pilot Malaria Vaccination Programs in Ghana, Kenya, and Malawi through a Complexity Lens: A Rapid Review Using a Consolidated Framework for Implementation Research
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Narrative Synthesis Using Qualitative Analysis
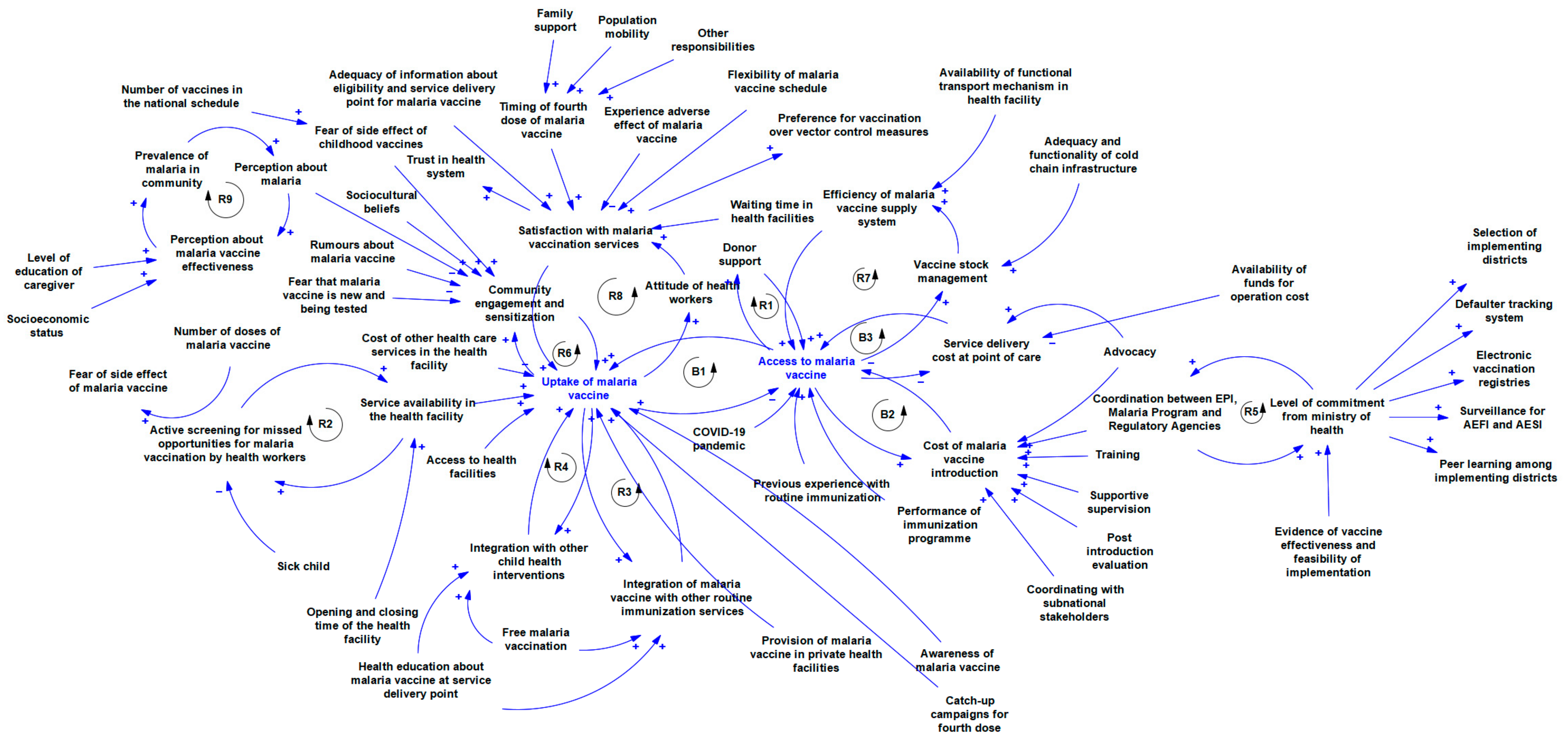
2.5. Development of the Causal Loop Diagram
3. Results
3.1. Characteristics of Included Studies
3.2. Implementation Determinants of Malaria Vaccine Pilot in Ghana, Kenya, and Malawi
4. Discussion
Implications for Policy and Practice
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Penny, M.A.; Maire, N.; Bever, C.A.; Pemberton-Ross, P.; Briët, O.J.T.; Smith, D.L.; Gething, P.W.; Smith, T.A. Distribution of malaria exposure in endemic countries in Africa considering country levels of effective treatment. Malar. J. 2015, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasites Vectors 2010, 3, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G.; Egan, A.; Keusch, G.T. The intolerable burden of malaria: A new look at the numbers. In The Intolerable Burden of Malaria: A New Look at the Numbers: Supplement to Volume 64 (1) of the American Journal of Tropical Medicine and Hygiene; American Society of Tropical Medicine and Hygiene: Springfield, IL, USA, 2001. [Google Scholar]
- Sarma, N.; Patouillard, E.; Cibulskis, R.E.; Arcand, J.-L. The Economic Burden of Malaria: Revisiting the Evidence. Am. J. Trop. Med. Hyg. 2019, 101, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Samba, E. The malaria burden and Africa. Am. J. Trop. Med. Hyg. 2001, 64, 14–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Malaria Eradication: Benefits, Future Scenarios and Feasibility: A Report of the Strategic Advisory Group on Malaria Eradication; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Malaria vaccine: WHO Position Paper—March 2022. Wkly. Epidemiol. Rec. 2022, 97, 60–78. [Google Scholar]
- World Health Organization. WHO Recommends R21/Matrix-M Vaccine for Malaria Prevention in Updated Advice on Immunization [Internet]. 2023. Available online: https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization (accessed on 28 November 2023).
- Beeson, J.G.; Kurtovic, L.; Valim, C.; Asante, K.P.; Boyle, M.J.; Mathanga, D.; Dobano, C.; Moncunill, G. The RTS,S malaria vaccine: Current impact and foundation for the future. Sci. Transl. Med. 2022, 14, eabo6646. [Google Scholar] [CrossRef]
- World Health Organization. Malaria: The Malaria Vaccine Implementation Programme (MVIP) [Internet]. 2020. Available online: https://www.who.int/news-room/questions-and-answers/item/malaria-vaccine-implementation-programme (accessed on 28 November 2023).
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malaria vaccine: WHO Position Paper–January 2016. Wkly. Epidemiol. Rec. 2016, 91, 33–52. [Google Scholar]
- World Health Organization. Malaria vaccine: WHO position paper, January 2016—Recommendations. Vaccine 2018, 36, 3576–3577. [Google Scholar] [CrossRef]
- Malaria Vaccine Pilot Launched in Ghana [Internet]. WHO|Regional Office for Africa. 2023. Available online: https://www.afro.who.int/news/malaria-vaccine-pilot-launched-ghana (accessed on 28 November 2023).
- RTS,S Malaria Vaccine Reaches More Than 650 000 Children in Ghana, Kenya and Malawi through Groundbreaking Pilot Programme [Internet]. Available online: https://www.who.int/news/item/20-04-2021-rts-s-malaria-vaccine-reaches-more-than-650-000-children-in-ghana-kenya-and-malawi-through-groundbreaking-pilot-programme (accessed on 28 November 2023).
- Mumtaz, H.; Nadeem, A.; Bilal, W.; Ansar, F.; Saleem, S.; Khan, Q.A.; Tango, T.; Farkouh, C.; Belay, N.F.; Verma, R.; et al. Acceptance, availability, and feasibility of RTS, S/AS01 malaria vaccine: A review. Immun. Inflamm. Dis. 2023, 11, e899. [Google Scholar] [CrossRef]
- Devi, S. 12 countries to get first doses of malaria vaccine. Lancet 2023, 402, 172. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Ferlie, E.B.; Shortell, S.M. Improving the Quality of Health Care in the United Kingdom and the United States: A Framework for Change. Milbank Q. 2001, 79, 281–315. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, P. Making sense of implementation theories, models and frameworks. Implement. Sci. 2015, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, L.J.; Reardon, C.M.; Widerquist, M.A.O.; Lowery, J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement. Sci. 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.A.; Kelley, C.; Yankey, N.; Birken, S.A.; Abadie, B.; Damschroder, L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement. Sci. 2015, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Borghi, J.; Chalabi, Z. Square peg in a round hole: Re-thinking our approach to evaluating health system strengthening in low-income and middle-income countries. BMJ Glob. Health 2017, 2, e000406. [Google Scholar] [CrossRef]
- Peters, D.H. The application of systems thinking in health: Why use systems thinking? Health Res. Policy Sys. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Roxas, F.M.Y.; Rivera, J.P.R.; Gutierrez, E.L.M. Locating Potential Leverage Points In A Systems Thinking Causal Loop Diagram Toward Policy Intervention. World Future 2019, 75, 609–631. [Google Scholar] [CrossRef]
- Tomoaia-Cotisel, A.; Kim, H.; Allen, S.D.; Blanchet, K. Causal Loop Diagrams: A Tool for Visualizing Emergent System Behaviour. In Applied Systems Thinking for Health Systems Research: A Methodological Handbook; McGraw-Hill: New York, NY, USA, 2017; pp. 97–114. [Google Scholar]
- Ganann, R.; Ciliska, D.; Thomas, H. Expediting systematic reviews: Methods and implications of rapid reviews. Implement. Sci. 2010, 5, 56. [Google Scholar] [CrossRef]
- Garritty, C.; Gartlehner, G.; Nussbaumer-Streit, B.; King, V.J.; Hamel, C.; Kamel, C.; Affengruber, L.; Stevens, A. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J. Clin. Epidemiol. 2021, 130, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Clarke, V.; Braun, V.; Hayfield, N. Thematic analysis. Qualitative psychology: A practical guide to research methods. Qual. Psychol. 2015, 3, 222–248. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psycholog. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Baral, R.; Levin, A.; Odero, C.; Pecenka, C.; Bawa, J.T.; Antwi-Agyei, K.O.; Amponsa-Achaino, K.; Chisema, M.N.; Jalango, R.E.; Mkisi, R.; et al. Cost of introducing and delivering RTS,S/AS01 malaria vaccine within the malaria vaccine implementation program. Vaccine 2023, 41, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, J.; Okello, G.; Bange, T.; Kariuki, S.; Jalloh, M.F.; Webster, J.; Hill, J. RTS,S/AS01 malaria vaccine pilot implementation in western Kenya: A qualitative longitudinal study to understand immunisation barriers and optimise uptake. BMC Public Health 2023, 23, 2283. [Google Scholar] [CrossRef] [PubMed]
- Adeshina, O.O.; Nyame, S.; Milner, J.; Milojevic, A.; Asante, K.P. Barriers and facilitators to nationwide implementation of the malaria vaccine in Ghana. Health Policy Plan. 2023, 38, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Okyere, J.; Bediako, V.B.; Ackah, J.A.; Acheampong, E.; Owusu, B.A.; Agbemavi, W.; Nwameme, A.U.; Kamau, E.M.; Asampong, E. RTS,S/AS01E vaccine defaults in Ghana: A qualitative exploration of the perspectives of defaulters and frontline health service providers. Malar. J. 2023, 22, 260. [Google Scholar] [CrossRef]
- Merle, C.S.; Affoukou, C.D.; Affo, S.Y.; Djogbenou, S.L.; Hounto, A.; Kompaore, S.C.B.; Ouedraogo, I.; Ateba, M.J.; Njoh, A.A.; Bomba, D.; et al. Implementation strategies for the introduction of the RTS,S/AS01 (RTS,S) malaria vaccine in countries with areas of highly seasonal transmission: Workshop meeting report. Malar. J. 2023, 22, 242. [Google Scholar] [CrossRef]
- Adjei, M.R.; Amponsa-Achiano, K.; Okine, R.; Tweneboah, P.O.; Sally, E.T.; Dadzie, J.F.; Osei-Sarpong, F.; Adjabeng, M.J.; Bawa, J.T.; Bonsu, G.; et al. Post introduction evaluation of the malaria vaccine implementation programme in Ghana, 2021. BMC Public Health 2023, 23, 586. [Google Scholar] [CrossRef]
- Bam, V.; Mohammed, A.; Kusi-Amponsah, A.; Armah, J.; Lomotey, A.Y.; Budu, H.I.; Poku, C.A.; Kyei-Dompim, J.; Dwumfour, C. Caregivers’ perception and acceptance of malaria vaccine for Children. PLoS ONE 2023, 18, e0288686. [Google Scholar] [CrossRef]
- Darkwa, S.; de Wildt, G.; Dalaba, M.; Vidzro, E.; Ansah, E.K. “I would have to sell things in order to get the money”: A qualitative exploration of willingness to pay for the RTS,S/AS01 malaria vaccine in the Volta region, Ghana. PLoS ONE 2022, 17, e0268009. [Google Scholar]
- Grant, J.; Gyan, T.; Agbokey, F.; Webster, J.; Greenwood, B.; Asante, K.P. Challenges and lessons learned during the planning and early implementation of the RTS,S/AS01E malaria vaccine in three regions of Ghana: A qualitative study. Malar. J. 2022, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Tabiri, D.; Ouédraogo, J.C.R.P.; Nortey, P.A. Factors associated with malaria vaccine uptake in Sunyani Municipality, Ghana. Malar. J. 2021, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, D.; Owusu-Marfo, J.; Agyeman, Y.N. Predictors of malaria vaccine uptake among children 6–24 months in the Kassena Nankana Municipality in the Upper East Region of Ghana. Malar. J. 2022, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Immurana, M.; Boachie, M.K.; Klu, D.; Dalaba, M.A.; Manyeh, A.K.; Alhassan, R.K. Determinants of willingness to accept child vaccination against malaria in Ghana. Int. J. Health Plan. Manag. 2022, 37, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Baral, R.; Levin, A.; Odero, C.; Pecenka, C.; Tabu, C.; Mwendo, E.; Bonsu, G.; Bawa, J.; Dadzie, J.F.; Charo, J.; et al. Costs of continuing RTS,S/ASO1E malaria vaccination in the three malaria vaccine pilot implementation countries. PLoS ONE 2021, 16, e0244995. [Google Scholar]
- Stetler, C.B.; Legro, M.W.; Wallace, C.M.; Bowman, C.; Guihan, M.; Hagedorn, H.; Kimmel, B.; Sharp, N.D.; Smith, J.L. The role of formative evaluation in implementation research and the QUERI experience. J. Gen. Intern. Med. 2006, 21, S1–S8. [Google Scholar] [CrossRef]
- Thomas-Henkel, C.; Schulman, M. Screening for social determinants of health in populations with complex needs: Implementation considerations. Cent. Health Care Strateg. 2017, 10, 6. [Google Scholar]
- A Flottorp, S.; Oxman, A.D.; Krause, J.; Musila, N.R.; Wensing, M.; Godycki-Cwirko, M.; Baker, R.; Eccles, M.P. A checklist for identifying determinants of practice: A systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement. Sci. 2013, 8, 35. [Google Scholar] [CrossRef]
- May, C.R.; Johnson, M.; Finch, T. Implementation, context and complexity. Implement. Sci. 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Naseem, R.; Salam, R.A.; Siddiqui, F.; Das, J.K. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 988. [Google Scholar] [CrossRef]
- Buonsenso, D.; Cinicola, B.; Kallon, M.N.; Iodice, F. Child Healthcare and Immunizations in Sub-Saharan Africa During the COVID-19 Pandemic. Front. Pediatr. 2020, 8, 517. [Google Scholar] [CrossRef]
- World Health Organization. 18 Million Doses of First-Ever Malaria Vaccine Allocated to 12 African Countries for 2023–2025: Gavi, WHO and UNICEF [Internet]. 2023. Available online: https://www.who.int/news/item/05-07-2023-18-million-doses-of-first-ever-malaria-vaccine-allocated-to-12-african-countries-for-2023-2025--gavi--who-and-unicef (accessed on 28 November 2023).

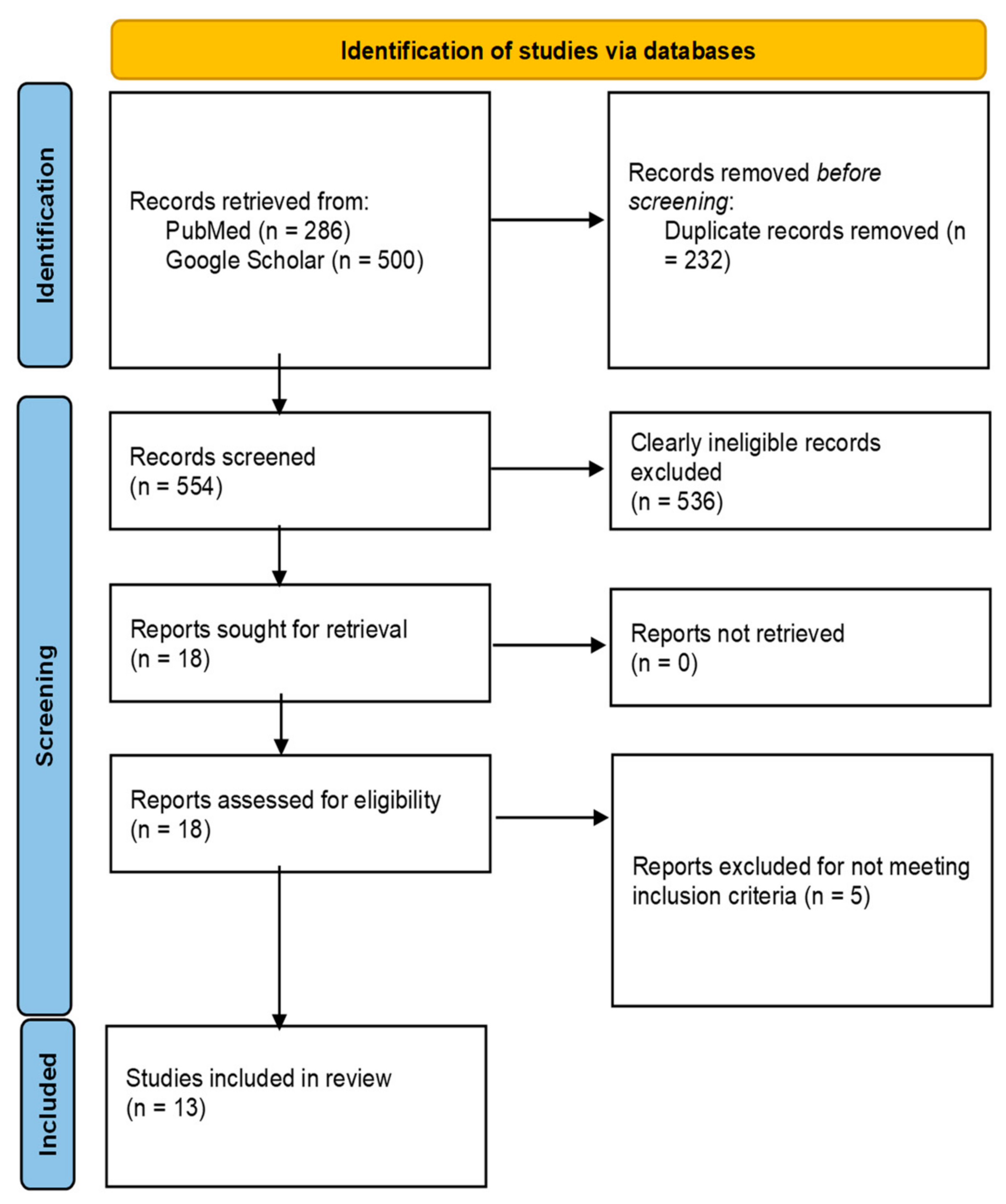

| S/No | Authors Name | Year of Publication | Country | Study Design | Target Population |
|---|---|---|---|---|---|
| 1 | Baral et al. [32] | 2023 | Ghana, Kenya, Malawi | Cross-sectional quantitative study | Government officials, health workers |
| 2 | Hoyt et al. [33] | 2023 | Kenya | Longitudinal qualitative study | Caregivers |
| 3 | Adeshina et al. [34] | 2023 | Ghana | Cross-sectional qualitative study | National program managers, research/academia, and program implementation partners |
| 4 | Okyere et al. [35] | 2023 | Ghana | Cross-sectional qualitative study | Health service providers and mothers |
| 5 | Merle et al. [36] | 2023 | Ghana, Kenya, Malawi | Cross-sectional qualitative study | Program managers |
| 6 | Adjei et al. [37] | 2023 | Ghana | Mixed methods study | EPI managers, coordinators and focal persons, healthcare workers, data managers, cold-chain managers, and caregivers |
| 7 | Bam et al. [38] | 2023 | Ghana | Cross-sectional qualitative study | Caregivers |
| 8 | Darkwa et al. [39] | 2022 | Ghana | Cross-sectional qualitative study | Caregivers of children that were involved in the RTSS pilot |
| 9 | Grant et al. [40] | 2022 | Ghana | Gross-sectional qualitative study | Regional and district health service managers and frontline health workers |
| 10 | Tabiri et al. [41] | 2022 | Ghana | Cross-sectional quantitative study | Caregivers |
| 11 | Yeboah et al. [42] | 2022 | Ghana | Cross-sectional quantitative study | Caregivers |
| 12 | Immurana et al. [43] | 2022 | Ghana | Cross-sectional quantitative study | Caregivers |
| 13 | Baral et al. [44] | 2021 | Ghana, Kenya, Malawi | Mixed-methods study | Ministry of Health officials at national and sub-national levels |
| Level of Influence | |||||||
|---|---|---|---|---|---|---|---|
| S/No | Determinants | Malaria Vaccine | Caregiver | Health Workers | Health Facilities | Health System | Society |
| 1 | Cost of malaria vaccine introduction | ||||||
| 2 | Service delivery cost at the point of care | ||||||
| 3 | Satisfaction with malaria vaccination services | ||||||
| 4 | Preference for vaccination over vector control measures | ||||||
| 5 | Perception about malaria | ||||||
| 6 | Free malaria vaccination | ||||||
| 7 | Awareness of the malaria vaccine | ||||||
| 8 | Previous experience with routine immunization | ||||||
| 9 | Access to health facilities | ||||||
| 10 | Perception about malaria vaccine effectiveness | ||||||
| 11 | Attitude of health workers | ||||||
| 12 | Adequacy of information about eligibility and the service delivery point for the malaria vaccine | ||||||
| 13 | Fear of side effects of childhood vaccines | ||||||
| 14 | Fear that the malaria vaccine is new and being tested | ||||||
| 15 | Other responsibilities | ||||||
| 16 | Service availability in the health facility | ||||||
| 17 | Socioeconomic status | ||||||
| 18 | Health education about malaria vaccine at service delivery point | ||||||
| 19 | Active screening for missed opportunities for malaria vaccination by health workers | ||||||
| 20 | COVID-19 pandemic | ||||||
| 21 | Community engagement and sensitization | ||||||
| 22 | Prevalence of malaria in the community | ||||||
| 23 | Evidence of vaccine effectiveness and feasibility of implementation | ||||||
| 24 | Availability of funds for operation cost | ||||||
| 25 | Population mobility | ||||||
| 26 | Number of vaccines in the national schedule | ||||||
| 27 | Number of doses of malaria vaccine | ||||||
| 28 | Family support | ||||||
| 29 | Flexibility of malaria vaccine schedule | ||||||
| 30 | Timing of the fourth dose of malaria vaccine | ||||||
| 31 | Selection of implementing districts | ||||||
| 32 | Fear of side effects of malaria vaccine | ||||||
| 33 | Experience adverse effects from malaria vaccine | ||||||
| 34 | Adequacy and functionality of cold-chain infrastructure | ||||||
| 35 | Availability of functional transport mechanism in health facility | ||||||
| 36 | Peer learning among implementing districts | ||||||
| 37 | Efficiency of the malaria vaccine supply system | ||||||
| 38 | Rumors about the malaria vaccine | ||||||
| 39 | Training | ||||||
| 40 | Level of commitment from the Ministry of Health | ||||||
| 41 | Supportive supervision | ||||||
| 42 | Post introduction evaluation | ||||||
| 43 | Coordination between EPI, the malaria program, and regulatory agencies | ||||||
| 44 | Coordinating with subnational stakeholders | ||||||
| 45 | Integration with other child health interventions | ||||||
| 46 | Defaulter tracking system | ||||||
| 47 | Catch-up campaigns for the fourth dose | ||||||
| 48 | Electronic vaccination registries | ||||||
| 49 | Vaccine stock management | ||||||
| 50 | Surveillance for AEFI and AESI | ||||||
| 51 | Trust in the health system | ||||||
| 52 | Waiting time in health facilities | ||||||
| 53 | Advocacy | ||||||
| 54 | Level of education of the caregiver | ||||||
| 55 | Sociocultural beliefs | ||||||
| 56 | Cost of other healthcare services in the health facility | ||||||
| 57 | Opening and closing times of the health facility | ||||||
| 58 | Sick child | ||||||
| 59 | Performance of the immunization program | ||||||
| 60 | Integration of the malaria vaccine into other routine immunization services | ||||||
| 61 | Provision of the malaria vaccine in private health facilities | ||||||
| 62 | Donor support | ||||||
 —Innovation;
—Innovation;  —Outer setting;
—Outer setting;  —Inner setting;
—Inner setting;  —Individuals;
—Individuals;  —Implementation process.
—Implementation process.| CFIR Domains | CFIR Constructs | Identified Determinants |
|---|---|---|
| Innovation | ||
| Innovation cost | Cost of malaria vaccine introduction | |
| Innovation cost | Service delivery cost at the point of care | |
| Innovation evidence base | Evidence of vaccine effectiveness and the feasibility of implementation | |
| Innovation complexity | Number of doses of the malaria vaccine | |
| Innovation adaptability | Flexibility of the malaria vaccine schedule | |
| Innovation design | Timing of the fourth dose of malaria vaccine | |
| Outer setting | ||
| Critical incidents | COVID-19 pandemic | |
| Local conditions | Prevalence of malaria in the community | |
| Local attitudes | Family support | |
| Policies and laws | Selection of implementing districts | |
| Local attitudes | Rumors about the malaria vaccine | |
| Local attitudes | Trust in the health system | |
| Local attitudes | Sociocultural beliefs | |
| Financing | Donor support | |
| Inner setting | ||
| Relative priority | Level of commitment from the Ministry of Health | |
| Available resources | Free malaria vaccination | |
| Available resources | Availability of funds for operation cost | |
| Available resources | Adequacy and functionality of the cold-chain infrastructure | |
| Available resources | Availability of the functional transport mechanism in health facility | |
| Structural characteristics | Access to health facilities | |
| Structural characteristics | Service availability in the health facility | |
| Compatibility | Number of vaccines in the national schedule | |
| Access to knowledge and information | Health education about the malaria vaccine at the service delivery point | |
| Communication | Peer learning among the implementing districts | |
| Structural characteristics | Efficiency of the malaria vaccine supply system | |
| Access to knowledge and information | Training | |
| Structural characteristics | Waiting times in health facilities | |
| Compatibility | Cost of other healthcare services in the health facility | |
| Structural characteristics | Opening and closing times of the health facility | |
| Structural characteristics | Performance of the immunization program | |
| Structural characteristics | Integration of the malaria vaccine with other routine immunization services | |
| Relational connections | Provision of the malaria vaccine in private health facilities | |
| Individuals | ||
| Capability | Active screening for missed opportunities for malaria vaccination by health workers | |
| Capability | Attitudes of health workers | |
| Motivation | Satisfaction with malaria vaccination services | |
| Need | Preference for vaccination over vector control measures | |
| Need | Perceptions about malaria | |
| Need | Awareness of the malaria vaccine | |
| Need | Previous experience with routine immunization | |
| Need | Perception about malaria vaccine effectiveness | |
| Opportunity | Adequacy of information about eligibility and service delivery points for the malaria vaccine | |
| Need | Fear of side effects of childhood vaccines | |
| Need | Fear that the malaria vaccine is new and being tested | |
| Need | Sick child | |
| Opportunity | Other responsibilities | |
| Opportunity | Socioeconomic status | |
| Opportunity | Population mobility | |
| Need | Fear of side effects of the malaria vaccine | |
| Motivation | Childexperienced adverse effects from the malaria vaccine | |
| Capability | Level of education of the caregiver | |
| Implementation process | ||
| Engaging | Community engagement and sensitization | |
| Engaging | Supportive supervision | |
| Assessing context | Post-introduction evaluation | |
| Teaming | Coordination between the EPI, the malaria control program, and regulatory agencies | |
| Teaming | Coordinating with subnational stakeholders | |
| Adapting | Integration with other child health interventions | |
| Performing | Defaulter tracking system | |
| Performing | Catch-up campaigns for the fourth dose | |
| Tailoring strategies | Electronic vaccination registries | |
| Performing | Vaccine stock management | |
| Assessing context | Surveillance for the AEFI and AESI | |
| Engaging | Advocacy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamu, A.A.; Jalo, R.I.; Ndwandwe, D.; Wiysonge, C.S. Assessing the Implementation Determinants of Pilot Malaria Vaccination Programs in Ghana, Kenya, and Malawi through a Complexity Lens: A Rapid Review Using a Consolidated Framework for Implementation Research. Vaccines 2024, 12, 111. https://doi.org/10.3390/vaccines12020111
Adamu AA, Jalo RI, Ndwandwe D, Wiysonge CS. Assessing the Implementation Determinants of Pilot Malaria Vaccination Programs in Ghana, Kenya, and Malawi through a Complexity Lens: A Rapid Review Using a Consolidated Framework for Implementation Research. Vaccines. 2024; 12(2):111. https://doi.org/10.3390/vaccines12020111
Chicago/Turabian StyleAdamu, Abdu A., Rabiu I. Jalo, Duduzile Ndwandwe, and Charles S. Wiysonge. 2024. "Assessing the Implementation Determinants of Pilot Malaria Vaccination Programs in Ghana, Kenya, and Malawi through a Complexity Lens: A Rapid Review Using a Consolidated Framework for Implementation Research" Vaccines 12, no. 2: 111. https://doi.org/10.3390/vaccines12020111
APA StyleAdamu, A. A., Jalo, R. I., Ndwandwe, D., & Wiysonge, C. S. (2024). Assessing the Implementation Determinants of Pilot Malaria Vaccination Programs in Ghana, Kenya, and Malawi through a Complexity Lens: A Rapid Review Using a Consolidated Framework for Implementation Research. Vaccines, 12(2), 111. https://doi.org/10.3390/vaccines12020111






