T Cell Responses to BA.2.86 and JN.1 SARS-CoV-2 Variants in Elderly Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Peptide Pools and Proteins
2.3. Ex Vivo Enzyme-Linked Immunospot Assay (ELISpot Assay)
2.4. Activation-Induced Marker (AIM) Assay
2.5. Antibody Measurement
2.6. Statistical Analysis
3. Results
3.1. Study Participants
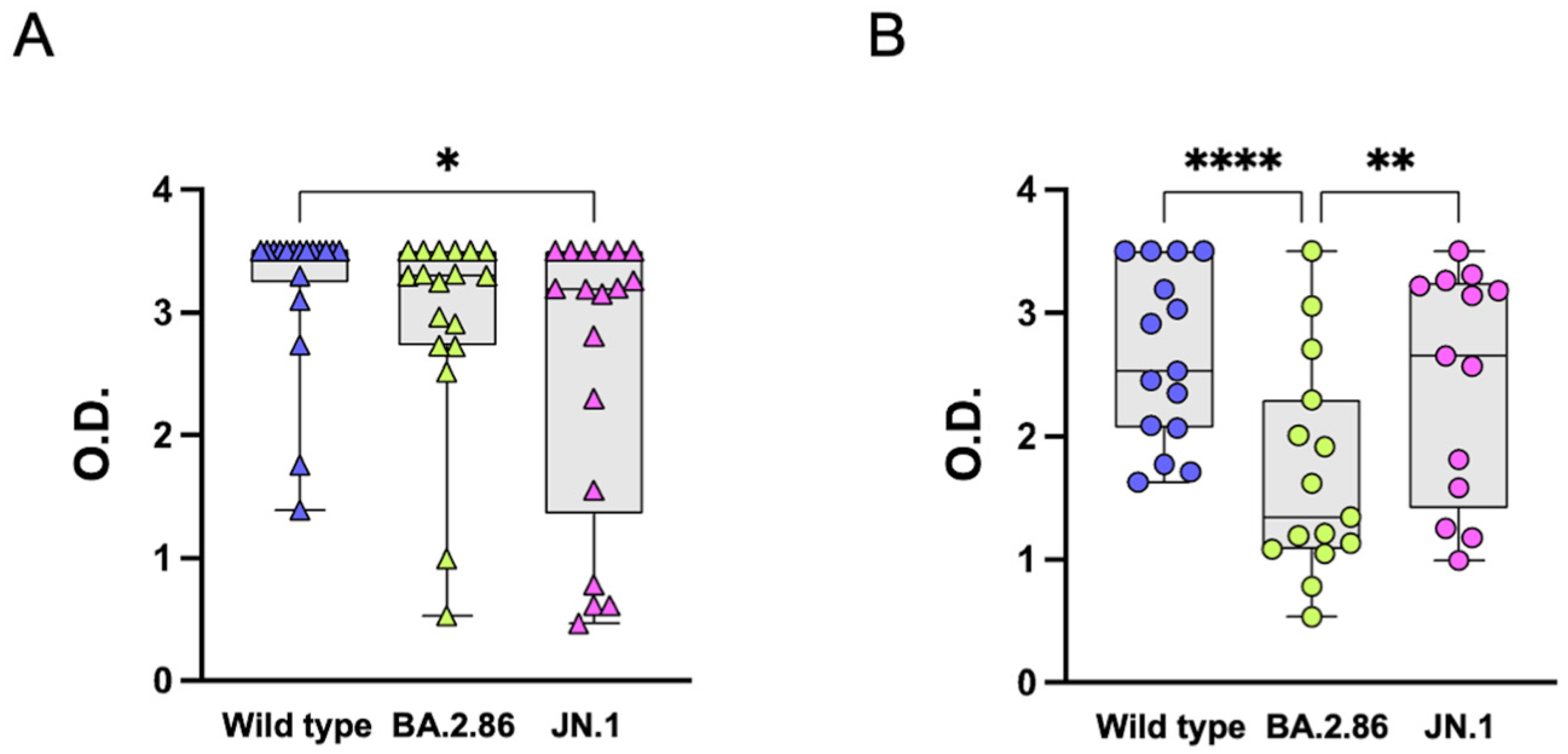
3.2. Anti-RBD IgG Antibodies
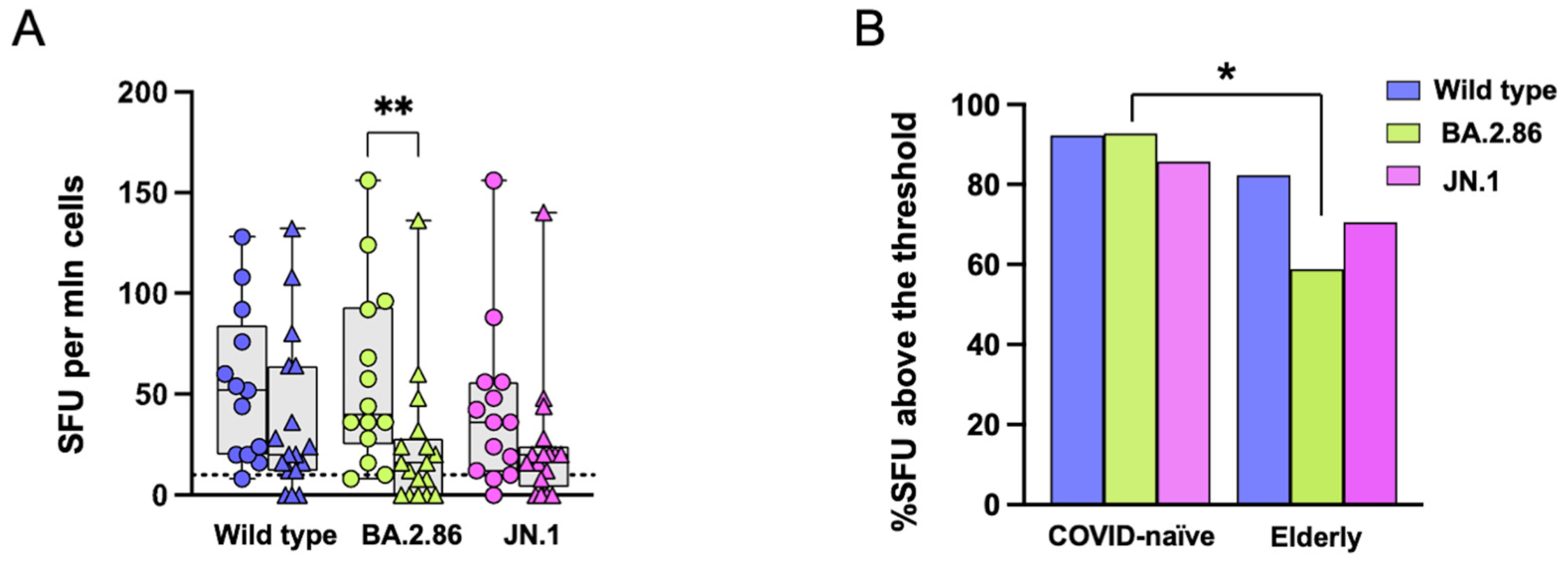
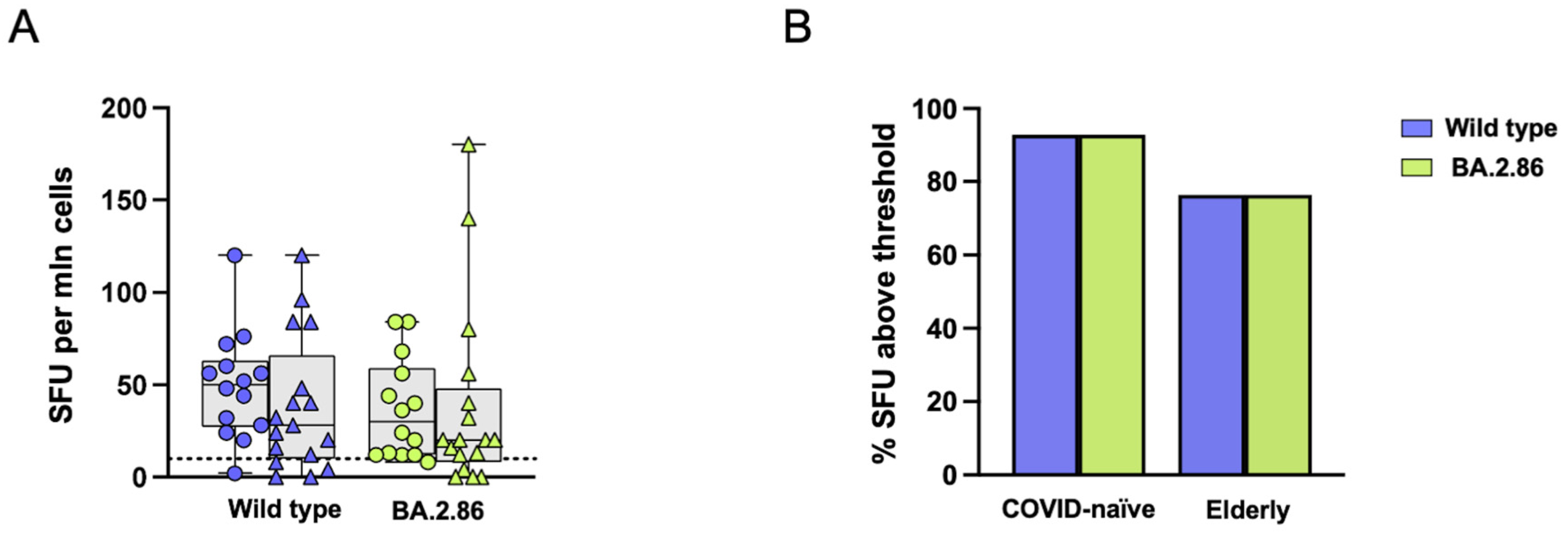
3.3. Reduced T Cell Responses to the BA.2.86 Variant in Elderly Subjects
3.4. CD4 T Cell Responses Against the JN.1 Protein Are Reduced in the COVID-19-Naïve Group Compared with Other Viral Proteins
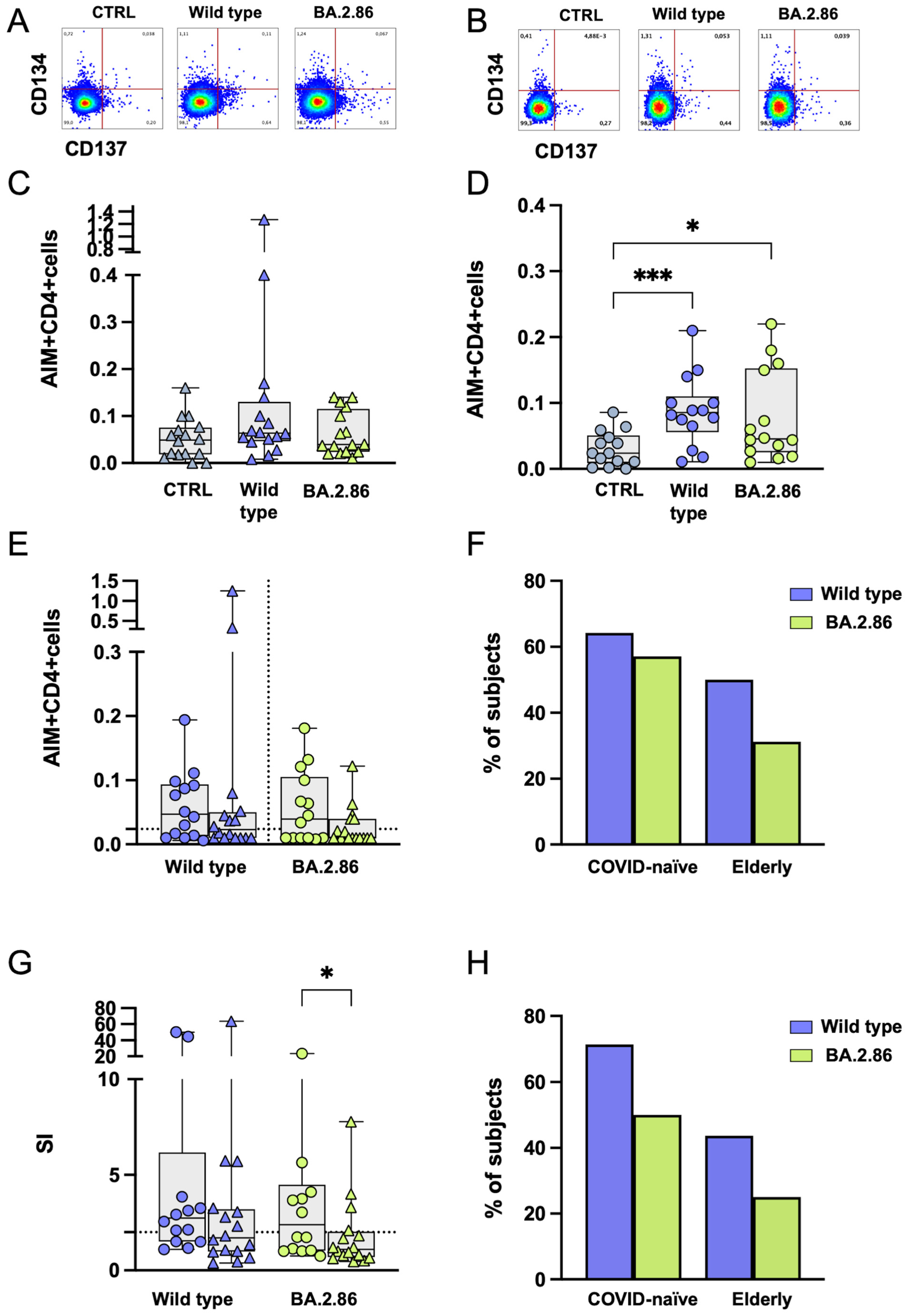
3.5. CD4+ T Cell Responses Against BA.2.86 Are Reduced in the Elderly Group
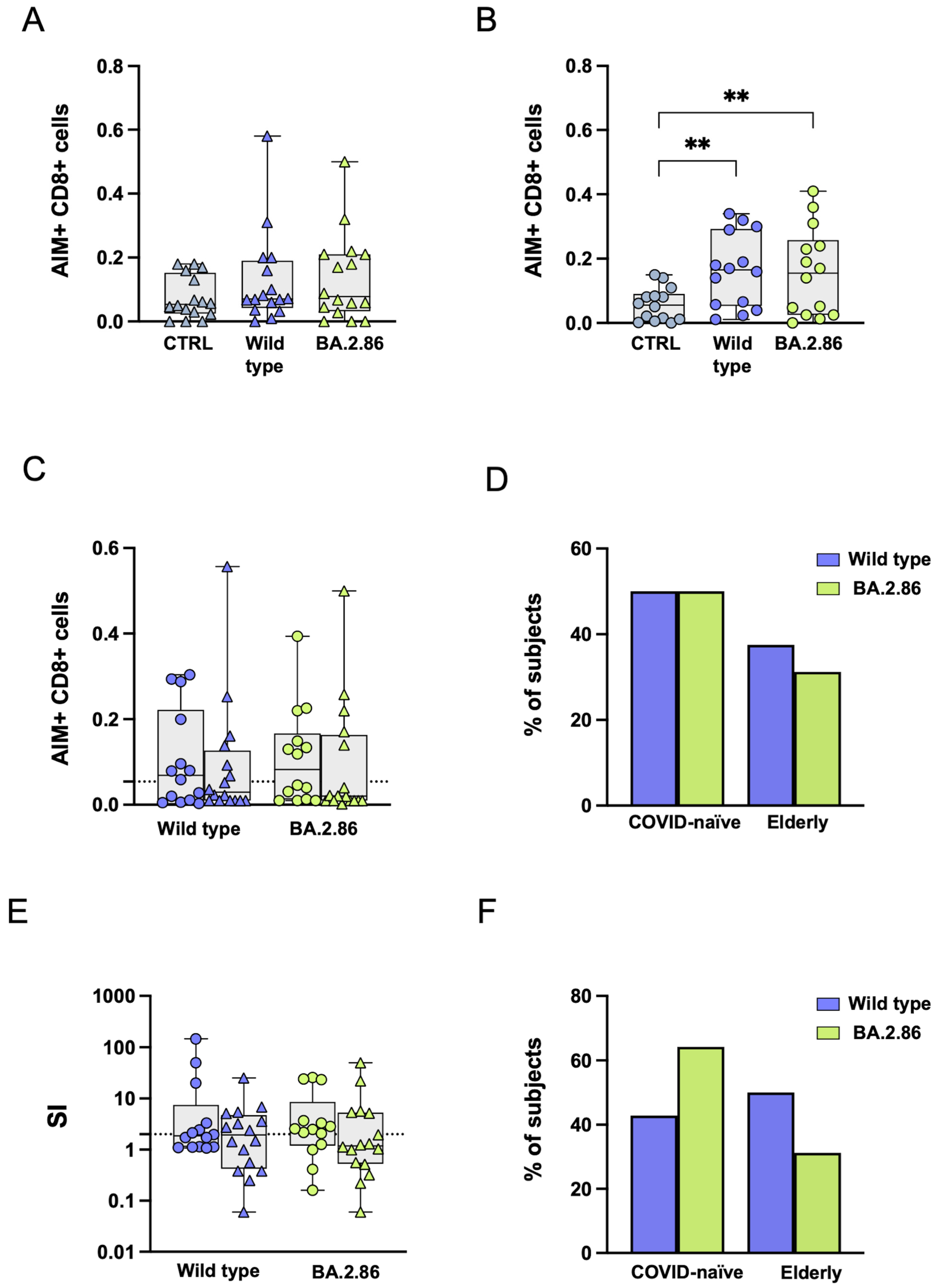
3.6. COVID-19 Vaccination Was Unable to Induce an Effective CD8+ T Cell Response Against the Ancestral Wild-Type or Current Variants in SARS-CoV-2-Unexposed and Elderly Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Favresse, J.; Gillot, C.; Closset, M.; Cabo, J.; Wauthier, L.; David, C.; Elsen, M.; Dogné, J.; Douxfils, J. Durability of humoral and cellular immunity six months after the BNT162b2 bivalent booster. J. Med. Virol. 2024, 96, e29365. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Lai, L.; Samaha, H.; Feng, Y.; Hu, M.; Hui, H.S.-Y.; Wali, B.; Ellis, M.; Davis-Gardner, M.E.; Huerta, C.; et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J. Clin. Investig. 2023, 133, e167955. [Google Scholar] [CrossRef]
- Chenchula, S.; Chandra, M.B.; Adusumilli, M.B.; Ghanta, S.N.; Bommasani, A.; Kuttiappan, A.; Padmavathi, R.; Amerneni, K.C.; Chikatipalli, R.; Ghanta, M.K.; et al. Immunogenicity, clinical efficacy and safety of additional second COVID-19 booster vaccines against Omicron and its subvariants: A systematic review. Rev. Med. Virol. 2024, 34, e2515. [Google Scholar] [CrossRef] [PubMed]
- Moustsen-Helms, I.R.; Bager, P.; Larsen, T.G.; Møller, F.T.; Vestergaard, L.S.; Rasmussen, M.; Hansen, C.H.; Christiansen, L.E.; Gubbels, S.; Trebbien, R.; et al. Relative vaccine protection, disease severity, and symptoms associated with the SARS-CoV-2 omicron subvariant BA.2.86 and descendant JN.1 in Denmark: A nationwide observational study. Lancet Infect. Dis. 2024, 24, 964–973. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Du, Y.; Xu, Y.; Paritala, S.; Donahue, M.; Maloney, P. Durability of XBB.1.5 Vaccines against Omicron Subvariants. N. Engl. J. Med. 2024, 390, 2124–2127. [Google Scholar] [CrossRef]
- Reuschl, A.-K.; Thorne, L.G.; Whelan, M.V.X.; Ragazzini, R.; Furnon, W.; Cowton, V.M.; De Lorenzo, G.; Mesner, D.; Turner, J.L.E.; Dowgier, G.; et al. Evolution of enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants. Nat. Microbiol. 2024, 9, 451–463. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Looi, M.K. Covid-19: Scientists sound alarm over new BA.2.86 “Pirola” variant. BMJ 2023, 382, 1964. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Ito, J.; Kosugi, Y.; Tanaka, Y.L.; Mugita, Y.; Guo, Z.; A Hinay, A.; Putri, O.; Kim, Y.; Shimizu, R.; et al. Transmissibility, infectivity, and immune evasion of the SARS-CoV-2 BA.2.86 variant. Lancet Infect. Dis. 2023, 23, e460–e461. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Xu, K.; Faraone, J.N.; Goodarzi, N.; Zheng, Y.-M.; Carlin, C.; Bednash, J.S.; Horowitz, J.C.; Mallampalli, R.K.; Saif, L.J.; et al. Immune evasion, infectivity, and fusogenicity of SARS-CoV-2 BA.2.86 and FLip variants. Cell 2024, 187, 585–595.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kempf, A.; Nehlmeier, I.; Cossmann, A.; Richter, A.; Bdeir, N.; Graichen, L.; Moldenhauer, A.-S.; Dopfer-Jablonka, A.; Stankov, M.V.; et al. SARS-CoV-2 BA.2.86 enters lung cells and evades neutralizing antibodies with high efficiency. Cell 2024, 187, 596–608.e17. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Y.; Xu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Wang, P.; Wang, J.; Liu, J.; Yu, L.; et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect. Dis. 2024, 24, e70–e72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Liu, L.; Schwanz, L.T.; Li, Z.; Nair, M.S.; Ho, J.; Zhang, R.M.; Iketani, S.; Yu, J.; et al. Antigenicity and receptor affinity of SARS-CoV-2 BA.2.86 spike. Nature 2023, 624, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; A Hinay, A.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Interim COVID-19 Vaccination Coverage in the EU/EEA During the 2023–24 Season Campaigns; ECDC: Stockholm, Sweden, 2024; ISBN 978-92-9498-720-4. [CrossRef]
- Gasperina, D.D.; Veronesi, G.; Castelletti, C.M.; Varchetta, S.; Ottolini, S.; Mele, D.; Ferrari, G.; Shaik, A.K.B.; Celesti, F.; Dentali, F.; et al. Humoral and Cellular Immune Response Elicited by the BNT162b2 COVID-19 Vaccine Booster in Elderly. Int. J. Mol. Sci. 2023, 24, 13728. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Rössler, A.; Rowe, M.; Collier, A.-R.Y.; Barouch, D.H. Neutralization of SARS-CoV-2 Omicron subvariant BA.2.87.1. Vaccine 2024, 42, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Bowen, A.; Mellis, I.A.; Valdez, R.; Gherasim, C.; Gordon, A.; Liu, L.; Ho, D.D. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe 2024, 32, 315–321.e3. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, S.; Ma, W.; Zhang, Y.; Wang, P. Robust neutralization of SARS-CoV-2 variants including JN.1 and BA.2.87.1 by trivalent XBB vaccine-induced antibodies. Signal Transduct. Target. Ther. 2024, 9, 123. [Google Scholar] [CrossRef]
- Müller, T.R.; Gao, Y.; Wu, J.; Ribeiro, O.; Chen, P.; Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; et al. Memory T cells effectively recognize the SARS-CoV-2 hypermutated BA.2.86 variant. Cell Host Microbe 2024, 32, 156–161.e3. [Google Scholar] [CrossRef]
- Nesamari, R.; Omondi, M.A.; Baguma, R.; Höft, M.A.; Ngomti, A.; Nkayi, A.A.; Besethi, A.S.; Magugu, S.F.; Mosala, P.; Walters, A.; et al. Post-pandemic memory T cell response to SARS-CoV-2 is durable, broadly targeted, and cross-reactive to the hypermutated BA.2.86 variant. Cell Host Microbe 2024, 32, 162–169.e3. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Lal, K.G.; A Balinsky, C.; Hontz, R.D.; Lin, J.; Beye, M.J.; Smith, L.; Pan, L.; Cheng, Y.; Fox, I.; et al. Informing the Need for a SARS-CoV-2 Booster Based upon the Immune Responses among Young Healthy Adults to Variants Circulating in Late 2023. J. Infect. Dis. 2024, 230, jiae249. [Google Scholar] [CrossRef] [PubMed]
- Vitallé, J.; Pérez-Gómez, A.; Ostos, F.J.; Gasca-Capote, C.; Jiménez-León, M.R.; Bachiller, S.; Rivas-Jeremías, I.; Silva-Sánchez, M.d.M.; Ruiz-Mateos, A.M.; Martín-Sánchez, M.; et al. Immune defects associated with lower SARS-CoV-2 BNT162b2 mRNA vaccine response in aged people. JCI Insight 2022, 7, e161045. [Google Scholar] [CrossRef] [PubMed]
- Gallerani, E.; Proietto, D.; Dallan, B.; Campagnaro, M.; Pacifico, S.; Albanese, V.; Marzola, E.; Marconi, P.; Caputo, A.; Appay, V.; et al. Impaired Priming of SARS-CoV-2-Specific Naive CD8+ T Cells in Older Subjects. Front. Immunol. 2021, 12, 693054. [Google Scholar] [CrossRef] [PubMed]
- Grubeck-Loebenstein, B.; Della Bella, S.; Iorio, A.M.; Michel, J.-P.; Pawelec, G.; Solana, R. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 2009, 21, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.C.; Toapanta, F.R.; Chen, W.; Tennant, S.M. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 2020, 38, 8264–8272. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Gelderloos, A.T.; Verheul, M.K.; Middelhof, I.; de Zeeuw-Brouwer, M.-L.; van Binnendijk, R.S.; Buisman, A.-M.; van Kasteren, P.B. Repeated COVID-19 mRNA vaccination results in IgG4 class switching and decreased NK cell activation by S1-specific antibodies in older adults. Immun. Ageing 2024, 21, 63. [Google Scholar] [CrossRef]
- Sohail, M.S.; Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Cross-Reactivity Assessment of Vaccine-Derived SARS-CoV-2 T Cell Responses against BA.2.86 and JN.1. Viruses 2024, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Piancone, F.; Marventano, I.; Hernis, A.; Trabattoni, D.; Invernizzi, M.; La Rosa, F.; Clerici, M. Innate immune responses to three doses of the BNT162b2 mRNA SARS-CoV-2 vaccine. Front. Immunol. 2022, 13, 947320. [Google Scholar] [CrossRef] [PubMed]
- Mele, D.; Ottolini, S.; Lombardi, A.; Conteianni, D.; Bandera, A.; Oliviero, B.; Mantovani, S.; Cassaniti, I.; Baldanti, F.; Gori, A.; et al. Long-term dynamics of natural killer cells in response to SARS-CoV-2 vaccination: Persistently enhanced activity postvaccination. J. Med. Virol. 2024, 96, e29585. [Google Scholar] [CrossRef] [PubMed]
- Jo, N.; Hidaka, Y.; Kikuchi, O.; Fukahori, M.; Sawada, T.; Aoki, M.; Yamamoto, M.; Nagao, M.; Morita, S.; Nakajima, T.E.; et al. Impaired CD4+ T cell response in older adults is associated with reduced immunogenicity and reactogenicity of mRNA COVID-19 vaccination. Nat. Aging 2023, 3, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Tan, H.-X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Mei, X.; Zhou, Y.; Tang, Z.; Li, G.; Zhong, J.; Yu, M.; Huang, M.; Su, X.; et al. SARS-CoV-2-specific CD4+ T cells are associated with long-term persistence of neutralizing antibodies. Signal Transduct. Target. Ther. 2022, 7, 132. [Google Scholar] [CrossRef]
- Liu, Z.; Long, W.; Tu, M.; Chen, S.; Huang, Y.; Wang, S.; Zhou, W.; Chen, D.; Zhou, L.; Wang, M.; et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef] [PubMed]


| Variable | Elderly (n = 18) | COVID-19-Naïve (n = 15) |
|---|---|---|
| Age, years | 81.5 ± 5.5 | 40.8 ± 13.3 |
| Sex, Male | 12 (66.6%) | 8 (53.3%) |
| Number of comorbidities | 1.6 ± 0.7 | None |
| Principal cause of hospitalization | ||
| Trauma | 8 (44.5%) | |
| Heart failure | 4 (22.2%) | |
| Stroke | 3 (16.7% | |
| Intestinal Obstruction | 2 (11.1%) | |
| COPD without infection | 1 (5.5%) | |
| Comorbidities | None | |
| CVD | 17 (94.4%) | |
| Diabetes | 4 (22.2%) | |
| COPD | 3 (16.6%) | |
| Chronic renal failure | 3 (16.6%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segato, I.; Mele, D.; Forlani, G.; Dalla Gasperina, D.; Mondelli, M.U.; Varchetta, S. T Cell Responses to BA.2.86 and JN.1 SARS-CoV-2 Variants in Elderly Subjects. Vaccines 2024, 12, 1451. https://doi.org/10.3390/vaccines12121451
Segato I, Mele D, Forlani G, Dalla Gasperina D, Mondelli MU, Varchetta S. T Cell Responses to BA.2.86 and JN.1 SARS-CoV-2 Variants in Elderly Subjects. Vaccines. 2024; 12(12):1451. https://doi.org/10.3390/vaccines12121451
Chicago/Turabian StyleSegato, Irene, Dalila Mele, Greta Forlani, Daniela Dalla Gasperina, Mario U. Mondelli, and Stefania Varchetta. 2024. "T Cell Responses to BA.2.86 and JN.1 SARS-CoV-2 Variants in Elderly Subjects" Vaccines 12, no. 12: 1451. https://doi.org/10.3390/vaccines12121451
APA StyleSegato, I., Mele, D., Forlani, G., Dalla Gasperina, D., Mondelli, M. U., & Varchetta, S. (2024). T Cell Responses to BA.2.86 and JN.1 SARS-CoV-2 Variants in Elderly Subjects. Vaccines, 12(12), 1451. https://doi.org/10.3390/vaccines12121451







